Abstract
Tomato paste processing wastes, an important by-product of the paste industry, is rich in potentially health-promoting compounds such as lycopene. In this study, extraction yield of lycopene from tomato paste processing wastes by application of ultrasound assisted extraction (UAE) was compared with conventional organic solvent extraction (COSE) method. BHT (butylated hydroxytoluene) 0.05 % (w/v) added hexane:acetone:ethanol (2:1:1) mixture was used as solvent. Three different solvent solid ratios; 50:1, 35:1 and 20:1, (v/w) were used in both COSE and UAE. COSE experiments were performed at 20 °C, 40 °C and 60 °C for 10, 20, 30 and 40 min. 50, 65 and 90 W of ultrasonic power were applied in UAE for 1, 2, 5, 10, 15, 20 and 30 min. Lycopene contents of the samples were determined by spectrophotometric method. The effects of different factors, including the temperature, solvent solid ratio and ultrasonic power on lycopene yield were investigated. It was determined that the most efficient application for COSE was extracting samples by 50:1 solvent solid ratio at 60 °C for 40 min run, for UAE, 35:1 (v/w) solvent solid ratio, 90 W ultrasonic power for 30 min run. It was showed that UAE of lycopene requires less time, lower temperature and lower solvent than COSE.
Keywords: Lycopene, Tomato waste, Ultrasound assisted extraction
Introduction
Tomato and tomato products are consumed in all over the world as in raw and processed form. Depending on their healthy composition these products are becoming more and more popular. Certain types of cancers such as epithelial cancer and prostate cancer, cardiovascular diseases and cataract can be reduced or prevented by consuming these products depending on their high lycopene content (Rao et al. 1998; Lianfu and Zelong 2008; Capanoglu et al. 2008; Krinsky et al. 1990; Tapiero et al. 2004; Stahl and Sies 2005; Arab and Steck 2000). For its protective properties against cancer and oxidants, lycopene is the important component in pharmaceuticals and cosmetic formulations. Additionally for its great solubility in oil and fat mediums, lycopene is used as a color agent in food industry (Sabio et al. 2003; Naviglio et al. 2008). Lycopene is characteristic red color of fruits and vegetables and has 11 conjugated and 2 non-conjugated double bonds so it can act as an antioxidant two folds than β-carotene and 100 folds than α-tocopherol (Shi and Le-Maguer 2000; Bohn 2008; George et al. 2004).
Extraction of lycopene is applied by COSE method traditionally (Lianfu and Zelong 2008). But in traditional extraction methods, long times and high amounts of solvent are needed. Decreasing the solvent consumption, shortening the extraction time, increasing the extraction yield, and enhancing the quality of extracts can be reached by new methods such as ultrasound assisted, microwave assisted and supercritical extraction (Fantin et al. 2007; Wang and Weller 2006; Wang et al. 2008).
Ultrasonic applications are efficient in shorter extraction time with lower liquid solid ratios by increasing mass transfer rate. Sound waves at frequencies greater than human hearing range can alter the materials both in physical and chemical ways applying sponge effect and cavitations (Wang et al. 2008; Jerman et al. 2010; Mason and Lorimer 2002). These effects enhance solvent penetration in solid and cease the release of extracts by destroying solid matrix. Implosions of cavitation bubbles generate high pressure and temperature and enough to obtain sufficient reaction energy (Vinatoru 2001; Khan et al. 2010). Some recent studies shows that ultrasound assisted application accelerates extraction rate and increase the yield about 10 % (Khan et al. 2010, Sivakumar et al. 2009; Xu 2008).
Ultrasonic assistance has been used successfully also in some process of food industry like emulsification, crystallization, filtration, separation, de-foaming, extrusion, fermentation and microbial inhibition etc. (Mason and Lorimer 2002; Patit and Bates 2008; Soria and Villamiel 2010).
In this study, UAE of lycopene was compared with COSE and the effects of factors such as temperature, time, ultrasonic intensity and liquid solid ratio were investigated.
Material and methods
Material
Tomato wastes used in this study were supplied from a hot break tomato paste manufacturing pilot plant (Ege University, Agricultural Faculty Izmir, Turkey). The raw material had a moisture content of 75 % was dried to 4.9 % moisture content in a vacuum drier at 40 °C for 24 h. Before extraction process dried raw material containing 48.80 ± 4.7 % skins and 51.20 ± 3.1 % seeds was ground in a lab scale hammer mill (Armfield, UK) then subjected to sieving. Particles in 286.6 μm average size was used in extraction experiments. Ground samples were packed under vacuum and stored at −40 °C.
Conventional organic solvent extraction (COSE) method
Lycopene extraction was carried out according to the method of Sadler et al. (1990) modified as described by Perkins-Veazie et al. (2001). For 50:1, 35:1 and 20:1 (v/w) liquid solid ratios 0.8 g, 1.14 g and 2.0 g dried sample weighed respectively. 40 ml mixture of solvents hexane: methanol: acetone (2:1:1 v/v), containing 0.05 % (w/v) BHT (butylated hydroxytoluene) to minimize oxidation was then added. The suspension was agitated continuously in a shaking water bath at various temperatures and duration times. Extractions were applied at 20 °C, 40 °C and 60 °C temperatures during 10, 20, 30 and 40 min. After extraction process 15 ml cold distilled water was added to accelerate separation and the suspension was agitated using separator by 1000 rpm at 5 °C. The solution was then allowed to stand for 5 min for separation of polar and non-polar layers. Polar layers were used for lycopene determination.
Ultrasound assisted extraction (UAE) method
The experiments were carried out in a high-intensity probe system of 200 W and 24 kHz (Model UP 400S, Dr. Hielscher, Germany) equipped with a H14 sonotrode (Dr. Hielscher, Germany). Solvent composition and liquid solid ratio were the same as applied in COSE. Ultrasonic probe was immersed in 150 ml flask, containing samples and solvent, by 7 cm from the top. Flasks were placed in water bath to keep temperature constant at 5 °C during extraction. To determine the effect of ultrasound power; 50, 65 and 90 W powers for 1, 2, 5, 10, 15, 20 and 30 min runs applied. For lycopene determination the same procedure was applied as in COSE method.
Statistical analysis
Analysis of variance (ANOVA) was carried out to compare the mean values and all significant differences were reported at P < 0.05. All measurements were carried out in triplicate. The results were expressed as the mean value ± the standard deviation. All data were subjected to statistical analysis using SPSS 16.0 (SPSS Inc. Chicago, IL, USA).
Lycopene determination
Optical density of the hexane extracts were measured by UV visible spectrophotometer (Varian, Cary 50 UV-VB, ABD). The absorbance peak of greatest magnitude of lycopene in hexane was not used because of minimizing to interference from other carotenoids. So absorbance peak of polar phase at 503 nm against a hexane blank was read. Concentration of lycopene was calculated using the extinction coefficient 17.2×104 M−1 · cm−1 (Fish et al. 2002; Zechmeister et al. 1943) at 503 nm. Lycopene content was expressed as mg/kg fresh weight (fw) and dry weight (dw) respectively (Sadler et al. 1990; Karakaya and Yilmaz 2007). Recovery of lycopene in the extracts was quantified using a calibration curve prepared by using a purchased lycopene standard (Sigma-Aldrich, Milan, Italy). For calculation of lycopene yield, maximum lycopene content obtained from COSE with 50:1 liquid solid ratio at 60 °C for 40 min was selected as reference.
Results and discussion
Conventional organic solvent extraction (COSE)
Statistical evaluation of the results are given in Table 1. All independent variables; temperature, liquid solid ratio, time and their composite effects were found to be significant.
Table 1.
Analysis of variance (ANOVA) for classical organic solvent extraction
| Source | DF | SS | MS | F | P |
|---|---|---|---|---|---|
| Temperature (°C) | 2 | 1285.66 | 1285.66 | 402.50 | <0.001 |
| Liquid/Solid (v/w) | 2 | 1645.42 | 1645.42 | 515.13 | <0.001 |
| Time (min) | 3 | 1711.10 | 1711.10 | 357.13 | <0.001 |
| Temperature (°C)* Liquid/Solid (v/w) | 4 | 175.87 | 175.87 | 27.53 | <0.001 |
| Temperature (°C)* Time (min) | 6 | 103.97 | 103.97 | 10.85 | <0.001 |
| Liquid/Solid (v/w)* Time (min) | 6 | 127.52 | 127.52 | 13.31 | <0.001 |
| Error | 84 | 134.16 | 134.16 | ||
| Total | 107 | 5183.69 |
DF degrees of freedom, SS sum of ssquares, MS mean square, α significant effects
α = 0.05
The experimental values of lycopene amount were between 57.13 and 93.92 ppm (Table 2) and maximum value (93.92 ppm) was used as reference for lycopene yield % calculation. In the literature, lycopene content of fresh tomato vary from 10 to 200 ppm (wet basis) with typical values of 40–90 ppm (wet basis) depending on many parameters such as genetic factors, environmental factors etc. (Nobre et al. 2009; Shi and Le-Maguer 2000). Al-Wandawi et al.(1985) found that lycopene content of tomato skins was 120 ppm (wet basis) and the seeds had much lower lycopene content. Lycopene extraction from tomato paste waste was studied by Baysal et al. (2000), Sabio et al.(2003), Kaur et al. (2008) and Nobre et al. (2009). However, there was a large difference between their results depending on the tomato paste processing method. These studies showed that extraction conditions had an important effect on lycopene extraction. The lycopene content of dried tomato processing waste used in this study was in the range of previously reported values.
Table 2.
Amount of Lycopene (mg/kg) obtained with application of classical organic solvent extraction
| Temperature (°C) | Liquid/Solid (v/w) | Extraction time (min) | |||
|---|---|---|---|---|---|
| 10 | 20 | 30 | 40 | ||
| 20 | 20:1 | 57.1 ± 0.34 | 72.1 ± 0.39 | 72.5 ± 0.30 | 71.1 ± 0.76 |
| 35:1 | 72.8 ± 0.74 | 78.5 ± 0.78 | 78.3 ± 0.34 | 76.3 ± 0.57 | |
| 50:1 | 74.1 ± 0.92 | 78.5 ± 0.84 | 79.3 ± 1.93 | 77.7 ± 0.38 | |
| 40 | 20:1 | 65.0 ± 1.13 | 76.5 ± 0.89 | 77.7 ± 0.59 | 77.1 ± 0.16 |
| 35:1 | 73.0 ± 1.05 | 81.5 ± 0.48 | 81.6 ± 0.54 | 80.3 ± 0.63 | |
| 50:1 | 76.9 ± 0.27 | 81.6 ± 0.61 | 84.0 ± 0.84 | 85.0 ± 1.44 | |
| 60 | 20:1 | 68.9 ± 0.46 | 79.5 ± 0.08 | 79.4 ± 0.64 | 79.1 ± 0.49 |
| 35:1 | 73.7 ± 1.18 | 83.6 ± 0.86 | 83.8 ± 0.53 | 83.2 ± 2.16 | |
| 50:1 | 78.9 ± 2.24 | 87.8 ± 2.22 | 92.6 ± 1.81 | 93.9 ± 0.56 | |
Number of replicates (n = 3)
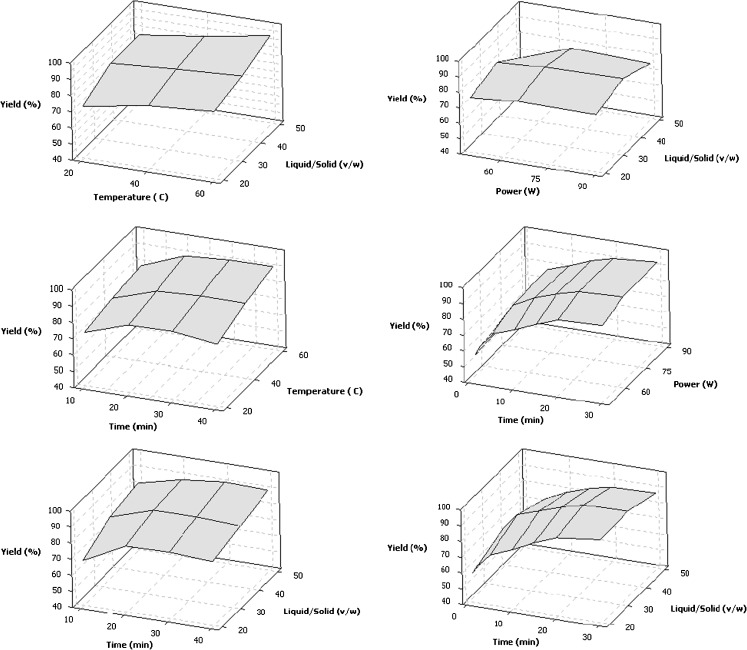
Figure 1 indicates effects of extraction time, ratio of liquid to solid and temperature on lycopene yield (%) by COSE. As shown in the Fig. 1 and Table 2, minimum yield was obtained at lowest extraction temperature (20 °C), the lowest liquid solid ratio (20:1v/w) for 10 min extraction duration and the maximum lycopene yield was obtained at highest extraction temperature (60 °C), highest liquid solid ratio (50:1 v/w) for the longest run time (40 min). Increases in all of the independent variables enhanced lycopene yield significantly. The lycopene yield increased with time at the beginning and did not vary significantly with time from 30 to 40 min. This can be explained by osmotic balance. The diffusing of lycopene in COSE was slow, so that the osmotic pressure between the inside and the outside of the cell easily reached equilibrium (Sun et al. 2011). Lycopene extraction rate decreased at this equilibrium situation even by longer extractions due to decreased driving force.
Fig. 1.
Surface plots indicating effects of extraction time, ratio of liquid to solid and temperature on lycopene yield by classical organic solvent extraction (COSE) and ultrasound assisted extraction (UAE)
Increases in the temperature, extraction time and volume of the extracting solvent (liquid solid ratio) increases the amount of lycopene to be extracted by the solvent. Increases in temperature had a positive effect on the lycopene extraction because of the reduction in the integrity of the cell comprising the lycopene and therefore higher accessibility of the lycopene contained within the cells to solvents (Baysal et al. 2000; Shi and Le-Maguer 2000; Kaur et al. 2008). Similar results have been reported in the literature; for lycopene extraction (Yaping et al. 2002) and for all-trans-β-carotene extraction from citrus peels (Sun et al. 2011) at higher temperatures. Temperature from 25 to 45 °C increased solubility and diffusivity of all-trans-β-carotene from citrus peels (Sun et al. 2011).
Ultrasound assisted extraction (UAE)
In this study applied ultrasonic powers and corresponding ultrasonic intensities were 50, 65, 90 W and 32.50, 42.25, 58.50 W/cm2, respectively. Results of the statistical analysis showed that all the terms including power, liquid solid ratio, time and their composite effects were significant as shown in Table 3. It was observed that time is the most important factor compared to the power and liquid solid ratio.
Table 3.
Analysis of variance (ANOVA) for ultrasound assisted extraction
| Source | DF | SS | MS | F | P |
|---|---|---|---|---|---|
| Power (W) | 2 | 798.00 | 798.00 | 209.00 | <0.001 |
| Liquid/Solid (v/w) | 2 | 2224.81 | 2224.81 | 582.69 | <0.001 |
| Time (min) | 6 | 25416.60 | 25416.60 | 2218.90 | <0.001 |
| Liquid/Solid (v/w)* Time (min) | 12 | 908.22 | 908.22 | 39.64 | <0.001 |
| Power (W)* Time (min) | 12 | 195.39 | 195.39 | 8.53 | <0.001 |
| Power (W)* Liquid/Solid (v/w) | 4 | 507.35 | 507.35 | 66.44 | <0.001 |
| Error | 150 | 286.36 | 286.36 | ||
| Total | 188 | 30336.74 |
DF degrees of freedom, SS sum of ssquares, MS mean square, α significant effects
α = 0.05
Experimental values of lycopene amounts obtained with different combinations of independent variables are given in Table 4.
Table 4.
Amount of Lycopene (mg/kg) obtained with application of ultrasound assisted extraction
| Power (W) | Liquid/Solid (v/w) | Extraction time (min) | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 5 | 10 | 15 | 20 | 30 | ||
| 50 | 20:1 | 55.8 ± 0.89 | 60.5 ± 0.41 | 66.5 ± 1.18 | 71.8 ± 0.57 | 77.4 ± 0.32 | 80.2 ± 0.92 | 82.8 ± 1.96 |
| 35:1 | 62.5 ± 1.75 | 66.1 ± 0.47 | 72.5 ± 0.16 | 76.5 ± 0.19 | 81.8 ± 0.89 | 86.9 ± 0.48 | 86.2 ± 1.01 | |
| 50:1 | 39.5 ± 0.04 | 46.3 ± 0.81 | 61.9 ± 0.31 | 66.1 ± 0.59 | 71.3 ± 0.13 | 77.2 ± 0.23 | 78.0 ± 0.48 | |
| 65 | 20:1 | 56.7 ± 0.52 | 60.1 ± 0.20 | 66.3 ± 0.75 | 73.8 ± 0.95 | 80.3 ± 0.81 | 85.7 ± 0.83 | 88.0 ± 0.32 |
| 35:1 | 59.9 ± 0.76 | 67.1 ± 0.54 | 76.7 ± 0.69 | 80.3 ± 0.38 | 84.1 ± 0.74 | 87.3 ± 0.24 | 87.0 ± 0.31 | |
| 50:1 | 46.3 ± 1.03 | 53.6 ± 0.87 | 72.9 ± 0.26 | 81.0 ± 1.02 | 83.9 ± 0.47 | 85.6 ± 0.29 | 86.5 ± 0.35 | |
| 90 | 20:1 | 52.7 ± 0.16 | 58.3 ± 0.09 | 70.2 ± 0.66 | 72.5 ± 0.30 | 78.9 ± 1.05 | 85.7 ± 132 | 90.1 ± 0.76 |
| 35:1 | 57.1 ± 1.67 | 67.3 ± 0.12 | 77.1 ± 0.53 | 82.1 ± 1.29 | 86.1 ± 1.39 | 88.4 ± 0.94 | 89.9 ± 0.87 | |
| 50:1 | 45.0 ± 0.41 | 59.1 ± 0.49 | 71.2 ± 0.39 | 76.5 ± 0.60 | 81.4 ± 0.46 | 82.4 ± 0.55 | 82.7 ± 0.79 | |
Number of replicates (n = 3)
Effects of extraction time, the ratio of liquid to solid and ultrasound power on lycopene yield (%) and lycopene amount (mg/kg) were given in Fig. 1 and Table 4, respectively. It has been observed that the lycopene yield increased with time till 20 min, later did not change significantly with time. Similar results were given in the literature; in ultrasonic extraction of phenolic compounds, time was found to be the most important factor (Wang and Weller 2006; Jerman et al. 2010). Although prolonged extraction time from 10 to 20 min improved the yield, after 30 min no more benefit could be observed because 95 % of phenolics could be already extracted up to 20–25 min extraction (Jerman et al. 2010; Ma et al. 2009).
The maximum lycopene yield obtained at 30 min extraction time, 35:1 liquid solid ratio while applied ultrasonic power was 90 W. The lycopene yield increased as the liquid solid ratio increased from 20:1 to 35:1 and a slight decrease was observed as liquid solid ratio increased from 35:1 to 50:1. Cavitation and thermal effects play important role in UAE. With increase in power more energy was getting transferred for cavitation. And this resulted into the increase in the lycopene yield. At low ultrasonic intensities thermal effect can be ignored because the heat produced by ultrasound may be completely diffused. As the ultrasonic intensity is further increased the cavitation effect starts decreasing and thermal effect becomes predominated. Sivakumar et al. (2009) worked on ultrasonic extraction of natural dye from beetroot. In that study although the maximum extraction yield was reached at 100 W ultrasonic power, they recommended 80 W power application because the excessive energy dissipation in the form of heat when 100 W was applied. This heat may cause degradation of substance. Ma et al. (2009) studied on phenolic acid extraction, up to 40 °C longer extraction times increased the yield but in higher temperatures yield was diminished by extended extraction time. It can be also explained by temperature increase during ultrasonic application by high ultrasonic power. Lycopene is relatively resistant to thermal degradation than other carotenoids like tocopherol and β-carotene (Shi and Le-Maguer 2000). During sonication the extreme physical conditions of temperature and pressure are appeared so that carotenoid isomerisation can be observed (Chen et al. 2009). Addition to this phenomenon hydroxyl radicals as hydrogen ions (H+), free radicals (O−, OH−,) and hydrogen peroxide (H2O2) are produced by cavitation effect. This situation results in the antioxidant degradation (Lianfu and Zelong 2008; Adekunte et al. 2010).
Conclusion
In this study various parameters affecting the COSE and UAE of lycopene were investigated. The results of this study indicate that each factor applied in UAE and COSE had a significant influence on the lycopene yield. Maximum lycopene yield was obtained in UAE using a 90 W ultrasonic power, 35:1 (v/w) liquid solid ratio and 30 min extraction time; while in COSE for a 50:1 (v/w) liquid solid ratio, 60 °C temperature and 40 min extraction time.
It is observed an initial sharp increase in the lycopene yield in both UAE and COSE with time; due to the large lycopene concentration gradient between the solvent and the cell at the beginning of the extraction process. The lycopene yield did not vary with time significantly between 30 and 40 min for COSE and 20 and 30 min for UAE, as the concentration gradient decreased compared to the beginning. In order to compare COSE and UAE, for both 30 min extraction time was selected. Lycopene yields obtained from UAE for all ultrasonic intensities and liquid solid ratios were found to be higher than values obtained from COSE or similar with COSE. By taking all the results into account, in UAE about 80 % of lycopene was extracted in 10 min while it required at least 20 min in COSE. The comparison of these two methods showed that UAE is more effective and requires shorter time and lower amounts of solvent than COSE at lower temperatures. Optimization of UAE is of great importance; if conditions are not correctly determined degradation of the desired component may occur due excessive heat and pressure produced by acoustic cavitation.
Acknowledgments
This research was financially supported by Ege University Scientific Research Project Commission (BAP) with Project no: 2009-MUH-082.
References
- Adekunte AO, Tiwari BK, Cullen PJ, ScannelL AG, O’Donnell CP. Effect of sonication on color, ascorbic acid and yeast inactivation in tomato juice. Food Chem. 2010;122:500–507. doi: 10.1016/j.foodchem.2010.01.026. [DOI] [PubMed] [Google Scholar]
- Al-Wandawi H, Abdul-Rahman M, Al-Shaikhly KJ. Tomato processing wastes as essential raw material sources. J Agr Food Chem. 1985;33:804–807. doi: 10.1021/jf00065a009. [DOI] [Google Scholar]
- Arab L, Steck S. Lycopene and cardiovascular disease. Am J Clin Nutr. 2000;71:1691–1695. doi: 10.1093/ajcn/71.6.1691S. [DOI] [PubMed] [Google Scholar]
- Baysal T, Ersus S, Starmans JDA. Supercritical CO2 extraction of β-carotene and lycopene from tomato paste waste. J Agr Food Chem. 2000;48:5507–5511. doi: 10.1021/jf000311t. [DOI] [PubMed] [Google Scholar]
- Bohn T. Bioavailability of non-provitamin A carotenoids. Curr Nutr Food Sci. 2008;4:240–258. doi: 10.2174/157340108786263685. [DOI] [Google Scholar]
- Capanoglu E, Beekwilder J, Boyacioğlu D, Hall R, De Vos R. Changes in antioxidant and metabolite profiles during production of tomato paste. J Agr Food Chem. 2008;6:964–973. doi: 10.1021/jf072990e. [DOI] [PubMed] [Google Scholar]
- Chen J, Shi J, Xue SJ, Ma Y. Comparison of lycopene stability in water and oil based food model systems under thermal- and light-irradiation treatments. Lwt-Food Sci Technol. 2009;42:740–747. doi: 10.1016/j.lwt.2008.10.002. [DOI] [Google Scholar]
- Fantin G, Fogagnolo M, Medici A, Perrone D. Isolation of lycopene from crude tomato extract via selective inclusion in deoxycholic acid. Tetrahedron Lett. 2007;48:9148–9150. doi: 10.1016/j.tetlet.2007.10.127. [DOI] [Google Scholar]
- Fish WW, Perkins-Veazie P, Collins JK. A quantitative assay for lycopene that utilizes reduced volumes of organic solvents. J Food Comp Anal. 2002;3:309–317. doi: 10.1006/jfca.2002.1069. [DOI] [Google Scholar]
- George B, Kaur C, Khurdiya DS, Kapoor HC. Antioxidants in tomato (Lycopersium esculentum) as a function of genotype. Food Chem. 2004;84:45–51. doi: 10.1016/S0308-8146(03)00165-1. [DOI] [Google Scholar]
- Jerman T, Trebše P, Mozetič Vodopivec B. Ultrasound-assisted solid liquid extraction (USLE) of olive fruit (Olea europaea) phenolic compounds. Food Chem. 2010;123:175–182. doi: 10.1016/j.foodchem.2010.04.006. [DOI] [Google Scholar]
- Karakaya S, Yilmaz N. Lycopene content and antioxidant activity of fresh and processed tomatoes and in vitro bioavailability of lycopene. J Agr Food Chem. 2007;87:2342–2347. doi: 10.1002/jsfa.2998. [DOI] [Google Scholar]
- Kaur D, Wani AA, Oberoi DPS, Sogi DS. Effect of extraction conditions on lycopene extractions from tomato processing waste skin using response surface methodology. Food Chem. 2008;108:711–718. doi: 10.1016/j.foodchem.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Khan MK, Vian MA, Tixier SF, Dangles O, Chemat F. Ultrasound-assisted extraction of polyphenols (flavanone glycosides) from orange (Citrus sinensis L.) peel. Food Chem. 2010;119:851–858. doi: 10.1016/j.foodchem.2009.08.046. [DOI] [Google Scholar]
- Krinsky NL, Russett MD, Handeman GJ, Snodderly DD. Structural and geometrical isomers of carotenoids in human plasma. J Nutr. 1990;120:1654–1662. doi: 10.1093/jn/120.12.1654. [DOI] [PubMed] [Google Scholar]
- Lianfu Z, Zelong L. Optimization and comparison of ultrasound/microwave assisted extraction (UMAE) and ultrasonic assisted extraction (UAE) of lycopene from tomatoes. Ultrason Sonochem. 2008;15:731–737. doi: 10.1016/j.ultsonch.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Ma Y, Chen J, Liu D, Ye X. Simultaneous extraction of phenolic compounds of citrus peel extracts: Effect of ultrasound. Ultrason Sonochem. 2009;16:57–62. doi: 10.1016/j.ultsonch.2008.04.012. [DOI] [PubMed] [Google Scholar]
- Mason TJ, Lorimer JP. Applied Sonochemistry. Weeinheim: Wiley-VCH; 2002. [Google Scholar]
- Naviglio D, Caruso T, Iannece P, Aragon A, Santini A. Characterization of high purity lycopene from tomato wastes using a new pressurized extraction approach. J Agr Food Chem. 2008;56:6227–6231. doi: 10.1021/jf703788c. [DOI] [PubMed] [Google Scholar]
- Nobre BP, Palavra AF, Pessoa FLP, Mendes RL. Supercriticcal CO2 extraction of trans lycopene from Portuguese tomato industrial waste. Food Chem. 2009;116:680–685. doi: 10.1016/j.foodchem.2009.03.011. [DOI] [Google Scholar]
- Patit A, Bates D. Ultrasonic innovations in the food industry: From the laboratory to commercial product. Innov Food Sci Emerg. 2008;9:147–154. doi: 10.1016/j.ifset.2007.07.004. [DOI] [Google Scholar]
- Perkins-Veazie P, Collins JK, Pair SD, Roberts W (2001) Lycopene content differs among red-fleshed watermelon cultivars. J Sci Food Agric 81:983–987
- Rao AV, Waseem Z, Aggarwal S. Lycopene content of tomatoes and tomato products and their contribution to dietary lycopene. Food Res Int. 1998;3:737–741. doi: 10.1016/S0963-9969(99)00053-8. [DOI] [Google Scholar]
- Sabio E, Lozano M, de Espinosa VM, Mendes RL, Pereira AP, Palavra AF, Coelho JA. Lycopene and beta-carotene extraction from tomato processing waste using supercritical CO2. Ind Eng Chem Res. 2003;42:6641–6646. doi: 10.1021/ie0301233. [DOI] [Google Scholar]
- Sadler G, Davis J, Dezman D. Rapid extraction of lycopene and β-carotene from reconstituted tomato paste and pink grapefruit homogenates. J Food Sci. 1990;55:1460–1461. doi: 10.1111/j.1365-2621.1990.tb03958.x. [DOI] [Google Scholar]
- Shi J, Le-Maguer M. Lycopene in tomatoes: chemical and physical properties affected by food processing. Crit Rev Food Sci. 2000;40:1–42. doi: 10.1080/10408690091189275. [DOI] [PubMed] [Google Scholar]
- Sivakumar V, Anna JL, Vijayeeswarri J, Swaminathan G. Ultrasound assisted enhancement in natural dye extraction from beetroot for industrial applications and natural dyeing of leather. Ultrason Sonochem. 2009;16:782–789. doi: 10.1016/j.ultsonch.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Soria AC, Villamiel M. Effect of ultrasound on the technological properties, bioactivity of food: a review. Trends Food Sci Technol. 2010;21:323–331. doi: 10.1016/j.tifs.2010.04.003. [DOI] [Google Scholar]
- Stahl W, Sies H. Bioactivity and protective effects of natural carotenoids. BBA-Mol Basis Dis. 2005;1740:101–107. doi: 10.1016/j.bbadis.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Sun Y, Liu D, Chen J, Ye X, Yu D. Effects of different factors of ultrasound treatment on the extraction yield of the all-trans-β-carotene from citrus peels. Ultrason Sonochem. 2011;18:243–249. doi: 10.1016/j.ultsonch.2010.05.014. [DOI] [PubMed] [Google Scholar]
- Tapiero H, Townsend DM, Tew KD. The role of carotenoids in the prevention of human pathologies. Biomed Pharmacother. 2004;58:100–110. doi: 10.1016/j.biopha.2003.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinatoru M. An overview of the ultrasonically assisted extraction of bioactive principles from herbs. Ultrason Sonochem. 2001;8:303–313. doi: 10.1016/S1350-4177(01)00071-2. [DOI] [PubMed] [Google Scholar]
- Wang J, Sun B, Cao Y, Tian Y, Li X. Optimization of ultrasound-assisted extraction of phenolic compounds from wheat bran. Food Chem. 2008;106:804–810. doi: 10.1016/j.foodchem.2007.06.062. [DOI] [Google Scholar]
- Wang L, Weller CL. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci Tech. 2006;17:300–312. doi: 10.1016/j.tifs.2005.12.004. [DOI] [Google Scholar]
- Xu Z. Comparison of extraction methods for quantifying vitamin E from animal tissues. Bioresource Technol. 2008;99:8705–8709. doi: 10.1016/j.biortech.2008.04.065. [DOI] [PubMed] [Google Scholar]
- Yaping Z, Suping Q, Wenli Y, Zheng X, Hong S, Side Y, Dapu W. Antioxidant activity of lycopene extracted from tomato paste towards tricholoromethyl peroxyl radical CCl3O2. Food Chem. 2002;77:209–212. doi: 10.1016/S0308-8146(01)00339-9. [DOI] [Google Scholar]
- Zechmeister L, Le Rosen AL, Schroeder WA, Polgar A, Pauling L. Spectral characteristics and configuration of some stereoisomeric carotenoids including pro lycopene and provitamin-A or antioxidant properties. Cancer Res. 1943;52:5707–5712. [Google Scholar]