Abstract
Casein and whey protein concentrate (WPC) films, plasticized with glycerol and sorbitol independently, were prepared by casting. The film thickness, water vapour and oxygen permeation and tensile and moisture sorption properties of the films were determined. The tensile strength (TS), tensile strain (TE) and elastic modulus (EM) of the films ranged from 0.71 to 4.58 MPa, 19.22 to 66.63 % and 2.05 to 6.93 MPa, respectively. The film properties were influenced by the type of biopolymer (casein and whey protein concentrate), plasticizer and its concentration. Increasing the plasticizer concentration, increased the film thickness, TE and water vapour permeability (WVP), but decreased the TS and EM. As the concentration of plasticizer increased to the highest level, the film thickness increased from 0.168 to 0.305 mm for glycerol-plasticized films and from 0.251 to 0.326 mm for sorbitol-plasticized films. The film thickness increased because the amount of plasticizer in the film network increased and the amount of biopolymer remained same. Casein films showed superior tensile properties as compared to WPC films. The WVP of both casein and WPC films lied between 3.87 and 13.97 g.mm./(m2.h.kPa). The moisture sorption isotherms of both films were typical of high-protein material, and were adequately described by the GAB model. The oxygen permeability of casein films was relatively lower than that of WPC films, regardless of the plasticizer used. The sensory data revealed that the organoleptic quality of Cheddar cheese was unaffected by milk-protein film packaging.
Keywords: Casein, Cheddar cheese, Edible packaging, Film properties, Whey-protein concentrate
Introduction
Packaging is the technique of using the most appropriate containers and components to protect as well as preserve the contained food from the manufacturing to its consumption. To achieve these objectives, synthetic polymers are mostly preferred because of their superior tensile strength, tensile strain and water vapour barrier properties. However, these plastics are non-biodegradable, and hence, they cause ecological pollution and toxicity. It is virtually impossible to collect the discarded plastics and recycle them. Therefore, there is a great need to develop environment-friendly packaging materials that are biodegradable. “An edible film or coating is simply defined as a thin continuous layer of edible material formed and/or placed on or between foods or food components” (Krochta and Mulder-Johnston 1997). Edible films or coatings improve the quality of food by limiting the migration of moisture, lipids, flavour and colour between food components (Krochta 1992).
Amongst the biopolymers, proteins possess excellent film-forming properties that make them useful in the development of edible films on a commercial scale. However, Maynes and Krochta (1994) reported that producing the films from total milk protein was difficult due to the presence of lactose together with milk protein, which crystallized during film drying to result in a non-homogeneous film that also adhered to the casting surfaces. Therefore, in most cases, milk protein films were prepared by using casein or whey protein individually. Casein and whey proteins thus have gained importance because they could form flavourless, flexible, and transparent films. In addition, milk proteins also possess excellent nutritional value. These films also may serve as carrier of additives such as antioxidants, antimicrobial agents, and colourants, thereby enhancing the organoleptic properties of packaged food products.
The formation of milk protein films involve heat denaturation in aqueous solution at 75–100 °C, which produce intermolecular disulfide bonds that might be partly responsible for film structure. The distribution of charged, polar and non-polar aminoacids along the protein chain creates chemical potential, and the resulting interactive forces produce a cohesive protein film matrix (Dangaran et al. 2009). Thus, proteins have multiple sites for chemical interaction as a function of their diverse aminoacid functional groups, which could allow for superior film properties.
Maynes and Krochta (1994) reported that transparent and functionally desirable edible films and coatings could be formed from components of milk protein. McHugh and Krochta (1994a) and Fairley et al. (1996) developed transparent whey protein films with good oxygen, aroma and barrier properties. Pérez-Gago et al. (1999) reported that native whey protein isolate (WPI) generally produced totally water-soluble films while heat-denatured solutions of WPI produce films in which the protein was insoluble. The authors also hypothesized that covalent cross-linking due to heat denaturation of whey protein was accountable for film water insolubility and higher tensile properties but it did not affect water vapour permeability (WVP) of the films. This was because the heat treatment generated cross-links in whey by disrupting the protein structure and exposing sulfhydryl and hydrophobic groups. Similarly, an extensive review of the properties of milk protein films cast from aqueous solutions of casein and caseinates and non-fat dry milk was presented by McHugh and Krochta (1994b).
Cheddar cheese, one of the important and popular products of the dairy industry, is a complex food product consisting mainly of casein, fat and water. In ripened cheese, spoilage can be induced by microorganisms such as yeast, moulds and anaerobic spore-forming bacteria which can oxidize lactate or utilize protein decomposition products. Application of edible film packaging may solve this problem of spoilage to some extent and extend the shelf-life of cheese. However, systematic scientific evaluations of biopackaging of dairy products, including Indian and Western dairy products, and covering both packaging material and food quality aspects, are scarce. Further limited are the studies on biopackaging of dairy products with edible milk protein films, and analysis of the quality changes in the food. There are a few reports that investigated the application of edible packaging for dairy products such as hydrocolloid coating of cheese (Kampf and Nussinovitch 2000; Holm et al. 2006) and other dairy products such as yoghurt (Frederiksen et al. 2003) with starch and non-edible but biodegradable polymers. Therefore, this work was done with the objectives of (a) development and characterization of edible films based on casein and whey proteins and (b) application and evaluation of these films for packaging of Cheddar cheese.
Materials and methods
Film preparation
Films were made from casein (HiMedia Laboratories Pvt. Ltd., Mumbai) and 80 % whey protein concentrate (WPC) (Nakoda Dairy Pvt. Ltd., Bangalore). Glycerol and sorbitol (LOBA Chemie Pvt. Ltd., Mumbai) were used independently as plasticizers. The biopolymer:plasticizer ratios were 1:0.25, 1:0.5 and 1:0.75 for glycerol and 1:1.0, 1:1.25 and 1:1.5 for sorbitol. The levels of glycerol and sorbitol were selected based on preliminary trials to obtain brittle-free films. The method developed by McHugh et al. (1994) for WPI films was followed for film preparation, though with minor modifications. Exactly 10 g of casein or WPC was weighed and dissolved in 200 mL of warm distilled water. The pH of the film-forming solution was adjusted to 5.6 by adding 2 N NaOH solution. The film-forming solution was heated and stirred at 85 °C for 15 min on a digital hot plate (Model: CMAG HS7, IKA, Germany). The plasticizer was added, and heating was continued for another five min. Potassium sorbate at 0.2 % (w/w) was then added, and the film-forming solutions were cooled to 40–45 °C. The solution was degassed by applying vacuum, and cast on Teflon-coated glass plates of 290 × 200 × 4 mm (LxBxH) size. The films were dried at 30 °C for 96 h, and tested for various functional properties.
Determination of film thickness and tensile properties
The thicknesses of casein and WPC films were measured using a digital caliper (Model: CD-6″CSX, Mitutoyo Corporation, Japan) at five places and the mean values were recorded. The tensile properties such as tensile strength (TS), % tensile strain at break (TE) and elastic modulus (EM) of the films were determined as per ASTM D882-97 standard using a texture analyzer (Model: TA.XT Plus, Stable Micro Systems, Goldaming, UK) equipped with 50 kg load cell. The films were cut into rectangular strips of 25 × 150 mm and mounted on the jaws using filter paper as grips. An initial grip distance of 100 mm and cross-head speed of 60 mm/min were used for the test. Prior to testing, the films were equilibrated for 48 h at 25 °C and 50 % RH. At least eight strips were tested in each sample, and the values of TS, TE and EM were calculated using the Texture Expert Exceed software supplied by the equipment manufacturer.
Water vapour permeability of milk protein films
Film specimens of 80x80 mm size were cut, and the thickness was measured on each side of the specimen using the digital caliper. The water vapor transmission rate (WVTR) was estimated gravimetrically using a modified ASTM E96-95 standard (Gennadios et al. 1994). The polycarbonate cups had a well of 46 mm diameter and 21 mm depth. The specimens were mounted on cups filled with distilled water to 10 mm from the film underside. The weight loss was monitored periodically at 2 h intervals, and the steady-state portion of weight loss (up to 10 h) versus time curve was used to compute the WVTR. The WVP of the films, expressed in g.mm./(m2.h.kPa), was calculated as:
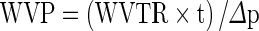 |
where ‘t’ was the mean thickness of the film specimen and ‘Δp’ was the water vapour partial pressure difference (kPa) between the two sides of the film specimen. Each sample was analyzed four times and the mean values of WVP were calculated.
Oxygen barrier properties of milk protein films
The oxygen permeabilities (OP) of select films were estimated at 27 °C and 65 % RH (ASTM D1434-82, 2009) using a permeability cell (Model: CS-135-139, Custom Scientific Instruments Inc., Easton, USA). Film specimens of 120 mm radius were cut using a template and mounted on a greased plate. The transmission rate of the gas through the film specimen was measured as an increase in pressure indicated in the glass capillary tube. The height of mercury in the tube was recorded at the start and then at 5 min intervals till steady state conditions were attained. The OP of the films was calculated as:
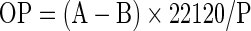 |
where, ‘B’ was the initial height of mercury, ‘A’ was the height of mercury after 20 min interval, 22,120 = constant and ‘P’ was pressure (atm). The OP was expressed in cc/(m2.atm.day).
Moisture sorption behaviour of milk protein films
The moisture sorption isotherms of select films were determined by gravimetric technique at 25 °C. Ten different relative humidity conditions, in the range of 0.112 to 0.98 aw, were achieved using saturated salt solutions. Exactly 1.0 g of film specimens, cut into small bits, were transferred into pre-weighed glass beakers and were equilibrated for 5 weeks. The samples were then weighed and dried at 70 °C to determine their final moisture contents. Three determinations of each sample were done, and the mean values were calculated.
Cheddar cheese packaging and physico-chemical changes during storage
Two-month ripened Cheddar cheese obtained from the experimental dairy of National Dairy Research Institute, Bangalore was used for packaging trials. Samples weighing 250 g were sealed using select casein and WPC films, wrapped with 50 μm (low density polyethylene) LDPE bag and stored at 5 ± 1 °C for 30 days. Samples packed simply in LDPE bags served as control. The samples were drawn at 10 day interval and analyzed for various physico-chemical and microbiological qualities.
The soluble nitrogen (SN) content of Cheddar cheese was determined by Micro-Kjeldahl method (BIS: SP-18 Part XI, 1981) with minor modifications. Approximately 3 g of sample was weighed and mixed with a small amount of Sharpe’s extraction solution in a mortar at 40 °C. The contents were transferred to a volumetric flask and the volume was made up to 100 mL with the same solution. The flask was kept in the water bath at 50–55 °C for 1 h and agitated. After 1 h, the contents were filtered through Whatman #42 filter paper. Then 10 mL of the filtrate was transferred to a 300 mL Kjeldahl digestion flask and the contents were digested using 12.5 mL of concentrated H2SO4 at 250 °C until a clear liquid was obtained. The rest of the procedure was same as for total protein determination (BIS: SP-18 Part XI, 1981).
The oxidative rancidity of packaged Cheddar cheese samples was determined in terms of thiobarbituric acid (TBA) value (King 1962). The titratable acidity of packaged Cheddar cheese samples was determined as per the method described in BIS: SP-18 Part XI (1981). The absorbance of the solutions was read at 532 nm using a spectrophotometer (Model: Anthelie 2, Secoman, France). The microbiological profile of Cheddar cheese samples packaged in casein and WPC films was done as described in BIS:SP-18 (1981). The samples were analyzed in duplicate for standard plate, yeast & mould and coliform counts. The organoleptic quality of Cheddar cheese was evaluated by an expert panel of judges on a 9-point hedonic scale. The samples were coded appropriately before serving to the judges.
Statistical analyses
The data on tensile and WVP properties of films were analyzed using SPSS v.15 software. The effects of type of biopolymer, plasticizer and biopolymer to plasticizer ratio on film thickness, tensile and WVP properties were analyzed using factorial design, which resulted in 12 formulations (2 × 2 × 3 levels). The effects of packaging material and storage time on the physico-chemical, microbiological and sensorial qualities of Cheddar cheese were analyzed using two-way ANOVA without interaction. The level of significance (α) was kept as 0.05. Pairwise comparisons of treatment means were performed using Tukey’s Test of Honesty Significant Difference. Duncan’s multiple range test was used to indicate the statistical differences between the treatments in Tables 1, 2 and 4. The Guggenheim-Anderson-de Boer (GAB) model parameters were estimated by regression analysis. In order to determine the precision of fit, coefficient of determination (R2) and mean relative percent deviation modulus (P%) were used.
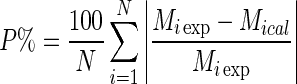 |
where, Miexp,Mical and ‘N’ were experimental moisture content, predicted moisture content and number of experimental values, respectively. The ‘P’ values are indicative of the mean departure of the experimental data from the predicted values. It is generally considered that ‘P’ values below 10 % gave a good fit.
Table 1.
Physical, mechanical and barrier properties of casein and whey protein concentrate films
| Composition | Polymer to plasticizer ratio | Thickness (mm) | Tensile strength, (MPa) | Tensile strain, % | Elastic modulus, (MPa) | Water vapour permeability, g.mm./(m2.h.kPa) | Oxygen permeability, (cc/(m2.atm.day)) |
|---|---|---|---|---|---|---|---|
| C:G | 1:0.25 | 0.19 ± 0.05ef | 3.4 ± 0.59b | 55.3 ± 1.83c | 5.5 ± 1.13b | 3.9 ± 0.37f | 856.3 |
| 1:0.50 | 0.24 ± 0.03de | 2.5 ± 0.27c | 65.2 ± 1.65a | 3.9 ± 0.46d | 7.0 ± 0.54e | – | |
| 1:0.75 | 0.31 ± 0.03ab | 1.4 ± 0.27e | 34.5 ± 0.69de | 2.1 ± 0.33e | 9.8 ± 0.59cd | – | |
| C:S | 1:1.0 | 0.25 ± 0.02cd | 4.6 ± 0.25a | 66.1 ± 0.56a | 6.9 ± 0.32a | 6.0 ± 1.01e | 829.5 |
| 1:1.25 | 0.27 ± 0.03cd | 3.1 ± 0.25b | 66.6 ± 0.02a | 4.6 ± 0.38cd | 10.2 ± 0.11cd | – | |
| 1:1.50 | 0.33 ± 0.02a | 2.5 ± 0.17c | 66.5 ± 0.16a | 3.7 ± 0.26d | 11.1 ± 0.12b | – | |
| WPC:G | 1:0.25 | 0.17 ± 0.03f | 3.1 ± 1.05b | 19.2 ± 2.85g | 5.1 ± 1.64cd | 6.8 ± 0.66e | 1964.5 |
| 1:0.50 | 0.27 ± 0.04cd | 1.6 ± 0.23de | 61.7 ± 1.62b | 2.6 ± 0.38e | 9.1 ± 1.10d | – | |
| 1:0.75 | 0.29 ± 0.01bc | 0.7 ± 0.11f | 54.7 ± 2.26c | 2.6 ± 0.56e | 10.4 ± 1.14bc | – | |
| WPC:S | 1:1.0 | 0.26 ± 0.04cd | 2.0 ± 0.07d | 22.0 ± 1.83f | 4.9 ± 1.74cd | 6.6 ± 0.92e | 1548.4 |
| 1:1.25 | 0.28 ± 0.05bc | 0.7 ± 0.08f | 32.7 ± 1.78e | 2.3 ± 0.25e | 11.0 ± 2.07b | – | |
| 1:1.50 | 0.33 ± 0.01a | 0.8 ± 0.07f | 35.7 ± 1.83d | 2.2 ± 0.19e | 14.0 ± 0.49a | – |
Each observation (mean ± S.D.) of film thickness and tensile properties was average of six replications while the WVP (mean + S.D.) values were average of four replications
C Casein; G Glycerol; S Sorbitol; WPC Whey protein concentrate
*Means with the same letter within a column for each parameter are not significantly different (p > 0.05) by Duncan’s multiple range test
Table 2.
Guggenheim-Anderson-de Boer (GAB) model parameters and precision of fit
| Composition | Mg | K | C | P% | R2 |
|---|---|---|---|---|---|
| C:G (1:0.25) | 7.856 | 0.991 | 2.921 | 7.031 | 0.983 |
| C:S (1:1) | 6.068 | 0.989 | 3.829 | 6.747 | 0.983 |
| WPC:G (1:0.25) | 10.232 | 0.963 | 15.728 | 5.360 | 0.977 |
| WPC:S (1:1) | 9.764 | 0.953 | 23.421 | 7.050 | 0.988 |
Refer Table 1 for abbreviations
Table 4.
Changes in microbial quality of Cheddar cheese during storage in various packaging materials
| Composition | Total bacterial count (log cfu/g) | Yeast and mould count (log cfu/g) | ||||
|---|---|---|---|---|---|---|
| 10 days | 20 days | 30 days | 10 days | 20 days | 30 days | |
| Initial value | –7.8 ± 0.01– | –1.1 ± 0.004– | ||||
| Control | 7.9 ± 0.006Xb | 8.0 ± 0.011Yab | 8.1 ± 0.004Zb | 1.4 ± 0.024Xb | 1.7 ± 0.055Yb | 1.9 ± 0.018Zb |
| C:G (1:0.25) | 7.9 ± 0.017Xab | 7.8 ± 0.017Yab | 8.1 ± 0.010Zab | 1.3 ± 0.007Xa | 1.7 ± 0.011Ya | 1.8 ± 0.017Za |
| C:S (1:1) | 7.9 ± 0.008Xa | 7.1 ± 0.008Ya | 8.1 ± 0.023Zab | 1.3 ± 0.014Xa | 1.7 ± 0.020Ya | 1.8 ± 0.007Za |
| WPC:G (1:0.25) | 7.9 ± 0.016Xa | 7.8 ± 0.016Yab | 8.1 ± 0.014Zab | 1.3 ± 0.028Xa | 1.7 ± 0.148Ya | 1.8 ± 0.026Za |
| WPC:S (1:1) | 7.9 ± 0.004Xab | 8.0 ± 0.004Yab | 8.1 ± 0.004Za | 1.3 ± 0.009Xa | 1.7 ± 0.013Ya | 1.8 ± 0.007Za |
cfu-colony forming units; Refer Table 1 for abbreviations
Each observation was a mean ± S.D. of two determinations
*For each parameter, means with same capital letters within a row are not significantly different (p > 0.05) by storage period and same small letters within a column are not significantly different (p > 0.05) by packaging material using Duncan’s multiple range test
Results and discussion
Thickness of casein and WPC films
Preliminary trials on edible film preparation revealed that at least 20 % glycerol and 75 % sorbitol (w/w of biopolymer) were required to produce flexible and brittle-free films from both casein and WPC. Therefore, three levels of each plasticizer, higher than the minimum required concentration, were selected. Both casein and WPC-based films were transparent and flexible at the plasticizer ratios tested. The thickness of both protein films plasticized with glycerol and sorbitol at different concentrations are presented in Table 1. The thickness of glycerol-plasticized films ranged from 0.17 to 0.31 mm and for sorbitol-plasticized films the range was from 0.25 to 0.33 mm. It was observed that the thickness of edible films was highly influenced (p < 0.05) by the type of biopolymer and by the type and amount of plasticizer used in film preparation. As the concentration of plasticizer increased, the film thickness also increased. Tukey’s pairwise comparisons also showed that glycerol-plasticized films showed significantly lower thickness (p < 0.05) than sorbitol-plasticized films for both biopolymers. It could be reasoned that higher concentrations of sorbitol were required to form brittle-free films than that of glycerol.
Tensile properties of casein and WPC films
The films formed using different ratios of biopolymer to plasticizer were tested for their tensile properties (Table 1). The tensile strength, tensile strain and elastic modulus of casein and WPC-based films ranged from 0.7 to 4.6 MPa, 19.2 to 66.6 % and 2.1 to 6.9 MPa, respectively. Maximum TS and EM were obtained at the lowest concentration of plasticizers.
The concentration of plasticizer had more influence on tensile properties than the type of biopolymer and plasticizer used. In general, it was observed that increase in plasticizer content yielded weaker and more pliable films. This resulted in a decrease in TS and EM and a consequent increase in TE. The tensile strain increased with increasing level of glycerol from 1:0.25 to 1:0.5 but decreased thereafter with further increase in concentration to 1:0.75. These results are in agreement with those of McHugh and Krochta (1994a) for glycerol-plasticized WPI films. Excess plasticization increased the free volume in the film-network and weakened the intermolecular forces between adjacent polymer chains, thereby reducing TS and EM (Banker 1966). Therefore, the tensile strain reduced and the film strip snapped before reaching the extensibility limits. In other words, the polymer chains were spread out in the presence of excess plasticizers, thereby weakening the stress—strain characteristics of the material. However, with increasing sorbitol concentration from 1.0 to 1.5, the tensile strain of casein films remained constant while that of WPC films continued to increase, indicating that saturation limits were not reached.
The plasticizing effect of a plasticizer in films is related to its size and molecular weight. As glycerol had smaller size and lower molecular weight than sorbitol, its effect on reduction in the number of interactions between the protein polymeric chains and on tensile properties was more intense. Sothornvit and Krochta (2001) also reported that plasticizers of smaller size were more efficient in interacting with protein molecules to decrease EM, TS and TE of films. Higher amounts of sorbitol than glycerol was needed to obtain similar tensile properties. The smaller-sized glycerol molecule permitted more influence on the film properties than the sorbitol molecule. Therefore, it could be concluded that glycerol was more effective as a plasticizer for casein and WPC films even at much lower concentrations than sorbitol.
Water vapour permeability and oxygen barrier properties of casein and WPC films
The WVP is the most extensively studied property of edible films mainly because of the importance of water and its migration in deteriorative reactions. The films formed using different ratios of casein and WPC to plasticizer were tested for their WVP (Table 1). At same plasticizer concentration, casein films had significantly lower values of WVP (p < 0.05) than WPC films. Similarly, it was observed that glycerol-plasticized films had better WVP barrier than sorbitol-plasticized films. The WVP values of glycerol-plasticized films ranged from 3.9 to 10.4 g.mm./(m2.h.kPa) and for sorbitol-plasticized films the range was from 6.0 to 14.0 g.mm./(m2.h.kPa). One of the reasons could be that lower concentrations of glycerol (minimum 1:0.25) were used for film formation than sorbitol (minimum 1:1). It is also presumed that the relatively thicker sorbitol-plasticized films offered lower resistance to permeation of water vapour. These values compare with those of 4.99 and 2.58 g mm/(m2 h kPa) obtained by McHugh et al. (1994) for 1.7:1 (biopolymer to plasticizer ratio) WPI films plasticized with sorbitol and glycerol, respectively. Even at low amount of plasticizers, the WVP’s of casein and WPC films were three orders of magnitude greater than that of LDPE (Pérez-Gago and Krochta 2002).
As the concentration of plasticizer increased, the WVP of films (p < 0.05) increased significantly. This was because increase in plasticizer concentration resulted in the formation of a much more porous and hydrophilic polymer matrix, making them susceptible to permeation of water vapour (Arvanitoyannis and Biliaderis 1998). Cuq et al. (1997) reported that increase in plasticizer concentration caused an increase in the WVP of hygroscopic films due to reorganization of the protein network and consequent increase of free volume. When the pore size in the film network increased with increasing concentration of plasticizer, the water molecules permeated easily through the water and plasticizer phase, resulting in high WVP (Anker et al. 2000). Statistical analysis revealed that the three-way interaction of biopolymer, plasticizer type and concentration significantly influenced (p < 0.05) the WVP. So it could be postulated that the effect of plasticizer on WVP was dependent on the levels of biopolymer and plasticizer. Similarly, the effect of plasticizer concentration on WVP was dependent on the type of biopolymer and plasticizer used. The OP of select casein and WPC films are presented in Table 1. It is evident that the OP of WPC films were nearly two times when compared with those of casein films. The differences in OP may be related to the differences in structure, cohesive energy density and free volume between polymer chains.
Moisture sorption behaviour of casein and WPC films
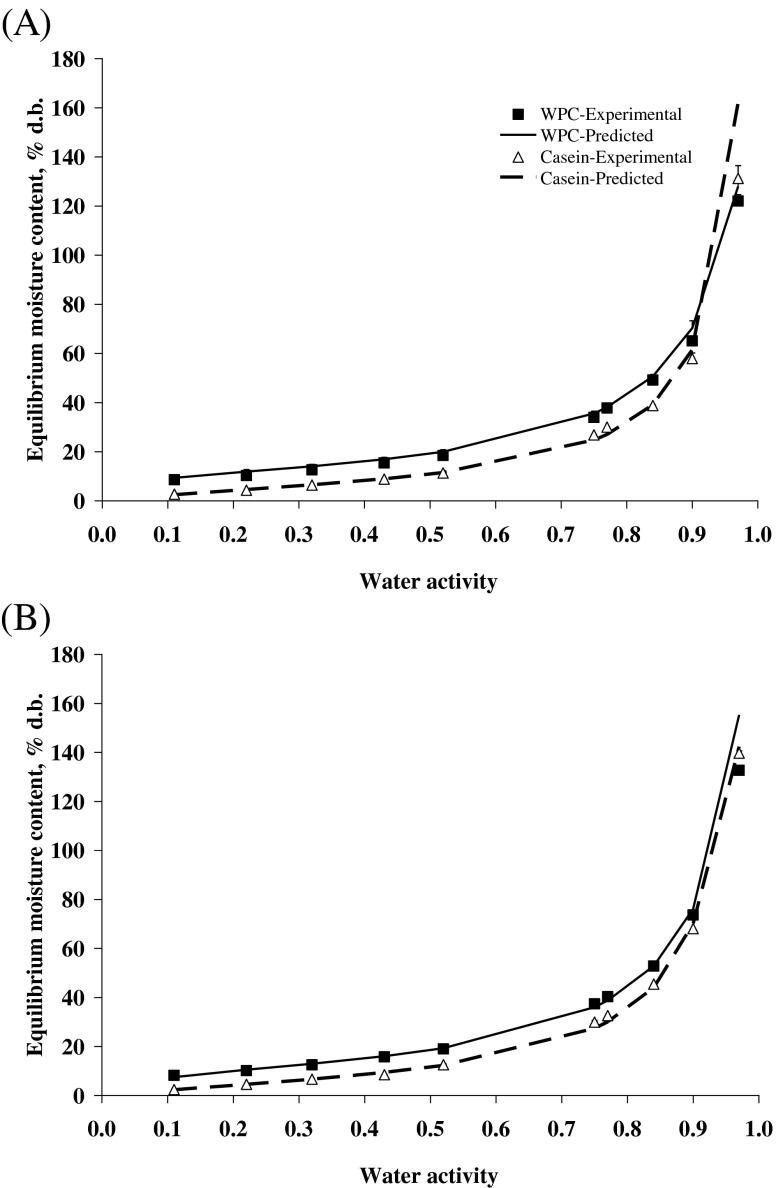
Select films that were used for packaging trials of Cheddar cheese were studied for their moisture sorption properties. As proteins are hydrophilic in nature, the moisture sorption behaviour of such films is very important for ascertaining their use as a packaging material at varying RH conditions. The sorption isotherm plots of casein and WPC films showed a classical sigmoid curve, typical of high-protein material and belonging to type II (Fig. 1a & b) of Brunauer-Deming-Deming-Teller (BDDT) classification. Initially for all samples, the increase in equilibrium moisture content (EMC) with increasing aw was slow up to 0.75, but thereafter, the EMC increased sharply. It could be presumed that increased moisture sorption caused the film matrix to swell, which consequently induced conformational changes in microstructure of the film that not only promoted further moisture sorption, but also increased the channels in polymeric structure to permit increase in permeability.
Fig. 1.
Moisture sorption isotherms of casein and whey protein concentrate films plasticized with (A) 25 % glycerol and (B) 100 % sorbitol at 25 °C (WPC-whey protein concentrate)
Glycerol-plasticized films adsorbed more moisture and had higher EMC than sorbitol-plasticized films at all aw investigated (Fig. 1a & b). This could probably due to the higher hygroscopicity of glycerol molecule than sorbitol. Similarly, at same plasticizer concentration, WPC films were found to adsorb more moisture than casein films. McHugh et al. (1994) also reported that casein-based films were more effective moisture barriers than WPI films plasticized with glycerol or sorbitol.
The experimental sorption data at 25 °C and the predicted GAB model are shown in Fig. 1a & b. The GAB model parameters, obtained by fitting the GAB equation to the experimental sorption data, are presented in Table 2. A good agreement between experimental and predicted data (R2 was 0.98–0.99) was obtained with the GAB model regardless of the plasticizer used. The %P value also was found to be less than 10 % in all cases. It is evident from Table 2 that values of Mg were higher for glycerol-plasticized films as compared to sorbitol-plasticized films. The Mg value is indicative of the maximum amount of water that can be adsorbed in a single layer of dry film and it is also a measure of number of sorbing sites. It is presumed that glycerol had more binding sites for chemi-sorption than sorbitol.
Changes in physico-chemical qualities of packaged Cheddar cheese
The SN content of Cheddar cheese was analyzed at 10 day interval to determine the extent of proteolysis that occurred during storage. The results are summarized in Table 3. The initial SN content of control was estimated as 125.7 mg N2/100 g, which increased to 151.2 mg N2/100 g at the end of 30 days of storage, mainly due to ripening of cheese. However, no significant difference in the SN content was observed between the control and protein film-wrapped samples for all storage intervals tested.
Table 3.
Physico-chemical changes in Cheddar cheese during storage in various packaging materials
| Sample | Soluble nitrogen (mg of N2/100 g) | Thiobarbituric acid | Titratable acidity, % lactic acid | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 10 days | 20 days | 30 days | 10 days | 20 days | 30 days | 10 days | 20 days | 30 days | |
| Initial value | –125.7 ± 0.67– | –0.01 ± 0.001– | –1.03 ± 0.002– | ||||||
| Control | 131.3 ± 0.69Xa | 142.1 ± 1.39Ya | 151.2 ± 0.68Za | 0.01 ± 0.001Xa | 0.035 ± 0.001Yb | 0.05 ± 0.001Zb | 1.07 ± 0.001Xab | 1.08 ± 0.001Yab | 1.09 ± 0.001Zab |
| C:G (1:0.25) | 130.2 ± 0.73Xa | 142.1 ± 0.66Ya | 151.1 ± 1.44Za | 0.01 ± 0.001Xa | 0.02 ± 0.002Ya | 0.04 ± 0.002Za | 1.05 ± 0.001Xa | 1.07 ± 0.002Ya | 1.08 ± 0.001Za |
| C:S (1:1) | 130.1 ± 0.68Xa | 142.1 ± 0.74Ya | 151.1 ± 0.69Za | 0.01 ± 0.001Xa | 0.02 ± 0.001Ya | 0.03 ± 0.001Za | 1.05 ± 0.001Xa | 1.07 ± 0.001Ya | 1.08 ± 0.001Za |
| WPC:G (1:0.25) | 130.2 ± 0.70Xa | 141.1 ± 0.69Ya | 151.2 ± 1.38Za | 0.01 ± 0.002Xa | 0.02 ± 0.001Ya | 0.04 ± 0.045Za | 1.05 ± 0.002Xa | 1.07 ± 0.001Ya | 1.08 ± 0.001Za |
| WPC:S (1:1) | 130.2 ± 0.69Xa | 142.0 ± 0.67Ya | 151.2 ± 0.72Za | 0.01 ± 0.001Xa | 0.02 ± 0.004Ya | 0.04 ± 0.001Za | 1.05 ± 0.001Xa | 1.07 ± 0.001Ya | 1.08 ± 0.001Za |
Refer Table 1 for abbreviations
Each observation was a mean ± S.D. of three determinations
*For each parameter, means with same capital letters within a row are not significantly different (p > 0.05) by storage period and same small letters within a column are not significantly different (p > 0.05) by packaging material using Duncan’s multiple range test
Oxidative rancidity
The TBA value is an indicator of the level of secondary oxidation compounds such as malonaldehydes that are responsible for off-flavours/odours. The TBA values of the stored samples were determined to monitor the oxidative deterioration in Cheddar cheese (Table 3). The initial TBA value of Cheddar cheese was determined as 0.01 which increased to 0.05 for control and 0.03 to 0.04 for samples wrapped in protein films at the end of 30 days. There was no significant difference in the TBA values of control and film-wrapped samples at the end of 10 days. However, as storage progressed, control Cheddar cheese samples exhibited considerably higher TBA value and consequently faster oxidation rate (p < 0.05) as compared with film-wrapped samples. The lower oxidation rate in edible film wrapped samples was attributed to the superior oxygen barrier property of the milk protein films over LDPE. Amongst the samples wrapped in milk protein films, higher TBA values were obtained for samples wrapped in WPC films as compared to casein samples. This could be attributed to the relatively higher OP of WPC films than casein films (Table 1). It could be argued that the overall packaging requirement could be reduced since the protein films acted as an efficient barrier to mass transfer.
Titratable acidity
The initial titratable acidity of Cheddar cheese was estimated as 1.03 % LA. The titratable acidity of Cheddar cheese increased during storage in all packages (Table 3). However, maximum increase in titratable acidity was observed in control. The increase in acidity suggested that the activity of internally produced enzymes that were responsible for the texture and flavour development during ripening would be more active in control. Tukey’s pairwise comparisons showed significant differences (p < 0.05) in titratable acidity values between control and film-wrapped samples for all storage intervals tested.
Changes in microbiological profile of Cheddar cheese
The changes in the bacterial microflora, expressed in log cfu/g, of Cheddar cheese during storage at 5 ± 1 °C are presented in Table 4. The initial TBC of Cheddar cheese was 7.8 log cfu/g, which increased to 8.1 log cfu/g in control and film-wrapped samples at the end of 30 days. Tukey’s pairwise comparisons revealed that there was a significant increase in TBC of control as compared to film-wrapped samples at different storage intervals.
The initial yeast and mould count of Cheddar cheese was 1.1 log cfu/g (Table 4), which subsequently increased to 1.9 log cfu/g in control and to 1.8 log cfu/g in film-wrapped samples at the end of 30 days. Tukey’s pairwise comparisons showed that there was significant increase (p > 0.05) in the yeast and mould population of control as compared to film-wrapped samples at any point of storage. However, coliforms were absent in all samples, regardless of the packaging used. From the microbiological data, it could be concluded that the protein films were effective in restricting the growth of spoilage microorganisms, and extended the shelf-life of Cheddar cheese during storage. It is presumed that part of the antimicrobial effect of casein and WPC films could be attributed to potassium sorbate present.
Sensory quality of Cheddar cheese
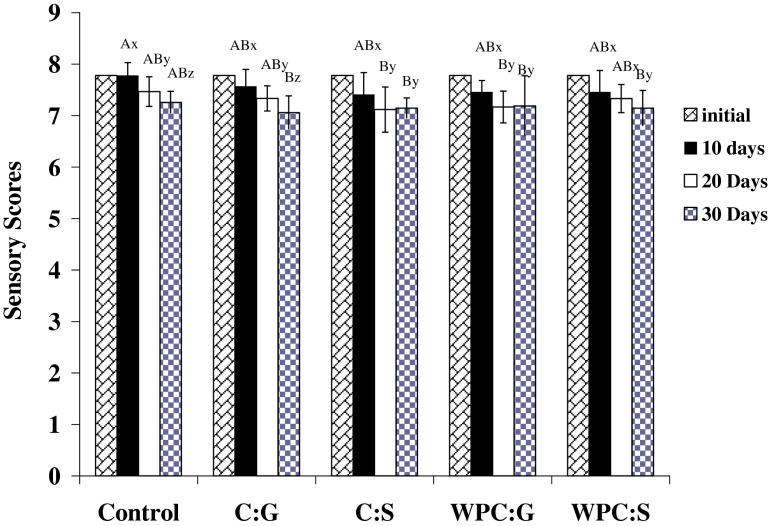
The overall acceptability scores of Cheddar cheese samples at different intervals of storage are represented in Fig. 2. The overall acceptability score was influenced by colour and appearance, as well as, body and texture characteristics. In all samples, a slight decline in overall acceptability scores was recorded with time. The initial score of control decreased from 7.8 to 7.2 at the end of 30 days. The corresponding decrease in film-wrapped samples was from 7.8 to 7.1. In general, Two-way ANOVA analysis (treatment and days were the factors) showed that there was no significant effect (p > 0.05) of the type of packaging, including control, on the sensory characteristics of the product. Therefore, it could be concluded that sensory characteristics of Cheddar cheese were not affected by packaging in casein and WPC films.
Fig. 2.
Changes in overall acceptability scores of Cheddar cheese during storage in various packaging materials (WPC Whey protein concentrate; G Glycerol; S Sorbitol). Scores with same capital letters are not significantly different (p > 0.05) by packaging material and those with same small letters are not statistically different by storage period using Duncan's multiple range test. Each score was a mean of at least ten observations
Conclusions
The film thickness, tensile and barrier properties of casein and WPC films were highly influenced by the type of biopolymer and the type and concentration of plasticizer used in film preparation. Casein films showed superior tensile and barrier properties than WPC films regardless of the plasticizer. Similarly, glycerol-plasticized films possessed better WVP barrier than sorbitol-plasticized films but their oxygen permeabilities were inferior. It was observed that Cheddar cheese packaged in LDPE deteriorated at faster rate than those sealed with additional layer of protein films. The physico-chemical and microbiological data revealed that it was possible to extend the shelf-life of milk products by packaging them with an additional layer of casein and WPC films. The overall packaging requirement could also be reduced since the protein films acted as barriers to oxygen transfer. The sensory qualities of Cheddar cheese was not affected by edible film packaging.
Acknowledgements
We gratefully acknowledge the valuable help extended by Dr. A.R. Indiramma, Principal Scientist, Food Packaging Technology Section and Mr. Srinivas Korra, Technical Officer, Central Food Technology Research Institute, Mysore in testing the oxygen permeability of the films. We also thank the technical assistance offered by Mr. Ravinder Singh, Dairy Engineering Section of NDRI, Bangalore in the preparation and tensile testing of films.
References
- Anker M, Stading M, Hermansson A-M. Relationship between the microstructure and the mechanical and barrier properties of whey protein films. J Agric Food Chem. 2000;48(9):3806–3816. doi: 10.1021/jf000040m. [DOI] [PubMed] [Google Scholar]
- Arvanitoyannis I, Biliaderis CG. Physical properties of polyol-plasticized edible films made from sodium caseinate and soluble starch blends. Food Chem. 1998;62(3):333–342. doi: 10.1016/S0308-8146(97)00230-6. [DOI] [Google Scholar]
- ASTM (2009) Standard test method for determining gas permeability characteristics of plastic film and sheeting. D1434-82. In: Annual Book of ASTM Standards, American Society for Testing and Materials, Philadelphia, PA
- Banker GS. Film coating, theory and practice. J Pharm Sci. 1966;55(1):81–89. doi: 10.1002/jps.2600550118. [DOI] [PubMed] [Google Scholar]
- BIS: SP-18 (1981) Handbook of food analysis Part XI: Dairy products. Indian Standards Institution, New Delhi
- Cuq B, Gontard N, Aymard C, Guilbert S. Relative humidity and temperature effects on mechanical and water vapor barrier properties of myofibrillar proteinbased films. Polym Gels Netw. 1997;5(1):1–15. doi: 10.1016/S0966-7822(96)00026-3. [DOI] [Google Scholar]
- Dangaran K, Tomasula PM, Qi P. Structure and function of protein-based edible films and coatings. In: Embuscado ME, Huber KC, editors. Edible films and coatings for food applications. Dordrecht: Springer; 2009. pp. 25–56. [Google Scholar]
- Fairley P, Monahan FJ, German JB, Krochta JM. Mechanical properties and water vapor permeability of edible films from whey protein isolate and sodium dodecyl sulfate. J Agric Food Chem. 1996;44(2):438–443. doi: 10.1021/jf9505234. [DOI] [Google Scholar]
- Frederiksen CS, Haugaard VK, Poll L, Becker EM. Light-induced quality changes in plain yoghurt packed in polylactate and polystyrene. Eur Food Res Technol. 2003;217(1):61–69. doi: 10.1007/s00217-003-0722-3. [DOI] [Google Scholar]
- Gennadios A, McHugh T, Weller C, Krochta JM. Edible films and coatings based on proteins. In: Krochta JM, Baldwin EA, Carriedo NB, editors. Edible films and coatings to improve food quality. Lancaster: Technomic; 1994. pp. 201–278. [Google Scholar]
- Holm VK, Risbo J, Mortensen G. Quality changes in semi-hard cheese packaged in a poly(lactic acid) material. Food Chem. 2006;97(3):401–410. doi: 10.1016/j.foodchem.2005.05.016. [DOI] [Google Scholar]
- Kampf N, Nussinovitch A. Hydrocolloid coating of cheeses. Food Hydrocoll. 2000;14:531–537. doi: 10.1016/S0268-005X(00)00033-3. [DOI] [Google Scholar]
- King RL. Oxidation of milk fat globule membrane material, thiobarbituric acid reaction as a measure of oxidized flavor in milk and model systems. J Dairy Sci. 1962;45(10):1165–1171. doi: 10.3168/jds.S0022-0302(62)89590-3. [DOI] [Google Scholar]
- Krochta JM. Control of mass transfer in foods with edible-coatings and films. In: Singh RP, Wirakarstakusumah MA, editors. Advances in food engineering. Boca Raton: CRC Press; 1992. pp. 517–538. [Google Scholar]
- Krochta JM, Mulder-Johnston CD. Edible and biodegradable polymer films: challenges and opportunities. Food Tech. 1997;51(2):61–74. [Google Scholar]
- Maynes JR, Krochta JM. Properties of edible films from total milk protein. J Food Sci. 1994;59(4):909–911. doi: 10.1111/j.1365-2621.1994.tb08155.x. [DOI] [Google Scholar]
- McHugh TH, Krochta JM. Sorbitol- vs. Glycerol-plasticized whey protein edible films: integrated oxygen permeability and tensile property evaluation. J Agric Food Chem. 1994;42(4):841–845. doi: 10.1021/jf00040a001. [DOI] [Google Scholar]
- McHugh TH, Krochta JM. Milk-protein-based edible films and coatings. Food Tech. 1994;48(1):97–103. [Google Scholar]
- McHugh TH, Aujard JF, Krochta JM. Plasticized whey protein edible films: water vapor permeability properties. J Food Sci. 1994;59(2):416–419. doi: 10.1111/j.1365-2621.1994.tb06980.x. [DOI] [Google Scholar]
- Pérez-Gago MB, Krochta JM. Formation and properties of whey protein films and coatings. In: Gennadios A, editor. Potein-based films and coatings. Boca Raton: CRC Press; 2002. pp. 159–180. [Google Scholar]
- Pérez-Gago MB, Nadaud P, Krochta JM. Water vapor permeability, solubility, and tensile properties of heat-denatured versus native whey protein films. J Food Sci. 1999;64(6):1034–1037. doi: 10.1111/j.1365-2621.1999.tb12276.x. [DOI] [Google Scholar]
- Sothornvit R, Krochta JM. Plasticizer effect on mechanical properties of beta-lactoglobulin films. J Food Eng. 2001;50(3):149–155. doi: 10.1016/S0260-8774(00)00237-5. [DOI] [Google Scholar]