Abstract
Banana (Musaceae) is one of the world’s most important fruit crops that is widely cultivated in tropical countries for its valuable applications in food industry. Its enormous by-products are an excellent source of highly valuable raw materials for other industries by recycling agricultural waste. This prevents an ultimate loss of huge amount of untapped biomass and environmental issues. This review discusses extensively the breakthrough in the utilization of banana by-products such as peels, leaves, pseudostem, stalk and inflorescence in various food and non-food applications serving as thickening agent, coloring and flavor, alternative source for macro and micronutrients, nutraceuticals, livestock feed, natural fibers, and sources of natural bioactive compounds and bio-fertilizers. Future prospects and challenges are the important key factors discussed in association to the sustainability and feasibility of utilizing these by-products. It is important that all available by-products be turned into highly commercial outputs in order to sustain this renewable resource and provide additional income to small scale farming industries without compromising its quality and safety in competing with other commercial products.
Keywords: Banana, Plantain, By-products processing, Agricultural waste, Value added products, Food and non-food application
Introduction
Banana is one of the earliest crops cultivated in the history of human agriculture. The origin of this particular plant family stretches from India to Papua New Guinea which includes the Southeast Asian region (Arvanitoyannis and Mavromatis 2009; De Lange et al. 2009). Its mass cultivation and consumption in the recent decades made it the world second largest fruit crop with an estimated gross production exceeds 139 million tones (FAO 2010a). World leading banana and plantain producers are India, China, Uganda, Ecuador, Philippines, and Nigeria. Most of the edible bananas are cultivated mainly for their fruits, thus banana farms could generate several tons of underused by-products and wastes. Therefore, without proper agricultural waste management practice, huge amount of valuable untapped commodity will be lost and causing serious ecological damages (Essien et al. 2005; Shah et al. 2005; Yabaya and Ado 2008). The native people have been utilizing these plants more than just for food purposes but have begun to explore the possibilities of utilizing banana plants in their daily life.
Banana by-products have been used for wrappings foods, clothes and used in various ceremonial occasions and the usage expands through cultural diversification (Kennedy 2009). Modern agriculture generally groups banana into fruit crop or cash crop commodities alongside with several other crops such as oil palm, sugarcane, pineapple, mangoes and rice. Similarly, some of these commodities do produce huge amount of cellulostic waste termed as agricultural waste or biomass. Innovation in managing such a vast amount of agricultural waste or biomass is a continuous challenge and recent trends favor the utilization of this biomass for value added purposes to fulfill the need in the areas such as renewable energy, fiber composites and textiles, food alternatives and livestock feed (Rosentrater et al. 2009). Studies on the cellulostic fibers from other agricultural wastes such as from the oil palm industries indicated the great potential of these by-products to become a commercial raw material in making highly demanded products such as paper and fiber composites (Bakar et al. 2007; Wan Rosli et al. 2007).
Numerous studies have been done to improve the usage of banana by-products to meet the escalating demand of raw materials supply in various industries (Clarke et al. 2008; Doran et al. 2005; Emaga et al. 2008a; Kuo et al. 2006). These researches paved new and alternative ways in creating new products and applications with value added approach at the cost of recycling banana agricultural wastes. There is a continuous need to create and invent new products with value-added applications from alternative bio-resources as means to develop a sustainable civilization. Due to the high demand for food products, energy, and other essential needs, gradual improvement in the current technological development towards utilizing alternative resources in many industries is necessary to cater the needs of the ever-increasing world population (Mohammadi 2006). Therefore, this paper aims to review the recent advances in the exploitation of banana by-products and wastes, the challenges in making these by-products a valuable commodity of the future.
Banana as an agricultural commodity
Classification and taxonomy
Banana is actually one of the largest herb groups in the world (Ploetz et al. 2007). The plant can grows up to 5–7 m consisting of a fleshy rhizome (corm), pseudostem (leaf petioles) and spirally arranged oblong leaves. The long oval shaped inflorescence, supported by a stalk, protrudes out from the tip of the pseudostem consisting of deep purple waxy bracts which enclosed the female (occupies the lower 5–15 rows) and male flowers (upper rows). The female flowers will eventually developed into “berry” fruits (hand) which will mature to be horned shaped with white or yellow flesh. Seeds are common in wild types but the cultivated varieties are generally seedless with almost invisible dots of ovules at the center (Arvanitoyannis and Mavromatis 2009). The term banana is commonly used to represent the dessert cultivar while the cooking cultivar is generally referred as plantain. They belong to the family Musaceae and various species of the genus Musa have been cultivated since time immemorial, and used as a source of fiber, foods, and ornaments (Kennedy 2009; Subbaraya 2006).
Today’s production and domestication of edible desert bananas and plantains involved a complex hybridization and polyploidy between two diploid species. Musa acuminata provides the “AA genome” while Musa balbisiana provides the “BB genome” (Heslop-Harrisons and Swarzacher 2007). The edible banana (eaten as dessert) and plantain (banana for cooking) may have combinations of these sets of genome which can range from triploid (AAA, BBB, AAB, ABB) to a diverse tetraploid blends. As such, they are grouped based on their ‘ploidy’ as Musa acuminata, Musa balbisiana or Musa acuminata x balbisiana, which is synonymous to the previous classification called Musa x paradisiaca that represents hybrids (Nelson et al. 2006). Hundreds of years of natural and selective cultivation made it possible to transform edible bananas into several hundred varieties with a number of improvements such as the reduction in their seed size, sterility, oversized pulp, and spontaneous development of fruit without the need for fertilization (Arvanitoyannis and Mavromatis 2009; Ploetz et al. 2007). There are approximately 1200 seedless fleshy fruits varieties and cultivars of banana and plantain in the world and mainly planted for food purposes (Aurore et al. 2009).
The advancement in the genomic identification of banana varieties using Polymerase Chain Reaction (PCR) technology and genetic based markers makes it possible for the accurate identification of banana within similar species (Brown et al. 2009; El-Khishin et al. 2009; Teo et al. 2005). However, morphological identification is still widely used to determine the variety of the cultivated banana although there are some difficulties associated with the used of whole-plant or floral morphology especially dealing with somaclonal variation and identifying clones (Brown et al. 2009). Agricultural bodies around the world especially in the banana producing countries do keep a live specimen and in vitro culture of banana plant varieties in case where genomic identification is required and for further improvement of banana cultivation and research (Mattos et al. 2010; Panis 2009).
Production and global market
Banana is grown in almost every country in the world especially in the tropical and subtropical countries where it has been sustainably cultivated and thus contributed to the country’s economy (Zhang et al. 2005). India remains the largest banana producing country in the world, which produce more than 25 % of the world’s banana production (Table 1) (FAO 2010a). Despite being the top ranked world’s banana producers, most of the banana produced in India are used for its domestic market and only about 0.04 % is exported. Thus, total exports of banana from India are dreadfully low as compared to other leading global banana exporting countries such as Ecuador, Costa Rica, Philippines, and Colombia, which accounts for more than 60 % of the world’s exports. The top importers of banana are the United States and the European Union. The largest plantain producing countries are mostly African countries where plantain is one of the staple foods in the region. According to FAO (2010a), Uganda is the largest plantain producer with an estimated production of 9.6 million tonnes, followed by Ghana and Rwanda.
Table 1.
Top ten major world producers of banana and plantain by country
| Rank | Banana | Plaintain | ||
|---|---|---|---|---|
| Country | Productiona | Country | Productiona | |
| 1 | India | 31,897,900 | Uganda | 9,550,000 |
| 2 | China | 9,848,895 | Ghana | 3,537,730 |
| 3 | Philippines | 1,101,340 | Rwanda | 2,749,150 |
| 4 | Equador | 7,931,060 | Nigeria | 2,733,300 |
| 5 | Brazil | 6,978,310 | Cameroon | 2,604,100 |
| 6 | Indonesia | 5,814,580 | Colombia | 2,815,050 |
| 7 | Tanzania | 2,924,700 | Peru | 2,007,280 |
| 8 | Guatemala | 2,621,500 | Cote d’Ivoire | 1,600,000 |
| 9 | Mexico | 2,103,360 | Congo | 1,250,690 |
| 10 | Colombia | 2,034,340 | Kenya | 791,579 |
FAO 2010a
aValues are expressed in metric tonne
Banana production is generally grouped into two different categories; the vast majorities being the small scale farmers that produce banana mainly for self-consumption and for the domestic market while the other group involves large plantations and companies that supplies both domestic as well as international markets. Based on the statistics by FAO (2010b), there are 93.3 million tonnes of dessert bananas and 34.3 million tonnes of plantains were produced in 2010 ahead of apple (70 million tonnes), orange (69 million tonnes) as well as grape (68 million tonnes). However, in the previous year’s statistics, less than 20 % of the total production is traded internationally, which valued around 7 billion Euros (Aurore et al. 2009). The common internationally traded dessert bananas are mostly from the cultivated AAA group varieties such as “Cavendish”, “Gros Michel”, and “Grande Naine”. Other well-known varieties which are endemic to a certain region includes “Yangambi Km5, AAA” found mostly in eastern Africa, “Red banana, AAA” and “Mysore AAB” found in southeast Asia as well as “Silk AAB” and “Bluggoe ABB” which are found across the tropical region (Ploetz et al. 2007).
Banana by-products
Conventional uses of banana by-products and waste
Banana is a unique perennial single harvest plant. Its visible part, the pseudostem and leaves dies after it bears fruit to make way for the young budding plant (suckers) to rejuvenate from the rhizome. The harvesting of the fruit in plantation requires the decapitation of the whole plant so that the young suckers can replace the mother plant and these cycles can continue for unlimited generations. Generally, banana by-products include the pseudostem, leaves, inflorescence, fruit stalk (floral stalk/rachis), rhizome and peels. Most of these by-products may serve as an undervalued commodity with a limited commercial value, application and in some cases, it is considered as an agricultural waste. The pseudostem and leaves are commonly left to rot in farms to replenish some of the nutrients in the soil. Young shoots, pseudostem piths and inflorescence, although be consumed as vegetables by the indigenous people in parts of Southeast Asia and Indo-Malesian Region (Kennedy 2009), they may not be able to compete with the common leafy vegetables due to its undesirable taste. The values of the banana inflorescences were quite low because of the inconsistent demand and limited acceptance. Banana leaves are still used as wrapping materials for traditional foods in Southeast Asia but its application only limited to some ethnic foods. A slightly better application of the banana waste was its utilization as an animal feed to minimize the cost of production (Akinyele and Agbro 2007), but additional processing is required due to its high water content that greatly reduces its nutritional density. Low cost agricultural wastes are generally poor in essential nutrient but at the same time high in fiber content (Ulloa et al. 2004).
In some places where “open fire burning” is still practiced, the burning of banana wastes may contribute to serious environmental issues. In addition, the piling up of banana waste in plantations is an eyesore, which will eventually obstruct farmers on their process to harvest mature and ripe fruits. Banana floral stalk and peels are not directly available at the farming site but may be available at the processing sites where the fruit is packaged or the fleshy pulp of the fruit is separated from its peels. Collectively, the waste that a single banana plant produces can make up to 80 % of the total plant mass. It is estimated that 220 tonnes of by-products are produced per hectare annually (Shah et al. 2005) indeed requires an innovative idea to turn these readily available resource into a value added products.
Banana by-products as potential renewable resource in promoting “green” technology
Renewable resource or biomass, are a naturally abundant resource, which may include any materials obtained from biological origin such as plants and animal materials, agricultural crops and biological residues or wastes (Xu et al. 2008). These resources can be turned into raw materials or products having the potential capacity of being recyclable and easily biodegradable which in turn having positive environmental acceptability or ‘green label’ attributes plus commercial viability (Mohanty et al. 2002). Renewable resources have paved way to the industry and have been used in decades to replace non-renewable resources especially petroleum and gas products, precious metals and minerals. It is important that the utilization of low cost agricultural by-products and biological wastes could be expanded to all possible industries in order to achieve a sustainable development of technology. This could contribute to an additional source of revenue to farmers and processing industries without adversely affecting soil fertility and reduce the depletion of the non-renewable resources (Reddy and Yang 2005). Additional valuable outputs from the existing farmland might save our precious forest from being destroyed to produce similar materials.
Green technology signifies an application, which is environmental friendly emphasizing on conserving the natural environment and resource as well as posing a minimal threat to the existing species on earth including humans. The technology should be independent from the existing agro-food commodity market, as the utilization of agro-food based products such as corn to drive green technology will eventually create food insecurity; ethical issues and unsustainable energy return (Pimentel and Patzek 2005). As an abundant biomass, banana by-products are readily available to be used as a source of raw materials for the green technology industry. The long history of human consumption of banana without any serious side effects reported provides somewhat a safety assurance in which these by-products do not contain hazardous phytochemicals. By-product harvesting, handling and storing perhaps require less precaution as compared to other plants with potent and hazardous chemical constituents. The utilization of banana by-products for the industrial application could promote “green technology” in which may not pose any food security and ethical issues as it is independent from the existing agro-food based market. Moreover, it does not require extra planting area apart from the current banana plantation for fruit.
Potential food and nutraceutical from banana by-products
Source of starch, pectin, and cellulose
Starch, pectin, and cellulose are used in the food industry as gelling agent, thickening agent and stabilizers. Starch, a group of carbohydrates, is commercially available and produced from plants such as corn, potato, rice, wheat, and cassava. Banana by-products that can be processed into edible starches includes the pith of pseudostem and the green culled banana which are removed during fruit selection and processing (Abdul Aziz et al. 2011; Da Mota et al. 2000; Zhang et al. 2005). Banana starches which are relatively low in amylase content, have high resistance to heating and amylase attack, low swelling properties, low solubility in water and low retrogradation, been proved slightly superior to modified and unmodified corn starch giving it a potentially higher market value (Zhang et al. 2005). Commercial pectin, a structural heteropolysaccharide classified under soluble dietary fiber, was produced mainly from fruits extract such as citrus peels, oranges, apples, and carrots. Comparing the quality of pectins isolated from various fruit wastes revealed that the pectin’s methoxyl composition and gelling quality of banana is slightly lower than the pectin isolated from citrus peels such as pomelo and lime (Madhav and Pushpalatha 2002). Pectin could be produced from discarded banana peels via acid extraction and precipitation by using alcohols or ammonium salts. A study by Emaga et al. (2008a) revealed that pectin content in banana peels was higher than plantain peels and both of these fruit peels provide a similar or slightly higher amount of extractable dietary fibers compared to other fruit peels implying a potential cheaper alternative source of pectins for banana producing countries, reducing their dependence on imported pectins. Emaga et al. (2008b) have successfully isolated and characterized pectins (87–248 kDa) from the banana peels bearing variable compositions of neutral sugars (galactose, arabinose and rhamnose), galacturonic acid and different degrees of esterification and the quality of pectin extracted could be improved by manipulating the extraction parameters as well as looking into the ripening stage of the fruit. The synthesis of sodium carboxymethylcellulose (CMC) utilizing banana pseudostem was made possible through the work by Adinugraha et al. (2005). However, the technical grade CMC obtained (98.63 %), may not be directly suitable yet for food application. Thus, further purification process is needed to obtain a food grade CMC (99.5 %) from banana pseudostem that will meet the specification requirement of the Food Chemical Codex (1996). Microcrystalline cellulose was also obtained from banana waste fibers through acid hydrolysis (Elanthikkal et al. 2010). However, it is indicated that banana by-products such as green culled banana, peels and pseudostem could be a potential low cost source and raw material of high quality starch, pectin and cellulose for the food industry (Table 2).
Table 2.
Starches, pectins and cellulose from banana by-products for food application
| Banana by-products (Species/variety) | Potential food application | References | |
|---|---|---|---|
| Starches | Green culled banana (variety not specified) | Food thickeners, gelling agent, reinforcing agent in tablets, fillers | Zhang et al. (2005) |
| Banana pith and pseudstem (Musa acuminata x balbisiana Colla cv. Awak) | Abdul Aziz et al. (2011) | ||
| Unripe fruit (Plantain bananas of the variety “Terra” (Musa paradisiaca) | Viscosity and swelling properties | Pelissari et al. (2012) | |
| Green banana (Musa AAA Cavendish) | Substitution of cassava starch for textural properties of snacks (crackers) | Wang et al (2012) | |
| Green banana plantain (Musa paradisiaca L.) | Production of starch based edible films | Zamudio-Flores et al (2006) | |
| Pectin | Banana peels (Musa acuminata AAA, Musa acuminata x balbisiana AAB | Food thickeners, gelling agent | Emaga et al. (2008a), Emaga et al. (2008b) |
| Cellulose | |||
| Carboxymethyl cellulose | Banana pseudostem (Musa acuminata cv Cavendish | Food thickeners, gelling agent | Adinugraha et al. (2005) |
| Microcrystalline cellulose | Not specified (variety not specified) | Water retainer, reinforcing agent in tablets | Elanthikkal et al. (2010) |
Natural bio-colorant
The anthocyanins, a subclass of flavonoids are an important pigment group that is responsible for the red, purple, and violet colors of the banana inflorescence (Kitdamrongsont et al. 2008). Anthocyanins are considered to be a good bio-colorant due to its attractive colors, moderately stable in food systems, water-soluble (Ozela et al. 2007; Torslangerpoll and Andersen 2005), and proven to be beneficial to health (Bagchi et al. 2004; Konzack and Zhang 2004). Previous studies showed that anthocyanins could be extracted from other plants including purple sweet potato (Fan et al. 2007), black chokeberry, strawberry, bilberry fruits (Pliszka et al. 2008) and some tropical fruits (Einbond et al. 2004). The abundance of anthocyanins reported in the banana inflorescence bracts (Musa acuminata and, Musa acuminata x balbisiana), ranging from 14–32 mg anthocyanin/100 g bracts, mainly comprising of cyanidin-3-rutinoside compound, could potentially be exploited as a cheap source of natural food colorant. The content is slightly higher than the commercially available anthocyanins from red cabbage and by looking into the abundance of bracts produced (mass per hectare of land), it may provide sufficient and sustainable market outlook (Jenshi et al. 2011; Pazmino Duran et al. 2001). Natural bio-colorant such as the anthocyanins remains in demand not only due to its health promoting properties but also the increase in demand on natural foods (Rymbai et al. 2011).
Biogeneration of flavor
Flavor plays a very important aspect in the food industry. They are formed through various chemical reactions during food processing and mostly through the reduction of carbon, nitrogen, and/or sulphur compounds along with the generation of volatile organic compounds (VOCs) such as aldehydes (Rappert and Muller 2005). Biogeneration of aldehydes and alcohols used in the flavor industry can be carried out naturally through enzymatic pathways utilizing enzymes such as lipase, alcohol dehydrogenase (ADH), lipooxygenase (LOX), hydroperoxide lyase (HPLS) (Gigot et al. 2010). Kuo et al. (2006) reported that banana leaves (Musa cv. Cavendish) contains a membrane-bound enzyme of 9-LOX, which is able to produce oolong tea-like, melon-like, and fruity cucumber-like flavor upon pickling or when treated with soybean oil, linoleic acid, linolenic acid and respectively; the kinetic properties were comparable to LOX obtained from canola seed and English pea. Thus, banana leaves could be used as a potential target for continuously generating natural flavors to be utilized in the food industry.
Source of dietary nutrients
There have been a few studies done on the by-products of banana and plantain in order to evaluate its nutrient content as a potential source of dietary food components such as carbohydrate, proteins, dietary fibers, and minerals for human consumption (Emaga et al. 2007; Mohapatra et al. 2010). Banana pith from the pseudostem has long been eaten as vegetables in some parts of the world such as India, Sri Lanka, and Malaysia (Kennedy 2009; Subbaraya 2006). It contains considerable amount of starch, sugars, and minerals (Mohapatra et al. 2010). In most of the Southeast Asian countries, banana inflorescence is consumed as vegetables and salad for a very long time. Emaga et al. (2007) reported that banana peels from three different genetic makeups namely AAA, AAB, ABB, and AAAB were rich in total dietary fibre (40–50 %), protein, and amino acids (8–11 %), lipids and fatty acids (2.2 % to 10.9 %). The dietary fibre content was slightly higher compared to wheat, barley, oats and rice brans (Sudha et al. 2007). The peels were reported to contain significant amount of potassium. An incorporation of banana peels at a ratio of 10 % into biscuits did not show significant differences in the overall color, aroma, and taste, which make it suitable for the production of low calorie food products with high dietary fiber content (Joshi 2007).
Source of nutraceutical and bioactive compounds
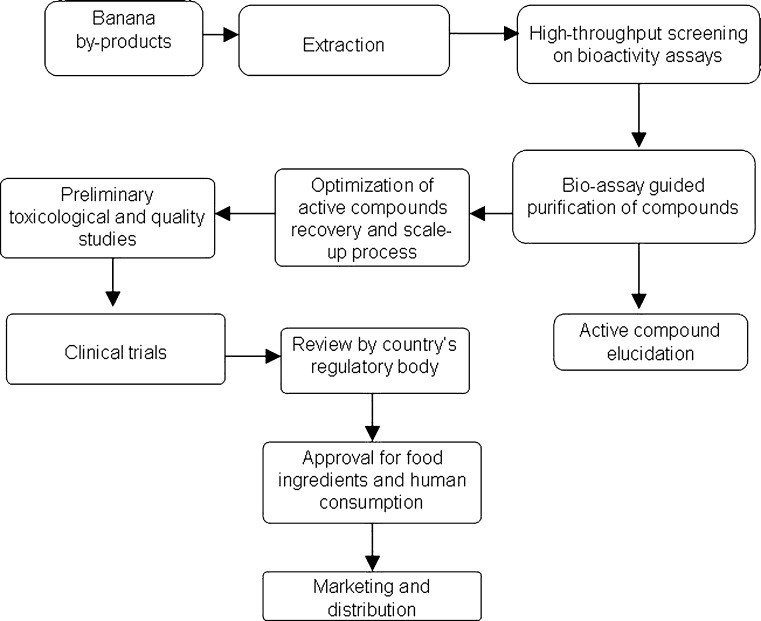
The term “nutraceutical”, was first coined in 1989 by Stephen DeFelice and the original term was defined as “A food or parts of food that provide medical or health benefits, including the prevention and/or treatment of disease" in which until now, the definition has not been regulated under the U.S. Food and Drug Administration (FDA) (Ameye and Chee 2006). Currently, the definition of nutraceutical is only regulated through the Dietary Supplement Health and Education Act of 1994 (DSHEA) in United States, which allows substantial flexibility between the definition of foods and medicines (Gulati and Ottaway 2006). The research and development on nutraceutical and bioactive compounds from plant sources often require laborious efforts and must undergo several stages before it can be safely marketed (Fig. 1). Banana by-products have been reported to contain compounds with nutraceutical properties that can potentially be commercialized in the pharmaceutical industry. Recently, Tin et al. (2010) have identified the presence of epigallocatechin and its derivatives form banana male flowers while in another study, Saravanan and Aradhya (2011) successfully isolated entisic acid, (+)-catechin, protocatechuic acid, caffeic acid, ferulic acid, and cinnamic acid from banana pseudostem. It has been accepted that polyphenolic compounds such as gallocatechin, caffeic acid, cinnamic acid and catechin posed antimicrobial activity (Chanwitheesuk et al. 2005; Shan et al. 2008), antioxidative (Chye and Sim 2009, Wong and Chye 2009), neuroprotective (Lu et al. 2005; Mandel et al. 2008), chemopreventive (Raina et al. 2008; Artali et al. 2009), anticancer (Faried et al. 2007; Shankar and Mulimani 2007) and antiproliferative capacities (Jagan et al. 2008).
Fig. 1.
Research and development of nutraceutical/bioactive components from banana/plantain by-products
Phytosterols are naturally occurring plant sterols that have been studied extensively. Several reports show their wide variety of positive health promoting effects including lowering of blood cholesterol (Moruisi et al. 2006; Racette et al. 2010; Weingärtner et al. 2008) and reducing the risk of coronary heart diseases (Miller and Nichols 2008). Several bioactive steryl glucosides, namely campesteryl 3-β-d-glucopyranoside, stigmasteryl 3-β-d-glucopyranoside and sitosteryl 3-β-d-glucopyranoside were identified from the dichloromethane extracts of Musa acuminata Colla cv. Cavendish (Oliveira et al. 2005). Sterols, steryl glucosides and steryl esters were found abundantly in banana (Musa cv. Dwarf Cavendish) indicate its potential application as a source of functional food products (Oliveira et al. 2008). A separate study done by Singh et al. (2007) revealed that oral administration of banana pseudostem juice (Musa paradisiaca) to the normal and hyperdiabetic (high glucose level in blood) rats shows a significant increase of blood glucose level, signify a possible treatment hypoglycemic patients due to low insulin level or hypoglycemic drugs.
Anthocyanins from fruits and vegetables are regarded as important components in human nutrition due to their antioxidative capacities (Mertens-Talcott et al. 2008; Zheng et al. 2011). Besides, anthocyanins also possessed anti-inflammatory (Bowen-Forbes et al. 2010; Karlsen et al. 2007), antiviral (Wang et al. 2006), anticarcinogenic (Kulma and Szopa 2007), antiproliferative effect (Mokbel and Hashinaga 2005), and cancer chemoprevention properties (Bowen-Forbes et al. 2010; Wang et al. 2011). Banana inflorescence from either cultivated and wild varieties are rich in anthocyanins (Jenshi Roobha et al. 2011; Kitdamrongsont et al. 2008) suggest it could potentially serves as a source of functional food components which are beneficial to health.
Dopamine, one of the catecholamines, was found at high concentration in the pulp of yellow banana (Musa acuminata), red banana (Musa sapientum var. baracoa) and peels of Cavendish banana (Adao and Gloria 2005; Kulma and Szopa 2007; Mokbel and Hashinaga 2005). These compounds were found to be free radical scavenger as well as posses strong antimicrobial activity against Salmonella enteritidis, Escherichia coli, Staphylococcus aureus, Bacillus cereus and Bacillus subtilis. Dopamine also displays anti-inflammatory activity when administered to brain-dead rats with a significant improvement of the rat’s blood system regulation and kidney function (Hoeger et al. 2007). Table 3 summarizes the known bioactive compounds that isolated from banana by-products with their respective nutraceutical properties.
Table 3.
Important bioactive compounds as potential nutraceuticals from banana/plantain by-products
| Bioactive compounds | Banana by-products (Species/variety) | Bioactivity | References |
|---|---|---|---|
| Cyanidin-3-rutinoside | Bracts (Musa paradisiaca) | Antioxidant, Anticancer | Jenshi Roobha et al. (2011) |
| Epigallocatechin and derivatives | Male flower (Musa paradisiaca) | Antibacterial, Antioxidant | Tin et al. (2008); Tin et al. (2010) |
| ß-sitosterol, malic acid, succinic acid, palmatic acid, 12-hydroxystrearic acid, glycoside | Peels (Musa acuminata cv. Cavendish) | Anti-inflamatory, Anti-cholesterol | Mokbel and Hashinaga (2005) |
| Antioxidants, Antibacterials | |||
| Campesteryl 3-β-d-glucopyranoside, stigmasteryl 3-β-d-glucopyranoside and sitosteryl 3-β-d-glucopyranoside | Petioles, leaves, floral stalk (Musa acuminata cv. Cavendish) | Anti-inflamatory, Anti-cholesterol | Oliveira et al. (2005) |
| Sterols, steryl glucosides, steril esters, tocopherols, phenolic compounds | Peels and pulp (Musa acuminata cv. Dwarf Cavendish) | Anti-inflamatory, Anti-cholesterol, Antioxidants | Oliveira et al. (2008) |
| Putrescine, spermidine, serotonin, dopamine, tyramine, spermine | Pulp (Musa acuminata x Musa balbisiana) | Stimulants | Adao and Gloria (2005); Lima et al. (2008) |
| Entisic acid, (+)-catechin, protocatechuic acid, caffeic acid, ferulic acid, and cinnamic acid | Pseudostem (Musa acuminata x balbisiana cv. Nanjanagudu Rasabale) | Antioxidants | Saravanan and Aradhya (2011) |
| Anthocyanins, catecholamines, tocopherols, phytosterols, ascorbic acid | Peel (Musa acuminata Colla AAA) | Antioxidants | González-Montelongo et al. (2010) |
Natural food preservative
Applications of bioactive constituents from banana by-product are not limited to nutraceuticals for direct human consumption, but should be further exploited as natural preservatives for foods. Food preservation plays a vital role in driving the food industry by extending the shelf life of foods. Current trends of industry show increase awareness towards the drawbacks of synthetic chemical preservatives and opt for minimally processed food or employing natural techniques in food preservation (Tiwari et al. 2009). Natural antimicrobials from numerous plant sources including spices and herbs have been well documented in suppressing food spoilage microbes and foodborne pathogens (Kumar and Tanwar 2011; Kumudavally et al. 2011; Pillay and Ramaswamy 2012; Padam et al. 2012a) further strengthens the concept of natural ingredients for food preservation. Antibacterial compounds such as ß-sitosterol, 12-hydroxystrearic acid and malic acid isolated from banana peels (Musa paradisiaca) shown to be a good suppressor of foodborne pathogens including Staphylococcus aureus, Bacillus cereus, Salmonella enteritidis and Escherichia coli (Mokbel and Hashinaga 2005) and could potentially be applied into food systems in the future. Devatkal et al. (2011) also added that the preservative capability of banana peel water extract from similar banana variety in reducing lipid oxidation process in raw meat was comparable to synthetic antioxidant such as butylated hydroxy toluene (BHT). This result was well anticipated due to the fact of the existence of known antioxidative substance in banana peels as reported by Mokbel and Hashinaga (2005) as well as González-Montelongo et al. (2010). Extracts from the male flowers of banana (Musa paradisiaca) were also demonstrated to contain antibacterial properties, which able to decontaminate and suppress the growth of Listeria monocytogenes and Staphylococcus aureus in chicken breast meat comparable to the commercial potassium sorbate (Tin et al. 2010; Padam et al. 2012b).
Animal feeds
The demand for food from animal sources has been escalating along with the increase of the world’s population, which will bring a few immediate consequences; there is a need to increase the productivity of domestic animals utilizing feed with higher nutritive value to overcome the limited and expensive source of raw materials for animal feed production. Therefore, it is important to utilize inexpensive materials not only to sustain the market of animal products but also an effort to search for new sources of animal feed by recycling underutilized wastes (Ulloa et al. 2004). Agricultural and food processing wastes such as rice bran, corncobs, pineapple waste and sugarcane bagasse have been utilized as an alternative source of animal feed production which are mostly dependent on grains and legumes (Sruamsiri 2007). The utilization of available raw material such as agricultural waste reduces the dependence on the costly imported feed and banana by-products could be one of the potential raw materials for feedstock production for most of the banana producing countries (Ulloa et al. 2004). Several notable studies in the current literature show promising results on the potential of converting banana by-products into livestock feed.
Banana peels are known to contain a substantial amount of protein, lipid, carbohydrate, fiber and a number of essential minerals such as potassium, sodium, calcium, iron and manganese, which serves as a promising raw material for feed production (Ahnwange 2008). It was reported earlier by Essien et al. (2005) that banana peels could be processed as a mycological medium for growing valuable micro-fungal biomass, enriching the protein and fatty acids content of the solid mixture. Protein content of banana peels increased up to 34 % through solid-state fermentation (SSF) by Aspergillus niger, Aspergillus flavus and Pennicilium sp. while the tremendous increase of sugar content (142 %) is possible through fermentation by Aspergillus flavus (Akinyele and Agbro 2007; Yabaya and Ado 2008). Enhancement of nutritional value of banana waste through microbial fermentation is an important step in order to create high nutritional quality feedstock from low quality materials; protein and sugar content can be increased comparable to the soybean meal, which is a common ingredient in most animal feeds (Hong et al. 2004).
Direct substitution of banana leaves and pseudostem (Musa paradisiaca) as forage in the diet of Ovin Martinik sheep shows no significant difference in carcasses quality compared to sheep feeding on normal hay forage diet (Marie-Magdeleine et al. 2009). The sheep carcass did not differ in terms of overall weight and chemical composition of the meat. In addition, it was reported that banana leaves are the best fodder because of its low partition factor, high ATP and high microbial biomass in a feeding experiment of pseudostem and leaves of banana (Musa paradisiaca) for ruminants (Amarnath and Balakrishnan 2007). Banana roots (rhizome) have been noted to treat rabbits with coccidiosis where the oral administration of crushed Musa paradisiaca roots significantly reduces the fecal worm egg count in just over 2 weeks of treatment (Matekaire et al. 2005).
Non-food usage of banana by-products
Natural fibers
Fiber industries have been eyeing on an alternative sustainable material that would eventually replace the usage of wood and pulp from the trees to make timbers, boards, textiles, and papers. Agricultural by-products from various sources are the main candidates because of its availability and mass production all year round (Reddy and Yang 2005). Fibers can be obtained from numerous sources of agricultural commodity and its by-products such as jute, cotton, rami, kenaf, sisal, palm oil, banana, sugar cane, corn and wheat. Fibers from the banana plant are comparable in physical strength and cellulose content to fibers obtained from other fibrous commodities by-products (Uma et al. 2005) and have been extensively characterized from their fruit stalk (Oliveira et al. 2006; Zuluaga et al. 2009), pseudostem (Cherian et al. 2008) and leaves (Oliveira et al. 2007). A few studies have been published emphasizing the potential of banana fibers as the raw materials in making composite boards (Chattopadhyay et al. 2010; Ibrahim et al. 2010; Idicula et al. 2005; Sapuan et al. 2007; Savastano et al. 2009). Maleque et al. (2005) demonstrated the usage of banana fibers from the pseudostem to reinforce epoxy composites. Their findings conclude that the banana fibers substantially increased the tensile strength of the virgin epoxy material by 40 %. The sturdiness of the banana fiber composites can also be enhanced by surface modification through acid treatment (Jannah et al. 2008) or the addition of adhesive (El-Meligy et al. 2004), which reduces their water absorption capacity. Quintana et al. (2008) made another innovative improvement in the banana fiberboard research without incorporating any adhesive or binding materials. Their technology comprised of utilizing steam explosion at high temperature and pressure that redistributes the lignin within the plant material itself and act as a binding agent into the structure. The fiberboard developed satisfied the minimal standard for high-density fiberboard (HDF) (ICONTEC, Columbia) although it was inferior in quality compared to the commercial fiberboard made from conifers.
Banana fibers obtained from the pseudostem have been used for decades as raw materials for textiles in the production of traditional handicrafts and clothes by several groups of people in the world (Kennedy 2009). Currently, the global textile and clothing industry is estimated to generate as much as USD395 billion export values (Chen et al. 2007), which signifies a great demand in fiber materials for textile purposes. Enzymatic degumming of raw plant fibers using microbial strains is a promising way to process textile fibers from natural plant sources including banana. Jacob and Prema (2008) employed polygalacturonase producing Streptomyces lydicus in a solid-state fermentation, which successfully converted raw fibers into processed banana fibers for textile purposes in a short period. They were able to extract polygalacturonase enzyme produced by Streptomyces lydicus in the solid mixture while at the same time obtaining processed banana fibers. John and Anandjiwala (2009) reviewed several methods on the surface modification of a wide range of natural fibers including banana fibers for textile purposes, which involved wet chemical processing and ionized gas treatments.
Paper production is one of the commercial applications of banana by-products. The initiative in utilizing available non-woody agricultural waste as raw materials for paper production offers a great potential in reducing the dependence on natural timbers, which is becoming more expensive due to the limited availability (Bastianello et al. 2009). It was found that pseudostem from Musa acuminata Colla, cv. Cavendish could be used for pulp and paper processing, where the fibres showed interesting potentialities in terms of burst index and breaking length either alone or in combination with other common pulps (Cordeiro et al. 2005). Banana pseudostem pulp from Musa paradisiaca L. shows increased burst index, tensile index, tear index and oil resistibility when combined with bamboo pulp in making greaseproof paper (Goswami et al. 2005). Ogunsile et al. (2006) compared the quality and yield of the pulp processed from different types of banana by-products. They found that the leaves (midrib part), pseudostem, and fruit stalk produced 34–49 % of pulp and the yield was heavily influenced by the pulping parameters such as pH, temperature, and pulping time. Pulping banana pseudostem (Musa acuminata cv. Cavendish) at low cooking temperature of 105 °C utilizing formic acid and acetic acid also yields better quality pulp for papermaking (Mire et al. 2005). Papers made from banana were reported to have a very low water absorption capacity making it more water resistant and stronger than wood-pulp paper (Jacob and Prema 2008).
Renewable fuel
The demand of hydrocarbon fuel as energy has been increasing rapidly throughout the years. Although the demand for energy is booming exponentially, the production and the discovery of new reserves of natural fossil fuels did not increase complementary fitting with the high demand. Moreover, the environmental effects of burning fossil fuels have been extensively debated around the world and the idea of using greener and more sustainable fuel to gradually reduce and replace fossil fuels were greatly considered (Hill et al. 2006). Other aspects such as food security issues, efficient agricultural land utilization and the usage of non-edible sugars as fermenting substrate further strengthens this concept (Corma et al. 2011). Biochemical conversion of biomass such as agricultural waste into renewable fuel is one of the preferred conversion pathways that provide environmental friendly approach and the common methods are based on enzymatic hydrolysis and microbial fermentation of solid matters (Saxena et al. 2009). A few notable current researches are discussed in the later section.
Ethanol is highly used in the industry not only as renewable fuel but also as a solvent. Natural bio-ethanol is produced generally from a fermentation process using either bacteria or yeast in which sugar derived from cellulosic sources is metabolized and converted into ethanol (Raposo et al. 2009). In order to make these natural sugars available for fermentation, breakdown of the macromolecules (polysaccharides and cellulose) through enzymatic or chemical reaction plays important roles (Doran-Peterson et al. 2008; Hahn-Hägerdal et al. 2006). Banana peels are noted to be a good substrate in producing ethanol and the contributing factors such as substrate concentration, fermentation parameters, and the type of fermenting organism do affect significantly the overall yield of ethanol (Manikandan et al. 2008). Banana pseudostem and leaves are also potential substrate for ethanol production through the utilization of cellulolytic thermophilic Clostridium thermocellum CT2 co-cultured with Clostridium thermosaccharolyticum HG8 (Reddy et al. 2009). The utilization of agricultural waste such as the banana by-products as raw materials for ethanol production could potentially reduce the cost of using staple food crops such as corn and wheat in conventional natural ethanol production.
Methane is an important fuel that powers many industries as well as household kitchen. It exists as gas and it is highly combustible compared to ethanol. Industrial methane is produced through extraction from natural gas fields and fermentation of organic matters such as sewage sludge, agricultural biomass and manure (Paepatung et al. 2009; Vijay et al. 2006). The conversion of banana waste cellular material into methane requires an anaerobic digestion of the plant matter in an airtight reactor with specific digestion parameter control. No special microbial inoculum is required as the digestion was done by the natural flora that exists within the plant material itself. Methane gas is produced generally starting on day 30 for 30–100 days (Chanakya et al. 2009; Clarke et al. 2008). The developed method in producing methane from banana waste is considered clean and safe, as it does not require addition of sewage sludge or manure.
Briquettes are made from a densification process that improves the handling properties of raw material and enhancing the energy content of the biomass. Most cellular plant waste including banana cannot be converted directly into energy through combustion because of their low density, high volume, high moisture content, and a very low energy density. This shortcoming directly affects the transportation and storage of these solid matters (Mani et al. 2006). Without proper processing, it is bulky and creates an incomplete combustion that may pollute the environment as well as may not be a viable source of energy. Conventional briquettes were made using sawdust with the addition of coal cake and in recent years, low cost agricultural by-products appeared in overcoming the shortage of wood-based products (Chou et al. 2009; Sotannde et al. 2009; Wilaipon 2007). Wilaipon (2009) reported that low cost banana peels bound with molasses under high press pressure are a potential raw material for making banana briquettes. These briquettes were made as an attempt to utilize agricultural waste such as the banana peel as a substitute for solid fossil fuels such as coals. Comparing to other agricultural waste briquettes such as sawdust, rice husk, peanut shell, coconut fibre and palm fibre in an earlier study by Ooi and Siddiqui (2000), briquettes made from banana peels had an outstandingly lower burning rate with equivalent briquette strength even when similar densification pressure is applied during processing.
Potential substrate for the production of non-food cellulose, cellulolytic enzymes, organic acids and edible mushrooms
Cellulose is considered the most abundant organic substrate on earth and the main building blocks of plants. Before natural cellulose can be utilized as raw materials to produce sugars, fuel and animal feed, it is necessary for it to be hydrolyzed either via acid hydrolysis or by enzymatic hydrolysis using cellulolytic enzymes such as cellulases. Commercial cellulases are produced by microorganism typically by bacteria and fungi. They are important group of enzymes that are required for the industrial scale cellulose processing (Shafique et al. 2004). Banana by-products have been identified to be a potential economical substrate for cellulolytic enzyme production and have been proven to support the growth of several microorganisms used in the production of cellulase in a solid-state fermentation system (SSF). Fungi are known organic waste decomposers and capable of hydrolyzing complex organic compounds as a major source of energy (Dinishi Jayasinghe and Parkinson 2008). Among various groups of microorganisms used in solid-state fermentation, the filamentous fungi are the most exploited due to their ability to grow on complete solid substrates and production of wide range of valuable extracellular enzymes (Boberga et al. 2008). The utilization of banana by-products for the production of cellulolytic enzymes is summarized in Table 4.
Table 4.
Utilization of banana/plantain by-products as substrates for the production of non-food cellulose, cellulolytic enzymes, and organic acids
| Products | Banana by-products used as substrate | Microorganism and macroorganism used | References |
|---|---|---|---|
| Non-food cellulose | Pseudostem | Mix cultures from banana plantation soil | Zainol and Abdul Rahman (2008) |
| Cellulolytic enzymes | |||
| Crude Cellulases | Pseudostem | Phanerochaete chrysosporium, Pleurotus ostreatus | Mena-Espino et al. (2011) |
| Exoglucanase | Floral stalk | Bacillus subtilis | Shafique et al. (2004) |
| a-amylase | Banana peels | Helminthosporium oxysporium, Aspergillus niger, Aspergillus fumigatus, Aspergillus flavus, and Penicillium frequestans. | Adeniran and Abiose (2009) |
| Floral stalk | Aspergillus oryzae | Ragunathan and Swaminathan (2005) | |
| Mix enzymes (Not specified) | Leaves and pseudostem | Trichoderma lignorum | Baig (2005) |
| Not specified | Banana floral stalk | Neurospora sitophila | Asad et al. (2006) |
| Laccase | Banana peels | Trametes pubescens | Osma et al. (2007) |
| laccase, lignin peroxidase, xylanase, endo-1,4-_-d-glucanase and exo-1,4-_-nglucanase | Leaves and pseudostem | Phylosticta spp. MPS-001 Aspergillus spp. MPS-002 | Shah et al. (2005) |
| Levansucrase | Not specified | Bacillus megaterium | Ahmed (2008) |
| Polygalacturonase | Leaf parts | Streptomyces lindicus | Jacob and Prema (2008) |
| Xylanase | Banana peels | Trichoderma harzianum 1073 | Seyis and Aksoz (2005) |
| Organic Acid | |||
| Citric Acid | Banana peels | Aspergillus niger | Vidhya and Neethu (2009) |
Apart from being used as a substrate for cellulase and cellulolyic enzyme production, by-products such as leaves and pseudostem of the banana were also noted to be a potential substrate for the cultivation of edible mushrooms (Table 5). Agricultural and wood industrial waste have been used tremendously as mushroom substrates because of its abundance, cheap and contains a high amount of cellulose materials in which are the keys in sustaining the cultivated mushroom industry (Mane et al. 2007). It is vital that available wastes are used and managed properly to ensure the efficient of recycling farm waste for environmental safety as well as to generate income for the country. Edible mushrooms are known to be a good agent in the degradation of cellulose. They are income generating, high in nutritional and pharmaceutical values (Wong and Chye 2009, Yim et al. 2011) as well as can grow rapidly on suitable substrates. Thus, they are considered as high value food products, which are capable for fast return of investments (Ukoima et al. 2009).
Table 5.
Banana/plantain by-products used as substrates for the cultivation of edible mushroom
| Banana/plantain by-products | Mushroom species | References |
|---|---|---|
| Leaves (Musa sapientum) | Straw mushroom (Volvariella volvacea) | Belewu and Belewu (2005) |
| Leaves and pseudostems (variety not specified) | Oyster mushroom (Pleurotus spp.) | Bonatti et al. (2004); Silveira et al. (2008) |
| Banana peels (variety not specified) | Shelf mushroom (Lentinus squarrosulus) | Adejoye and Masewonrun (2009) |
| Leaves (variety not specified) | Agaric mushroom (Psathyrella atroumbonata Pegler) | Ayodele and Okhuoya (2007) |
Heavy metals and dye absorbers
Heavy metals are regarded as a threat to the environment and the availability of these hazardous metals in wastewater such lead, chromium, cadmium, mercury and zinc pose a great health threat to humans as it might contaminate the drinking water system. Heavy metals are hardly biodegradable and can easily accumulate in living tissues making it concentrated as it goes up the food chain (Metcheva et al. 2010). Numerous agricultural wastes were explored and most are found to have the potential as low-cost heavy metal absorbers (Kumar 2006). Cleaning the environment from the contamination of heavy metals is very costly and thus cheaper alternative absorbers from agricultural waste particularly from banana are highly considered. Noeline et al. (2005) showed the formaldehyde polymerized banana pseudostem is an effective absorbent in cleaning lead (II) in batch reactors. Removal of lead (II) up to 99.0 % and above is achievable when all the required absorptive conditions are met. Banana pseudostem processed into carboxylated functionalized banana pseudostem (CBS), was also being reported to be a good mercury (II) absorber even in the presence of other ions and comparable in absorption capacity and binding energy to the commercially available carboxylic acid functionalized cation exchanger Amberlite IRC-50 (Anirudhan et al. 2007). Other notable innovations include the reported heavy metals sorption capacity of banana peels in removing cromium (III) (Memon et al. 2008) and cromium (IV) (Park et al. 2008; Memon et al. 2009a). Banana fruit stalk was also discovered to be potential cobalt (II) and cadmium (II) remover (Anirudhan and Shibi 2007; Memon et al. 2009b).
Synthetic dyes are commonly used in some chemical assays, textile industry, and commercial products. A number of commercially used synthetic dyes have been reported contributing to health problems, which justified the need to remove these dyes from wastewaters. Mas Harris and Sathasivam (2009) demonstrated the capacity of banana pseudostem (Musa paradisiaca cv. ‘Pisang Awak’ ABB) as a potential absorber of methyl red in aqueous solutions. This recent finding further compliments earlier studies that show the capability of banana stalk waste as an absorbent to remove methylene blue from aqueous solutions (Hameed et al. 2008). Natural heavy metal and dye absorbers made from renewable low-cost banana by-products are relatively cheaper compared to synthetic and inorganic absorbers but may not work well in extreme conditions (high pH and high temperature) (Mas Harris and Sathasivam 2009).
Source of bioactive compounds for non-food purposes
A few literatures emphasize on the existence of bioactive compounds, which may not be directly applicable for human consumption readily available or available through induction from the banana by-products (Luque-Ortega et al. 2004; Otalvaro et al. 2007). These bioactive components are potential substitutes for industrial chemicals and functional compounds in pharmaceuticals as well as could be further exploited into numerous applications. These isolated bioactive compounds, which are generally secondary metabolites (produced naturally or induced) of the banana by-products, were reported to have antiprotozoan, antifungal, and antiviral activity (Table 6).
Table 6.
Other bioactive compounds found in banana/plantain by-product either exists naturally or obtained through artificial induction
| Bioactive compounds | Banana by-products | Bioactivity reported | Potential application | References |
|---|---|---|---|---|
| Anigorufone, 2-hydroxy-9-phenyl-phenalen-1-one, 2-hydroxy-9-(p-methoxyphenyl)-phenalen-1-one, 2,3-epoxy-9-(p-methoxyphenyl)phenalen-1-one, 2,3-epoxy9-phenyl-phenalen-1-one | Rhizomes infected with fungus Fusarium oxysporum (Musa acuminata) | Antileishmanial | Antiprotozoan agent | Luque-Ortega et al. (2004) |
| 2-hydroxy-1H-phenalen-1-one and 2-methoxy-1H-phenalen-1-one | Rhizomes (Musa acuminata var. “Yangambi km 5”, | Antifungal | Antifungal agent | Otalvaro et al. (2007) |
| Thaumatin-like protein (TLP) | Ripe fruits (Musa basjoo cv. Emperor banana) | Antifungal | Antifungal agent | Ho et al. (2007) |
| 31-norcyclolaudenone | Rhizome (Musa acuminata var. errans) | Antimicrobial | Antimicrobial agent | Ragasa et al. (2007) |
Organic fertilizers and bio-fertilizers
The usage of organic fertilizers and bio-fertilizers have gained momentum as a substitute to chemically synthesized fertilizers due to its reported effectiveness, the increasing cost of some chemical fertilizers and the awareness towards the hazardous effects of chemical fertilizers to human and the environment (Aseri et al. 2008; Doran et al. 2005). The technology in developing organic fertilizers also aims to manage, recycle and convert biodegradable solid waste into nutrient rich plant growth medium or soil amender (Sim and Wu 2010). Bio-fertilizers are often distinguished from normal organic fertilizers by its capacity to deliver and retain a high number of beneficial soil enhancing microorganisms to the soil instead of just having processed fertile organic biomass (Phirke and Kothari 2005). Organic fertilizers and bio-fertilizers from plant and animal materials are generally processed through composting and solid state fermentation (SSF). A microbial starter consists of mixture of decomposing microorganisms and soil enhancing bacteria or selected worm species is generally added to initiate the composting process. Traditional method allows banana waste to decompose naturally in the farm to replenish soil nutrients or to act as an organic fertilizer. Recently, the utilization of banana waste as organic fertilizers and bio-fertilizers have been greatly improved by incorporating biotechnological methods (Doran et al. 2005).
Phirke and Kothari (2005) discovered that turning banana waste into growth stimulating soil conditioner through solid-state fermentation and recycling it as fertilizers for banana farming greatly reduced the planted suckers’ mortality, improving plant biomass and increasing fruit yield. It has been confirmed by Doran et al. (2005) that organic fertilizer prepared from composting banana waste also stand out to be a cheaper and economical fertilizer with a significant effect on growth and yield of banana crop compared to chemical fertilizers and poultry manure. Banana waste was also reported to be a suitable carrier of Azospirillum, Azotobacter and phosphate-solubilizer bacteria to the soil cultivated with banana gave positive effects towards the availability of soil and banana foliar phosphorus content (Rivera-Cruz et al. 2008).
Challenges and future prospects
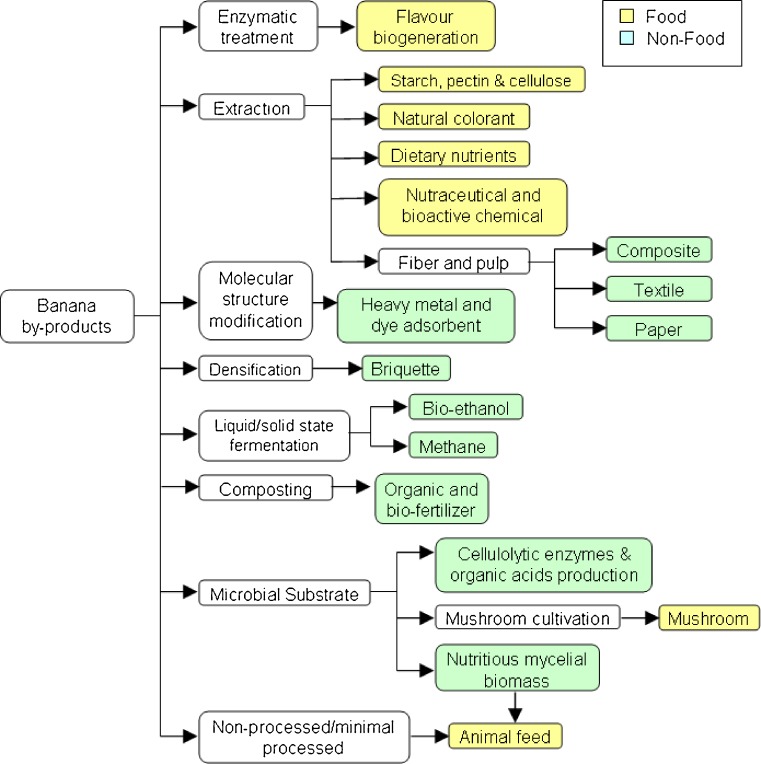
As a newly emerging candidate for industry-driven application, a number of challenges have been identified prompting immediate attention before banana by-products can become a sustainable agricultural commodity. The focus on the utilization of any by-products or waste should always be transformed into high valued processed raw materials or products that meet market demands and creating substantial economic impacts (Jayathilakan et al. 2012). This is also the most important key aspects in the management of agricultural waste, as it will greatly determine the sustainability and viability of the by-product itself as a future commodity (Adinugraha et al. 2005; Uyen and Schnitzer 2009). In other words, the market value of the newly developed products must be able to cover internal and external expenses of its production. The quality of the product and processed raw materials from banana by-products must be comparable or better than its counterparts to ensure market competitiveness. The technology and innovation through creative improvement of the existing processes may also be a key to guarantee the survival of the by-products (Lew et al. 2011). Figure 2 provides an overview of the potential utilization of banana by-products with the developed processing technology towards creating products for the food and non-food industries.
Fig. 2.
Schematic illustrating the utilization of banana/plantain by-product processing for food and non-food
Developed products, purified raw materials and biochemicals or any research outcomes from the banana by-products which may adhere to regulations or applied for human consumption and usage requires immediate assessment for their toxicity or any negative environmental impacts. The purity of food additives and components derived from banana by-products should be conformed to the regulations and standards stipulated by international bodies such as Codex. Toxicological assessment and clinical trials are mandatory for medicinal foods and food designated for health promotion and disease prevention, following their governing bodies around the world. Scientific advances, awareness of personal health deterioration, increasing healthcare costs, busy lifestyle, and technical advances in the food industry have stimulated the field of designer foods. Today, foods are not intended to only satisfy hunger and provide sufficient nutrients for human, but also to prevent degenerative disorders, which is a significant public health issue worldwide.
High value nutraceuticals and processed functional products are greatly influence by the quality of the raw materials. Producing high quality banana by-products as raw material is another challenge to be looked into. Uma et al. (2005) reported that there are significance differences in the quality of fibers obtained from banana by-products across different varieties and cultivars, which prompt their study to look into these issues. Moreover, the enormous varieties and cultivars within the banana (Musaceae) family itself is a potential area to be explored in order to obtain better-quality by-products as raw material for the food and non-food applications. There are limited reports on the effects of geographical location, climate, and the level of plant maturity on the quality of banana by-products. For instance, it has been documented that some bioactive constituents in a similar plant can vary significantly due to seasonal changes (Shah et al. 2010) and geographical location (Baraldi et al. 2008; Dinchev et al. 2008) as well as different stages of growth (Baima 2005). The previous studies on banana by-product focused on a limited number of commercial varieties and cultivars create substantial uncertainties in the potential of less studied by-products from other varieties. Other important areas such as the cost of technology in converting these wastes into valuable products are still less studied; an important aspect in order to evaluate its sustainability and feasibility.
There are also limitations that need to be resolved at the plantation level especially in establishing a proper collecting facility for these by-products to be kept and sorted according to the types and quality and a handling system that would prevent the degradation of the biomass and valuable components. Lignocellulosic materials, for instance, bio-fibrous from agricultural waste degrade after a storage time reducing the quality of the bio-fibers (Adinugraha et al. 2005). Standardized storage and handling procedures of the by-products are needed in order to ensure the quality of the by-products remain stable prior to further processing. Moreover, the numerous banana varieties that exist in the world today may not be easily identified due to the almost identical morphologies between species and cultivars. Thus, the development of molecular markers for the identification of banana varieties to aid in the verification process could potentially be expanded further encompassing lesser-known varieties, intra and inter species. Using marker assisted breeding and the application of genetic engineering, new products with potentially better characteristics and optimized benefits to human and animal health can be developed over relatively short periods of time (Jing et al. 2010; Laroche 2007). Training and education needed to be given to farmers and plantation administrators on the varieties of banana, which its by-products have been proven to be of potentially valuable. Some technologies require large initial setup, which may not be sustainable for under developed and developing countries may prompt the translocation of raw materials from one place to another.
In the future, natural biomasses such as the banana by-products are potential substitutes for our depleting nonrenewable resources such hydrocarbon fuel and plastics (Jing et al. 2010). Currently, there is an ongoing trend in utilizing low cost renewable agricultural waste as a raw material in making value added products to curb land degradation, increasing agricultural productivity, and reducing waste (Mohammadi 2006). Banana by-products, which are available abundantly around the world, are renewable and sustainable as long as the global banana industry maintains its momentum. Shifting towards the utilization of agricultural wastes such as the banana by-products is also seen as an environmental friendly approach to reduce environmental problems due to the improper management of the wastes. Its versatility and usefulness as raw materials in many food and non-food industries provides good and solid prospects as the potential income generating commodity of the future. As a commodity, not only it will benefit both banana farmers and the industry but also provide alternatives in terms of generated products to consumers.
Conclusions
Recycling and the utilization of agricultural by-products and waste for the creation of commercially viable and income generating products is not a new topic, however, the need to utilize available and abundant resources to the fullest such as the banana by-products is deemed important as to reduce the emission of solid waste and loss of valuable untapped biomass. This is an ongoing issue that require constant attention and monitoring in order to be a developed nation, improving the standard of living while preserving as much as our natural resources. There are unlimited possibilities of utilizing these renewable resources innovatively in fulfilling the need in the areas, which have been previously discussed as well as identifying new areas yet to be explored. Bearing in mind, the immediate challenge would always be the innovation of research towards creating high value and quality products with economical impacts.
Banana, consisting of numerous well-known varieties and cultivars, has been explored and the by-products such as pseudostem, rhizome, leaves, fruit stalks, and peels from the common varieties to some extent are potential raw materials in areas of food and non-food industries, providing each different application. Banana by-products which have been assessed and found to have potential application for food additives, nutraceuticals, food supplements, feeds, renewable fuel, fibers, source of bioactive and other organic chemicals, fertilizers as well as contaminant absorbers should be further addressed for its safety aspects to meet the market requirement. Standardized collection procedure and processing of banana by-products needed to be resolved in order to create a viable setting for these unprocessed raw materials to be available for industrial scale processing. The exponential increase of world’s population and the trend towards the utilization of eco-friendly and viable agricultural by-products creates a steady platform for the continuation of innovation on development of products from the banana by-products and waste, thus, making it a sustainable income generating commodity. Generating wealth from waste such as from the banana by-products should be regarded as one of the ways to create an eco-friendly environment for the future generations.
Acknowledgments
The authors would like to acknowledge the financial support by the Ministry of Science, Technology and Innovation, Malaysia for the Science Fund (02-01-10-SF0061). The technical support given by the Agriculture Department of Sabah is greatly appreciated.
References
- Abdul Aziz NA, Ho LH, Azahari B, Bhat R, Cheng LH, Ibrahim MNM. Chemical and functional properties of the native banana (Musa acuminata x balbisiana Colla cv. Awak) pseudostem and pseudostem tender core flours. Food Chem. 2011;128:748–753. [Google Scholar]
- Adao RC, Gloria MBA. Bioactive amines and carbohydrate shanges during ripening of ‘Prata’ banana (Musa acuminata x Musa balbisiana) Food Chem. 2005;90:705–711. [Google Scholar]
- Adejoye OD, Masewonrun O (2009) Growth and yield of Lentinus squarrosulus (M.) Singer a Nigerian edible mushroom as affected by supplements. The Internet Journal of Nutrition and Wellness 8(2) http://www.ispub.com/journal/the_internet_journal_of_nutrition_and_wellness/volume_8_number_1_15/article/growth-and-yield-of-lentinus-squarrosulus-m-singer-a-nigerian-edible-mushroom-as-affected-by-supplements.html [Assessed 2 July 2011]
- Adeniran AH, Abiose SH. Amylolytic potentiality of fungi isolated from some Nigerian agricultural wastes. Afr J Biotechnol. 2009;8(4):667–672. [Google Scholar]
- Adinugraha MP, Marceno DW, Haryadi Synthesis and characterization of sodium carboxymethylcellulose (CMC) from Cavendish banana pseudostem (Musa cavendishii LAMBERT) Carbohydr Polym. 2005;62:164–169. [Google Scholar]
- Ahmed S. Optimization of production and extraction parameters of Bacillus megaterium levansucrase using solid state fermentation. J App Sci Res. 2008;4(10):1199–1204. [Google Scholar]
- Ahnwange BA. Chemical composition of Musa sapientum (banana) peels. J Food Technol. 2008;6(6):263–266. [Google Scholar]
- Akinyele BJ, Agbro O. Increasing the nutritional value of plantain wastes by the activities of fungi using the solid state fermentation technique. Res J Microbiol. 2007;2(2):117–124. [Google Scholar]
- Amarnath R, Balakrishnan V. Evaluation of the banana (Musa paradisiaca) plant by-product’s fermemtantion characteristics to assess their fodder potential. Int J Dairy Sci. 2007;2(3):217–225. [Google Scholar]
- Ameye LG, Chee WSS. Osteoartritis and nutrition. From nutraceuticals to functional foods: a systematic review of the scientific evidence. Arthrit Res Ther. 2006;8:R127. doi: 10.1186/ar2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aniridhan TS, Shibi IG. Preparation of a cation exchanger containing carboxyl groups from banana stalk and its utilization as chelating agent. Infomusa. 2007;16(1&2):7–11. [Google Scholar]
- Anirudhan TS, Senan P, Unnithan MR. Sorptive potential of a cationic exchange resin of carboxyl banana stem for mercury(II) from aqueous solutions. Sep Purif Technol. 2007;52:512–519. [Google Scholar]
- Artali R, Beretta G, Morazzoni P, Bombardelli E, Meneghetti F. Green tea catechins in chemoprevention of cancer: a molecular docking investigation into their interaction with glutathione S-transferase (GST P1-1) J Enzyme Inhib Med Chem. 2009;24(1):287–295. doi: 10.1080/14756360802177282. [DOI] [PubMed] [Google Scholar]
- Arvanitoyannis S, Mavromatis A. Banana cultivars, cultivation practices, and physicochemical properties. Crit Rev Food Sci Nutr. 2009;49(2):113–135. doi: 10.1080/10408390701764344. [DOI] [PubMed] [Google Scholar]
- Asad MJ, Asgher M, Sheikh MA, Sultan JI. Production of Neurospora sitophila cellulases in solid state cultures. J Chem Soc Pak. 2006;28(6):590–595. [Google Scholar]
- Aseri GK, Jain N, Panwar J, Rao AV, Meghwal PR. Biofertilizers improve plant growth, fruit yield, nutrition, metabolism and rhizosphere enzyme activities of pomegranate (Punica granatum L.) in Indian thar dessert. Sci Hortic. 2008;117:130–135. [Google Scholar]
- Aurore G, Parfait B, Fahrasmane L. Bananas, raw materials for making processed food products. Trends Food Sci Technol. 2009;20(2):78–91. [Google Scholar]
- Ayodele SM, Okhuoya JA. Cultivation studied on Psathyrella atroumbonata Pegler. A Nigerian edible mushroom on different agro industrial wastes. Int J Bot. 2007;3(4):394–397. [Google Scholar]
- Bagchi D, Sen CK, Bagchi M, Atalay M. Review: antiangiogenic, antioxidant, and anticarcinogenic properties of a novel anthocyanin-rich berry extract formula. Biochem. 2004;69:95–102. doi: 10.1023/b:biry.0000016355.19999.93. [DOI] [PubMed] [Google Scholar]
- Baig MMV. Cellulostic enzymes of Trichoderma lignorum produced on banana agro-waste: Optimization of culture medium and conditions. J Sci Ind Res. 2005;64:57–60. [Google Scholar]
- Baima S. Plant genomics ad plant breeding: at the root of human nutrition and health. Curr Top Nutraceut Res. 2005;3(2):95–112. [Google Scholar]
- Bakar MA, Natarajan VD, Kalam A, Kudiran NH (2007) Mechanical properties of oil palm fibre reinforced epoxy for building short span bridge. Experimental Analysis Of Nano And Engineering Materials And Structures Proceeding of the 13th International Conference on Experimental Mechanics, Alexandroupolis, Greece, B, 2T6, pp 97–98
- Baraldi R, Isacchi B, Predieri S, Marconi GF, Vincieri F, Bilia AR. Distribution of artemisinin and bioactive flavonoids from Artemisia annua L. during plant growth. Biochem Syst Ecol. 2008;36(5–6):340–348. [Google Scholar]
- Bastianello SF, Testa RC, Pezzin APT, Silva DAK. Evaluation of physical and mechanical properties of handmade recycled papers reinforced with pulp of banana tree or rice straw. Rev Mater. 2009;14(4):1172–1178. [Google Scholar]
- Belewu MA, Belewu KY. Cultivation of mushroom (Volvariella volvaceae) on banana leaves. Afr J Biotechnol. 2005;4(12):1401–1403. [Google Scholar]
- Boberga J, Finlay RD, Stenlida J, Nasholm T, Lindahl BD. Glucose and ammonium additions affect needle decomposition and carbon allocation by the litter degrading fungus Mycena epipterygia. Soil Biol Biochem. 2008;40:995–999. [Google Scholar]
- Bonatti M, Karnopp P, Soares HM, Furlan SA. Evaluation of Pleurotus ostreatus and Pleurotus sajor-caju nutritional characteristics when cultivated in different lignocellulosic wastes. Food Chem. 2004;88:425–428. [Google Scholar]
- Bowen-Forbes CS, Zhang Y, Nair MG. Anthocyanin content, antioxidant, anti-inflammatory and anticancer properties of blackberry and raspberry fruits. J Food Compos Anal. 2010;23(6):554–560. [Google Scholar]
- Brown N, Venkatasamy S, Khittoo G, Bahorun T, Jawaheer S. Evaluation of genetic diversity between 27 banana cultivars (Musa spp.) in Mauritius using RAPD markers. Afr J Biotechnol. 2009;8(9):1834–1840. [Google Scholar]
- Chanakya HN, Sharma I, Ramachandra TV. Micro-scale anaerobic digestion of point source components of organic fraction of municipal solid waste. Waste Manage. 2009;29(4):1306–1312. doi: 10.1016/j.wasman.2008.09.014. [DOI] [PubMed] [Google Scholar]
- Chanwitheesuk A, Teerawutgulrag A, Kilburn JD, Rakariyatham Antimicrobial gallic acid from Caesalpinia mimosoides Lamk. Food Chem. 2005;100:1044–1048. [Google Scholar]
- Chattopadhyay SK, Khandal RK, Uppaluri R, Goshal AK. Mechanical, thermal, and morphological properties of maleic anhydride-g-polypropylene compatibilized and chemically modified banana-fiber-reinforced polypropylene composites. J Appl Polym Sci. 2010;117(3):1731–1740. [Google Scholar]
- Chen J, Wang Q, Hua Z, Du G. Research and application of biotechnology in textile industries in China. Enzyme Microb Technol. 2007;40(7):1651–1655. [Google Scholar]
- Cherian BM, Pothan LA, Chung TN, Mennig G, Kottaisamy M, Thomas S. A novel method for the synthesis of cellulose nanofibril whiskers from banana fibers and characterization. J Agric Food Chem. 2008;56:5617–5627. doi: 10.1021/jf8003674. [DOI] [PubMed] [Google Scholar]
- Clarke WP, Radnidge P, Lai TE, Jensen PD, Hardin MT. Digestion of waste bananas to generate energy in Australia. Waste Manage. 2008;28:527–533. doi: 10.1016/j.wasman.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Chou CS, Lin SH, Peng CC, Lu WC. The optimin conditions for preparing solid fuel briquette of rice straw by a piston-mold process using the Taguchi method. Fuel Process Technol. 2009;90(7–8):1041–1046. [Google Scholar]
- Chye FY, Sim KY. Antioxidative and antibacterial activities of Pangium edule seed extracts. Int J Pharmacol. 2009;5:285–297. [Google Scholar]
- Cordeiro N, Belgacem MN, Chaussy D, Moura JCVP. Pulp and paper properties from dwarf cavendish pseudostems. Cellul Chem Technol. 2005;39:517–529. [Google Scholar]
- Corma A, Torre O, Renz M, Villandier N. Production of high-quality diesel from biomass waste products. Angew Chem Int Ed. 2011;50:2375–2378. doi: 10.1002/anie.201007508. [DOI] [PubMed] [Google Scholar]
- Da Mota RV, Lajolo FM, Cordenunsi BR, Ciacco C. Composition and functional properties of banana flour from different varieties. Food Chem. 2000;52(2–3):63–68. [Google Scholar]
- De Lange E, Vrydaghs L, Maret PE, Perrier X, Denham T. Why bananas matter: an introduction to the history of banana domestication. Ethnobotany Research and Application. 2009;7:165–178. [Google Scholar]
- Devatkal SK, Kumboj R, Paul D (2011) Comparative antioxidant effect of BHT and water extracts of banana and sapodilla peels in raw poultry meat. J Food Sci Technol. Article in press [DOI] [PMC free article] [PubMed]
- Dinchev D, Janda B, Evstatieva L, Oleszek W, Aslani MR, Kostova I. Distribution of steroidal saponins in Tribulus terrestris from different geographical regions. Phytochemistry. 2008;69(1):176–186. doi: 10.1016/j.phytochem.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Dinishi Jayasinghe BAT, Parkinson D. Actinomycetes as antagonists of litter decomposer fungi. Appl Soil Ecol. 2008;38:109–118. [Google Scholar]
- Doran I, Sen B, Kaya Z. The effects of compost prepared from waste material of banana on the growth, yield and quality properties of banana plants. J Environ Biol. 2005;26(1):7–12. [PubMed] [Google Scholar]
- Doran-Peterson J, Cook DM, Brandon SK. Microbial conversion of sugars from plant biomass to lactic acid or ethanol. Plant J. 2008;54:582–592. doi: 10.1111/j.1365-313X.2008.03480.x. [DOI] [PubMed] [Google Scholar]
- Einbond LS, Reynertson KA, Luo XD, Basile MJ, Kennelly EJ. Anthocyanin antioxidants from edible fruits. Food Chem. 2004;84:23–28. [Google Scholar]
- Elanthikkal S, Gopalakrishnapanicker U, Varghese S, Guthrie J. Cellulose microfibres produced from banana wastes: Isolation and characterization. Carbohydr Polym. 2010;80(3):852–859. [Google Scholar]
- El-Khishin DA, Belatus EL, El-Hamid AA, Radwan KH. Molecular characterization of banana cultivars (Musa Spp.) from Egypt using AFLP. Res J of Agric Biol Sci. 2009;5(3):272–279. [Google Scholar]
- El-Meligy MG, El-Zawawy WK, Ibrahim MM. Lignocellulosic composite. Polym Adv Technol. 2004;15(12):738–745. [Google Scholar]
- Emaga TH, Andrianaivo RH, Wathelet B, Tchango TJ, Paquot M. Effects of the stage of maturation and varieties on the chemical composition of banana and plantain peels. Food Chem. 2007;103:590–600. [Google Scholar]
- Emaga TH, Roberta C, Ronkart SN, Wathelet B, Paquot M. Characterization of pectins extracted from banana peels (Musa AAA) under different conditions using an experimental design. Food Chem. 2008;108:463–471. doi: 10.1016/j.foodchem.2007.10.078. [DOI] [PubMed] [Google Scholar]
- Emaga TH, Roberta C, Ronkart SN, Wathelet B, Paquot M. Dietary fibre components and pectin chemical features of peels during ripening in banana and plantain varieties. Biores Technol. 2008;99(10):4346–4354. doi: 10.1016/j.biortech.2007.08.030. [DOI] [PubMed] [Google Scholar]
- Essien JP, Akpan EJ, Essien EP. Studies on mould growth and biomass production using waste banana peel. Biores Technol. 2005;96:1451–1456. doi: 10.1016/j.biortech.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Fan G, Han Y, Gu Z, Chen D. Optimizing conditions for anthocyanins extractions from purple sweet potato using reponse surface methodology (RSM) LWT- Food Sci Technol. 2007;41(1):155–160. [Google Scholar]
- FAO (2010a) FAOSTAT: Banana Production by Countries 2010 http://faostat.fao.org/site/339/default.aspx [Assessed 27 May 2012]
- FAO (2010b) FAOSTAT: Total World Banana Production 2010 http://faostat.fao.org/site/567/DesktopDefault.aspx?PageID=567#ancor [Assessed 27 May 2012]
- Faried A, Kurnia D, Faried LS, Usman N, Miyazaki T, Kato H, Kuwano H. Anticancer effects of gallic acid isolated from Indonesian herbal medicine, Phaleria macrocarpa (Schedd.) Boerl, on human cancer cell lines. Int J Oncol. 2007;30:605–613. doi: 10.3892/ijo.30.3.605. [DOI] [PubMed] [Google Scholar]
- Codex FC. Revised monograph—sodium carboxymethylcellulose. Washington: Institute of Medicine Food and Nutrition Board; 1996. pp. 1–3. [Google Scholar]
- Gigot C, Ongena M, Fouconnier ML, Wathelet JP, Du Jardin P, Thonart P. The lipooxygense metabolic pathway in plants: potential for industrial production of natural green leaf volatiles. Biotechnol Agron Soc Environ. 2010;14(3):451–460. [Google Scholar]
- González-Montelongo R, Lobo MG, González M. Antioxidant activity in banana peel extracts: testing extraction conditions and related bioactive compounds. Food Chem. 2010;119(13):1030–1039. [Google Scholar]
- Goswami T, Kalita D, Rao PG. Greaseproof paper from banana (Musa paradisiaca L.) pulp fiber. Indian J Chem Technol. 2005;15:457–461. [Google Scholar]
- Gulati OP, Ottaway PB. Legislation relating to nutraceuticals in the European Union with a particular focus on botanical-sourced products. Toxicology. 2006;221:75–87. doi: 10.1016/j.tox.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Hahn-Hägerdal B, Galbe M, Gorwa-Grauslund MF, Lidén G, Zacchi G. Bio-ethanol—the fuel of tomorrow from the residues of today. Trends Biotechnol. 2006;24(12):549–556. doi: 10.1016/j.tibtech.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Hameed BH, Mahmoud DK, Ahmad AL. Sorption equilibrium and kinetics of basic dye from aqueous solution using banana stalk waste. J Hazard Mater. 2008;158(2–3):499–506. doi: 10.1016/j.jhazmat.2008.01.098. [DOI] [PubMed] [Google Scholar]
- Heslop-Harrisons JS, Swarzacher T. Domestication, genomics and the future for banana. Ann Bot. 2007;100:1073–1084. doi: 10.1093/aob/mcm191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J, Nelson E, Tilman D, Polasky S, Tiffany D. Environmental, economic, and energetic costs and benefits of biodiesel and ethanol biofuels. Proc Natl Acad Sci USA. 2006;103(30):11206–11210. doi: 10.1073/pnas.0604600103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho VSM, Wong JH, Ng TB. Thaumatin-like antifungal protein from the emperor banana. Peptides. 2007;28:760–766. doi: 10.1016/j.peptides.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Hoeger S, Gottmann U, Liu Z, Schnuelle P, Birck R, Braun C, Van Der Woude FJ, Yard BA. Dopamine treatment in brain dead rats mediates anti-inflammatory effects: the role of hemodynamic and D-receptor stimulation. Transpl Int. 2007;20(9):790–799. doi: 10.1111/j.1432-2277.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- Hong KJ, Lee CH, Kim SW. Aspergillus oryzae GB-107 fermentation improves nutritional quality of food soybean and feed soybean meals. J Med Food. 2004;7(4):430–435. doi: 10.1089/jmf.2004.7.430. [DOI] [PubMed] [Google Scholar]
- Ibrahim MM, Dufresne A, El-Zawawy WK, Agblevor FA. Banana fibers and microfibrils as lignocellulosic reinforcements in polymer composites. Carbohydr Polym. 2010;81:811–819. [Google Scholar]
- Idicula M, Neelakantan NR, Oommen Z, Joseph K, Thomas S. A study of the mechanical properties of randomly oriented short banana and sisal hybrid fiber reinforced polyester composites. J Appl Polym Sci. 2005;96(5):1699–1709. [Google Scholar]
- Jacob N, Prema P. Novel process for the simultaneous extraction and degumming of banana fibers under solid state cultivation. Braz J Microbiol. 2008;32:320–326. doi: 10.1590/S1517-838220080001000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagan S, Ramakrishnan GP, Anandakumar KS, Davaki T. Antiproliferative potential of gallic acid against diethylnitrosamine-induced rat hepatocellular carcinoma. Mol Cell Biochem. 2008;319:51–59. doi: 10.1007/s11010-008-9876-4. [DOI] [PubMed] [Google Scholar]
- Jayathilakan K, Sultana K, Radhakrisna K, Bawa AS. Utilization of byproducts and waste materials from meat, poultry and fish processing industries: a review. J Food Sci Technol. 2012;29(3):278–293. doi: 10.1007/s13197-011-0290-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jannah M, Mariatti M, Abu Bakar A. Effect of chemical surface modifications on the properties of woven banana-reinforced unsaturated polyester composites. J Reinf Plas Compos. 2008;28(12):1519–1532. [Google Scholar]
- Jenshi RJ, Saravanakumar M, Aravinthan KM, Suganya Devi P. Antioxidant analysis of anthocyanin extracted from Musa acuminata bract. J Pharm Res. 2011;4(5):1488–1492. [Google Scholar]
- Jing YT, Chin LL, Keat TL, Kok TT, Bhatia S. Banana biomass as potential renewable energy resource: a Malaysian case study. Renew Sustain Energy Rev. 2010;14(2):798–805. [Google Scholar]
- John M, Anandjiwala RD (2009) Surface modification and preparation techniques for textile materials. Surface modification of textiles, Woodhead Pub. Ltd, Cambridge, pp.1–25
- Joshi RV. Low calorie biscuits from banana peel pulp. J Solid Waste Technol Manage. 2007;33(3):142–147. [Google Scholar]
- Karlsen A, Retterstol L, Laake P, Paur I, Kjolsrud-Bohn S, Sandvik L, Blomhoff R. Anthocyanins inhibit nuclear factor-xB activation in monocytes and reduce plasma concentrations of pro-inflammatory mediators in healthy adults. J Nutr. 2007;137:1951–1954. doi: 10.1093/jn/137.8.1951. [DOI] [PubMed] [Google Scholar]
- Kennedy J. Bananas and people in the homeland of genus Musa: not just pretty fruit. Ethnobotany Research and Application. 2009;7:179–197. [Google Scholar]
- Kitdamrongsont K, Pothavorn P, Swangpol S, Wongniam S, Atawongsa K, Svasti J, Somana J. Anthocyanin composition of wild bananas in Thailand. J Agric Food Chem. 2008;56:10853–10857. doi: 10.1021/jf8018529. [DOI] [PubMed] [Google Scholar]
- Konzack I, Zhang W. Anthocyanins-More than nature’s colors. J Biomed Biotechnol. 2004;5:239–240. doi: 10.1155/S1110724304407013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulma A, Szopa J. Catecholamines are active compounds in plants. Plant Sci. 2007;172(3):433–440. [Google Scholar]
- Kumar D, Tanwar VK. Effects of incorporation of ground mustard on quality attributes of chicken nuggets. J Food Sci Technol. 2011;48(6):759–762. doi: 10.1007/s13197-010-0149-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar U. Agricultural products and by-products as a low cost adsorbent for heavy metal removal from water and wastewater: a review. Sci Res Essays. 2006;1(2):033–037. [Google Scholar]
- Kumudavally KV, Tabassum A, Radhakrishna K, Bawa AS. Effect of ethanolic extract of clove on the keeping quality of fresh mutton during storage at ambient temperature (25±2°C) J Food Sci Technol. 2011;48(4):466–471. doi: 10.1007/s13197-010-0181-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo JM, Hwang A, Yeh DB, Pan MH, Tsai ML, Pan BN. Lipoxygenase from banana leaf: purification and characterization of an enzyme that catalyzes linoleic acid oxygenation and the 9-position. J Agric Food Chem. 2006;54:3151–3156. doi: 10.1021/jf060022q. [DOI] [PubMed] [Google Scholar]
- Laroche A. Biotechnology and medicinal plants. In: Acharya SN, Thomas JE, editors. Advances in medicinal plant research. Kerala: Research Signpost; 2007. pp. 339–356. [Google Scholar]
- Lew LC, Bhat R, Mat Easa A, Liong MT. Development of probiotic carriers using microbial Transglutaminase-crosslinked Soy Protein Isolate incorporated with agrowastes. J Sci Food Agric. 2011;91(8):1406–1415. doi: 10.1002/jsfa.4325. [DOI] [PubMed] [Google Scholar]
- Lima GPP, Da Rocha SA, Takaki M, Ramos PRR, Ono EO. Comparison of polyamine, phenol and flavonoid contents in plants grown under conventional and organic methods. Int J Food Sci Technol. 2008;43(10):1838–1843. [Google Scholar]
- Lu Z, Nie G, Belton PS, Tang H, Zhao B. Structure-activity relationship analysis of antioxidant ability and neuroprotective effect of gallic acid derivatives. Neurochem Int. 2005;48:263–274. doi: 10.1016/j.neuint.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Luque-Ortega JR, Martinez S, Saugar JM, Izquerdo LR, Abad T, Luis JG, Pinero J, Valladares B, Rivas L. Fungus-elicited metabolites from plants as an enriched source for new leishmanicidal agents: Antifungal phenyl-phenalenone phytoalexins from the banana plant (Musa acuminata) target mitochondria of Leishmania donovani promastigotes. Antimicrob Agents Chemother. 2004;48(5):1534–1540. doi: 10.1128/AAC.48.5.1534-1540.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhav A, Pushpalatha PB. Characterization of pectin extracted from different fruit waste. J Trop Agric. 2002;40:53–55. [Google Scholar]
- Maleque MA, Belal FY, Sapuan SM. Mechanical properties study of pseudo-stem banana fiber reinforced epoxy composite. Arab J Sci Eng. 2005;32(2B):359–364. [Google Scholar]
- Mandel SA, Limor Kalfon TA, Reznichenko L, Youdim MBH. Targeting multiple neurodegenerative diseases etiologists with multimodal-acting green tea catechins. J Nutr. 2008;138:1578–1583. doi: 10.1093/jn/138.8.1578S. [DOI] [PubMed] [Google Scholar]
- Mane VP, Patil SS, Syed AA, Baig MMV. Bioconversion of low quality lignocellulosic waste into edible protein by Pleurotus sajor-caju (Fr.) singer. J Zhejiang Univ (Sci B) 2007;8(10):745–751. doi: 10.1631/jzus.2007.B0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani S, Tabil LG, Sokhansanj S. Effects of compressive force, particle size and moisture content on mechanical properties of biomass pallets from grasses. Biomass Bioenergy. 2006;30(7):648–654. [Google Scholar]
- Manikandan K, Saravanan V, Viruthagiri T. Kinetics studies on ethanol production from banana peel waste using mutant strain of Saccharomyces cerevisiae. Indian J Biotechnol. 2008;7:83–88. [Google Scholar]
- Marie-Magdeleine C, Limea L, Etienne T, Lallo CHO, Archimede H, Alexandre G. The effects of replacing Dichantium hay with banana (Musa paradisiaca) leaves and pseudostem in carcass traits of Ovin Martinik sheep. Trop Anim Health Prod. 2009;41:1531–1538. doi: 10.1007/s11250-009-9344-5. [DOI] [PubMed] [Google Scholar]
- Mas Harris MR, Sathasivam K. The removal of methyl red from aqueous solutins using banana pseudostem fibers. Am J Appl Sci. 2009;6(9):1690–1700. [Google Scholar]
- Matekaire T, Mupangwa JF, Kanyamura EF. The efficacy of banana plant (Musa paradisiaca) as a coccidiostat in rabbits. Int J Appl Res Vet Med. 2005;3(4):326–331. [Google Scholar]
- Mattos LA, Amorim EP, Cohen KO, Amorim TB, Oliveira S. Agronomic, physical and chemical characterization of banana fruits. Crop Breed Appl Biotechnol. 2010;10:225–231. [Google Scholar]
- Memon JR, Memon SQ, Bhanger I, Khuhawar MY. Banana peel: a green and economical sorbent for Cr(III) removal. Pak J Anal Environ Chem. 2008;9(1):20–25. [Google Scholar]
- Memon JR, Memon SQ, Bhanger I, El-Turki A, Hallam KR, Allen GC. Banana peel: a green and economical sorbent for the selective removal of Cr(VI) from industrial wastewater. Colloids Surf B. 2009;70:232–237. doi: 10.1016/j.colsurfb.2008.12.032. [DOI] [PubMed] [Google Scholar]
- Memon JR, Memon SQ, Bhanger I, El-Turki A, Memon GZ, Allen GC. Characterization of banana peel by scanning electron microscopy and FT-IR spectroscopy and its use for cadmium removal. Colloids Surf B. 2009;66:260–265. doi: 10.1016/j.colsurfb.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Mena-Espino X, Barahona-Perez F, Alzate-Gaviria L, Rodriguez-Vazquez R, Tzec-Sima M, Dominguez-Maldonado J, Canto-Canche BB. Saccharification with Phanerochaete chrysosporium and Pleurotus ostreatus enzymatic extracts of pretreated banana waste. Afr J Biotechnol. 2011;10(19):3824–3834. [Google Scholar]
- Mertens-Talcott SU, Rios J, Jilma-Stohlawetz P, Pacheco-Palencia LA, Meibohm B, Talcott ST, Derendorf H. Pharmacokinetics of anthocyanins and antioxidant effects after the consumption of anthocyanin-rich acai juice and pulp (Euterpe oleraceaMart.) in human healthy volunteers. J Agric Food Chem. 2008;56(17):7796–7802. doi: 10.1021/jf8007037. [DOI] [PubMed] [Google Scholar]
- Metcheva R, Yurukova L, Bezrukov V, Beltcheva M, Yankov Y, Dimitrov K. Trace and toxic elements accumulation on food chain representatives at Livingston Island (Antartica) Int J Biol. 2010;2(1):155–161. [Google Scholar]
- Miller MR, Nichols PD. The digestibility and accumulation of dietary phytosterols in atlantic salmon (Salmo salar L.) smolt fed with diets with replacement plant oils. Lipids. 2008;43:549–557. doi: 10.1007/s11745-008-3175-4. [DOI] [PubMed] [Google Scholar]
- Mire M, Mlayah BB, Delmas M, Bravo R. Formic acid/acetic acid pulping of banana stem (Musa Cavendish) Appita J. 2005;58(5):393–396. [Google Scholar]
- Mohammadi IM. Agricultural waste management extension education (AWMEE) The ultimate need for intellectual productivity. Am J Environ Sci. 2006;2(1):10–14. [Google Scholar]
- Mohanty AK, Misra M, Drzal LT. Sustainable bio-composites from renewable resources: opportunities and challenges in the green materials. World J Polym Environ. 2002;10(1/2):19–26. [Google Scholar]
- Mohapatra D, Mishra S, Sutar N. Banana and its by-product utilization: an overview. J Sci Ind Res. 2010;69:323–329. [Google Scholar]
- Mokbel MS, Hashinaga F. Antibacterial and antioxidant activities of banana (Musa AAA cv. Cavendish) fruit peels. Am J Biochem Biotechnol. 2005;1(3):125–131. [Google Scholar]
- Moruisi KG, Oosthuizen W, Opperman M. Phytosterols/Stanols lower cholesterol concentrations on familial hypercholesterolemic subjects: A systematic review with meta analysis. J Am Coll Nutr. 2006;25(1):41–48. doi: 10.1080/07315724.2006.10719513. [DOI] [PubMed] [Google Scholar]
- Nelson SC, Ploetz RC, Kepler AK (2006) Musa species (bananas and plantains), ver. 2.2. In: Elevitch CR (ed) Species profiles for pacific island agroforestry. Permanent Agriculture Resources (PAR), Hōlualoa, Hawai‘i. http://www.traditionaltree.org [20 July 2011]
- Noeline BF, Manohar DM, Anirudhan TS. Kinetic and equilibrium modeling of Lead(II) sorption from water and wastewater by polymerized banana stem in a batch reactor. Sep Purif Technol. 2005;45:131–140. [Google Scholar]
- Ogunsile BO, Omotoso MA, Onilude MA. Comparative Soda Pulps from the Mid-Rib Pseudostem and Stalk of Musa paradisiaca. J Biol Sci. 2006;6(6):1047–1052. [Google Scholar]
- Oliveira L, Evtuguin DV, Cordeiro N, Silvestre AJD, Silva AMS, Torres IC. Structural characterization of lignin from leaf sheaths of “Dwarf cavendish” banana plant. J Agric Food Chem. 2006;54:2598–2605. doi: 10.1021/jf0528310. [DOI] [PubMed] [Google Scholar]
- Oliveira L, Cordeiro N, Evtuguin D, Torresa IC, Silvestre AJD. Chemical composition of different morphological parts from ‘Dwarf Cavendish’ banana plant and their potential as a non-wood renewable source of natural products. Ind Crop Prod. 2007;26:163–172. [Google Scholar]
- Oliveira L, Freire CSR, Silvestre AJD, Cordeiro N. Lipohilic extracts from banana fruit residues: a source of valuable phytosterols. J Agric Food Chem. 2008;56:9520–9524. doi: 10.1021/jf801709t. [DOI] [PubMed] [Google Scholar]
- Oliveira L, Freire CSR, Silvestre AJD, Cordeiro N, Torres IC, Evtuguin D. Steryl glucosides from banana plant Musa acuminata Colla var Cavendish. Ind Crops Prod. 2005;22(3):187–192. [Google Scholar]
- Ooi CC, Siddiqui KM. Characteristics of some biomass briquettes prepared under modest die pressure. Biomass Bioenerg. 2000;18(3):220–228. [Google Scholar]
- Osma JF, Toca Herrera JL, Rodriguez Couto S. Banana skin: A novel waste for laccase production by Trametes pubescens under solid-state conditions. Application to synthetic dye decolouration. Dyes Pigm. 2007;75(1):32–37. [Google Scholar]
- Otalvaro F, Nanclares J, Vásquez LE, Quiñones W, Echeverri F, Arango R, Schneider B. Phenalenone-type compounds from Musa acuminata var. “Yangambi km 5” (AAA) and their activity against Mycosphaerella fijiensis. J Nat Prod. 2007;70(5):887–890. doi: 10.1021/np070091e. [DOI] [PubMed] [Google Scholar]
- Ozela EF, Stringheta PC, Chauca MC. Stability of anthocyanin in spinach vine (Basella rubra) fruits Cien. Inv Agr. 2007;34(2):115–120. [Google Scholar]
- Padam BS, Tin HS, Chye FY, Abdullah MI. Antibacterial and antioxidant activities of various solvent extracts of Banana (Musa paradisiacal cv Mysore) Inflorescence. J. Biological Sci. 2012;12(2):62–73. [Google Scholar]
- Padam BS, Tin HS, Chye FY, Abdullah MI (2012b). Inhibitory activity of semi-purified banana inflorescence (Musa paradisiaca cv. Mysore) extract on foodborne pathogens in juice model. In Proceedings International Conference on Food Science and Nutrition (ICFSN 2012). Sutera Pacific Hotel, Kota Kinabalu, Sabah. 1–4 April 2012. p 584–596
- Paepatung N, Nopharatana A, Songkasiri W. Bio-methane potential of biological solid materials and agricultural as wastes. Asian J Energ Environ. 2009;10(01):19–27. [Google Scholar]
- Panis B (2009) Cryopreservation of Musa germplasm, 2nd edn. Technical Guidelines No. 9. In: Engelmann F, Benson E (eds) Bioversity International, Montpellier, France
- Park D, Lim SR, Yun YS, Park JM. Development of a new Cr(VI)-biosorbent from agricultural biowaste. Biores Technol. 2008;99:8810–8818. doi: 10.1016/j.biortech.2008.04.042. [DOI] [PubMed] [Google Scholar]
- Pazmino Duran EA, Giusti MM, Wrolstad RE, Gloria MBA. Anthcyanins from banana bract (Musa x paradisiacal) as potential food colorant. Food Chem. 2001;73(3):327–332. [Google Scholar]
- Pelissari FM, Andrade-Mahecha MM, Sobral PJA, Menegalli FC. Isolation and characterization of the flour and starch of plantain bananas (Musa paradisiaca) Starch/Stärke. 2012;64:382–391. [Google Scholar]
- Phirke NV, Kothari RM. Conservation and recycling of banana orchard waste: the need of time for Indian banana growers. Ecol Environ Conserv. 2005;11:211–218. [Google Scholar]
- Pillay P, Ramaswamy K. Effect of naturally occurring antimicrobials and chemical preservatives on the growth of Aspergillus parasiticus. J Food Sci Technol. 2012;49(2):228–233. doi: 10.1007/s13197-011-0275-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimentel D, Patzek TW. Ethanol production using corn, switchgrass and wood; Biodiesel production using soybean and sunflower. Nat Resour Res. 2005;14(1):65–76. [Google Scholar]
- Pliszka B, Huszcza-Ciokowska G, Wierzbicka E. Effects of extraction conditions on the content of anthocyanins and bioelements in berry fruit extracts. Commun Soil Sci Plant Anal. 2008;39(5 & 6):753–762. [Google Scholar]
- Ploetz RC, Kepler AK, Daniells J, Nelson SC. Banana and plantain—an overview withemphasis on Pacific island cultivars, ver. 1. In: Elevitch CR, editor. Species profiles for Pacific Island agroforestry. Hōlualoa: Permanent Agriculture Resources (PAR); 2007. [Google Scholar]
- Quintana G, Velasquez J, Betancourt S, Ganan P. Binderless fiberboard from steam exoloded banana bunch. Ind Crops Prod. 2008;29(1):60–66. [Google Scholar]
- Racette SB, Lin X, Lefevre M, Spearie CA, Most MM, Ma L, Ostlund RE., Jr Dose effects of dietary phytosterols on cholesterol metabolism: a controlled feeding study. Am J Clin Nutr. 2010;91(1):32–38. doi: 10.3945/ajcn.2009.28070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragasa CY, Martinez AT, Chua JEY, Rideout JA. A triterpene from Musa errans. Philipp J Sci. 2007;136(2):167–171. [Google Scholar]
- Ragunathan R, Swaminathan K. Growth and amylase production by Aspergillus oryzae during solid state fermentation using banana waste as substrate. J Environ Biol. 2005;26(4):653–656. [PubMed] [Google Scholar]
- Raina K, Rajamanickam S, Deep G, Singh M, Agarwal R, Agarwal C. Chemopreventive effects of oral gallic acid feeding on tumor growth and progression in TRAMP mice. Mol Cancer Ther. 2008;7:1258–1267. doi: 10.1158/1535-7163.MCT-07-2220. [DOI] [PubMed] [Google Scholar]
- Raposo S, Pardao JM, Diaz I, Lima-Costa ME. Kinetic modelling of bioethanol production using agro-industrial by-products. Int J Energ Environ. 2009;1(3):1–8. [Google Scholar]
- Rappert S, Muller R. Odor compounds in waste gas emissions from agricultural operations and food industries. Waste Manage. 2005;25:887–907. doi: 10.1016/j.wasman.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Reddy N, Yang YQ. Biofibers from agricultural by-products for industrial applications. Trends Biotechnol. 2005;23(1):22–27. doi: 10.1016/j.tibtech.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Reddy HKY, Srijana M, Reddy MD, Reddy G. Co-culture fermentation of banana agro-waste to ethanol by cellulolytic thermophilic Clostridium thermocellum CT2. Afr J Biotechnol. 2009;9(13):1926–1934. [Google Scholar]
- Rivera-Cruz MC, Narcia AT, Ballona GC, Kohler J, Caravaca F, Antonio R. Poultry manure and banana wastes are effective biofertilizer carriers for promoting plant growth and soil sustainability in banana crops. Soil Biol Biochem. 2008;40:3092–3095. [Google Scholar]
- Rosentrater K, Todey D, Persyn R (2009) Quantifying total and sustainable agricultural biomass resources in South Dakota—A preliminary assessment. Agricultural Engineering International: the CIGR Journal of Scientific Research and Development Manuscript 1059–1058–1
- Rymbai H, Sharma RR, Srivastav M. Biocolorants and its implications in health and food industry. Int J Pharmtech Res. 2011;3(4):2228–2244. [Google Scholar]
- Sapuan SM, Harun N, Abbas KA. Design and fabrication of a multipurpose table using a composite of epoxy and banana pseudostem fibres. J Trop Agric. 2007;45(1–2):66–68. [Google Scholar]
- Saravanan K, Aradhya SM. Polyphenols of pseudostem of different banana cultivars and their antioxidant activities. J Agric Food Chem. 2011;59(8):3613–3623. doi: 10.1021/jf103835z. [DOI] [PubMed] [Google Scholar]
- Savastano HJ, Santos SF, Radonjic M, Soboyejo WO. Fracture and fatigue of natural fiber-reinforced cementitious composites. Cement Concr Compos. 2009;31:232–243. [Google Scholar]
- Saxena RC, Adhikari DK, Goyal HB. Biomass-based energy fuel through biochemical routes: a review. Renew Sustain Energy Rev. 2009;13(1):167–178. [Google Scholar]
- Seyis I, Aksoz N. Xylanase production from Trichoderma harzianum 1073 D3 with alternative carbon and nitrogen sources. Food Technol Biotechnol. 2005;43(1):37–40. [Google Scholar]
- Shafique S, Asgher M, Sheik MA, Asad MJ. Solid state fermentation of banana stalk for exoglucanase production. Int J Agric Biol. 2004;3(3):488–491. [Google Scholar]
- Shah MP, Reddy GP, Banerjee R, Ravindra Babu P, Kothari IL. Microbial degradation of banana waste under solid state bioprocessing using two lignocellulolytic fungi (Phylosticta spp. MPS-001 and Aspergillus spp. MPS-002) Process Biochem. 2005;40:445–451. [Google Scholar]
- Shan B, Cai Y, Brooks JD, Corke H. Antibacterial properties of Polygonum cuspidatum roots and their major bioactive constituents. Food Chem. 2008;109:530–537. [Google Scholar]
- Shah S, Saravanan R, Gajbhiye NA. Phytochemical and physiological changes in Ashwagandha (Withania somnifera Dunal) under soil moisture stress. Braz J Plant Physiol. 2010;22(4):255–261. [Google Scholar]
- Shankar SK, Mulimani VH. α-Galactosidase production by Aspergillus oryzae in solid-state fermentation. Biores Technol. 2007;98(4):958–961. doi: 10.1016/j.biortech.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Silveira MLL, Furlan SA, Ninow JL. Development of an alternative technology for the oyster mushroom production using liquid inoculum. Ciênc Tecnol Aliment. 2008;28(4):858–862. [Google Scholar]
- Sim EY, Wu TY. The potential reuse of biodegradable municipal solid wastes (MSW) as feedstocks in vermicomposting. J Sci Food Agric. 2010;90(13):2153–2162. doi: 10.1002/jsfa.4127. [DOI] [PubMed] [Google Scholar]
- Singh SK, Kesari AN, Rai PK, Watal G. Assessment of glycemic potential of Musa paradisiaca stem juice. Indian J Clin Biochem. 2007;22(2):48–52. doi: 10.1007/BF02913313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotannde OA, Oluyege AO, Abah GB. Physical and combustion properties of charcoal briquettes from neem wood residues. Int Agrophysics. 2009;24:189–194. [Google Scholar]
- Sruamsiri S. Agricultural wastes as dairy feed in Chiang Mai. Anim Sci J. 2007;78(4):335–341. [Google Scholar]
- Subbaraya U (2006) Potential and constraints of using wild Musa. In: Farmers’ knowledge of wild Musa in India. Food and Agriculture Organization of the United Nations, pp 33–36
- Sudha ML, Vetrimani R, Leelavathi K. Influence of fibre from different cereals on the rheological characteristics of wheat flour dough and on biscuit quality. Food Chem. 2007;100(4):1365–1370. [Google Scholar]
- Teo CH, Tan SH, Ho CH, Faridah OZ, Othman YR, Heslop-Harrison JS, Kalendar R, Schulman AH. Genome constitution and classification using retrotransposon-based markers in the orphan crop banana. J Plant Biol. 2005;48(1):96–105. [Google Scholar]
- Tin HS, Padam BS, Chye FY, Abdullah MI (2008) Screening of antimicrobial and antioxidant activity of banana (Musa spp) by-products: a potential source of natural bio-preservatives. In: Proceeding of the 30th Symposium of Malaysian Society for Microbiology, 16th–19th August, Kuantan, Malaysia
- Tin HS, Padam BS, Chye FY, Abdullah MI (2010) Study on the potential of antimicrobial compounds from banana/plantain by-products against foodborne pathogens. In: National Biotechnology Seminar, 24th–26th May, Kuala Lumpur, Malaysia
- Tiwari BK, Valdramidis VP, O’Donnel CP, Muthukumarappan K, Bourke P, Cullen PJ. Application of natural antimicrobials for food preservation. J Agric Food Chem. 2009;57:5987–6000. doi: 10.1021/jf900668n. [DOI] [PubMed] [Google Scholar]
- Torslangerpoll K, Andersen OM. Color stability of anthocyanins in aquous solutions at various pH. Food Chem. 2005;89(3):427–440. [Google Scholar]
- Ukoima HN, Ogbonnaya LO, Arikpo GE, Ikpe FN. Cultivation of mushroom (Volvariella volvacea) on various farm wastes in obubra local government of Cross River State, Nigeria. Pak J Nutr. 2009;8(7):1059–1061. [Google Scholar]
- Ulloa JB, Weerd JH, Huisman EA, Varreth JAJ. Tropical agricultural residues and their potential uses in fish feeds: the Costa Rican situation. Waste Manage. 2004;24(1):87–97. doi: 10.1016/j.wasman.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Uma S, Kalpana S, Sathiamoorthy KV. Evaluation of commercial cultivars of banana (Musa) for their suitability for the fiber industry. Plant Genetic Research Newsletter. 2005;142:29–35. [Google Scholar]
- Uyen NN, Schnitzer H. Sustainable solutions for solid waste management in Southeast Asian countries. Waste Manage. 2009;29:1982–1995. doi: 10.1016/j.wasman.2008.08.031. [DOI] [PubMed] [Google Scholar]
- Vidhya R, Neethu V. Agroindustrial banana wastes as inexpensive substrate for citric acid production by Aspergillus niger. Res J Biotechnol. 2009;4(3):51–55. [Google Scholar]
- Vijay VK, Chandra R, Subbarao PMV, Kapdi SS (2006) Biogas purification and bottling into CNG cylinders: Producing bio-CNG from biomass for rural automotive applications. The 2nd Joint Int. Conference on “Sustainable Energy and Environment (SEE 2006)” 21st–23rd November, Bangkok, Thailand, C-003 (O):1–6
- Wan Rosli WD, Law KN, Zainuddin Z, Asro R. Effect of pulping variables on the characteristics of oil-palm frond-fiber. Biores Technol. 2007;93(3):233–240. doi: 10.1016/j.biortech.2003.11.016. [DOI] [PubMed] [Google Scholar]
- Wang X, Jia W, Zhao A, Wang X. Anti-influenza agents from plants and traditional Chinese medicine. Phytother Res. 2006;20(5):335–341. doi: 10.1002/ptr.1892. [DOI] [PubMed] [Google Scholar]
- Wang LS, Dombkowski AA, Seguin C, Rocha C, Cukovic D, Mukundan A, Henry C, Stoner GD. Mechanistic basis for the chemopreventive effects of black raspberries at a late stage of rat esophageal carsinogenesis. Mol Carcinog. 2011;50(4):291–300. doi: 10.1002/mc.20634. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang M, Mujumdar AS. Influence of green banana flour substitution for cassava starch on the nutrition, color, texture and sensory quality in two types of snacks. LWT- Food Sci Technol. 2012;47(1):175–182. [Google Scholar]
- Weingärtner O, Lütjohann D, Ji S, Weisshoff N, List F, Sudhop T, Bergmann K, Gertz K, König J, Schäfers HJ, Endres M, Böhm M, Laufs U. Vascular effects of diet supplementation with plant sterols. J Am Coll Cardiol. 2008;51(6):1553–1561. doi: 10.1016/j.jacc.2007.09.074. [DOI] [PubMed] [Google Scholar]
- Wilaipon P. Physical characteristics of maize cob briquette under moderate die pressure. Am J App Sci. 2007;4:995–998. [Google Scholar]
- Walaiporn P. The effects of briquetting Pressure on banana peel briquettes and the banana waste in northern Thailand. Am J Appl Sci. 2009;6(1):167–171. [Google Scholar]
- Wong JY, Chye FY. Antioxidant properties of selected tropical wild edible mushrooms. J Food Compos Anal. 2009;22:269–277. [Google Scholar]
- Xu YX, Hanna MA, Isom L. “Green” Chemicals from renewable agricultural biomass—a mini review. Open Agr J. 2008;2:54–61. [Google Scholar]
- Yabaya A, Ado SA. Mycelial protein production by Aspergillus niger using banana peels. Sci World J. 2008;3(4):9–12. [Google Scholar]
- Yim HS, Chye FY, Rao V, Low JY, Matanjun P, How SE, Ho CH (2011) Optimization of extraction time and temperature on antioxidant activity of Schizophyllum commune aqueous extract using response surface methodology. J Food Sci Technol. Article in press [DOI] [PMC free article] [PubMed]
- Zainol N, Abdul Rahman R (2008) Anaerobic cellulose recovery from banana stem waste. Proceedings of the 1st International Conference of the IET Brunei Darussalam Network, 26–27 May
- Zamudio-Flores PB, Vargas-Torres A, Perez-Gonzalez J, Bosquez-Molina E, Bello-Perez LA. Films prepared with oxidized banana starch: mechanical and barrier properties. Starch. 2006;58:274–282. [Google Scholar]
- Zhang P, Whistler RL, BeMiller JN, Hamaker BR. Banana starch: production, physicochemical properties and digestibility—a review. Carbohydr Polym. 2005;59:443–458. [Google Scholar]
- Zheng J, Ding C, Wang L, Li G, Shi J, Li H, Wang H, Suo Y. Anthocyanins composition and antioxidant activity of wild Lycium ruthenicum Murr. from Qinghai-Tibet plateau. Food Chem. 2011;126(3):859–865. [Google Scholar]
- Zuluaga R, Putaux JL, Cruz J, Vélez J, Mondragon I, Ganan P. Cellulose microfibrils from banana rachis: effect of alkaline treatments on structural and morphological features. Carbohydr Polym. 2009;76(1):51–59. [Google Scholar]