Abstract
Probiotics are the class of beneficial microorganisms that have positive influence on the health when ingested in adequate amounts. Probiotic fermented milk is one of the dairy products that is prepared by using probiotic lactic acid bacteria. The study comprised preparation of fermented milk from various sources such as cow, goat and camel. Pediococcus pentosaceus which is a native laboratory isolate from cheese was utilized for the product formation. Changes in functional properties in the fermented milks obtained from three different species were evaluated. Antioxidant activity determined by DPPH assay showed activity in probiotic fermented milk obtained from all the products being highest in goat milk (93 %) followed by product from camel milk (86 %) and then product from cow milk (79 %). The composition of beneficial fatty acids such as stearic acid, oleic acid and linoleic acid were higher in fermented milk than the unfermented ones. Results suggested that probiotic bacteria are able to utilize the nutrients in goat and camel milk more efficiently compared to cow milk. Increase in antioxidant activity and fatty acid profile of fermented milks enhances the therapeutic value of the products.
Keywords: Probiotics, Fermented milk, Antioxidant, Fatty acid
Introduction
Probiotics as defined by World Health Organization (WHO) are the “live microorganisms which when ingested in adequate amounts confer health benefits on the host”. One of the potential benefits of ingesting probiotics is their antagonistic effect against harmful microorganisms (Midolo et al. 1995). There are innumerable articles that list the effects of probiotics in decreasing gastrointestinal disorder, intestinal bowel syndrome, diarrhea, lactose intolerance etc. Due to the array of health benefits offered by these microorganisms, there is an ever-growing increase in the production of foods fermented using probiotic species. Most widely used microorganisms in probiotic food preparation include Lactobacillus bulagaricus, L.plantarum, Streptococcus thermophilus, Bifidobacterium sp., Pediococcus sp. (Smith 1991).
Pediococci are gram positive cocci, salt tolerant, non-motile, homofermentative bacteria that belong to the family Lactobacillacea. This organism is used as an acid producing starter culture in sausage fermentations, cucumber and green bean fermentations, soy milk fermentations and silage (Wood and Holzapfel. 1995). Pediococcus pentosaceus play an important role in food biopreservation as they produce distinct pediocins which are active against pathogenic microbes including Listeria monocytogenes, Staphylococcus aureus, Clostridium perfringens and Clostridium botulinum. They have the ability to ferment different types of carbohydrates such as glucose, galactose, maltose and lactose. Fermentation of lactose by Pediococcus sp. is strain specific. The strains that have potential to ferment lactose are P.acidilactici, P.pentosaceus. (Raccach 1987; Agrawal et al. 2000). Pediococcus sp. are also capable of metabolizing citrate and malate into acetoin and diacetyl which help in enriching the flavors of cheese, butter and other dairy products (Papagianni and Anastasiadou 2009). These properties of Pediococcus sp. pave the way for their use in dairy fermentations.
Fermented milk is one of the dairy products obtained by the use of appropriate probiotic bacteria which results in lowering of pH with or without coagulation of milk (Abdelbasset and Djamila 2008). The beneficial activity of probiotic bacteria in milk can vary with milk source and composition. The study deals with the comparative differences in the antioxidant activity and fatty acid profile in fermented milk prepared with P. pentosaceous from camel, goat and cow milk.
Materials and methods
Mineral estimation of milk
The total mineral content of milk was determined by atomic absorption spectrometry according to the method described by Jacobs (1951) and modified by Miller-Ihli (1996). The milk samples (50 ml) were dried at 100 °C in a crucible for 6 h. After charring, samples were incinerated in a muffle furnace at 460 °C for 24 h. The ash obtained was dissolved in concentrated H2SO4 (2 ml) and warmed for 5 min at 40 °C in a water bath. The mixture was then made up to 30 ml with double-distilled water and analyzed by atomic absorption spectrometer.
Preparation of fermented milks
To prepare the fermented milk, raw cow, goat and camel milk was pasteurized at 71 °C for 15 min and cooled to room temperature. All different milks were inoculated with P.pentosaceus, native laboratory isolate from cheese (initial concentration of 1 × 1011 cfu/ml; 1 % inoculum) and incubated at 37 °C for 24 h. Unfermented milk was the pasteurized milk without the addition of culture. After incubation period, both unfermented and fermented milk samples were evaluated for pH, antioxidant activity and fatty acid profile.
The pH of the milk sources was measured using digital pH meter (GENEI Control Dynamic pH meter, model: APX 175). Both the initial pH and pH of the fermented milks after 24 h were determined. All the experiments were carried out in triplicates.
Antioxidant activity
The effect of fermentation upon free radicals was estimated by 1, 1 Diphenyl 2 picrylhydrazyl (DPPH) assay. The method offers advantages of being rapid, simple and inexpensive and provides first-hand information on the overall antioxidant capacity (Kedare and Singh 2011). The scavenging of DPPH by the culture was according to the method of Brand-Williams et al. (1995), which was modified by Pyo et al. (2005). After an incubation period of 24 h, the fermented and unfermented milk samples were macerated with methanol separately and centrifuged at 8000 rpm for 20 mins at 4 °C. A stock solution of DPPH was prepared by dissolving in methanol (0.1 mM in methanol). DPPH (1 ml) and methanol (1.5 ml) were added to the supernatant (0.5 ml) obtained after centrifugation. Control was prepared by adding DPPH (1.5 ml) to methanol (1.5 ml). The antioxidant activity was analyzed for both unfermented and fermented milks after 30 mins by reading the absorbance at 517 nm. Blank sample contained deionized water. Per cent scavenging activity was calculated using the following equation. The experiments were conducted in triplicate and the mean values were used.
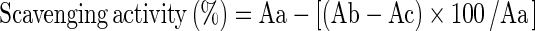 |
- Aa
Absorbance of DPPH solution without sample
- Ab
Absorbance of mixture containing sample and DPPH
- Ac
Absorbance of blank solution without DPPH
Fatty acid profile
In order to determine the fatty acid profile of milk, samples were derivatized by alcoholysis procedure based on methanolic potassium hydroxide. This was according to the method of Bligh and Dyer (1959). The sample (5 ml), was dissolved in non-polar solvent such as hexane. To this methanolic KOH (2 N; 0.1 ml) was added and heated in a water bath at 45 °C for 1 h. After esterification reaction, sample was cooled and hexane (1 ml) was added and mixed thoroughly to extract the fatty acid esters. Upper hexane layer containing the fatty acid methyl ester (FAME) was dried with sodium sulfate. For FAME analysis, the dried residues were then dissolved in 0.1 ml of hexane and analyzed by GC (Shimadzu model 14B, Shimadzu, Kyoto, Japan) using BP1 packed column with following operating conditions: injection temperature 220 °C and detector temperature 250 °C. The temperature was programmed from 100 to 220 °C. The temperature rise was 10 °C/min and held at 220 °Cfor 10 min. Carrier gas N2 was used at a flow rate of 1.5 ml/min. Injection volume was 1 μl. The identification of peaks was based on the comparison with the standards and fragmentation pattern of GCMS chromatograms with GCMS library data base and also GCMS volumes by Noever et al. (1988). A FAME mix of known standard compounds (Sigma-Aldrich, USA) was analyzed under the same GC operating conditions as those of the samples for peak identification.
Sensory analysis
Sensory analysis of the fermented milks was carried out in 5 point hedonic scale with 1, dislike very much, 2, dislike moderately, 3 neither like nor dislike, 4, like moderately and 5, like very much for overall acceptability. Twenty untrained members rated the sensory attributes in the hedonic scale.
Statistical analysis
The measurements for pH, antioxidant activity and fatty acids were conducted in triplicates. Results were subjected to Analysis of Variance (ANOVA) and Tukey’s HSD (honestly significant difference) procedure to determine significant differences (P < 0.05) among treatments (unfermented and fermented) and also among milk sources (cow, goat and camel).
Results and discussion
Ash (%), which represents minerals, was 0.7 ± 0.15, 0.8 ± 0.30, 0.8 ± 0.10 in cow, goat and camel milk respectively. Minerals in milk may influence growth of probiotic bacteria, since it is part of enzymatic complexes of lactose fermentation (Slacanac et al. 2007). Initial amount of P.pentosaceus that was used for fermentation of the milk sources was 1011 cfu/ml in all the cases. The minimum number of probiotic bacteria for preparation of fermented food is 106 cfu/ml and minimum therapeutic dose per day is 108–109 cells (Kailasapathy and Chin 2000). In this study, the concentration of P.pentosaceus used for fermentation is more than the minimum number of viable cells taken as 1 × 1011 cfu/ml to 3 × 1011 cfu/ml required for obtaining beneficial effect. The pH variability of fermented milks prepared using different milk sources in Table 1 shows that pH declined by 1.5 units after 24 h of fermentation. The values confirm the growth of P.pentosaceus and production of lactic acid which led to reduction in pH. In all the products, the viable count increased by one log after 24 h. Based on statistical analysis, there was no significant difference in the pH of different milk sources (cow, goat and camel), though there was a difference among treatments (fermented and unfermented).
Table 1.
Antioxidant activity and pH of cow, goat and camel milk before and after 24 h of fermentation with Pediococcus pentosaceus (n = 3)
| Fermentation | Cow milk | Goat milk | Camel milk | |||
|---|---|---|---|---|---|---|
| pH | Antioxidant activity (%) | pH | Antioxidant activity (%) | pH | Antioxidant activity (%) | |
| Before | 6.5 ± 0.01I | 38.7 ± 1.53f | 6.5 ± 0.01I | 54.0 ± 1.02e | 6.5 ± 0.01I | 64.4 ± 01.42d |
| After | 5.0 ± 0.02II | 78.0 ± 0.74c | 5.0 ± 0.01II | 92.7 ± 1.53a | 5.0 ± 0.01II | 84.3 ± 2.09b |
All values are expressed as mean ± standard deviation (n = 3). Mean values with in the column with different letters are significantly different according to Tukey’s HSD at p > 0.05
Antioxidant activity
Antioxidant activity was measured by ability of the P.pentocaseus in fermented milk to scavenge the free radicals. Table 1 shows the radical scavenging activity exhibited by unfermented and fermented milks after 24 h. The fermented milks demonstrated higher activity compared to the unfermented ones. Based on ANOVA results, fermented cow, goat and camel milk had significantly higher antioxidant activity than their respective unfermented ones. Nishino et al. (2000) reported that increased radical scavenging activity was due to the protein peptides present in the fermented milks. Radical scavenging activities of fermented milks suggest that they could be used as natural antioxidant supplement for improving human health.
Composition of fatty acids in fermented milks
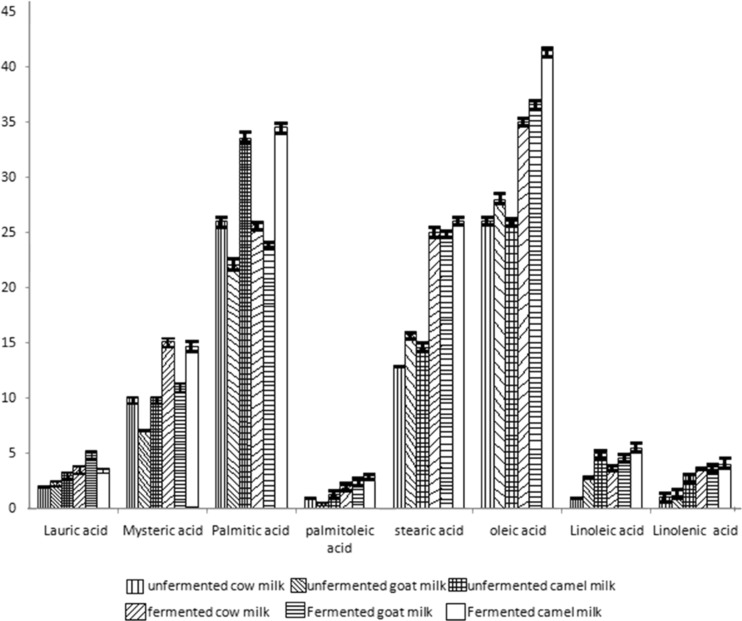
Fatty acid composition was studied and the yield (%) was calculated by comparing with the standard fatty acid compounds. Yield (%) of fatty acids in both unfermented and fermented milks is shown in Figure 1. Yield (%) refers to percent composition of each fatty acid component in milk (after estimating with the area of the peak in chromatogram and the standard area). Though the yield of fermented milk was slightly higher than their unfermented counterpart in both unsaturated and saturated fatty acids, there was no statistical significant difference between the treatments (p > 0.05). The major fatty acids formed were myristic acid (C14:0), palmitic in acid (C16:0), stearic acid (C18:0) and oleic acid (C18:1). However, lauric (C12:0), linoleic (C18:2) and linolenic (C18:3) were present in minor amounts. Lauric acid was three folds in fermented goat milk and more than one and a half times in camel milk as compared to the cow milk. Lauric acid is known to have antibacterial activity and antiviral activities (Shobharani and Agrawal 2009). Stearic acid was 1.89, 1.58 and 1.82 folds higher in fermented cow, goat and camel milk, respectively compared to their corresponding unfermented milk. It has been reported that saturated stearic acid does not increase the serum cholesterol level and is not atherogenic (Ebringer et al. 2008). Oleic acid showed highest yield among all the fatty acids that was found in fermented milks. Yield of oleic acid in fermented camel milk was 1.15 and 1.08 times higher compared to fermented cow and goat milks. Monounsaturated oleic acid lowers plasma cholesterol, LDL (low density lipoprotein) cholesterol and triacylglycerols and prevents coronary heart disease (Mensink et al. 2003). Though linoleic and linolenic acids were found in minor amounts, presence of these acids reduces cholesterol, protects against ischemic stroke and exerts positive effects in reducing heart disease (Djoussé et al. 2001; Zock and Katan 1998). Yield of palmitic acid and myristic acid was higher in fermented milks (Fig. 1). These two fatty acids are associated with raising total cholesterol levels in plasma, but their individual effects are variable-both toward raising low-density lipoproteins and raising the level of beneficial high-density lipoproteins (Ebringer et al. 2008). Increase in HDL (high density lipoprotein) cholesterol helps to fight against heart disease and stroke (Tall 2007). Therefore, presence of these fatty acids improves the therapeutic value of fermented milks.
Fig. 1.
Yield of fatty acids of both unfermented and fermented cow, goat and camel milk after an incubation time of 24 h. (Mean ± S.D; No significant difference between treatments (fermented and unfermented) and between milk sources p > 0.05 (n = 3))
Apart from the nutritional benefits, fatty acids are also precursors of aldehydes, ketones and esters—the volatile compounds that are responsible for aroma in fermented dairy products (De la Fuente et al. 1993).
Sensory analysis
The mean score for overall acceptability of fermented cow, goat and camel milk in a 5 point hedonic scale was 4.1 ± 0.91, 4.0 ± 0.79 and 4.3 ± 0.86 respectively. Two-way ANOVA was performed to determine significant difference in overall acceptability of fermented milks. All the three fermented milks were accepted by the panelists to the same extent (p > 0.05). Since the average values on overall acceptability for the milks were above 4.0 in a 5 point scale for all three milks, it can be concluded that all the milks had excellent sensory properties.
Conclusion
This study determined the functional properties of P.pentosaceus in different sources of fermented milk. Higher antioxidant activity and fatty acid profile of fermented goat and camel milks suggest that nutrients present in the milk are utilized more efficiently by the probiotic bacteria than from cow’s milk. Maximum antioxidant activity was observed in fermented goat’s milk. Stearic and oleic acid were formed in significant amounts in all the fermented milks, though it was comparatively higher in goat and camel milk.
References
- Abdelbasset M, Djamila K. Antimicrobial activity of autochthonous lactic acid bacteria isolated from Algerian traditional fermented milk “Raïb”. Afr J Biotechnol. 2008;7:2908–2914. [Google Scholar]
- Agrawal R, RatiRao E, Vijayendra SVN, Varadaraj MC, Prasad MS, Nand K. Flavour profile of idli batter prepared from defined microbial starter cultures. World J Microbiol Biotechnol. 2000;16:687–690. doi: 10.1023/A:1008939807778. [DOI] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Brand-Williams W, Cuvelier M, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- De la Fuente MA, Fontecha J, Juárez M. Fatty acid composition of the triglyceride and free fatty acid fractions in different cows-, ewes-and goats-milk cheeses. Z Lebensm Unters Forsch A. 1993;196:155–158. doi: 10.1007/BF01185577. [DOI] [Google Scholar]
- Djoussé L, Pankow JS, Eckfeldt JH, Folsom AR, Hopkins PN, Province MA, Hong Y, Ellison RC. Relation between dietary linolenic acid and coronary artery disease in the National Heart, Lung, and Blood Institute Family Heart Study. Am J Clin Nutr. 2001;74:612–619. doi: 10.1093/ajcn/74.5.612. [DOI] [PubMed] [Google Scholar]
- Ebringer L, Ferenčik M, Krajčovič J. Beneficial health effects of milk and fermented dairy products. Review. Folia Microbiol (Praha) 2008;53:378–394. doi: 10.1007/s12223-008-0059-1. [DOI] [PubMed] [Google Scholar]
- Jacobs MB. The chemical analysis of foods and food products. 3. Princeton: D. Van Nastrand; 1951. [Google Scholar]
- Kailasapathy K, Chin J. Survival and therapeutic potential of probiotic organisms with reference to Lactobacillus acidophilus and Bifidobacterium spp. Immunol Cell Biol. 2000;78:80–88. doi: 10.1046/j.1440-1711.2000.00886.x. [DOI] [PubMed] [Google Scholar]
- Kedare SB, Singh R. Genesis and development of DPPH method of antioxidant assay. J Food Sci Technol. 2011;48:412–422. doi: 10.1007/s13197-011-0251-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mensink RP, Zock PL, Kester ADM, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr. 2003;77:1146–1155. doi: 10.1093/ajcn/77.5.1146. [DOI] [PubMed] [Google Scholar]
- Midolo P, Lambert J, Hull R, Luo F, Grayson M. In vitro inhibition of Helicobacter pylori NCTC 11637 by organic acids and lactic acid bacteria. J Appl Microbiol. 1995;79:475–479. doi: 10.1111/j.1365-2672.1995.tb03164.x. [DOI] [PubMed] [Google Scholar]
- Miller-Ihli N. Trace element determinations in foods and biological samples using inductively coupled plasma atomic emission spectrometry and flame atomic absorption spectrometry. J Agric Food Chem. 1996;44:2675–2679. doi: 10.1021/jf950616l. [DOI] [Google Scholar]
- Nishino T, Shibahara-Sone H, Kikuchi-Hayakawa H, Ishikawa F. Transit of radical scavenging activity of milk products prepared by maillardreaction and Lactobacillus casei Strain Shirotafermentation through the hamster intestine. J Dairy Sci. 2000;83:915–922. doi: 10.3168/jds.S0022-0302(00)74954-X. [DOI] [PubMed] [Google Scholar]
- Noever D, Bouman J, Gramberg L, Lavos G (1988) Compilation of mass spectra of volatile compounds in food: TNO Institute CIVO-Food Analysis, vol 1:18. Zeist, The Netherlands
- Papagianni M, Anastasiadou S. Pediocins: the bacteriocins of pediococci. Sources, production, properties and applications. Microbial Cell Factories. 2009;8:1–16. doi: 10.1186/1475-2859-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyo YH, Lee TC, Lee YC. Effect of lactic acid fermentation on enrichment of antioxidant properties and bioactive isoflavones in soybean. J Food Sci. 2005;70:S215–S220. doi: 10.1111/j.1365-2621.2005.tb07160.x. [DOI] [Google Scholar]
- Raccach M. Pediococci and biotechnology. Crit Rev Microbiol. 1987;14:291–309. doi: 10.3109/10408418709104442. [DOI] [PubMed] [Google Scholar]
- Shobharani P, Agrawal R. Supplementation of adjuvants for increasing the nutritive value and cell viability of probiotic fermented milk beverage. Int J Food Sci Nutr. 2009;60:70–83. doi: 10.1080/09637480802668463. [DOI] [PubMed] [Google Scholar]
- Slacanac V, Hardi J, Curzik D, Pavlovic H, Lucan M, Vlainic M. Inhibition of the in vitro growth of Salmonella enteritidis D by goat and cow milk fermented with probiotic bacteria Bifidobacterium longum Bb-46. Czech J Food Sci. 2007;25:351–358. [Google Scholar]
- Smith J. Probiotics—fact or fiction? J Chem Technol Biotechnol. 1991;51:539–540. doi: 10.1002/jctb.280510411. [DOI] [Google Scholar]
- Tall AR. CETP inhibitors to increase HDL cholesterol levels. N Engl J Med. 2007;356:1364–1366. doi: 10.1056/NEJMe078029. [DOI] [PubMed] [Google Scholar]
- Wood BJB, Holzapfel W. Thelactic acid bacteria: the genera of lactic acid bacteria. Glasgow: Springer; 1995. [Google Scholar]
- Zock PL, Katan MB. Linoleic acid intake and cancer risk: a review and meta-analysis. Am J Clin Nutr. 1998;68:142–153. doi: 10.1093/ajcn/68.1.142. [DOI] [PubMed] [Google Scholar]