Abstract
Flours and starches isolated from traditional tubers and roots grown in Indonesia have physical and chemical properties suitable for certain food applications. Compared to other flour samples, cassava and canna flours contained the highest amount of total starch (TS) (77.4 and 77.1 %, respectively). Taro starch had the lowest amount of TS among other starch samples with 75.4 %. The highest amount of amylose was observed from yam and canna flours (25.2 and 23.2 %, respectively). Among starch samples, canna starch contained the highest amylose content (30.4 %), while taro had the lowest (7.6 %). In terms of protein content, arrowroot flour had the highest amount (7.7 %), in contrast to cassava flour which had the lowest (1.5 %). Compared to other flours, canna and konjac flour were the most slowly digested which indicated by their high amount of resistant starch (RS). Canna starch had the highest swelling power and viscosity than other starches and flours. The clearest paste was observed from cassava flour and starch as opposed to konjac starch which was the most opaque paste.
Keywords: Physico-chemical properties, Taro, Yam, Sweet potato, Canna, Arrowroot, Konjak, Cassava, Flour, Starch
Introduction
Starch presents the principal energy reserve in plants. As such it is deposited in storage organs of many starchy crops, such as wheat, corn, rice, potato, wheat, and serves as the most important energy source for humans. Due to its biodegradability and well-defined physico-chemical properties, it offers a number of different opportunities as a versatile renewable resource for various material applications. Although a great number of native starches with different functionalities is available on the market, increasing demand for specific starch properties requires new strategies or, alternatively, novel sources. Chemical derivatization has extended the range of functional properties available; however, due to consumers’ opposition starches from other botanical sources including tropical sources are now being assessed for required functional properties thus avoiding the need for chemical modifications (Tetchi et al. 2007).
Indonesia harbors many plants, out of which traditional starchy tubers and roots have the potential to be used as sources of flours and starch, and replace commercial starches in various industrial applications. Some of these plants are cassava, sweet potato, taro, arrowroot, canna, konjac and sago. These tubers and roots have carbohydrate content and nutritional value very similar to that of rice and wheat. In comparison to cereals, tubers and roots contain relatively high amounts of resistant starch, an important form of starch which is non-digestible and therefore acts as fiber (Liu et al. 2006). Therefore, these traditional starchy plants may be important in the human diet to reduce the risks of obesity, cardiovascular diseases and diabetes. In addition to this, these tubers and roots are free of gluten and therefore may also replace wheat in certain food applications to reduce the incidence of celiac disease (CD) or other allergic reactions to gluten (Hung and Morita 2005)
Flour and starch from tubers and roots can be used to substitute wheat flour in certain food applications (Raja and Sindhu 2000; Chen et al. 2003a, b; Charles et al. 2007; Mepba et al. 2007). However, the potential of these plants has not been utilized fully, mainly due to lack of general knowledge on processability and functional properties of these materials. The application of these underutilized starchy materials may also be driven by current world policies and demographic demand. By 2050 food production must increase by about 70 % above current levels to feed the anticipated 9 billion people. Another challenge is the large, growing food security gap in certain places around the world. In this context, diversification and sustainability of agricultural resources will play an important part in addressing these important issues. Thus, there is a need to explore diverse plant materials including underutilized starchy roots and tubers and transform these commodities into more acceptable forms such as flour or starch (Liu et al. 2006). Such forms could be fortified with other nutrients, thus improving their low protein or vitamin content, or processed further to increase the stability and consequently shelf life of these commodities to achieve a sustainable food supply. Moreover, these materials could be applied wider either in food or non-food industries.
Enhancing our knowledge of the properties of different starchy underutilized tubers and roots from Indonesia may result in different applications in the food or non-food industry. The aim of this study was to assess the important physical and chemical properties of the main compounds, namely flours and starches, of underutilized tubers and roots traditionally produced in Indonesia, for their applications in the food industry.
Materials and methods
Materials and sample preparation
Matured tubers and roots of taro (Colocasia esculenta var Bentul), yam (Dioscorea alata var Krimbang), sweet potato (Ipomoea batatas var Kalasan), canna (Canna edulis var Ganyong merah), konjac (Amorphophallus campanulatus var Mutiara), arrowroot (Marantha arundinaceae var Creole), and cassava (Manihot utillisima var Muntilan) were collected from Tulung Agung (East Java, Indonesia). The tubers and roots were processed by peeling, washing, cutting these materials into 1–2 cm cubes, and slicing into thick chips. These were then oven dried at 30 °C for 40 h until the final moisture content of approximately 13 % was reached.
Flour was obtained by grinding dried chips and sifting coarse materials through a 300 μm sieve. The yield of flour based on the weight of the raw tubers was then determined. For starch isolation, a flour sample (100 g) was dispersed in 300 ml of sodium metabisulphite (0.075 %) and stored at 4 °C overnight. Subsequently, this dispersion was passed through a 150 μm sieve. The residue was washed with sodium metabisulphite (0.075 %). The resulting slurry was left to stand overnight at 4 °C, centrifuged (Sorvall RC 5, Beckman, MN, USA) at 14,000xg for 20 min and the supernatant was discarded. The colored layer was manually scraped off from the starch. This step was repeated until the supernatant layer was almost colorless. After the last centrifugation, the supernatant was decanted and sodium hydroxide (0.1 M) was added to the remining sediment (starch). Deionized water was then added to wash the pellets until their pH was neutral. Recovered starch was dried using an air oven at ~35 °C for 30 h, ground and sieved using a 250 μm sieve. The yield of starch based on the weight of its respective flour (100 g) was determined. Starch was stored in an air tight container under dry conditions.
Proximate analysis of extracted components
Moisture and protein content of flours and starches were analyzed following established protocols (#44-15A and #46-12, respectively). Total amylose content within the flour and starch samples was determined after lipid removal with hot 75 % n-propanol for 7 h in a Soxhlet extractor (Hoover and Ratnayake 2005). Amylose and amylopectin contents were expressed relative to the total starch content. Amylopectin content was calculated as the difference between total starch content and the amylose content. Total starch was measured using Total Starch assay kit (Megazyme, Ireland). A total starch assay kit (Megazyme, Ireland) and a Megazyme resistant starch assay (Megazyme, Ireland) kit were used to measure total starch and the resistant starch content, respectively. Digestibility of samples was determined based on the ratio of non-resistant starch to total amount of resistant and non-resistant starch (Hung and Morita 2005).
Particle size distribution
A Coulter counter (LS130, Coulter Corporation, FL, USA) was used for assessment of particle size distribution of samples. Background reading for water was recorded before each measurement and sample was added until an obscuration of 18–20 % was achieved. Sonication for 5 min was performed to disperse any agglomeration.
Swelling power
Flour and starch dispersions (0.5 %) were prepared in falcon tubes and heated in a water bath at 60, 70, 80, or 90 °C for 30 min with constant agitation to avoid sedimentation. This was followed by centrifugation (Sorvall) at 1,000xg for 15 min at 20 °C. The sedimented fraction was weighed and its mass related to the mass of dry starch was expressed as swelling power (w/w) (Santacruz et al. 2003).
Paste clarity
Paste clarity of flour samples was determined based on the percentage of their light transmittance at 650 nm against the water blank using a spectrophotometer (Cary IE; Varian Australia Pty. Ltd., Melbourne, Australia) (Craig et al. 1989). The flour and starch dispersions (1 %) were prepared in a screw cap tube and heated at 100 °C for 30 min with intermittent mixing to avoid sedimentation. The tubes were then cooled down at room temperature for 1 h and the percentage of light transmittance was measured. To monitor retrogradation tendency, the tubes were stored at 4 °C for 7 days and the percentage of light transmittance was measured each day.
Pasting properties
The pasting properties of flour and starch were investigated using a starch cell (Physica SmartStarch analyzer, Anton Paar) attached to a CR/CS rheometer (Physica MCR 33011, Anton Paar, GmbH, Germany) using the method described elsewhere (Jayakody et al. 2007). An aqueous sample containing 7 % w/w of either flour or starch was prepared and equilibrated at 50 °C for 1 min, then heated from 50 to 95 °C at 6 °C/min, held at 95 °C for 5 min, cooled to 50 °C at 6 °C/min, and held at 50 °C for 2 min. The speed was 960 rpm for the first 10s, then 160 rpm for the reminder of the experiment. The pasting properties of each sample were inferred from acquired diagrams.
Thermal properties
Differential scanning calorimetry (DSC-7, Perkin Elmer, Norwalk, CT, USA) was used to assess the gelatinisation temperatures including onset (To), peak (Tp), and endset (Te) temperature, as well as gelatinization enthalpy (∆H) of each sample. Flour and starch samples were prepared by adding deionized water (11 μL) to 3 mg of sample in an aluminum pan (BO160932, Perkin Elmer), which was allowed to stand overnight at room temperature before analysis to ensure equilibration of sample and water. The sample was heated from 20 to 100 °C at 10 °C/min heating rate. An empty aluminum pan was used as reference in each measurement (Ratnayake et al. 2001).
Statistical analysis
A randomized block design was applied in the design of all experiments, with tubers and replications (block) as the main effects. This block structure was repeated at least three times with at least 2 sub samplings. Results were analyzed using General Linear Model procedure of the SAS systems. The level of significance was preset at p < 0.05. Relationships between the different properties of flours and starches were determined using Pearson correlation analysis.
Results and discussion
Physicochemical properties
Proximate composition of flours and their isolated starches is shown in Table 1. Cassava and canna flours contained similar amounts of total starch (TS) (77.4 % and 77.1 %, respectively), which was significantly higher (p < 0.05) than for other sources (Table 1). Further purification has increased the amount of TS. Besides taro starch that contained less TS (75.4 %), other starches had a similar content of TS. Lower TS content for taro and sweet potato flours has been reported previously (60.7 % and 64.4 %, respectively) (Hung and Morita 2005). In contrast, TS of yam and arrowroot flours determined in the present study was lower than in other reported studies i.e. 88.7 % and 85 %, respectively (Raja and Sindhu 2000; Chen et al. 2003a, b). Overall, TS of starch samples in this study was lower than other studies (Chen et al. 2003a, b; Piyachomkwan et al. 2002; Moorthy 2002). The observed differences in raw materials could be caused by multiple factors including tuber and root maturity at harvesting, botanical origin, analytical methods, and high endogenous α-amylase activity (Yadav et al. 2006; Srichuwong et al. 2005a, b).
Table 1.
Chemical composition (%) of flours and starches extracted from taro, yam, sweet potato, canna, arrowroot, konjac and cassava
| Source | Protein | Total starch | Amylose | Amylopectin | Moisture | Yield |
|---|---|---|---|---|---|---|
| Flours | ||||||
| Taro | 5.5 ± 0.20c | 65.4 ± 3.30b | 17.3 ± 2.50d | 48.1 ± 1.91e | 8.9 ± 0.60c | 19.0 ± 2.50d |
| Yam | 5.3 ± 0.10c | 70.2 ± 5.70b | 33.1 ± 1.20a | 37. 0 ± 7.32d | 9.5 ± 0.11bc | 14.1 ± 1.80a |
| Sweet potato | 3.3 ± 0.20e | 64.0 ± 1.80b | 26.8 ± 2.70b | 37.2 ± 4.40b | 9.9 ± 0.31b | 30.0 ± 2.30b |
| Canna | 4.2 ± 0.00d | 77.1 ± 3.20b | 32.7 ± 2.00a | 44.4 ± 1.41c | 10.9 ± 0.11a | 25.0 ± 1.70a |
| Arrowroot | 7.7 ± 0.00a | 62.3 ± 0.30b | 29.4 ± 1.40b | 32.8 ± 1.70c | 9.4 ± 0.20bc | 32.0 ± 1.60b |
| Konjac | 6.2 ± 0.00b | 68.5 ± 0.80b | 21.7 ± 2.30c | 46.7 ± 1.79b | 9.5 ± 0.11bc | 12.1 ± 2.00b |
| Cassava | 1.4 ± 0.02f | 77.4 ± 0.90a | 13.1 ± 2.60d | 64.4 ± 3.99b | 9.5 ± 0.58bc | 40.2 ± 2.50a |
| Starch | ||||||
| Taro | 0.60 ± 0.10b | 75.40 ± 2.10b | 10.1 ± 1.61e | 65.2 ± 0.71a | 9.8 ± 0.41bc | 21.1 ± 1.60b |
| Yam | 0.60 ± 0.20b | 82.10 ± 3.50a | 23.7 ± 2.41b | 58.4 ± 2.90b | 10.8 ± 0.10b | 15.2 ± 2.40d |
| Sweet potato | 0.50 ± 0.10b | 87.10 ± 3.80a | 15.9 ± 0.91cd | 71.2 ± 4.62b | 10.1 ± 0.60bc | 18.1 ± 1.70d |
| Canna | 0.80 ±0.10b | 88.10 ± 0.80a | 35.0 ± 0.49a | 53.0 ± 1.11c | 12.3 ± 0.51a | 16.0 ± 2.80d |
| Arrowroot | 0.60 ±0.20b | 84.20 ± 4.40a | 21.9 ± 1.39c | 62.3 ± 2.20b | 10.2 ± 0.40bc | 12.0 ± 2.60d |
| Konjac | 1.2 ± 0.20b | 82.40 ± 3.80a | 21.3 ± 2.51c | 61.1 ± 5.59b | 10.6 ± 0.20b | 18.1 ± 2.54d |
| Cassava | 0.40 ± 0.10b | 85.50 ± 2.80a | 14.6 ± 1.50d | 70.1 ± 2.51e | 8.9 ± 0.59bc | 36.0 ± 1.56d |
n = 3. Values followed by the same superscript in each column are not significantly different (p < 0.05)
Canna and yam flour contained similar amounts of amylose (32.7 % and 33.1 %, respectively), followed by arrowroot (29.4 %), sweet potato (26.8 %) and konjac (21.7 %). Taro and cassava had the lowest amounts of amylose of all with 17.3 % and 13.1 %, respectively. Among the starch samples, canna contained the highest amount of amylose (35.0 %) followed by similar amounts for yam (23.7 %), arrowroot (21.9 %) and konjac (21.3 %). Sweet potato and cassava also contained comparable concentrations of amylose (15.9 % and 14.6 %, respectively) while taro was the lowest with only 10.1 %. Similar amylose contents of canna and yam starch have been reported in other studies (Puncha-arnon et al. 2007; Woolfe 1992). On the contrary, amylose content of sweet potato, arrowroot, konjac, and taro starches obtained from this study was lower than previous reports (Liu et al. 2006; Puncha-arnon et al. 2007; Zaidul et al. 2007). Amongst the flour samples analyzed in this study, canna and yam flours contained similar amounts of amylose to that of wheat flour (Mbofung et al. 2006)
Amongst flour samples, arrowroot contained the highest amount of protein (7.7 %), followed by konjac (6.2 %), taro, yam, canna (5.5, 5.3 and 4.2 %, respectively) and sweet potato (3.3 %). Cassava flour contained the lowest amount of protein with only 1.4 %. The protein content of taro and yam flour was lower than in previous reports i.e. 2.7–5.4 % and 6.9 %, respectively (Chen et al. 2003a, b; Tattiyakul et al. 2006). As expected, the level of protein content within the flour samples was slightly decreased after starch isolation. The protein values of starches are not significantly different. The protein content of taro was slightly lower than what has been reported earlier, i.e. 0.2–1.3 % (Freitas et al. 2004). For yam and sweet potato, the protein level was similar to that previously reported (0.5 % and 0.45 %, respectively) (Moorthy 2002), while the protein levels of canna and cassava starches were higher than that reported in other studies (0.05–0.2 % and 0.1 %, respectively) (Piyachomkwan et al. 2002; Jane et al. 1992). This difference might be due to the starch isolation method, variation in botanical origin and local climate during cultivation (Radley 1976).
All flours and starches observed here have a lower protein content compared to that of wheat flour (Zaidul et al. 2007). For some food product applications, the protein content of wheat flour is too high and can be diluted with other starches of lower protein content. This is the case with biscuit making. The protein content of the mixture required is around 7.0–8.5 % for sweet biscuits or 8.4–10 % for biscuit sponge (Snow and O’Dea 1981). Therefore, the flours and starches studied here have the potential to partially substitute wheat flour to obtain composite flours with acceptable protein contents for certain food applications.
Canna and konjac flour had similar amounts of resistant starch (56.4 % and 51.7 %) and the highest amount of resistant starch amongst all the flour samples examined (Table 2). Arrowroot contained a lower amount of resistant starch (33.2 %) and cassava contained 19.3 %. Taro and yam had comparable amounts of resistant starch (11.9 % and 10.8 %, respectively) while sweet potato had the lowest amount with only 4.7 %. A low amount of resistant starch in sweet potato flour has been previously reported4 this being 3.4 %. Since having the lowest amount of resistant starch, sweet potato flour would be the most digested among our flours. On the other hand, canna starch contained the highest amount of resistant starch (70.8 %) and therefore would be the least digested. This percentage of resistant starch in the canna starch was much higher than other starches such as konjac (20.9 %), arrowroot (15.9 %), yam (13.2 %), cassava (10.4 %), sweet potato (10.2 %) and taro (3.3 %).
Table 2.
The content (%) of resistant starch, non-resistant starch, and digestibility of flours and starches extracted from taro, yam, sweet potato, canna, arrowroot, konjac and cassava
| Source | Resistant starch | Non resistant starch | Digestibility |
|---|---|---|---|
| Flours | |||
| Taro | 10.8 ± 1.50D | 30.8 ± 1.70D | 74.0 ± 1.50BC |
| Yam | 11.9 ± 0.30D | 56.8 ± 5.90C | 82.6 ± 1.81AB |
| Sweet potato | 4.7 ± 0.71D | 84.6 ± 1.21A | 94.7 ± 0.70A |
| Canna | 56.4 ± 4.40A | 28.1 ± 1.20D | 33.3 ± 2.70D |
| Arrowroot | 33.2 ± 0. 80BC | 62.0 ± 3.50BC | 65.1 ± 3.81C |
| Konjac | 51.7 ± 0.11A | 29.6 ± 2.90D | 36.4 ± 3.30D |
| Cassava | 19.3 ± 3.80CD | 72.1 ± 2.90AB | 78.9 ± 4.00B |
| Starch | |||
| Taro | 3.3 ± 1.20D | 91.2 ± 2.60A | 96.5 ± 1.30A |
| Yam | 13.2 ± 0.80CD | 80.6 ± 4.31BC | 85.9 ± 1.41BC |
| Sweet potato | 10.2 ± 0.40D | 86.8 ± 4.10AB | 89.5 ± 0.80B |
| Canna | 70.8 ± 1.70A | 22.1 ± 1.80E | 23.7 ± 1.90E |
| Arrowroot | 15.9 ± 0.11C | 77.1 ± 4.40BC | 82.8 ± 0.80CD |
| Konjac | 20.9 ± 0.60B | 73.9 ± 4.01C | 77.8 ± 1.21D |
| Cassava | 10.4 ± 1.21D | 41.4 ± 2.90D | 79.9 ± 2.50D |
n = 3. Values followed by the same superscript in each column are not significantly different (p < 0.05)
The differences in the degree of digestibility among the samples assessed in this study are more likely due to the differences in their crystallinity. Starch with A-type crystallinity has inferior crystals due to the high proportion of short-branch amylopectin chain. Thus, this type of starch is more susceptible to digestion by α-amylase. As a consequence, the A-type starch has a higher digestibility compared to the B-type starch (Radley 1976). Results obtained in this study are in agreement with this statement. In general, starches with B-type of crystallinity such as sweet potato, taro, arrowroot and cassava have higher digestibility compared to canna that has A-type (Puncha-arnon et al. 2007). Others factors that might also influence digestibility are amylose content, particle size and amylopectin chain-length distribution (Srichuwong et al. 2005a, b; Gelencser et al. 2008).
In this study, resistant starch content was negatively correlated (r = −0.974) with digestibility of the samples. A similar result has been reported previously (Wickramasinghe and Noda 2009), arguing that interaction between resistant starches and other starch components may influence the digestibility by α-amylase. A significant negative correlation was also observed between amylose content and digestibility (r =−0.868). It was proposed that the low amount of amylose might cause reduction in the compactness of the amorphous region of starch granules and subsequently increase susceptibility of starch toward α-amylase digestion (Taggart 2004).
Based on their proximate analysis, flours and starches studied here could bring benefits for some food applications. Having high amount of amylose, canna flour and starch could partially replace wheat flour in snack food formulations to obtain products with a crunchy texture. Amylose within flour or starch could strengthen the dough, which in turn improves the forming and cutting properties of dough to produce snack foods with a crunchy texture (Huang et al. 2006). As mentioned earlier, the low protein content of these flours and starches is beneficial to dilute protein content of wheat flour to produce composite flours that fulfill the requirement of protein content for certain food applications, for example biscuits making (Snow and O’Dea 1981).
The high digestibility of some of these starchy materials might be beneficial for food preparations especially for infants and the elderly who require more readily digestible food (Snow and O’Dea 1981). On the other hand, the low digestibility of canna flour and starch could be important in the prevention of obesity, diabetes and other related diseases, when they are used as food ingredients (Hung and Morita 2005). Besides imparting nutritional benefits, incorporation of flours or starches that contain resistant starch might also offer processing benefits. For example, in low-water systems due to low water-binding capacity and negligible impact on dough viscosity and rheology. Moreover, in such a system, the presence of resistant starch might also bring textural benefits. A more expanded, light, and crispy texture could be obtained for snacks containing resistant starch (Huang et al. 2006).
Particle size
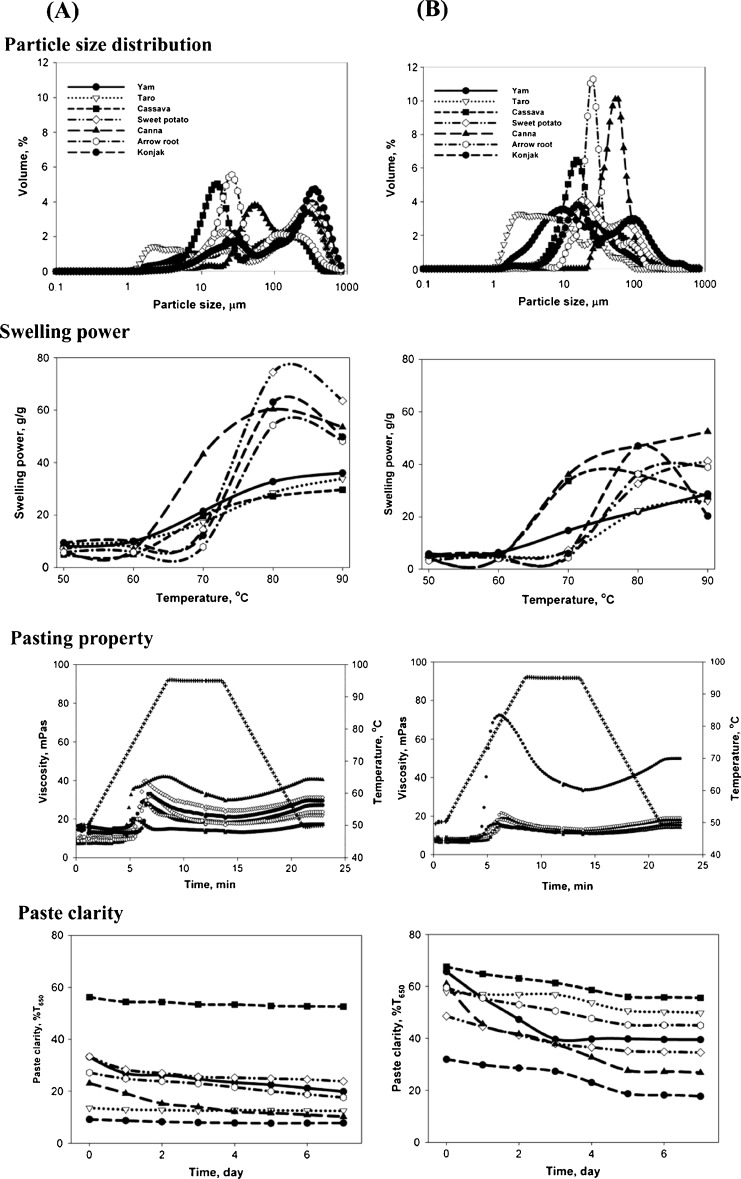
Particle size distribution patterns of the flour samples are represented in Fig. 1a. In general, all flour samples had two distinct populations of granule. Sweet potato, taro, yam and konjac flours had a similar pattern of distribution with a high proportion of larger granules centered at 288, 315, 345 and 378 μm, respectively. A small proportion of small granules was also observed, indicated by the presence of the second peak at 19 μm for sweet potato, 2 μm for taro and 28 μm for both yam and konjac flours. The range of granule size of sweet potato and yam was not clearly different but was slightly lower than for taro and konjac flours. Thus, these two flours were more homogenous than taro and konjac flour. In contrast to cassava and arrowroot flours, which had a different distribution pattern, these flours had a high amount of small granules (17 and 27 μm, respectively). Cassava flour had a narrower size range than that of arrowroot, and the proportion of small (53 μm) and large granules (316 μm) within the canna flour was not significantly different. It was predicted that the presence of smaller granules in sweet potato, taro, arrowroot, cassava and canna flour was mainly associated with starch granules. For yam and konjac flour, the smaller granule could be related to particle clusters.
Fig. 1.
Particle size distribution, Swelling power, Pasting properties and Paste clarity of flours (a) and starches (b) extracted from taro, yam, sweet potato, canna, konjac, arrowroot and cassava
Among the starches, canna had the largest mean diameter of granule size (56 μm) followed by arrowroot (27 μm) and sweet potato (19 μm) (Fig. 1b). Konjac and cassava had the same mean diameter (15 μm) while taro had the smallest (2 μm). Results obtained for canna, taro, sweet potato, konjac were in agreement with previous studies (Liu et al. 2006; Santacruz et al. 2003; Mbofung et al. 2006; Gelencser et al. 2008). However, the mean diameter for arrowroot and yam starch was lower than previously reported (Gelencser et al. 2008). As expected, further purification of flour samples eliminated the presence of other components such as lipids and proteins. Thus, purification resulted in starches with more homogenous granular-size distribution (Fig. 1b). Arrowroot starch was the most homogenous whereas taro starch was the least homogenous. In general, there were two types of distribution patterns in the starch samples. Canna and arrowroot starches showed a unimodal distribution whereas taro, yam, sweet potato, konjac and cassava showed a bimodal pattern.
The size of starch granule is important in determining the suitability of starch for certain food applications (Freitas et al. 2004; Leon et al. 2006). In this study, taro starch, which has a small particle size, could be used for several food products especially those requiring a smooth texture (Freitas et al. 2004). In snack food production, the fine granule of taro starch could improve binding and reduce breakage of the final product (Leon et al. 2006). The fine particle size of taro starch might allow a better light reflection on the porous structure of bread yielding white breadcrumbs that is preferred by the consumer.
Swelling power
In general, there were two patterns of swollen granules of the flour samples shown during heating from 50 to 90 °C (Fig. 1a). The first group consisted of yam, taro and cassava. Their granules started to swell at ~60 °C, then, the swelling power increased steadily with temperature from 70 to 90 °C. The swelling powers of these flours were not significantly different throughout this temperature range and at 90 °C granules maintained their integrity. The second group consists of sweet potato, canna, konjac and arrowroot. Besides canna, granules of these flours start to swell sharply at ~70 °C and reach their maximum swelling power at 80 °C. In contrast to the first group, further heating to 90 °C caused these granules to lose their integrity. Overall, swelling power of flours within the second group was high, which may be due to the extensive and strong intermolecular bonding within the granules of flours in the first group (Tester and Morrison 1990).
The amount of other components such as amylose, protein, lipids, amylopectin, phosphorous, and particle size may influence the swelling pattern (Thitipraphunkul et al. 2003; Fu 2008). Amylose could reinforce the internal network and restrict the swelling ability (Snow and O’Dea 1981). Proteins may lower the swelling power by being embedded in the starch granules forming a stiff matrix that limits the access of water into the starch granule. Despite of their reducing effects on swelling power as mentioned above, protein and amylose contents showed a positive but weak correlation with swelling power (r = 0.109 and r = 0.498, respectively).
Among the starch samples, canna showed the highest swelling power (Fig. 1b). Sweet potato and arrowroot had an intermediate value while yam, taro and konjac had the lowest swelling power. The high swelling power of canna starch has been reported in other studies (Taggart 2004). It was proposed that the high phosphorous content, the large granule size and the high number of hydrogen bonds formed between the very long-branch chains of amylopectin and water would contribute to the high swelling power of canna starch.
Canna starch is suitable for food applications that require a high swelling ability. In noodle making, this starch might be suitable for products that require a soft and smooth texture with a high elasticity such as yellow alkaline noodle (YAN) and Japan noodle. However, this starch is not suitable for Chinese wet noodles with a firm bite and springy texture (Collado et al. 1999).
Pasting properties
Canna and sweet potato had similar peak viscosity values, which were higher than other flours. Taro, yam, arrowroot and konjac had similar peak viscosities while cassava had the lowest (Table 3; Fig. 1a). Cassava flour had the lowest peak and breakdown viscosity and was the most resistant regarding the heat and stirring treatment. The highest setback viscosity was observed in yam and canna flour indicating the high retrogradation tendency of these flours (Mbofung et al. 2006; Tester and Morrison 1990). Overall viscosity of canna starch was the highest amongst our samples. The viscosity of canna starch was even higher than that of its respective flour. The presence of other components such as lipids and protein, which interfere with the pasting process, and the lower amount of starch in the flour, are behind reported differences. This trend was not observed for the other starch samples that had lower viscosities than their respective flour.
Table 3.
Pasting characteristics of flours and starches extracted from taro, yam, sweet potato, canna, arrowroot, konjac, and cassava
| Source | Pasting characteristics | |||||||
|---|---|---|---|---|---|---|---|---|
| Peak time (min) | Past temp (°C) | Peak vis (cP) | H strength (cP) | B down (cP) | SFP (cP) | SFT (cP) | Final vis (cP) | |
| Flours | ||||||||
| Taro | 389.7 ± 0.10C | 62.8 ± 1.31BC | 30.0 ± 0.72B | 16.8 ± 0.51C | 13.2 ± 0.48C | −7.9 ± 0.43BC | 5.3 ± 0.1 C | 22.1 ± 0.42C |
| Yam | 377.8 ± 0.01C | 58.5 ± 0.92C | 31.0 ± 2.3B | 18.1 ± 1.61C | 12.9 ± 1.62C | −2.6 ± 0.62A | 10.3 ± 1.5 AB | 28.4 ± 1.84B |
| Sweet potato | 385.7 ± 6.91C | 66.6 ± 1.61AB | 41.2 ± 1.4A | 23.3 ± 1.20B | 17.9 ± 0.71A | −10.5 ± 3.11C | 7.4 ± 2.5 BC | 30.7 ± 2.21B |
| Canna | 485.8 ± 11.92A | 65.2 ± 0.81B | 41.7 ± 2.4A | 29.8 ± 2.50A | 13.8 ± 0.82C | −2.7 ± 1.20A | 9.1 ± 1.2 AB | 38.9 ± 2.60A |
| Arrowroot | 415.5 ± 3.71B | 70.1 ± 0.41A | 32.1 ± 1.8B | 16.3 ± 0.40C | 15.7 ± 1.43AB | −8.2 ± 0.51BC | 7.5 ± 0.9 BC | 23.8 ± 1.40C |
| Konjac | 407.7 ± 0.10B | 66.1 ± 2.71AB | 33.7 ± 0.5B | 21.4 ± 0.59B | 15.5 ± 0.19AB | −5.1 ± 0.79AB | 9.2 ± 1.0 B | 28.6 ± 1.12B |
| Cassava | 317.8 ± 0.10D | 64.4 ± 2.42B | 20.21 ± 0.42C | 12.0 ± 0.19D | 8.2 ± 0.31 D | −2.8 ± 0.11A | 5.4 ± 0.2 C | 17.4 ± 0.53D |
| Starch | ||||||||
| Taro | 378.1 ± 0.0AB | 73.6 ± 9.40A | 14.9 ± 0.9E | 9.4 ± 0.1C | 5.5 ± 0. 83D | −1.0 ± 1.02A | 4.5 ± 0.3B | 13.9 ± 0.11D |
| Yam | 381.0 ± 21.2AB | 66.8 ± 6.82B | 18.3 ± 0.6CD | 9.8 ± 0.3C | 8.5 ± 0.81BC | −3.17 ± 1.33A | 5.4 ± 0. 5B | 15.4 ± 0.72CD |
| Sweet potato | 380.8 ± 3.12AB | 59.8 ± 5.51D | 21.8 ± 0.2B | 12.1 ± 0.0BC | 9.7 ± 0.22B | −2.7 ± 0.21A | 6.9 ± 0.0B | 19.1 ± 0.01B |
| Canna | 373.8 ± 6.90B | 66.2 ± 1.32B | 71.3 ± 1.2A | 35.2 ± 1.7A | 36.1 ± 1.01A | −23.1 ± 1.50B | 13.1 ± 2.1A | 48.3 ± 2.62A |
| Arrowroot | 383.9 ± 0.00AB | 68.7 ± 1.51B | 18.9 ± 0.3C | 11. 7 ± 0.3BC | 7.3 ± 0.04CD | −0.8 ± 0.21A | 6.5 ± 0.2B | 18.1 ± 0.50 BC |
| Konjac | 399.8 ± 3.41A | 62.6 ± 0.91C | 18.9 ± 0.8C | 13.6 ± 1.8B | 5.4 ± 1.32D | −0.9 ± 0.40A | 4.4 ± 1.6B | 17.9 ± 1.13BC |
| Cassava | 361.8 ± 6.91B | 61.4 ± 0.93C | 16.2 ± 0.7DE | 10.4 ± 0.7C | 5.8 ± 0.51D | −1.2 ± 0.70A | 4.6 ± 0.9B | 15.1 ± 0.50CD |
n = 3. Values followed by the same superscript in each column are not significantly different (p < 0.05). Peak T peak time; Past T pasting temperature; Peak vis peak viscosity; H strength holding strength; B down breakdown; SFP setback from peak; SFT set back from through; Final vis final viscosity
Swelling power and amylose content appeared to be the major factors affecting pasting properties of flour and starch samples. A significant positive correlation was observed between swelling power and peak (r = 0.783, p ≤ 0.05), breakdown (r = 0.815), and final viscosities (r = 0.785). Similarly, amylose content was also significantly positively correlated with peak viscosity, breakdown, and final viscosity (r = 0.817, 0.788, and 0.820, respectively). Swelling power and amylose content could influence some of the pasting properties of starch, since the pasting process itself involves granular swelling, leaching out of amylose, and disruption of granules during heating (Thitipraphunkul et al. 2003).
In general, the viscosity of samples obtained was substantially lower than what it has been reported elsewhere (Gelencser et al. 2008; Martin and Fitzgerald 2002). Activity of α-amylase could be a cause of this drastic viscosity reduction (Srichuwong et al. 2005a, b). Another factor that might have contributed to the lower viscosities in this study was the effect of storage time. Increasing disulphide-bond formation in protein networks that occurs during starch aging could lower the pasting viscosity of starch (Muyonga et al. 2001).
All flours and starches examined in this study have lower viscosity (peak, breakdown, setback and final viscosities) compared to that of wheat flour (Mbofung et al. 2006). The low viscosity of the flour and starches is beneficial for some food applications including confectionary (soft candy and gum drops), weaning foods, and other liquid foods (Snow and O’Dea 1981; Hoover 2001). In addition, low-viscosity flours and starches can be used to avoid the use of chemically modified starch, which was introduced to produce low-viscosity oxidized starch and thin boiling starch (Cornell 2004). The low setback value of these samples indicates their low retrogradation tendency and is important for frozen or cold storage foods. Low breakdown viscosity of these samples, as compared to that of wheat flour, reflected the stability of these materials toward heat and mechanical processing. This property is crucial for food production that involves heat and mechanical treatment in canned foods (Snow and O’Dea 1981).
Thermal properties
Thermal properties of the flour and starch samples are presented in Table 4. The onset and peak temperature of gelatinisation of konjac starch were the highest among the six starches, which indicated high crystallite stability. The perfectness of konjac starch was also reflected in the high ΔH (gelatinisation enthalpy) value. A number of factors may influence gelatinisation temperature, including the molecular architecture of amylopectin, the formation of lipid complexes, degrees of crystallinity, and the proportion of crystalline regions. In the current study, protein content had a negative correlation with gelatinization temperature (r = −0.025, −0.061 and −0.061 for To, Tp, and Tc, respectively). Similar correlation between amylopectin content and To, Tp, and Tc was also observed (r = −0.025, −0.061 and −0.061, respectively). This could be due to a decrease in amylopectin crystallinity caused by cleavage of the long-branch chain of the polysaccharide into short-straight chains (Radley 1976). This would require less energy to initiate gelatinisation and consequently a lower gelatinization temperature was observed (Hoover and Ratnayake 2005).
Table 4.
Thermal properties of flours and starches extracted from taro, yam, sweet potato, canna, arrowroot, konjac and cassava
| Source | Gelatinisation characteristics | |||
|---|---|---|---|---|
| To (°C) | Tp (°C) | Tc (°C) | ΔH (J/g) | |
| Flours | ||||
| Taro | 51.0 ± 1.51b | 51.8 ± 2.53a | 55.8 ± 0.93b | 2.8 ± 0.51a |
| Yam | 62.0 ± 1.72b | 73.9 ± 0.94b | 81.8 ± 1.12a | 7.4 ± 0.82b |
| Sweet potato | 71.2 ± 1.62b | 77.3 ± 1.7b | 82.8 ± 1.32b | 4.1 ± 0.71b |
| Canna | 66.9 ± 1.23b | 70.5 ± 1.21a | 79.4 ± 1.61b | 4.7 ± 0.9b |
| Arrowroot | 69.6 ± 1.41b | 74.8 ± 1.51b | 80.9 ± 1.21b | 2.9 ± 0.61a |
| Konjac | 73.9 ± 1.52b | 81.2 ± 1.30b | 89.6 ± 1.83a | 1.8 ± 1.12b |
| Cassava | 64.9 ± 1.81a | 71.4 ± 1.90b | 76.5 ± 1.40a | 2.2 ± 1.23b |
| Starch | ||||
| Taro | 69.7 ± 2.13b | 75.4 ± 2.52a | 80.2 ± 1.91b | 7.6 ± 1.52a |
| Yam | 61.4 ± 1.86b | 69.4 ± 1.73b | 76.6 ± 1.51b | 7.7 ± 0.72b |
| Sweet potato | 62.9 ± 1.76b | 73.5 ± 1.52a | 80.5 ± 1.72b | 11.3 ± 0.83b |
| Canna | 64.1 ± 1.62b | 69.8 ± 1.41b | 77.5 ± 1.42a | 11.4 ± 1.11a |
| Arrowroot | 65.0 ± 1.40b | 72.5 ± 1.22b | 84.0 ± 1.13b | 11.7 ± 0.90b |
| Konjac | 71.1 ± 2.01b | 77.7 ± 1.10a | 82.6 ± 1.91b | 11.1 ± 0.70a |
| Cassava | 61.1 ± 1.73b | 68.2 ± 1.43b | 74.2 ± 2.00a | 8.1 ± 0.62b |
n = 3. Values followed by the same superscript in each column are not significantly different (p < 0.05). To, Tp, and Tc indicate temperature of onset, midpoint and end of gelatinisation, respectively. ΔH indicates enthalpy of gelatinisation (J/g dry starch)
Overall, the gelatinisation temperature and ΔH of the starch samples was lower than previously reported (Liu et al. 2006; Craig et al. 1989; Gelencser et al. 2008). Differences in genetic, environmental factors, time of harvest, and seasonal variations might cause this discrepancy. The lower gelatinization temperatures of flour and starch samples could bring benefits in some foods containing ingredients that are heat-labile at high temperature. Flours and starches with lower gelatinisation temperature could be applied in food processing that involves low temperature such as batter coating or processed meat products. Low gelatinisation temperature allows easier cooking and the efficiency of food processing could be increased by reducing the time and heat during cooking (Snow and O’Dea 1981).
Paste clarity
Paste clarity of the flour and starch samples is presented in Fig. 1a and b. Cassava had the highest paste clarity compared to the other flours. In addition, the clarity of cassava paste was stable during storage at cold temperatures. The high clarity and stability of cassava paste might be due to its low amount of amylose. Also, the retrogradation of amylose may cause rapid opacification, aggregation and phase separation, which in turn decreases paste clarity (Craig et al. 1989; Achille et al. 2007). Other factors that influence paste clarity are phosphate, protein and lipid content (Craig et al. 1989).
Upon starch extraction, paste clarity of all starches increased, as compared to their respective flours (Fig. 1b). Cassava and yam starch were the clearest, whereas konjac starch was the most opaque paste. Canna, yam and konjac were the least stable during cooling storage. This might be due to their relatively high amylose content compared to the other starches. Based on their paste clarity, cassava and yam could be used for food applications that require paste clarity such as fruit fillings and jellies. Konjac flour and starch that forms an opaque pasta might be used for puddings, sauces, gravies, dressings and mayonnaise (Craig et al. 1989).
Conclusions
Flours and starches extracted from tubers and roots collected from Indonesia exhibit differences in their physicochemical properties. The relatively high starch content of these flours and starches makes them potential alternative sources of carbohydrate. Low digestibility of canna and konjac flour offers health benefits with potential applications in the prevention of obesity, hypertension and other related diseases. High digestibility of the remaining flours and starches could find use in the diets of the elderly and young children. These high-resistant starches are promising for textural benefits in low-water systems, especially if this property is combined with low-viscosity product applications such as soft candy, weaning foods and others liquid preparations. The low retrogradation of flours and starches examined in this study compared to that of wheat flour is important in frozen and cold-storage food products. Depending on system, high clarity pastes can be produced that find application in fruit filling, candy, and Turkish delight, whereas flour and starches that produce opaque paste could be used for salad dressings, sauces, mayonnaises and puddings. Given the functional versatility of the materials examined presently, direct technologically based research is required in the future to establish tuber and root starches as a wheat-flour substitute in composite materials with added value applications.
Acknowledgments
Financial support from AusAID in the form of an Australian Development Scholarship for the first named author is acknowledged.
References
- Achille TF, Georges AN, Alphonse K. Contribution to light transmittance modelling in starch media. Afr J Biotechnol. 2007;6:569–575. [Google Scholar]
- Charles AL, Huang TC, Lai PY, Chen CC, Lee PP, Chang YH. Study of wheat flour-cassava starch composite mix and the function of cassava mucilage in Chinese noodles. Food Hydrocoll. 2007;21:368–378. doi: 10.1016/j.foodhyd.2006.04.008. [DOI] [Google Scholar]
- Chen Z, Schols HA, Voragen AGJ. Physicochemical properties of starches obtained from three different varieties of Chinese sweet potatoes. J Food Sci. 2003;68:431–437. doi: 10.1111/j.1365-2621.2003.tb05690.x. [DOI] [Google Scholar]
- Chen Z, Schols HA, Voragen AGJ. Starch granule size strongly determines starch noodle processing and noodle quality. J Food Sci. 2003;68:1584–1589. doi: 10.1111/j.1365-2621.2003.tb12295.x. [DOI] [Google Scholar]
- Collado LS, Mabesa RC, Corke H. Genetic variation in the physical properties of sweet potato starch. J Agric Food Chem. 1999;47:4195–4201. doi: 10.1021/jf990110t. [DOI] [PubMed] [Google Scholar]
- Cornell H. The functionality of wheat starch. In: Elliason AC, editor. Starch in food. England: CRC Press; 2004. pp. 211–238. [Google Scholar]
- Craig SAS, Maningat CC, Seib PA, Hoseney RC. Starch paste clarity. Cereal Chem. 1989;66:173–182. [Google Scholar]
- Freitas RA, Paula RC, Feitosa JPA, Rocha S, Sierakowski MR. Amylose contents, rheological properties and gelatinisation kinetics of yam (Dioscorea alata) and cassava (Manihot utilisima) starches. Carbohydr Polym. 2004;55:3–8. doi: 10.1016/S0144-8617(03)00142-5. [DOI] [Google Scholar]
- Fu BX. Asian noodles: history, classification, raw materials, and processing. Food Res Int. 2008;41:888–902. doi: 10.1016/j.foodres.2007.11.007. [DOI] [Google Scholar]
- Gelencser T, Juhász R, Hódsági M, Gergely S, Salgó A. Comparative study of native and resistant starches. Acta Aliment. 2008;37:255–270. doi: 10.1556/AAlim.37.2008.2.11. [DOI] [Google Scholar]
- Hoover R. Composition, molecular structure and physicochemical properties of tuber and root starches – a review. Carbohydr Polym. 2001;45:253–267. doi: 10.1016/S0144-8617(00)00260-5. [DOI] [Google Scholar]
- Hoover R, Ratnayake WS, et al. Determination of total amylose content of starch. In: Wrolstad RE, Acree TE, Decker EA, et al., editors. Handbook of food analytical chemistry: water, proteins, enzymes, lipids, and carbohydrates. New Jersey: Wiley; 2005. pp. 689–693. [Google Scholar]
- Huang CC, Lin MC, Wang CCR. Changes in morphological, thermal, and pasting properties of yam (Dioscorea alata) starch during growth. Carbohydr Polym. 2006;64:524–531. doi: 10.1016/j.carbpol.2005.11.009. [DOI] [Google Scholar]
- Hung PV, Morita N. Physicochemical properties and enzymatic digestability of starch from edible canna (Canna edulis) grown in Vietnam. Carbohydr Polym. 2005;61:314–321. doi: 10.1016/j.carbpol.2005.04.021. [DOI] [Google Scholar]
- Jane JL, Shen L, Lim S, Kasemsuwan T, Nip WK. Physical and chemical studies of taro starches and flours. Cereal Chem. 1992;69:528–535. [Google Scholar]
- Jayakody L, Hoover R, Liu Q, Donner E. Studies on tuber starches. II. Molecular structure, composition and physicochemical properties of yam (Dioscorea sp) starches grown in Sri Lanka. Carbohydr Polym. 2007;69:148–163. doi: 10.1016/j.carbpol.2006.09.024. [DOI] [Google Scholar]
- Leon AE, Barrera GN, Perez GT, Ribotta PD, Rosell CM. Effects of damaged starch levels on flour-thermal behaviour and bread staling. Eur Food Res Technol A. 2006;2242:187–192. doi: 10.1007/s00217-006-0297-x. [DOI] [Google Scholar]
- Liu Q, Donner E, Yin Y, Huang RL, Fan MZ. The physicochemical properties and in vitro digestibility of selected cereals, tubers, and legumes grown in China. Food Chem. 2006;99:470–477. doi: 10.1016/j.foodchem.2005.08.008. [DOI] [Google Scholar]
- Martin M, Fitzgerald MA. Proteins in rice grains influence cooking properties. J Cereal Sci. 2002;36:285–294. doi: 10.1006/jcrs.2001.0465. [DOI] [Google Scholar]
- Mbofung CMF, Aboubakar, Njintang YN, Abdou Bouba A, Balaam FF. Physicochemical and functional properties of six varieties of taro (Colocasia esculenta L. Schott) flour. J Food Technol. 2006;4:135–142. [Google Scholar]
- Mepba HD, Eboh L, Nwaojigwa SU. Chemical composition, functional and baking properties of wheat-plantain composite flours. Afr J Food Agric Nutr Dev. 2007;7:1–22. [Google Scholar]
- Moorthy SN. Physicochemical and functional properties of tropical tuber starches: a review. Starch. 2002;54:559–592. doi: 10.1002/1521-379X(200212)54:12<559::AID-STAR2222559>3.0.CO;2-F. [DOI] [Google Scholar]
- Muyonga JH, Ramteke RS, Eipeson WE. Prehydration steaming change-physicochemical properties of unripe banana flour. J Food Process Preserv. 2001;25:35–47. doi: 10.1111/j.1745-4549.2001.tb00442.x. [DOI] [Google Scholar]
- Piyachomkwan K, Chotineeranat S, Kijkunasatian C, Tonwitowat R, Prammanee S, Oates CG, Sriroth K. Edible canna (Canna edulis) as a complementary starch source to cassava for the starch industry. Ind Crop Prod. 2002;16:11–21. doi: 10.1016/S0926-6690(02)00003-1. [DOI] [Google Scholar]
- Puncha-arnon S, Puttanlek C, Rungsardthong V, Pathipanawat W, Uttapap D. Changes in physicochemical properties and morphology of canna starches during rhizomal development. Carbohydr Polym. 2007;70:206–217. doi: 10.1016/j.carbpol.2007.03.020. [DOI] [Google Scholar]
- Radley JA (1976) Industrial uses of starch and its derivatives. In: Radley JA (ed). Applied Science Publishers, 268 pg
- Raja MKC, Sindhu P. Properties of steam-treated arrowroot (Maranta arundinacea) starch. Starch. 2000;52:471–476. doi: 10.1002/1521-379X(200012)52:12<471::AID-STAR471>3.0.CO;2-U. [DOI] [Google Scholar]
- Ratnayake WS, Hoover R, Shahidi F, Parera C, Jane J. Composition, molecular structure and physicochemical properties of starches from four field pea (Pisum sativum L.) cultivars. Food Chem. 2001;74:189–202. doi: 10.1016/S0308-8146(01)00124-8. [DOI] [Google Scholar]
- Santacruz S, Rualesa J, Eliasson AC. Three under-utilised sources of starch from the Andean region in Ecuador. Part II. Rheological characterization. Carbohydr Polym. 2003;51:85–92. doi: 10.1016/S0144-8617(02)00140-6. [DOI] [Google Scholar]
- Snow P, O’Dea K. Factor affecting the rate of hydrolysis of starch in food. Am J Clin Nutr. 1981;43:2721–2727. doi: 10.1093/ajcn/34.12.2721. [DOI] [PubMed] [Google Scholar]
- Srichuwong S, Sunarti TC, Mishima T, Isono N, Hisamatsu M. Starches from different botanical sources II: contribution of amylopectin fine structure to thermal properties and digestibility. Carbohydr Polym. 2005;62:25–34. doi: 10.1016/j.carbpol.2005.07.003. [DOI] [Google Scholar]
- Srichuwong S, Sunarti TC, Mishima T, Isono N, Hisamatsu M. Starches from different botanical sources II: contribution of starch structure to swelling and pasting properties. Carbohydr Polym. 2005;62:25–34. doi: 10.1016/j.carbpol.2005.07.003. [DOI] [Google Scholar]
- Taggart P. Starch as an ingredient: manufacture and application. In: Elliason AC, editor. Starch in food. England: CRC Press; 2004. pp. 363–392. [Google Scholar]
- Tattiyakul J, Asavasaksakul S, Pradipasena P. Chemical and physical properties of flour extracted from taro Colocasia esculenta (L.) Schott grown in different regions in Thailand. Sci Asia. 2006;32:279–284. doi: 10.2306/scienceasia1513-1874.2006.32.279. [DOI] [Google Scholar]
- Tester RF, Morrison WR. Swelling and gelatinisation of cereal starches I. Effect of amylopectin, amylose and lipids. Cereal Chem. 1990;67:551–559. [Google Scholar]
- Tetchi FA, Rolland-Sabate A, Amani GN, Colonna P. Molecular and physicochemical characterisation of starches from yam. cocoyam, cassava, sweet potato and ginger produced in the Ivory Coast. J Sci Food Agric. 2007;87:1906–1916. doi: 10.1002/jsfa.2928. [DOI] [Google Scholar]
- Thitipraphunkul K, Uttapap D, Piyachomkwan K, Takeda Y. A comparative study of edible canna (Cannae edulis) starch from different cultivars. Part I. Chemical composition and physicochemical properties. Carbohydr Polym. 2003;53:317–324. doi: 10.1016/S0144-8617(03)00081-X. [DOI] [Google Scholar]
- Wickramasinghe H, Noda T. Comparative analysis of starch properties of different root and tubers crops of Sri Lanka. Food Chem. 2009;112:98–103. doi: 10.1016/j.foodchem.2008.05.046. [DOI] [Google Scholar]
- Woolfe JA. Sweet potato: an untapped food source. Cambridge: Cambridge University Press; 1992. pp. 292–313. [Google Scholar]
- Yadav AR, Guha M, Tharanathan RN, Ramteke RS. Changes in characteristics of sweet potato flour prepared by different drying techniques. LWT. 2006;39:20–26. doi: 10.1016/j.lwt.2004.12.010. [DOI] [Google Scholar]
- Zaidul ISM, Norulaini NAN, Omar AKM, Yamauchi H, Noda T. RVA analysis of mixtures of wheat flour and potato, sweet potato, yam, and cassava starches. Carbohydr Polym. 2007;69:784–791. doi: 10.1016/j.carbpol.2007.02.021. [DOI] [Google Scholar]