Abstract
Freshness of mushrooms (Agaricus bisporus) was related to the internal atmosphere composition during modified atmosphere packaging (MAP) experiments using polyvinyl chloride (PVC) wrap, polyethylene-1 (PE-1) and PE-2 films. The packaged mushrooms were stored at 12 °C for 7 days and lightness value, browning index, weight loss and maturity index were also measured. The results obtained showed that the whiteness of whole mushrooms varied significantly with the type of coating (chitosan and CaCl2), but not with the type of packaging films. It was evident that the extent of darkening in whole mushroom was greater than in sliced ones after coated. In addition, mushroom in PE-2 package exhibited the lowest weight loss due to the lower permeability of film. And the type of packaging films significantly affected the maturity index of mushroom, where PE-2 packaging most effectively lowered maturity index for both whole and sliced mushrooms. By considering the overall quality, it was obvious that PE-2 packaging combined with coating treatment was the most effective to improve the preservation of mushrooms stored at 12 °C up to 7 days and satisfy consumer acceptance.
Keywords: Modified atmosphere packaging, Agaricus bisporus, Whole mushroom, Sliced, Coating
Introduction
Mushroom (Agaricus bisporus) is highly perishable and nutritional in nature, containing several bioactive compounds, including polysaccharides (such as mannose and trehalose), antioxidants, dietary fiber, ergosterol, vitamin B1, B2 and C, folates, niacin and minerals. However, due to the high respiration rate, its rapid postharvest degradation and quality maintenance still remains an economic issue (Mahajan et al. 2008). It was reported that after only 1 day of storage at ambient temperature, the cap was opened and colored, stem was elongated, and texture became soft and spongy, resulting in shortening the shelf-life of mushroom (Guillaume et al. 2010). Besides, the sliced mushrooms, usually marketed in trays overwrapped with plastic films and have drawn the attention of industry as novel lightly processing and consuming convenience. Therefore, it is critical and important to optimizing postharvest quality and improve shelf-life of fresh mushroom (Xing et al. 2008).
The use of synthetic chemical fungicides has been the main method for reducing postharvest disease of fruits and vegetables. However, consumers concerning over pesticide residues on foods, along with pathogen resistance to many currently used pesticides, has increased the need to find alternative methods for decay control. Recently, biologically active natural products are becoming an effective alternative to chemical fungicides (Tripathi and Dubey 2004). So far, packaging, refrigeration, coating and dipping are the most common approaches used for extending the shelf life of mushrooms (Eissa 2007; Mau et al. 1993; Roy et al. 1993).
Modified atmosphere packaging (MAP) has become increasingly common in recent years that is created by altering normal air composition, in order to provide an appropriate atmosphere surrounding the produce for decreasing its deterioration rate and prolonging its shelf life (Farber et al. 2003; Tano et al. 1999). Shelf-life extension due to modified atmosphere can be mainly attributed to low O2 and high CO2 concentration in package, which causes a decrease in respiration rate and also inhibits microbial growth of fresh produce (Farber et al. 2003). Several works reported some beneficial effects of MAP on the postharvest storage potential of Agaricus sp. and Pleurotus sp. mushrooms (Ares et al. 2007; Villaescusa and Gil 2003). Appropriate packaging can delay development of postharvest deterioration and senescence of this highly perishable commodity.
Most recently, it has been reported that chitosan [poly-β-(1,4)N-acetyl-D-glucosamine], due to its non-toxic nature, antibacterial activity and biocompatibility, has attracted much attention as a natural food additive (Dutta et al. 2009; Ravi Kumar 2000). Chitosan and its derivatives have been processed excellent film-forming properties used in fruits and vegetables (Tamer and Çopur 2010), and have been successfully used to prolong the shelf-life and maintain the quality contributes of many fresh-cut produce, such as litchi (Dong et al. 2004), mango (Chien et al. 2007), blueberry (Duan et al. 2011) and water caltrop fruit (Zhan et al. 2011) and mushroom (Eissa 2007). Furthermore, calcium ions play an essential role in the structural maintenance of membranes and cell walls. Preharvest and postharvest treatments with calcium salts have been effective in controlling several physiological disorders, reducing the incidence of fungal pathogens and maintaining fruit firmness (Bakshi et al. 2005). It has been reported that postharvest coating treatments with or without MAP could result in the improvement of shelf-life and quality maintenance (Del Nobile et al. 2009; Eissa 2007).
Therefore, the main objective of the present research was to evaluate combined effect of MAP and coating treatments on the shelf-life improvement of whole and sliced mushroom (Agaricus bisporus) stored at 12 °C, investigating headspace gas composition, color, weight loss and maturity index as indicators of mushroom quality.
Materials and methods
Sample preparation and coating treatment
Fresh mushrooms (Agaricus bisporus) were purchased from a local orchard after harvest, and then were transported to the laboratory in one hour. Mushrooms were selected, based on the pileus size of 30 to 40 mm, the absence of mechanical damage and fungal infection, and refrigerated in darkness at 4 °C before slicing to 5 mm thickness and processing. Coating solutions were prepared by dissolving 0.3 % chitosan in 0.5 % acetic acid solution, and 2.0 % calcium chloride in deionized water, respectively. The pH value of chitosan solution was adjusted to pH 5.0 with NaOH solution. Whole and sliced mushrooms were spray-coated with solution of chitosan and CaCl2. Then the surface was allowed to dry using a fan at ambient temperature.
Modified atmosphere packaging
The following packaging films were investigated: polyvinyl chloride (PVC) wrap (28 μm thickness with a permeability to O2 and CO2 of 1.6 × 10−6 mL m−2 s−1 Pa−1 and 4.8 × 10−6 mL m−2 s−1 Pa−1 at 23 °C and 0 % RH, respectively), and two types of polyethylene films: PE-1 film (25 μm thickness with a permeability to O2 and CO2 of 1.4 × 10−6 mL m−2 s−1 Pa−1 and 5.6 × 10−6 mL m−2 s−1 Pa−1 at 23 °C and 0 % RH, respectively) and PE-2 (30 μm thickness with a permeability to O2 and CO2 of 1.1 × 10−6 mL m−2 s−1 Pa−1 and 2.5 × 10−6 mL m−2 s−1 Pa−1 at 23 °C and 0 % RH, respectively). Both coated and uncoated mushrooms were randomly divided into the three above package groups. Each package (22.0 × 30.0 cm) containing 180 g of mushrooms was heat-sealed and stored at 12 °C in a static-conditioned incubator (Shellab, USA) for 7 days. The mushrooms without coating were also packaged and stored as control. For each treatment, three replicates were performed.
Package headspace gas analysis
The gas composition of the package headspace was determined with a gas chromatograph (Shimadzu, GC-14A) based on the method reported by Ishikawa et al. (1997) with some modifications. O2 and CO2 concentration was measured by using Molecular Sieve 5A column and Propaq-Q column, respectively. Internal gas atmospheres in packages were withdrawn by means of a gas tight syringe. The chromatograph was a dual detector type and thermal conductivity detector was used during the measurements. The carrier gas was helium. The package headspace gas composition was analyzed by use of the analyzer (Chromato-pack, Shimadzu, Japan).
Color evaluation
The ground color of whole and sliced mushroom was measured taken at random locations on the sliced site of the cap using a Hunterlab MiniScan XE colorimeter (Hunterlab, Reston VA, USA). Lightness value (L*) parameter in the L*a*b* mode of CIE, since it was considered as the best CIE parameter correlated with the market value of mushroom (Lopez Briones et al. 1992). The parameter L* was a measure of brightness/whiteness that ranged from 0 to 100 (white if L* = 100, black if L* = 0). Color was also assessed in terms of the following browning index (BI):
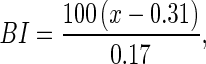 |
where x = (a − 1.75 × L)/(5.645 × L) + (a − (3.012 × b)). The colorimeter was standardized using a white reflectance plate (L* = 98.9, a* = −0.44 and b* = −0.30).
Weight loss (WL) determination
Weight loss for each package was determined by dividing the weight change during the storage period by the original weight.
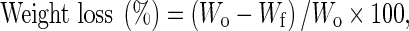 |
where Wo is the weight on the original weight on the first day and Wf is the final weight on the end of storage period.
Maturity index (MI) determination
For each package, the maturity index was assigned to whole and sliced mushrooms selected randomly based on the extend of cap opening on a 7-point scale: (1) veil intact tightly, (2) veil intact stretched, (3) veil partially broken (< half), (4) veil partially broken (> half), (5) veil completely broken, (6) cap open and gills well exposed, and (7) cap open and gill surface flat (Roy et al. 1995). Overall acceptability based on sensory attributes viz. appearance, flavor, taste and firmness was done by a trained panel of twelve judges using 5-point scale where, (1) excellent, (2) good, (3) fair, (4) poor and (5) not been acceptance at all. For each treatment five pieces of mushrooms were selected randomly, coded and served to judges.
Statistical analysis
All experiments were replicated three times and data were analyzed by a Duncan’s multiple-range test with SAS statistical software. The experimental data were subjected to a one-way analysis of variance (ANOVA) and the least significant difference (LSD) was calculated to compare significant effects at 5 % level and only significant differences were discussed unless stated otherwise.
Results and discussion
Changes in headspace gas composition of mushrooms
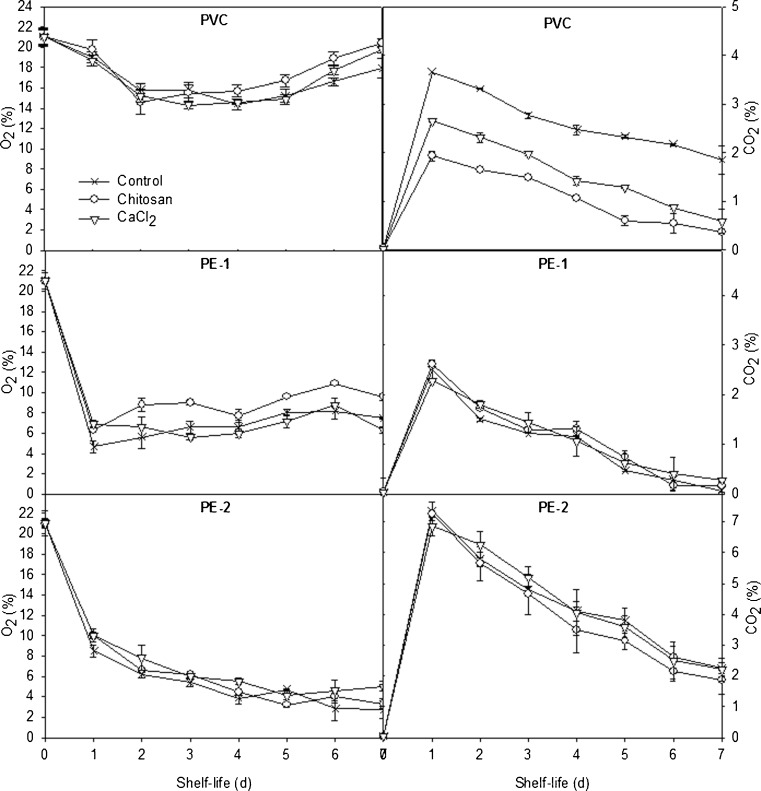
As shown in Figs. 1 and 2, a steady state period occurred after a transient state period where O2 concentration decreased whereas CO2 concentration increased, whatever the packaging films used, in agreement well with results previously observed in MAP experiments for fresh fruits and vegetables (Barron et al. 2002; Charles et al. 2008). The steady state was reached when gas diffusion through the film exactly compensates O2 consumption and CO2 production by mushrooms (Guillaume et al. 2010). In the present study, the whole mushroom in PVC wrap depleted O2 in packages was achieved in 2 to 5 days of storage, then a slight production afterwards. The evolution of CO2 concentration, however, in packages of both whole and sliced mushrooms during storage was similar for the three packaging films (Figs. 1 and 2). The CO2 concentration around whole and sliced mushrooms in different types of coating treatments and package films increased sharply on day 1 except for the sliced mushroom without coating treatment in PVC wrap packaging (Fig. 2). The peak on day 1 was believed to be a result of a sharp rise in postharvest respiration that occurs with fresh fruits and vegetables (Eskin et al. 1971), especially the mushroom which has higher respiration rate. Both PE-1 and PE-2 packages showed peak concentrations of CO2 within a day, and after that, sliced mushroom packages sustained higher CO2 and lower O2 concentrations than whole ones (Figs. 1 and 2), which indicated that the respiration rate of the mushrooms was increased by increasing surface areas.
Fig. 1.
O2 and CO2 concentrations of whole mushrooms in different coating treatments and packaging films during storage at 12 °C for 7 days. Data shown are mean ± standard deviation (n = 3). PVC polyvinyl chloride wrap; PE-1 polyethylene-1 film, PE-2 polyethylene-2 film
Fig. 2.
O2 and CO2 concentrations of sliced mushrooms in different coating treatments and packaging films during storage 12 °C for 7 days. Data shown are mean ± standard deviation (n = 3). PVC polyvinyl chloride wrap, PE-1 polyethylene-1 film, PE-2 polyethylene-2 film
The patterns of change in gas composition were also in agreement with the results of Lopez Briones et al. (1992) and Roy et al. (1995) who reported that the CO2 concentration around mushroom sharply increased after a few hours in modified atmosphere package. Considering the relatively higher respiration rate of fresh mushroom, the modification of the package headspace readily occurred according to different gas permeabilities of package films. The rates of O2 depletion and CO2 production by mushroom were much greater than the permeation rates of these gases through PE-1 and PE-2 films. The CO2 concentration decreased after 1 day and gas compositions were controlled by O2 and CO2 transmission rates through packaging films (Kang et al. 2001). And according to Guillaume et al. (2010), the generated equilibrium atmosphere obtained in MAP experiments should be compared with the optimal atmosphere recommended for mushrooms. The O2 level in package should not be lower than 2 % otherwise aerobic respiration turns into anaerobic, resulting in off-flavors (acetaldehyde and ethanol) and a sharp increase in tissue breakdown (Cliffe-Byrnes and O'Beirne 2007). It was also worthy to mention that the CO2 concentration higher than 5 % could enhance overall browning or yellowing, and induce a phytotoxic effect, which was marked by an increase in the respiration rate when the produce returned to normal air composition (Lopez Briones et al. 1992; Guillaume et al. 2010). Taken together, it is evident that the O2 and CO2 concentrations varied in the safe ranges when reached to the steady state during storage in the present study.
Change in color
The browning degree of whole and sliced mushrooms in different types of coating treatments and packaging films was expressed as Lightness value (L*) and browning index (BI) (Table 1). The whiteness of whole mushrooms varied significantly with the type of coating, but not with the type of films (P < 0.05). It was evident that the extent of darkening in whole mushrooms was greater than in sliced ones after coating treatment (Table 1). It has been reported that the L* value of mushroom may not be considered as commercially acceptable if a L* value of 80 for wholesalers was take into account (Lopez Briones et al. 1992). It was reported that calcium chloride and chitosan coating could significantly maintain L* value and free from browning of fruits and vegetables during storage (Qi et al. 2011; Oms-Oliu et al. 2008).
Table 1.
Lightness value (L*) and browning index (BI) of whole and sliced mushrooms in different types of coating treatments and MAP packages stored at 12 °C for 7 days
| L* | BI | |||||
|---|---|---|---|---|---|---|
| Control | Chitosan | CaCl2 | Control | Chitosan | CaCl2 | |
| Whole mushroom | ||||||
| PVCA | 87.6 ± 1.16a | 82.0 ± 1.24b | 80.5 ± 0.74b | 13.9 ± 0.42a | 12.3 ± 0.40b | 14.3 ± 0.52a |
| PE-1A | 89.7 ± 0.82a | 74.2 ± 1.07c | 81.2 ± 1.83b | 16.1 ± 0.17a | 12.7 ± 0.24c | 14.4 ± 0.33b |
| PE-2A | 89.3 ± 1.25a | 76.9 ± 0.88b | 77.5 ± 0.92b | 15.4 ± 0.54a | 11.1 ± 0.55b | 15.1 ± 0.04a |
| Sliced mushroom | ||||||
| PVCB | 79.0 ± 1.02a | 72.5 ± 1.11b | 75.2 ± 1.02ab | 14.9 ± 0.29a | 12.9 ± 0.57b | 14.0 ± 0.86ab |
| PE-1B | 77.7 ± 1.25a | 73.4 ± 0.72b | 73.4 ± 0.87b | 14.5 ± 0.33a | 10.7 ± 0.26a | 13.9 ± 0.45a |
| PE-2A | 89.1 ± 0.85a | 79.3 ± 1.35b | 82.5 ± 0.90b | 13.0 ± 0.84a | 10.5 ± 0.22b | 11.2 ± 0.30b |
Data shown are mean ± standard deviation (n = 3). Different superscripts within a column (A, B) between packaging films and within a row (a, b) between coating treatments differ significantly (P < 0.05)
PVC polyvinyl chloride wrap, PE-1 polyethylene-1 film, PE-2 polyethylene-2 film
A different browning index (BI) was observed in whole and sliced mushrooms stored with different types of package films, with values ranged from 10.5 to 16.1, that was attributed to a generalized enzymatic browning in mushroom during storage. The browning index (BI) of uncoated mushrooms was significantly higher than either chitosan or CaCl2 coating ones, except for the sliced mushroom packaged in PE-1 film (P < 0.05). Generally, both the whole mushroom in PE-1 film and the sliced mushroom in PE-2 film coated chitosan had lower browning index (BI) than other treatments. It is obvious that coating treatment did have a positive effect on inhibiting browning of mushrooms. Similarly, it was reported that the BI value of the uncoated mushroom was significantly higher than the mushroom coated with chitosan and the chitosan coating resulted in a good color of mushroom (P < 0.05) (Eissa 2007). It appeared that chitosan coatings could delay postharvest senescence of fruits and vegetables associated with color changes and dehydration (Cliffe-Byrnes and O'Beirne 2008; Hernández-Muñoz et al. 2008).
In the present study, there was no significant difference in BI values of mushrooms between PVC and PE packages (P > 0.05). However, previous study has reported mushroom (Agaricus bisporus) packaged in stretchable PVC film led to a detrimental deterioration of mushrooms after only one day of storage at 20 °C and both the blotches browning and veil opening were also exhibited (Guillaume et al. 2010).
Change in weight loss (WL)
Figure 3 shows the effect of coating treatments on weight loss of whole and sliced mushrooms in three MAP packages stored at 12 °C for 7 days. Weight loss occurred in all packages and the weight loss varied from about 3 % to above 8 % after 7 days of storage. Those results were almost similar to the values that were reported with a weight loss of 5 to 7 % in mushroom (Agaricus bisporus) after 5 days at 18 °C (Nichols and Hammond 1973). Generally, the sliced mushrooms showed significantly higher values of weight loss than whole ones. Weight loss from the mushroom surface, may be caused by loss of water from the package to the surrounding atmosphere, that resulting from the vapor pressure difference across the packaging film, and to the loss of carbon upon formation of CO2 during respiration.
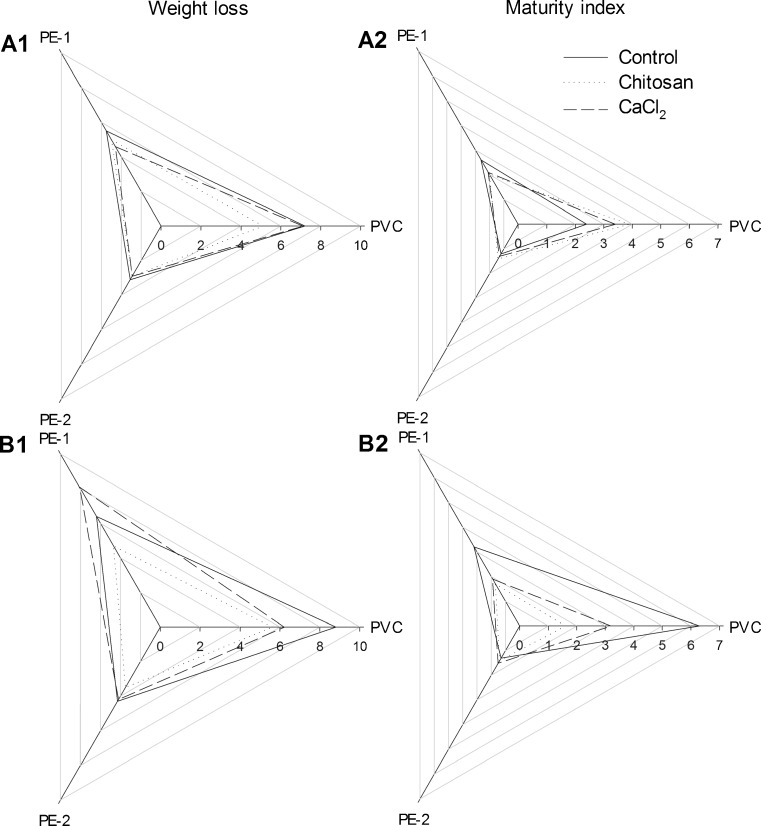
Fig. 3.
Weight loss (WL) and maturity index (MI) of whole (A1, A2) and sliced (B1, B2) mushrooms in different types of coating treatments and MAP packages stored at 12 °C for 7 days. Data shown are mean ± standard deviation (n = 3). PVC polyvinyl chloride wrap, PE-1 polyethylene-1 film; PE-2 polyethylene-2 film
As also shown in Fig. 3, the weight loss of whole and sliced mushroom without coating treatment in PVC film up to 7.2 % and 8.8 %, respectively, suggesting that dehydration was an important process in mushroom quality loss during postharvest storage. This could be attributed to the fact that mushrooms were only protected by a thin and porous epidermal structure, which does not prevent a quick superficial dehydration (Singer 1986). Due to lower permeability, mushrooms in PE-2 package had the lowest weight loss, for both whole and sliced samples. Furthermore, the lower water vapor transmission rate of PE films, combined with the higher respiration rate of mushrooms, developed a nearly saturated condition in the packages, which was responsible for the smaller weight loss (Antmann et al. 2008). In general, chitosan coating did significantly reduce the weight loss both in whole and sliced mushrooms, with the exception of the sliced mushroom packaged in PE-2 films (P < 0.05).
Change in maturity index (MI)
Postharvest development of mushrooms is a function of storage duration and atmosphere composition. The maturity index of sliced mushroom was significantly higher in all coating treatments than whole mushroom (Fig. 3). As appeared in Fig. 3, delay in cap opening of both whole and sliced mushrooms could be classified significantly according to the following descending order: mushrooms packaged in PE-2 film, PE-1 film and PVC wrap. Lower O2 and CO2 permeability of PE-2 film resulted in significantly smaller values of maturity index (MI) than PVC wrap and PE-1 film (P < 0.05). The type of coating did not appear to affect the maturity index (MI) significantly except for the PVC wrap package where chitosan coating markedly lowered the maturity index (MI) of sliced mushroom.
Several researchers attributed to study the effect of O2 and CO2 compositions in package on postharvest development of mushrooms, which was controversial. Murr and Morris (1975) stated that the cap opening of mushrooms in storage at 10 °C was promoted by O2 tension lower than air, and that the stimulatory effect was maximum when mushroom was stored at 5 % O2. For Hammond and Wood (1985), CO2 acted as a regulator for mushroom morphogenesis; but for Lopez Briones et al. (1992), the delay in cap opening was attributed to a physiological response to a CO2 stress rather than a regulatory action. Guillaume et al. (Guillaume et al. 2010) reported that the rate of cap opening was reduced when decreasing O2 in combined with increasing CO2. Taken together, by considering that unbroken veil is a major quality criterion for consumers (Gormley 1975), both whole and sliced mushrooms stored in PE-2 would satisfy visual perception of consumers and also effectively retard mushroom senescence after 7 days of storage at 12 °C (Fig. 3).
Conclusions
The headspace gas composition of modified atmosphere package was highly related to the quality of both whole and sliced mushrooms. Slicing and coating treatments could cause physical stress, resulting in changes of color, weight loss and maturity index. By maintaining fair color, weight loss and unbroken veil of whole and sliced mushrooms, only PE-2 film combined coating treatments provided a successful package that extended the shelf life at 12 °C up to 7 days and satisfied consumer acceptance.
Acknowledgements
The authors gratefully acknowledge the financial support from the Chinese Government, under Key Project of the National Eleventh-Five/Twelveth-Five Year Research Programs of China (No. 2008BADA1B07; No. 2012BAD38B02), in the framework of All China Federation of Supply and Marketing Cooperatives, managed by Jinan Fruit Research Institute.
References
- Antmann G, Ares G, Lema P, Lareo C. Influence of modified atmosphere packaging on sensory quality of shiitake mushrooms. Postharvest Biol Technol. 2008;49(1):164–170. doi: 10.1016/j.postharvbio.2008.01.020. [DOI] [Google Scholar]
- Ares G, Lareo C, Lema P. Modified atmosphere packaging for postharvest storage of mushrooms. a review. Fresh Produce. 2007;1(1):32–40. [Google Scholar]
- Bakshi P, Masoodi FA, Chauhan GS, Shah TA. Role of calcium in post-harvest life of temperate fruits: a review. J Food Sci Technol. 2005;42(1):1–8. [Google Scholar]
- Barron C, Varoquaux P, Guilbert S, Gontard N, Gouble B. Modified atmosphere packaging of cultivated mushroom (Agaricus bisporus L.) with hydrophilic films. J Food Sci. 2002;67(1):251–255. doi: 10.1111/j.1365-2621.2002.tb11393.x. [DOI] [Google Scholar]
- Charles F, Guillaume C, Gontard N. Effect of passive and active modified atmosphere packaging on quality changes of fresh endives. Postharvest Biol Technol. 2008;48(1):22–29. doi: 10.1016/j.postharvbio.2007.09.026. [DOI] [Google Scholar]
- Chien PJ, Sheu F, Yang FH. Effects of edible chitosan coating on quality and shelf life of sliced mango fruit. J Food Eng. 2007;78(1):225–229. doi: 10.1016/j.jfoodeng.2005.09.022. [DOI] [Google Scholar]
- Cliffe-Byrnes V, O'Beirne D. Effects of gas atmosphere and temperature on the respiration rates of whole and sliced mushrooms (Agaricus bisporus)—Implications for film permeability in modified atmosphere packages. J Food Sci. 2007;72(4):E197–E204. doi: 10.1111/j.1750-3841.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- Cliffe-Byrnes V, O'Beirne D. Effects of washing treatment on microbial and sensory quality of modified atmosphere (MA) packaged fresh sliced mushroom (Agaricus bisporus) Postharvest Biol Technol. 2008;48(2):283–294. doi: 10.1016/j.postharvbio.2007.10.012. [DOI] [Google Scholar]
- Del Nobile MA, Gammariello D, Conte A, Attanasio M. A combination of chitosan, coating and modified atmosphere packaging for prolonging Fior di latte cheese shelf life. Carbohyd Polym. 2009;78(1):151–156. doi: 10.1016/j.carbpol.2009.03.017. [DOI] [Google Scholar]
- Dong H, Cheng L, Tan J, Zheng K, Jiang Y. Effects of chitosan coating on quality and shelf life of peeled litchi fruit. J Food Eng. 2004;64(3):355–358. doi: 10.1016/j.jfoodeng.2003.11.003. [DOI] [Google Scholar]
- Duan J, Wu R, Strik BC, Zhao Y. Effect of edible coatings on the quality of fresh blueberries (Duke and Elliott) under commercial storage conditions. Postharvest Biol Technol. 2011;59(1):71–79. doi: 10.1016/j.postharvbio.2010.08.006. [DOI] [Google Scholar]
- Dutta PK, Tripathi S, Mehrotra GK, Dutta J. Perspectives for chitosan based antimicrobial films in food applications. Food Chem. 2009;114(4):1173–1182. doi: 10.1016/j.foodchem.2008.11.047. [DOI] [Google Scholar]
- Eissa HAA. Effect of chitosan coating on shelf life and quality of fresh-cut mushroom. J Food Qual. 2007;30(5):623–645. doi: 10.1111/j.1745-4557.2007.00147.x. [DOI] [Google Scholar]
- Eskin N, Henderson H, Townsend R. Biochemistry of foods. New York: Academic; 1971. [Google Scholar]
- Farber JN, Harris LJ, Parish ME, Beuchat LR, Suslow TV, Gorney JR, Garrett EH, Busta FF. Microbiological safety of controlled and modified atmosphere packaging of fresh and fresh-cut produce. Compr Rev Food Sci Food Safety. 2003;2(1 suppl):142–160. doi: 10.1111/j.1541-4337.2003.tb00032.x. [DOI] [Google Scholar]
- Gormley R. Chill storage of mushrooms. J Sci Food Agr. 1975;26(4):401–411. doi: 10.1002/jsfa.2740260404. [DOI] [Google Scholar]
- Guillaume C, Schwab I, Gastaldi E, Gontard N. Biobased packaging for improving preservation of fresh common mushrooms (Agaricus bisporus L.) Innov Food Sci Emerg. 2010;11(4):690–696. doi: 10.1016/j.ifset.2010.05.007. [DOI] [Google Scholar]
- Hammond J, Wood D. Metabolism, biochemistry and physiology. In: Flegg P, Spencer D, Wood D, editors. The Biology and technology of cultivated mushroom. Chichester: Willey; 1985. pp. 63–80. [Google Scholar]
- Hernández-Muñoz P, Almenar E, Valle VD, Velez D, Gavara R. Effect of chitosan coating combined with postharvest calcium treatment on strawberry (Fragaria × ananassa) quality during refrigerated storage. Food Chem. 2008;110(2):428–435. doi: 10.1016/j.foodchem.2008.02.020. [DOI] [PubMed] [Google Scholar]
- Ishikawa Y, Hirata T, Hasegawa Y. Development of simple Gas permeability measurement for polymeric films-on high Gas permeability films for MA packaging of fresh produce. J Packag Sci Technol. 1997;6:213–220. [Google Scholar]
- Kang JS, Park WP, Lee DS. Quality of enoki mushrooms as affected by packaging conditions. J Sci Food Agric. 2001;81(1):109–114. doi: 10.1002/1097-0010(20010101)81:1<109::AID-JSFA789>3.0.CO;2-Y. [DOI] [Google Scholar]
- Lopez Briones G, Varoquaux P, Chambroy Y, Bouquant J, Bureau G, Pascat B. Storage of common mushroom under controlled atmospheres. Int J Food Sci Technol. 1992;27(5):493–505. doi: 10.1111/j.1365-2621.1992.tb01216.x. [DOI] [Google Scholar]
- Mahajan PV, Rodrigues FAS, Motel A, Leonhard A. Development of a moisture absorber for packaging of fresh mushrooms (Agaricus bisporous) Postharvest Biol Technol. 2008;48(3):408–414. doi: 10.1016/j.postharvbio.2007.11.007. [DOI] [Google Scholar]
- Mau J, Miklus M, Beelman R. The shelf life of agaricus mushrooms. In: Charalambous C, editor. The shelf life of foods and beverages. Amsterdam: Elsevier Publishing Co.; 1993. pp. 255–288. [Google Scholar]
- Murr D, Morris L. Effect of storage atmosphere on postharvest growth of mushrooms. J Amer Soc Hort. 1975;100(3):298–301. [Google Scholar]
- Nichols R, Hammond JBW. Storage of mushrooms in pre packs: the effect of changes in carbon dioxide and oxygen on quality. J Sci Food Agric. 1973;24(11):1371–1381. doi: 10.1002/jsfa.2740241108. [DOI] [Google Scholar]
- Oms-Oliu G, Soliva-Fortuny R, Martín-Belloso O. Edible coatings with antibrowning agents to maintain sensory quality and antioxidant properties of fresh-cut pears. Postharvest Biol Technol. 2008;50(1):87–94. doi: 10.1016/j.postharvbio.2008.03.005. [DOI] [Google Scholar]
- Qi H, Hu W, Jiang A, Tian M, Li Y. Extending shelf-life of fresh-cut ‘Fuji’apples with chitosan-coatings. Innov Food Sci Emerg Technol. 2011;12(1):62–66. doi: 10.1016/j.ifset.2010.11.001. [DOI] [Google Scholar]
- Ravi Kumar MNV. A review of chitin and chitosan applications. React Funct Polym. 2000;46(1):1–27. doi: 10.1016/S1381-5148(00)00038-9. [DOI] [Google Scholar]
- Roy S, Anantheswaran RC, Beelman RB. Fresh mushroom quality as affected by modified atmosphere packaging. J Food Sci. 1995;60(2):334–340. doi: 10.1111/j.1365-2621.1995.tb05667.x. [DOI] [Google Scholar]
- Roy S, Anantheswaran RC, Shenk JS, Westerhaus MO, Beelman RB. Determination of moisture content of mushrooms by Vis-NIR spectroscopy. J Sci Food Agric. 1993;63(3):335–360. doi: 10.1002/jsfa.2740630314. [DOI] [Google Scholar]
- Singer R. The agaricales in modern taxonomy. Koeningstein: Koeltz Scientific Books; 1986. [Google Scholar]
- Tamer CE, Çopur ÖU. Chitosan: An edible coating for fresh-cut fruits and vegetables. Acta Hortic. 2010;877:619–626. [Google Scholar]
- Tano K, Arul J, Doyon G, Castaigne F. Atmospheric composition and quality of fresh mushrooms in modified atmosphere packages as affected by storage temperature abuse. J Food Sci. 1999;64(6):1073–1077. doi: 10.1111/j.1365-2621.1999.tb12285.x. [DOI] [Google Scholar]
- Tripathi P, Dubey NK. Exploitation of natural products as an alternative strategy to control postharvest fungal rotting of fruit and vegetables. Postharvest Biol Technol. 2004;32(3):235–245. doi: 10.1016/j.postharvbio.2003.11.005. [DOI] [Google Scholar]
- Villaescusa R, Gil MI. Quality improvement of Pleurotus mushrooms by modified atmosphere packaging and moisture absorbers. Postharvest Biol Technol. 2003;28(1):169–179. doi: 10.1016/S0925-5214(02)00140-0. [DOI] [Google Scholar]
- Xing Z, Wang Y, Feng Z, Tan Q. Effect of different packaging films on postharvest quality and selected enzyme activities of Hypsizygus marmoreus mushrooms. J Agric Food Chem. 2008;56(24):11838–11844. doi: 10.1021/jf8024387. [DOI] [PubMed] [Google Scholar]
- Zhan L, Hu J, Zhu Z. Shelf life extension of minimally processed water caltrop (Trapa acornis Nakano) fruits coated with chitosan. Int J Food Sci Tech. 2011;46(12):2634–2640. doi: 10.1111/j.1365-2621.2011.02794.x. [DOI] [Google Scholar]