Abstract
Nisin is a widely used bacteriocin active against gram positive bacteria and is also reported to be active against some gram negative bacteria. Incorporation of nisin into food systems is another challenge as directly added nisin is prone to inactivation by food constituents. Encapsulation of nisin has been done so far in liposomes which is rather an expensive technology involving multiple processes. Other cost effective alternatives with good encapsulation efficiency and better control release properties are sought. Alginate is useful as a matrix for entrapment of bioactive compounds. Present study was aimed at optimizing conditions for microencapsulation of nisin using calcium alginate as primary wall material and guar gum as filler at different air pressures using response surface methodology. The optimum conditions were: sodium alginate concentration (2 % w/v), guar gum concentration (0.4 % w/v), and air pressure (0.5 bar gauge). The encapsulation efficiency of nisin in microcapsules produced under optimal conditions was 36.65 %.
Keywords: Alginate, Guar gum, Encapsulation efficiency, nisin, bacteriocins, RSM
Introduction
Microencapsulation is a process by which small droplets or particles of liquid or solid core material are surrounded or coated with a continuous film of polymeric material. Microencapsulation improves the performance of biologically active substance and enhances its shelf life. It offers a means to convert liquids to solids, to alter colloidal and surface properties, to provide environmental protection and to control the release characteristics or availability of core materials. A number of above characteristics can be attained by macro-packaging techniques however the exclusivity of microencapsulation is the compactness of the coated particles and their subsequent use and adaptation to a wide variety of dosage forms (Bansode et al. 2010).
Nisin is a small, cationic, hydrophobic peptide that is of special interest to the food and dairy industry, because it has been conferred GRAS (generally regarded as safe) status by the Food and Drug Administration (FDA) (Federal Register 1988). Nisin is primarily active against Gram-positive bacteria but when combined with a chelator, nisin can inhibit growth of some Gram-negative bacteria also and is thus potentially effective against a broad spectrum of bacteria (Dawson et al. 2005). Methods to deliver antimicrobials to food can vary from direct addition to incorporation into packaging materials. Although instant addition yields immediate inhibition of microorganisms, its efficacy is lost in short span. The antimicrobials are consumed in the reaction to kill microbes or become ineffective due to complex interactions with the food matrix and by natural degradation over time. Thus the protection ceases and results in challenged microbial safety and quality (Balasubramanian et al. 2011). The direct application of nisin to food systems will also result in development of bacteriocin resistance of bacteria (Kaur et al. 2011). Microencapsulation is an efficient technique to minimize nisin resistance development and achieve controlled release of nisin. Nisin has so far been encapsulated in liposome which is rather an expensive technology (Laridi et al. 2003). Biopolymers like zein, cellulose have also been explored for making micro particles and films for controlled release of nisin (Guiga et al. 2010; Xiao et al. 2011), but microencapsulation using alginate was less exploited. Although micro particles of alginate were prepared by Wan et al. (1997), but optimization has not been done since then and its scale up is also not available.
Alginate is perhaps the most widely used material for bioencapsulation (Chan et al. 2011). Alginate, a linear polysaccharide extracted from brown seaweed, is composed of variable proportions of β-D-mannuronic acid (M block) and α-L-guluronic acid (G block) linked by 1–4 glycosidic bonds (Fu et al. 2011; Hay et al. 2010). Sodium alginate is a polyelectrolyte with negative charges on its backbone (Zhong et al. 2011). Alginate forms a thermally stable and biocompatible hydrogel in the presence of di- or tri-cations. Alginate beads can be easily produced by dropping an alginate solution in a calcium chloride bath. Alginate has been used in many encapsulation applications, including various fields, such as biomedical, bioprocess, pharmaceutical, food, and feed (Chan et al. 2011). Alginate is useful as a matrix for immobilization of plant, animal and microbial cells as well as entrapment of bioactive compounds and drugs (Goh et al. 2012; Narsaiah et al. 2011). The material encapsulated within the inert alginate environment could be delivered at a desired rate in a controlled release system. Encapsulant is released from alginate by diffusional processes through pores and the release is facilitated by the degradation of the polymeric network (Goh et al. 2012). Guar gum is naturally occurring galactomannan polysaccharide consisting of a linear chain of β-d-mannospyronose joined by β-(1–4) linkage with α-D-galactopyranosyl units attached by 1,6 linkage in the ratio of 1:2. The hydration property of guar gum is an important characteristic in many applications where solutions of these polymers often need to be prepared, for example in the pharmaceutical industry; it is used for controlled release (Wang et al. 2002). Present study was aimed at optimising the process parameters of microencapsulation of nisin to achieve maximum encapsulation efficiency in calcium alginate as wall material and guar gum as filler using response surface methodology.
Material and methods
Present study was carried out in the laboratory of Agricultural Structures and Environmental Control, CIPHET, Ludhiana.
Microencapsulation
Microcapsules were prepared using air atomization technique with an in house developed encapsulator. Sodium alginate + guar gum solution was prepared by mixing the dry powders of sodium alginate (High viscosity, > 2000 cP for 2 % solution) and guar gum (SD Fine Chemicals, India) and then dissolving these polymers in distilled water overnight using magnetic stirrer (Labco Instruments, New Delhi, India) with mild heating (30 °C). Nisin solution (0.1 % in 0.02 N HCl) (Danisco, Germany) was added at the rate of 10 % to polymer solution. The aqueous solution of sodium alginate-guar gum containing nisin was delivered from a digitally controlled peristaltic pump (Ravel Hiteks Pvt. Ltd., Chennai, India) at controlled flow rate (120 ml/min) into a concentric two fluid glass nozzle and sprayed under pressurized air into a reaction vessel containing 0.1 M calcium chloride solution. The distance between the nozzle tip and the liquid level in reaction vessel was fixed at 12 in. The size of orifice of inner nozzle (carrying polymer solution/ matrix fluid) is 1 mm. It has 1.3 mm annular space (carrying pressurized air) between inner and outer nozzle. The schematic representation of experimental set up is shown in Fig. 1. The kinetic energy of high pressure air was used for breakup of matrix fluid jet. The spray of small droplets falls in reaction vessel. The divalent calcium ions in reaction vessel replaced sodium ions and cross linked alginate polymer chains and formed calcium alginate-guar gum microcapsules by ionotropic gelification. Calcium chloride solution was continuously stirred at 1000 rpm during spraying of polymer solution and was stirred for 30 min after spray for hardening. The hardened microcapsules were sieved and washed with deionised water.
Fig. 1.
Schematic representation of microencapsulator set up with two fluid glass nozzle
Encapsulation efficiency
Content of nisin was measured using Lowry method. Microcapsules were macerated in phosphate buffer (0.2 M/7.4pH) and centrifuged at 10000 rpm for 30 min. Nisin content of supernatant was estimated. Nisin content of polymer solution of alginate + guar gum before spraying was also estimated. Encapsulation efficiency (EE) was calculated using following formula:
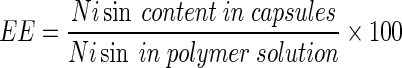 |
Experimental design for response surface methodology
There were many factors that can affect the encapsulation efficiency; therefore response surface methodology was applied for optimizing the encapsulation efficiency. Box-Behnken design was used to statistically optimize the processing parameters and evaluate the main effects, interaction effects and quadratic effects of the processing parameters on encapsulation efficiency (Box and Behnken 1960). A 3-factor, 3-level design was used to explore the quadratic response surfaces and for constructing second order polynomial models using Design Expert® (Version 8.0.2, Stat-Ease, Minneapolis, MN). The independent variables used for study are shown in Table 1. Levels of independent variables were selected on the basis of literature available and preliminary screening experiments. The quadratic polynomial regression model was assumed for predicting Y variable (EE = Encapsulation efficiency). The model proposed for the response of Y fitted equation as follows:
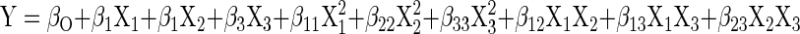 |
Table 1.
Actual and predicted values of encapsulation efficiency for experimental runs of Box-Behnken design.
| Run | Guar Gum (%w/v) | Sodium Alginate (%w/v) | Pressure (Bar gauge) | Encapsulation Efficiency (%) (Actual) | Encapsulation Efficiency (%) (Predicted) |
|---|---|---|---|---|---|
| 1 | 0.4 | 2.5 | 0.25 | 37.415 | 36.270 |
| 2 | 0.6 | 2.0 | 0.25 | 37.989 | 37.842 |
| 3 | 0.4 | 2.0 | 0.50 | 36.942 | 36.653 |
| 4 | 0.4 | 2.0 | 0.50 | 36.535 | 36.653 |
| 5 | 0.4 | 2.5 | 0.75 | 37.107 | 36.528 |
| 6 | 0.2 | 2.0 | 0.75 | 30.259 | 30.406 |
| 7 | 0.2 | 2.0 | 0.25 | 28.157 | 28.870 |
| 8 | 0.4 | 1.5 | 0.25 | 24.929 | 25.508 |
| 9 | 0.2 | 1.5 | 0.50 | 22.081 | 20.789 |
| 10 | 0.4 | 2.0 | 0.50 | 36.733 | 36.653 |
| 11 | 0.2 | 2.5 | 0.50 | 30.415 | 30.847 |
| 12 | 0.6 | 2.0 | 0.75 | 36.432 | 35.719 |
| 13 | 0.4 | 1.5 | 0.75 | 23.521 | 24.666 |
| 14 | 0.4 | 2.0 | 0.50 | 36.891 | 36.653 |
| 15 | 0.6 | 1.5 | 0.50 | 27.110 | 26.678 |
| 16 | 0.6 | 2.5 | 0.50 | 37.951 | 39.243 |
| 17 | 0.4 | 2.0 | 0.50 | 36.162 | 36.653 |
Where βO is the constant coefficient of intercept and are regression coefficients computed from the observed experimental values of Y from experimental runs.
The best conditions for the production of microcapsules were obtained by desirability analysis. The goal was to obtain maximum encapsulation efficiency. Validation of the correlation was done by comparing the size of the microcapsules experimentally obtained with that predicted from the regression equation.
Statistical analysis was performed with the software package ‘Design Expert’ (Version 8.0.2, Stat-Ease, Minneapolis, MN). The adequacy of response surface model was investigated using regression coefficient analysis and the Analysis of Variance (ANOVA) with the lack of fit test. ANOVA was performed to assess the significance of the effect of the independent variables on response variable i.e. encapsulation efficiency and statistical model.
Characteristics of microcapsules
Particle size of microcapsules was measured with particle size analyzer (Horiba Instruments, Japan). Morphology of microcapsules was studied under compound microscope (Motic, Hong Kong) and optical research microscope having provision of phase contrast mode (Leica-5000, Lieca Microsystems, Germany).
Results and discussion
Optimization of encapsulation efficiency
A 17 run Box-Benhken design with three factors and three levels, including five runs replicated at the centre point, was used for the fitting a second order response surface. The five centre point runs were added to provide as a measure of process stability and inherent variability. The actual encapsulation efficiency obtained in experiments and predicted encapsulation efficiency produced by the model are given in Table 1. The mathematical equation expressing relationship of encapsulation efficiency with variables Xguar gum, Xalginate and Xpressure is given below in terms of coded factors.
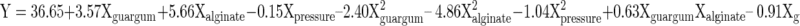 |
In order to determine significance of the quadratic model, it was necessary to run ANOVA analysis. The ANOVA of quadratic regression model demonstrated that the model to be significant, as is evident from Fisher’s F-test value being 46.08. The P-value was used as a tool for checking the significance of each coefficient. It also indicated the interaction strength of each parameter. The smaller the P-value, the larger is the significance of corresponding coefficient (Murthy et al. 2000). Here the P-value was smaller than 0.0001which indicated that the model was suitable for use. The fitness of the model was further confirmed by a satisfactory value of coefficient of determination (R2) which was calculated to be 0.983. The value of adjusted coefficient of determination (R2adjusted) was calculated to be 0.962 which established high significance of the model. At the same time relatively low value of the coefficient of variation (3.36 %) indicated greater accuracy and reliability of the experiment. The linear terms Xguar gum, and Xalginate as well as Xalginate2 were significant model terms with p-value less than 0.0001. Xguar gum2 was also significant model term with p-value less than 0.01. Xguar gum, Xalginate and Xpressure had largest effect on encapsulation efficiency. Xpressure was non-significant as revealed by high P-value.
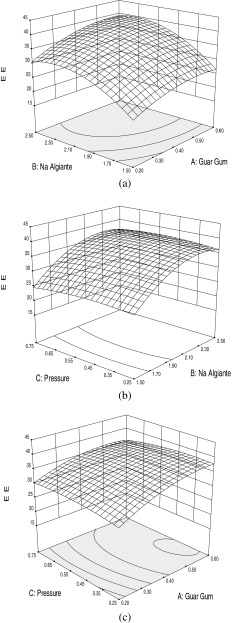
The 3D plots (Fig. 2) are the graphical representations of the regression equation from which the value of encapsulation efficiency for different variables can be predicted. These graphs are plotted as a function of two of factors while keeping the third variable as constant at its mean level. There was increase in encapsulation efficiency with increase in both alginate and guar gum concentration (Fig. 2a). The maximum encapsulation efficiency was achieved with highest concentrations of alginate and guar gum. This is apparently due to the fact that on increasing the alginate concentration, there is an increase in the number of biopolymer molecule per unit volume which in turn increases the number of binding sites for the calcium ions. As a result more densely packed gel structure forms that entrap more nisin (Ana et al. 2000). Guar gum has been used for encapsulation of various bioactive components (Wang et al. 2002), its addition to alginate further enhanced the encapsulation efficiency by increasing the density of the gel. Pressure had no significant effect on encapsulation efficiency as can be observed from the surface plots (Fig. 2b and c).
Fig. 2.
Response surface plots for encapsulation efficiency (EE) with respect to (a) sodium alginate and guar gum, (b) sodium alginate and air pressure and (c) Guar gum and air pressure
A numerical procedure was carried out for predicting the optimum level of alginate concentration, guar gum concentration and air pressure leading to the desirable encapsulation efficiency. The optimization procedure showed that the optimum values were: sodium alginate concentration (2%w/v), guar gum concentration (0.4 % w/v), and air pressure (0.5 bar gauge). Under these optimum conditions, the predicted response value for encapsulation efficiency was 36.653 % which was close to experimental encapsulation efficiency (36.652 %) obtained by testing the microcapsules prepared according to the optimized conditions. The prediction error for encapsulation efficiency for three variables was found to be 1.2 %. Although the encapsulation efficiency is not very high but encapsulated nisin will be effective for longer duration of time due to controlled release rather than the direct addition of nisin (Balasubramanian et al. 2011). These results represents that the regression equation was a suitable model to describe the response of experimental parameters to the encapsulation efficiency of microcapsules. Although concentrations of alginate beyond 2 % (w/v) may yield higher encapsulation efficiency but handling issues crop up. Solution of alginate used in the present study became highly viscous beyond 2 % and thus pose difficulty in pumping it.
Characteristics of microcapsules
The mean particle size of the microcapsules was 233.41 μm. Morphologically capsules were spherical in shape, were shiny and whitish in colour (Fig. 3). Surface of the capsules was not very smooth with small pits on its walls. Similar results have been found by Nochos et al. (2008) in alginate beads.
Fig. 3.

Morphology of microcapsules (produced at optimized conditions) (a) 10 X magnification, (b) 40X- bright field, and (c) 40X-dark field
Conclusion
The optimum conditions for microcapsules with maximum encapsulation efficiency were: sodium alginate concentration (2 % w/v), guar gum concentration (0.4 % w/v), and air pressure (0.5 bar gauge). The encapsulation efficiency of nisin microcapsules produced under optimal conditions was 36.65 %. Although the encapsulation efficiency is not very high but encapsulated nisin offers possibility of controlled release and sustained activity. These results suggested that the combination of alginate and guar gum can be used as wall material for encapsulation of nisin to increase the encapsulation efficiency.
Acknowledgment
This work was supported by National fund for Basis, Strategic and Frontier Application Research in Agriculture (NFBSFARA), Indian Council of Agricultural Research (ICAR), New Delhi, through project entitled “Microencapsulation methods for bacteriocins for their controlled release”.
References
- Ana B, Manuel M, Domingo C. Glucose oxidase release from calcium alginate gel capsules. Enzyme Microb Technol. 2000;27:319–324. doi: 10.1016/S0141-0229(00)00204-0. [DOI] [PubMed] [Google Scholar]
- Balasubramanian A, Lee DS, Chikindas ML, Yam KL. Effect of nisin’s controlled release on microbial growth as modeled for Micrococcus luteus. Probiotics Antimicro Prot. 2011;3:113–118. doi: 10.1007/s12602-011-9073-8. [DOI] [PubMed] [Google Scholar]
- Bansode SS, Banarjee SK, Gaikwad DD, Jadhav SL, Thorat RM. Microencapsulation: a review. I J Pharm Sci Rev Res. 2010;1(2):38–43. [Google Scholar]
- Box GEP, Behnken DW. Some new three level designs for study of quantitative variables. Technometrics. 1960;2:455–476. doi: 10.1080/00401706.1960.10489912. [DOI] [Google Scholar]
- Chan ES, Wong SL, Lee PP, Lee JS, Ti TB, Zhang Z, Poncelet D, Ravindra P, Phan SH, Yim ZH. Effects of starch filler on the physical properties of lyophilized calcium–alginate beads and the viability of encapsulated cells. Carbohydr Polym. 2011;83(1):225–232. doi: 10.1016/j.carbpol.2010.07.044. [DOI] [Google Scholar]
- Dawson PL, Sotthibandhu H, Han IY. Antimicrobial activity of nisin-adsorbed silica and corn starch powders. Food Microb. 2005;22:93–99. doi: 10.1016/j.fm.2004.04.001. [DOI] [Google Scholar]
- Fu S, Thacker A, Sperger D, Boni R, Buckner I, Velankar S. Relevance of rheological properties of sodium alginate in solution to calcium alginate gel properties. AAPS Pharm Sci Technol. 2011;12(2):1–8. doi: 10.1208/s12249-011-9587-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh CH, Heng PWS, Chan LW. Alginates as a useful natural polymer for microencapsulation and therapeutic applications. Carbohydr Polym. 2012;88:1–12. doi: 10.1016/j.carbpol.2011.11.012. [DOI] [Google Scholar]
- Guiga W, Swesi Y, Galland S, Peyrol E, Degraeve P, Sebti I. Innovative multilayer antimicrobial films made with Nisaplin® or nisin and cellulosic ethers: physico-chemical characterization, bioactivity and nisin desorption kinetics. Innov Food Sci Emerg Tech. 2010;11:352–360. doi: 10.1016/j.ifset.2010.01.008. [DOI] [Google Scholar]
- Hay ID, Rehman ZU, Ghafoor A, Rehm BHA. Bacterial biosynthesis of alginates. J Chem Tech Biotech. 2010;85(6):752–759. doi: 10.1002/jctb.2372. [DOI] [Google Scholar]
- Kaur G, Singh TP, Malik RK, Bhardwaj A, De S (2011) Antibacterial efficacy of nisin, pediocin 34 and enterocin FH99 against L. monocytogenes, E. faecium and E. faecalis and bacteriocin cross resistance and antibiotic susceptibility of their bacteriocin resistant variants. J Food Sci Technol. doi:10.1007/s13197-011-0500-3 [DOI] [PMC free article] [PubMed]
- Laridi R, Kheadr EE, Benech RO, Vuillemard JC, Lacroix C, Fliss I. Liposome encapsulated nisin Z: optimization, stability and release during milk fermentation. I Dairy J. 2003;13:325–336. doi: 10.1016/S0958-6946(02)00194-2. [DOI] [Google Scholar]
- Murthy MSRC, Swaminathan T, Rakshit SK, Kousugi Y. Statistical optimization of lipase catalysed hydrolysis of methyloleate by response surface methodology. Bioproc eng. 2000;22:35–39. doi: 10.1007/PL00009097. [DOI] [Google Scholar]
- Narsaiah K, Jha SN, Bhardwaj R, Sharma R, Kumar R. Optical biosensors for food quality and safety assurance - a review. J Food Sci Technol. 2011;49(4):383–406. doi: 10.1007/s13197-011-0437-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nochos A, Douroumis D, Bouropoulos N. In vitro release of bovine serum albumin from alginate/HPMC hydrogel beads. Carbohydr Polym. 2008;74:451–457. doi: 10.1016/j.carbpol.2008.03.020. [DOI] [Google Scholar]
- Register F. Nisin preparation: affirmation of GRAS status as a direct human food ingredient. Federal Register. 1988;53:11247–11251. [Google Scholar]
- Wan J, Gordon JB, Muirhead K, Hickey MW, Coventry MJ. Incorporation of nisin in micro particles of calcium alginate. Lett appl microbiol. 1997;24:153–158. doi: 10.1046/j.1472-765X.1997.00294.x. [DOI] [PubMed] [Google Scholar]
- Wang Q, Ellis PR, Ross-Murphy SB. Dissolution kinetics of guar gum powders. I. Methods for commercial polydisperse samples. Carbohydr Polym. 2002;49(1):131–137. doi: 10.1016/S0144-8617(01)00327-7. [DOI] [Google Scholar]
- Xiao D, Davidson PM, Zhong Q. Release and antilisterial properties of nisin from zein capsules spray-dried at different temperatures. LWT - Food Sci Tech. 2011;44:1977–1985. doi: 10.1016/j.lwt.2011.07.017. [DOI] [Google Scholar]
- Zhong D, Huang X, Yang H, Cheng R. New insights into viscosity abnormality of sodium alginate aqueous solution. Carbohydr Polym. 2011;81(4):948–952. doi: 10.1016/j.carbpol.2010.04.012. [DOI] [Google Scholar]