Abstract
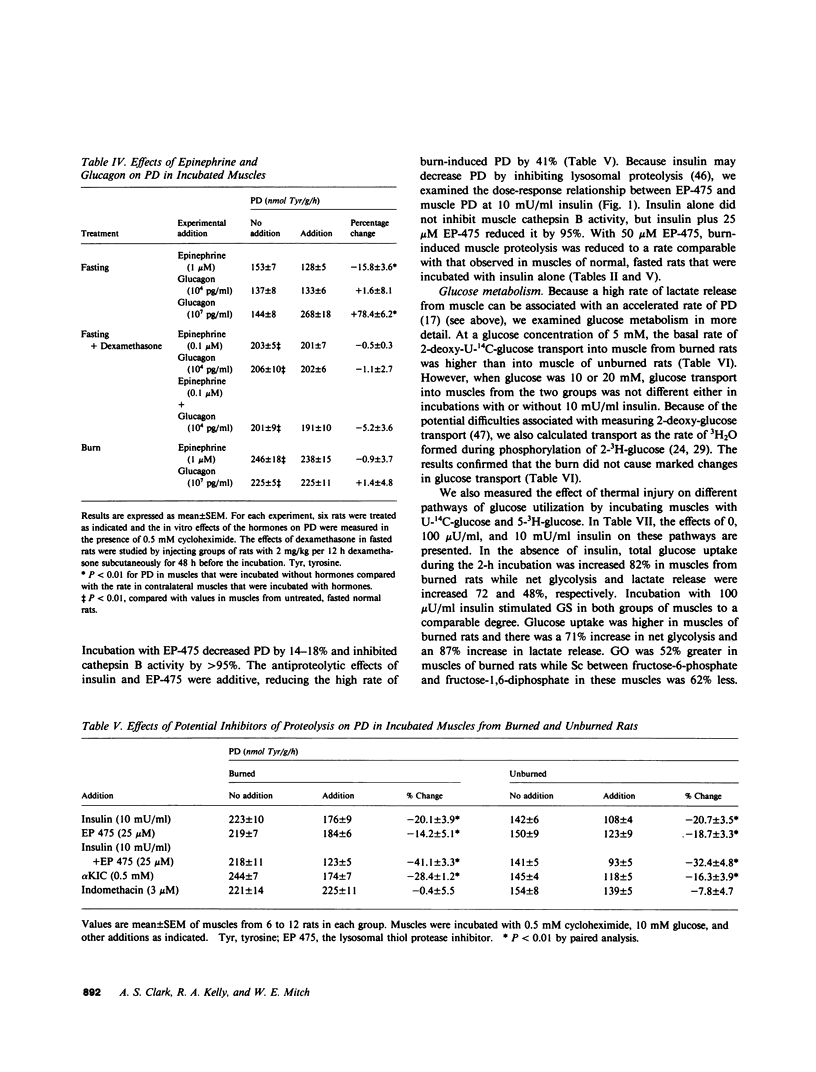
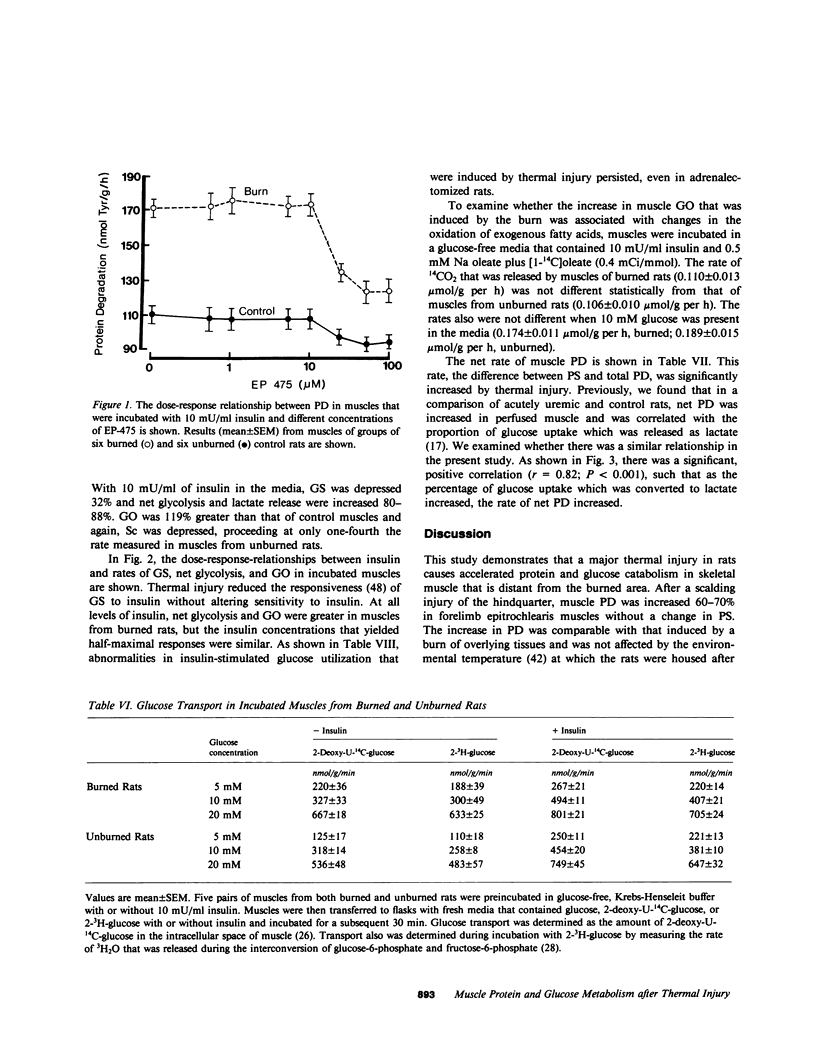
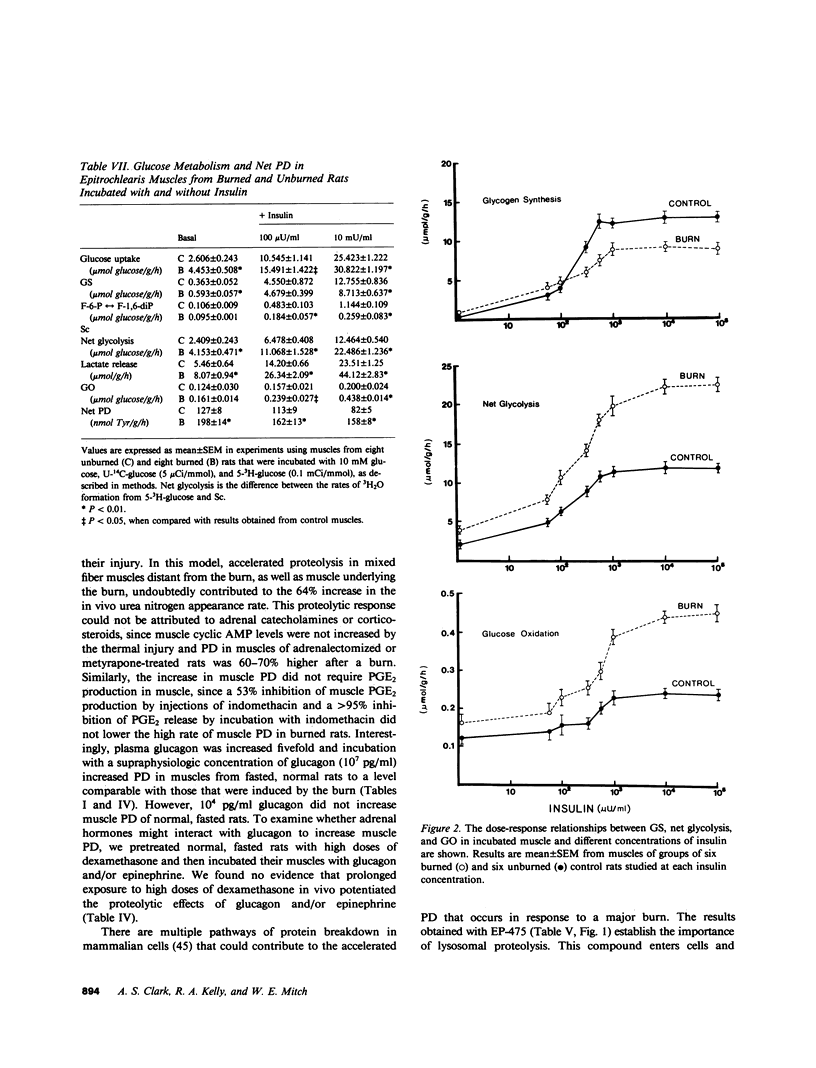
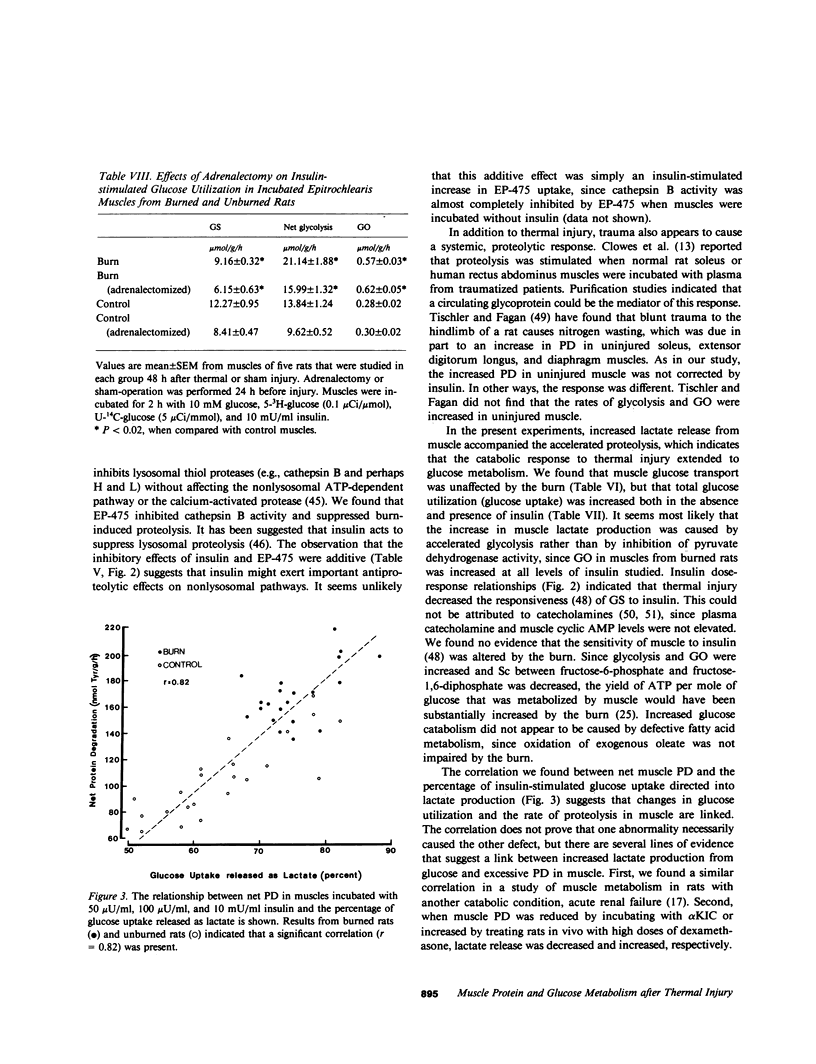
Negative nitrogen balance and increased oxygen consumption after thermal injury in humans and experimental animals is related to the extent of the burn. To determine whether defective muscle metabolism is restricted to the region of injury, we studied protein and glucose metabolism in forelimb muscles of rats 48 h after a scalding injury of their hindquarters. This injury increased muscle protein degradation (PD) from 140 +/- 5 to 225 +/- 5 nmol tyrosine/g per h, but did not alter protein synthesis. Muscle lactate release was increased greater than 70%, even though plasma catecholamines and muscle cyclic AMP were not increased. Insulin dose-response studies revealed that the burn decreased the responsiveness of muscle glycogen synthesis to insulin but did not alter its sensitivity to insulin. Rates of net glycolysis and glucose oxidation were increased and substrate cycling of fructose-6-phosphate was decreased at all levels of insulin. The burn-induced increase in protein and glucose catabolism was not mediated by adrenal hormones, since they persisted despite adrenalectomy. Muscle PGE2 production was not increased by the burn and inhibition of prostaglandin synthesis by indomethacin did not inhibit proteolysis. The increase in PD required lysosomal proteolysis, since inhibition of cathepsin B with EP475 reduced PD. Insulin reduced PD 20% and the effects of EP475 and insulin were additive, reducing PD 41%. An inhibitor of muscle PD, alpha-ketoisocaproate, reduced burn-induced proteolysis 28% and lactate release 56%. The rate of PD in muscle of burned and unburned rats was correlated with the percentage of glucose uptake that was directed into lactate production (r = +0.82, P less than 0.01). Thus, a major thermal injury causes hypercatabolism of protein and glucose in muscle that is distant from the injury, and these responses may be linked to a single metabolic defect.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews T. M., Goldthorp R., Watts R. W. Fluorimetric measurement of the phenylalanine content of human granulocytes. Clin Chim Acta. 1973 Feb 12;43(3):379–387. doi: 10.1016/0009-8981(73)90477-4. [DOI] [PubMed] [Google Scholar]
- Ashcroft S. J., Weerasinghe L. C., Bassett J. M., Randle P. J. The pentose cycle and insulin release in mouse pancreatic islets. Biochem J. 1972 Feb;126(3):525–532. doi: 10.1042/bj1260525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baracos V., Rodemann H. P., Dinarello C. A., Goldberg A. L. Stimulation of muscle protein degradation and prostaglandin E2 release by leukocytic pyrogen (interleukin-1). A mechanism for the increased degradation of muscle proteins during fever. N Engl J Med. 1983 Mar 10;308(10):553–558. doi: 10.1056/NEJM198303103081002. [DOI] [PubMed] [Google Scholar]
- Bilbrey G. L., Faloona G. R., White M. G., Knochel J. P. Hyperglucagonemia of renal failure. J Clin Invest. 1974 Mar;53(3):841–847. doi: 10.1172/JCI107624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke J. F., Wolfe R. R., Mullany C. J., Mathews D. E., Bier D. M. Glucose requirements following burn injury. Parameters of optimal glucose infusion and possible hepatic and respiratory abnormalities following excessive glucose intake. Ann Surg. 1979 Sep;190(3):274–285. doi: 10.1097/00000658-197909000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell F. T., Jr Energy metabolism following thermal burns. Arch Surg. 1976 Feb;111(2):181–185. doi: 10.1001/archsurg.1976.01360200087017. [DOI] [PubMed] [Google Scholar]
- Clark A. S., Mitch W. E. Comparison of protein synthesis and degradation in incubated and perfused muscle. Biochem J. 1983 Jun 15;212(3):649–653. doi: 10.1042/bj2120649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. S., Mitch W. E. Muscle protein turnover and glucose uptake in acutely uremic rats. Effects of insulin and the duration of renal insufficiency. J Clin Invest. 1983 Sep;72(3):836–845. doi: 10.1172/JCI111054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark M. G., Bloxham D. P., Holland P. C., Lardy H. A. Estimation of the fructose diphosphatase-phosphofructokinase substrate cycle in the flight muscle of Bombus affinis. Biochem J. 1973 Jun;134(2):589–597. doi: 10.1042/bj1340589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowes G. H., Jr, George B. C., Villee C. A., Jr, Saravis C. A. Muscle proteolysis induced by a circulating peptide in patients with sepsis or trauma. N Engl J Med. 1983 Mar 10;308(10):545–552. doi: 10.1056/NEJM198303103081001. [DOI] [PubMed] [Google Scholar]
- Durkot M. J., Wolfe R. R. Effects of adrenergic blockade on glucose kinetics in septic and burned guinea pigs. Am J Physiol. 1981 Sep;241(3):R222–R227. doi: 10.1152/ajpregu.1981.241.3.R222. [DOI] [PubMed] [Google Scholar]
- Foley J. E., Foley R., Gliemann J. Glucose-induced acceleration of deoxyglucose transport in rat adipocytes. Evidence for a second barrier to sugar entry. J Biol Chem. 1980 Oct 25;255(20):9674–9677. [PubMed] [Google Scholar]
- Foley J. E., Foley R., Gliemann J. Rate-limiting steps of 2-deoxyglucose uptake in rat adipocytes. Biochim Biophys Acta. 1980 Jul;599(2):689–698. doi: 10.1016/0005-2736(80)90210-2. [DOI] [PubMed] [Google Scholar]
- Fulks R. M., Li J. B., Goldberg A. L. Effects of insulin, glucose, and amino acids on protein turnover in rat diaphragm. J Biol Chem. 1975 Jan 10;250(1):290–298. [PubMed] [Google Scholar]
- Garcia-Barreno P., Balibrea J. L. Metabolic response in shock. Surg Gynecol Obstet. 1978 Feb;146(2):182–189. [PubMed] [Google Scholar]
- Goldberg A. L., Baracos V., Rodemann P., Waxman L., Dinarello C. Control of protein degradation in muscle by prostaglandins, Ca2+, and leukocytic pyrogen (interleukin 1). Fed Proc. 1984 Apr;43(5):1301–1306. [PubMed] [Google Scholar]
- Hammerstedt R. H., Lardy H. A. The effect of substrate cycling on the ATP yield of sperm glycolysis. J Biol Chem. 1983 Jul 25;258(14):8759–8768. [PubMed] [Google Scholar]
- Harper J. F., Brooker G. Femtomole sensitive radioimmunoassay for cyclic AMP and cyclic GMP after 2'0 acetylation by acetic anhydride in aqueous solution. J Cyclic Nucleotide Res. 1975;1(4):207–218. [PubMed] [Google Scholar]
- Herndon D. N., Wilmore D. W., Mason A. D., Jr Development and analysis of a small animal model simulating the human postburn hypermetabolic response. J Surg Res. 1978 Nov;25(5):394–403. doi: 10.1016/s0022-4804(78)80003-1. [DOI] [PubMed] [Google Scholar]
- Higgins A. J., Morville M., Burges R. A., Gardiner D. G., Page M. G., Blackburn K. J. Oxfenicine diverts rat muscle metabolism from fatty acid to carbohydrate oxidation and protects the ischaemic rat heart. Life Sci. 1980 Sep 15;27(11):963–970. doi: 10.1016/0024-3205(80)90106-x. [DOI] [PubMed] [Google Scholar]
- Kagan R. J., Matsuda T., Hanumadass M., Castillo B., Jonasson O. The effect of burn wound size on ureagenesis and nitrogen balance. Ann Surg. 1982 Jan;195(1):70–74. doi: 10.1097/00000658-198201001-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn C. R. Insulin resistance, insulin insensitivity, and insulin unresponsiveness: a necessary distinction. Metabolism. 1978 Dec;27(12 Suppl 2):1893–1902. doi: 10.1016/s0026-0495(78)80007-9. [DOI] [PubMed] [Google Scholar]
- Katz J., Rognstad R. The metabolism of glucose-2-T by adipose tissue. J Biol Chem. 1969 Jan 10;244(1):99–106. [PubMed] [Google Scholar]
- Katz J., Wals P. A., Golden S., Rognstad R. Recycling of glucose by rat hepatocytes. Eur J Biochem. 1975 Dec 1;60(1):91–101. doi: 10.1111/j.1432-1033.1975.tb20979.x. [DOI] [PubMed] [Google Scholar]
- Le Marchand-Brustel Y., Jeanrenaud B., Freychet P. Insulin binding and effects in isolated soleus muscle of lean and obese mice. Am J Physiol. 1978 Apr;234(4):E348–E358. doi: 10.1152/ajpendo.1978.234.4.E348. [DOI] [PubMed] [Google Scholar]
- McLane J. A., Holloszy J. O. Glycogen synthesis from lactate in the three types of skeletal muscle. J Biol Chem. 1979 Jul 25;254(14):6548–6553. [PubMed] [Google Scholar]
- Meguid M. M., Brennan M. F., Aoki T. T., Muller W. A., Ball M. R., Moore F. D. Hormone-substrate interrelationships following trauma. Arch Surg. 1974 Dec;109(6):776–783. doi: 10.1001/archsurg.1974.01360060046013. [DOI] [PubMed] [Google Scholar]
- Mitch W. E. Amino acid release from the hindquarter and urea appearance in acute uremia. Am J Physiol. 1981 Dec;241(6):E415–E419. doi: 10.1152/ajpendo.1981.241.6.E415. [DOI] [PubMed] [Google Scholar]
- Mitch W. E., Walser M., Sapir D. G. Nitrogen sparing induced by leucine compared with that induced by its keto analogue, alpha-ketoisocaproate, in fasting obese man. J Clin Invest. 1981 Feb;67(2):553–562. doi: 10.1172/JCI110066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson K. M., Turinsky J. Analysis of postburn insulin unresponsiveness in skeletal muscle. J Surg Res. 1981 Nov;31(5):404–414. doi: 10.1016/0022-4804(81)90081-0. [DOI] [PubMed] [Google Scholar]
- Nelson K. M., Turinsky J. Local effect of burn on skeletal muscle insulin responsiveness. J Surg Res. 1981 Oct;31(4):288–297. doi: 10.1016/0022-4804(81)90051-2. [DOI] [PubMed] [Google Scholar]
- Newsholme E. A., Crabtree B. Substrate cycles in metabolic regulation and in heat generation. Biochem Soc Symp. 1976;(41):61–109. [PubMed] [Google Scholar]
- Nonaka K., Foà P. P. A simplified glucagon immunoassay and its use in a study of incubated pancreatic islets. Proc Soc Exp Biol Med. 1969 Jan;130(1):330–336. doi: 10.3181/00379727-130-33549. [DOI] [PubMed] [Google Scholar]
- Odessey R., Parr B. Effect of insulin and leucine on protein turnover in rat soleus muscle after burn injury. Metabolism. 1982 Jan;31(1):82–87. [PubMed] [Google Scholar]
- Parnham M. J., Adolfs M. J., Bonta I. L. The effect of metyrapone on granuloma induced by carrageenan-impregnated sponges in normal and essential fatty acid deficient rats. J Pharm Pharmacol. 1977 Nov;29(11):670–673. doi: 10.1111/j.2042-7158.1977.tb11432.x. [DOI] [PubMed] [Google Scholar]
- Peuler J. D., Johnson G. A. Simultaneous single isotope radioenzymatic assay of plasma norepinephrine, epinephrine and dopamine. Life Sci. 1977 Sep 1;21(5):625–636. doi: 10.1016/0024-3205(77)90070-4. [DOI] [PubMed] [Google Scholar]
- Rocha D. M., Santeusanio F., Faloona G. R., Unger R. H. Abnormal pancreatic alpha-cell function in bacterial infections. N Engl J Med. 1973 Apr 5;288(14):700–703. doi: 10.1056/NEJM197304052881402. [DOI] [PubMed] [Google Scholar]
- Rodemann H. P., Goldberg A. L. Arachidonic acid, prostaglandin E2 and F2 alpha influence rates of protein turnover in skeletal and cardiac muscle. J Biol Chem. 1982 Feb 25;257(4):1632–1638. [PubMed] [Google Scholar]
- Rodemann H. P., Waxman L., Goldberg A. L. The stimulation of protein degradation in muscle by Ca2+ is mediated by prostaglandin E2 and does not require the calcium-activated protease. J Biol Chem. 1982 Aug 10;257(15):8716–8723. [PubMed] [Google Scholar]
- Roman R. J., Bonventre J. V., Lechene C. P. Fluorometric assay for urea in urine, plasma, and tubular fluid. Anal Biochem. 1979 Sep 15;98(1):136–141. doi: 10.1016/0003-2697(79)90717-6. [DOI] [PubMed] [Google Scholar]
- Sapir D. G., Stewart P. M., Walser M., Moreadith C., Moyer E. D., Imbembo A. L., Rosenshein N. B., Munoz S. Effects of alpha-ketoisocaproate and of leucine on nitrogen metabolism in postoperative patients. Lancet. 1983 May 7;1(8332):1010–1014. doi: 10.1016/s0140-6736(83)92643-0. [DOI] [PubMed] [Google Scholar]
- Shikama H., Chiasson J. L., Exton J. H. Studies on the interactions between insulin and epinephrine in the control of skeletal muscle glycogen metabolism. J Biol Chem. 1981 May 10;256(9):4450–4454. [PubMed] [Google Scholar]
- Smits J. F., van Essen H., Struyker-Boudier H. A. Propranolol in conscious spontaneously hypertensive rats. I. Cardiovascular effects after subcutaneous and intracerebroventricular administration. Naunyn Schmiedebergs Arch Pharmacol. 1979 Oct;309(1):13–18. doi: 10.1007/BF00498751. [DOI] [PubMed] [Google Scholar]
- Tischler M. E., Fagan J. M. Response to trauma of protein, amino acid, and carbohydrate metabolism in injured and uninjured rat skeletal muscles. Metabolism. 1983 Sep;32(9):853–868. doi: 10.1016/0026-0495(83)90198-1. [DOI] [PubMed] [Google Scholar]
- Turinsky J., Patterson S. A. Proximity to a burn wound as a new factor in considerations of postburn insulin resistance. J Surg Res. 1979 Feb;26(2):171–174. doi: 10.1016/0022-4804(79)90096-9. [DOI] [PubMed] [Google Scholar]
- Vaughan G. M., Becker R. A., Allen J. P., Goodwin C. W., Jr, Pruitt B. A., Jr, Mason A. D., Jr Cortisol and corticotrophin in burned patients. J Trauma. 1982 Apr;22(4):263–273. doi: 10.1097/00005373-198204000-00001. [DOI] [PubMed] [Google Scholar]
- WAALKES T. P., UDENFRIEND S. A fluorometric method for the estimation of tyrosine in plasma and tissues. J Lab Clin Med. 1957 Nov;50(5):733–736. [PubMed] [Google Scholar]
- Wilmore D. W., Aulick L. H., Mason A. D., Pruitt B. A., Jr Influence of the burn wound on local and systemic responses to injury. Ann Surg. 1977 Oct;186(4):444–458. doi: 10.1097/00000658-197710000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmore D. W., Long J. M., Mason A. D., Jr, Skreen R. W., Pruitt B. A., Jr Catecholamines: mediator of the hypermetabolic response to thermal injury. Ann Surg. 1974 Oct;180(4):653–669. doi: 10.1097/00000658-197410000-00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmore D. W., Moylan J. A., Jr, Lindsey C. A., Faloona G. R., Unger R. H., Pruitt B. A., Jr Hyperglucagonemia following thermal injury: insulin and glucagon in the posttraumatic catabolic state. Surg Forum. 1973;24:99–101. [PubMed] [Google Scholar]
- Wolfe R. R., Durkot M. J., Allsop J. R., Burke J. F. Glucose metabolism in severely burned patients. Metabolism. 1979 Oct;28(10):1031–1039. doi: 10.1016/0026-0495(79)90007-6. [DOI] [PubMed] [Google Scholar]
- Wolfe R. R., Goodenough R. D., Burke J. F., Wolfe M. H. Response of protein and urea kinetics in burn patients to different levels of protein intake. Ann Surg. 1983 Feb;197(2):163–171. doi: 10.1097/00000658-198302000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawalich W. S., Rognstad R., Pagliara A. S., Matschinsky F. M. A comparison of the utilization rates and hormone-releasing actions of glucose, mannose, and fructose in isolated pancreatic islets. J Biol Chem. 1977 Dec 10;252(23):8519–8523. [PubMed] [Google Scholar]



