Abstract
Pink flesh guava (Psidium guajava L) is an important tropical fruit widely cultivated in different parts of India. The fruit apart from its characteristic pink flesh color is a good source of ascorbic acid, reducing sugars and pectin. Pink color of guava pulp is attributed to the presence of carotenoid pigment lycopene. Incorporation of lycopene in the form of tomato puree to the guava pulp resulted in changes in the quality characteristics of the guava beverage formulations. Lycopene in guava beverage improved the color and appearance and also the nutritional quality of the beverage. Guava beverage having 6 % tomato puree had acceptable color, flavor and overall quality. Increasing levels of tomato puree in the beverage affected the flavor and decreased the sensory acceptability. Beverage formulations showed increase in lycopene concentration from 760 μg/100 g to 2010 μg/100 g with increase in concentration of tomato puree. Ascorbic acid and lycopene decreased by 25.7 % and 12.23 % respectively in beverage stored at room temperature. Guava beverage fortified with lycopene was stable with acceptable sensory quality during the storage of 6 months at room temperature.
Keywords: Pink flesh guava, Lycopene, Fortified beverage, Nutritional quality
Introduction
Guava (Psidium guajava L) is an important tropical fruit with a characteristic pleasing flavor and is a good source of natural vitamin C. Guava pulp is commercially manufactured from pink and white flesh guava varieties. Pink flesh color of guava is attributed to the presence of the carotenoid pigment lycopene. Red guavas grown in Brazil were reported to contain 16 types of carotenoids. Phytofluene, betacarotene, gamma carotene, lycopene, beta cryptoxanthin, rubixanthin, cryptoflavin, lutein and neochrome are the major carotenoids isolated and characterized from guava (Mercadante et al. 1999). Guava beverage formulated from pink guava pulp has attractive color and appearance apart from the characteristic flavor of guava. Pink color of guava pulp is directly proportional to the lycopene concentration in the guava pulp. Color of the guava beverage is dependent on the concentration of lycopene pigment and fruit pulp content in the beverage formulation. Ready to serve fruit juice beverages are commercially formulated with a fruit pulp content ranging from 15 to 30 %, the rest being water, sugar, acidulants, coloring and flavoring agents. Beverages formulated at this pulp levels may not have desirable natural color imparted from the pigments present in the fruit pulp. These commercial beverages are added with permitted synthetic food colors to enhance the color of the beverage. Use of natural colorants, nutraceutical compounds is being preferred in the soft drinks manufacture. Anthocyanins, micro encapsulated natural colorants and natural carotene are the main colorants used in the manufacture of soft drinks. It is expected that more food and beverage manufacturers will shift from using synthetic colors to natural colorants (Villadsen 1999). Consumers across the world are showing preference towards fruit juice beverages free from preservatives and added colors. Most of these fruit beverages processed from tropical fruits contain either carotenes or ascorbic acid, and both these nutritional compounds are seldom present in significant quantities in a single fruit. Blending of fruit juices from different fruits to develop a wide variety of colors and flavors was reported by different workers. Guava juice with its dominant flavor has a distinct space in the fruit juice beverage market and renders itself well for blending with other fruit juices (Floribeth and Lastreto 1981). Ready to serve beverages prepared from the guava concentrate were comparable in colour, flavour and cloudiness with those prepared from fresh guava juice (Sandhu and Bhatia 1985). Fortification of fruit juice beverages by blending of guava, banana, galagal, pineapple and apple with carrot juice reportedly enhanced beta carotene content in the beverage (Khan et al. 1988).
Tomato (Lycopersicum esculentum L) is a rich source of carotenoid pigment lycopene which is attributed to the red color of tomato. Tomato juice consumption was reported to increase the lycopene and betacarotene in the plasma. Lycopene supplementation from tomato juice may protect the oxidation of phospholipids in low density lipoprotein from oxidation (Maruyama et al. 2001). Lycopene was also attributed with many health benefit properties such as antioxidant, photo protective effects and other therapeutic properties. Consumption of tomato drink was reported to reduce the DNA damage in plasma and lymphocytes exposed to oxidative stress. Carotenoids intake even at very low concentrations from tomato products improved cell antioxidant protection. It was also reported that a fruit based drink containing bio active compounds from tomato could be an alternative source of bioactive carotenoids for people who do not consume tomato products (Porrini et al. 2005).
Therefore, the present study was aimed to fortify pink guava pulp with carotenes from tomato puree to develop fortified guava beverage with improved sensory and nutritional quality. The main objective of the present study was to fortify the pink guava beverage with lycopene from tomato puree and to study its effect on the physico chemical and sensory quality.
Materials and methods
Physical characteristics
Pink flesh guava fruits were harvested from an orchard at Yeswanthpur, near Bangalore, Karnataka, India. The fruits were sorted, washed and used for experimental studies. Mean values of physical characteristics viz., fruit weight, length, diameter, pulp, peel, seed content and visual colour of ten randomly selected fruits were reproted. Hunter color values were measured using Shimadzu Color Measuring system, (Model No. UV – 2100, Japan). The color values were expressed as L, a, b, where L = lightness, a (+) = redness, a (−) = greenness, b (+) = yellowness.
Chemical composition
Total soluble solids (TSS) of the pulp was determined using a digital refractometer (Model No. RX 5000, ATAGO, Japan). Titrable acidity, reducing and total sugars were determined as described in AOAC (2000). Ascorbic acid was determined by 2,6- Dichlorophenol indophenol dye method based on the reduction of ascorbic acid by the dye in the pH range of 1 to 3.5. Tannin content in pulp was determined by Spectrophotometric method based on the measurement of blue color formed by the reduction of phospho tungstomolybdic acid by tannin in alkaline solution (AOAC 2000). Lycopene and pectin content in pulp were determined as per method suggested by Ranganna (1995). All the analyses were carried out in triplicate and the values were expressed as mean ± S.D values.
Lycopene content
Ten grams of sample was weighed and extracted repeatedly with acetone using pestle and mortor till the residue became colorless. The acetone extract was transferred to a separating funnel containing 10–15 ml of petroleum ether and mixed gently. Carotenoid pigments are collected in the petroleum ether fraction by diluting the acetone with water. The lower phase was transferred to another separating funnel and the petroleum ether extract containing pigments to an amber colored bottle. Extraction was repeated similarly with petroleum ether until it is colorless. To the petroleum ether extract small quantity of anhydrous Na2So4 was added and the volume made up to 50 ml. The color was measured in a quartz cuvet at 503 nm in a spectrophotometer using petroleum ether as a blank. Lycopene content of sample was calculated by using the relationship that optical density (OD) of 1.0 = 3.1206 μg of lycopene per ml.
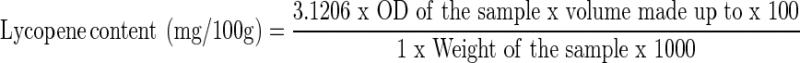 |
Tomato puree preparation
Mature, ripe tomatoes of deep red color were procured from the local fruit market at Mysore, Karnataka, India. Tomatoes were washed and crushed in a fruit mill. Crushed tomato pulp was immediately heated to 95 °C for 5 min and passed through a pulper extractor fitted with stainless steel sieve of pore diameter of 0.4 mm to obtain tomato juice. Tomato juice was concentrated in a forced circulation evaporator at 47 °C and 24″Hg vacuum to total soluble solids of 12°brix.
Preparation of fortified guava beverage formulations
Mature, ripe pink flesh guava fruits were selected, washed in tap water, trimmed to remove the stock and passed through a fruit mill. Crushed pulp was passed through pulper extractor fitted with a sieve having a pore diameter of 0.4 mm, to separate the seeds and pulp. The guava pulp thus obtained was used for the development of guava beverage formulations.
Guava beverages were formulated to contain 20 g of pink guava pulp per 100 g of beverage with a total soluble solids of 12°brix and acidity of 0.28 %. Fortified guava beverages were prepared by substituting pink guava pulp with tomato puree at concentrations ranging from 2 to 8 g per 100 g of beverage. The total pulp content including guava pulp and tomato puree of the beverage formulations was maintained at 20 %. Sugar syrup was prepared by weighing cane sugar, citric acid and water in a stainless steel steam jacketed kettle, boiling and filtering through a muslin cloth. Pink guava pulp alone or in combination with tomato puree were weighed according to the formulation and added to the syrup. The beverage formulation was blended in a homogenizer, pasteurized at 90 °C, hot filled into sterilized glass bottles of 200 ml capacity and sealed with a crown cork. The bottles were stored at room temperature (25 ± 5 °C) for further analysis.
Sensory analysis
Sensory analysis is carried out using Quantitative difference tests—‘Numerical scoring test’. The samples were assessed for color, flavor, texture and overall quality by a 15 member trained panel, on a 10 point scale, where 1–2 = poor, 3–4 = fair, 5–6 = good, 7–8 = very good, and 9–10 = excellent (Askar and Treptow 1993). Samples receiving an overall quality score of 7 or above were considered acceptable and those receiving below 7 were considered unacceptable.
Statistical analysis was carried out for physical, chemical and sensory quality characteristics parameters. Data was subjected to two way analysis of variance and the significance of the data was reported (Steel and Torrie 1980). All the analyses were carried out in triplicate.
Results and discussion
Quality indices of pink guava
External color of the pink flesh guava fruits was predominantly yellow with green tinge as indicated by hunter color reflectance values (Table 1). Physical characteristics of guava pulp such as mean fruit weight and pulp yield were 162 g and 79.4 % respectively. Pulp yield of 54 % and 49 % were earlier reported in red and white fleshed guavas (Harnanan et al., 1980). The lower pulp yield obtained in could be due to the cultivar differences and extraction procedure.
Table 1.
Physical characteristics of pink flesh guava fruit
| Fruit weight (g) | 162.9 ± 32.84 |
| Length (cm) | 7.2 ± 0.64 |
| Diameter (cm) | 6.4 ± 0.50 |
| Shape of fruit | Oval |
| External colour of fruit | Light greenish yellow |
| Pulp content (g/100 g) | 79.4 |
| Seeds (g/100 g) | 10.1 |
| Peels (g/100 g) | 10.5 |
| Hunter color values of peel | |
| L | 66.5 ± 1.23 |
| a | −2.3 ± 0.78 |
| b | 35.1 ± 0.47 |
| a/b | 0.07 |
Mean ± SD (n = 10), L = Lightness; a = Redness; b = Yellowness; a/b = ratio of redness and yellowness
Composition of guava pulp is shown in Table 2. Pink guava pulp had total soluble solids of 9.1°brix with a titrable acidity of 0.4 %. Ascorbic acid content was found to be 96.41 mg /100 g and lycopene content was 6900 μg/100 g. Guava pulp contained significant quantity of pectin which is responsible for the viscous nature of the pulp. Tomato puree contained ascorbic acid and lycopene contents of 18.75 mg/100 g and 15500 μg/100 g respectively. Lycopene fortification of increased color intensity of guava beverage.
Table 2.
Quality characteristics of lycopene fortified guava beverage formulations
| Parameters | Fortified guava beverage formulations1 | ||||||
|---|---|---|---|---|---|---|---|
| Guava pulp (g) : Tomato puree (g) | |||||||
| Pink guava pulp | Tomato puree | A (20:0) | B (18:2) | C (16:4) | D (14:6) | E (12:8) | |
| Total soluble solids (°Brix) | 9.1 ± 0.10 | 12 ± 0.10 | 12.1a | 12.2a | 12.1a | 12.2a | 12.1a |
| pH | 4.1 ± 0.12 | 3.5 ± 0.10 | 3.1a | 3.1a | 3.1a | 3.1a | 3.1a |
| Acidity (g/100 g) | 0.40 ± 0.01 | 0.46 ± 0.01 | 0.29 ± 0.01a | 0.28 ± 0.01a | 0.3 ± 0.01b | 0.31 ± 0.01b | 0.27 ± 0.01a |
| Reducing sugars (g/100 g) | 6.2 ± 0.30 | 6.3 ± 0.30 | 4.4 ± 0.08a | 4.5 ± 0.06a | 4.6 ± 0.07b | 4.8 ± 0.07c | 5.0 ± 0.09d |
| Total sugars (g/100 g) | 7.7 ± 0.30 | 7.4 ± 0.30 | 11.6 ± 0.08b | 11.5 ± 0.07a | 11.8 ± 0.08b | 11.4 ± 0.08a | 11.3 ± 0.10a |
| Ascorbic acid (mg/100 g) | 96.4 ± 2.10 | 18.75 ± 1.50 | 19.58 ± 0.15c | 18.09 ± 0.17c | 15.01 ± 0.13b | 12.75 ± 0.15a | 10.67 ± 0.12a |
| Lycopene (μg/100 g) | 6900 ± 80 | 15500 ± 85 | 760 ± 15a | 1400 ± 18b | 1850 ± 20c | 1880 ± 20c | 2010 ± 25d |
| Hunter colour Values2 | |||||||
| L | 50.6 ± 0.42 | ND | 32.7 ± 0.05b | 32 ± 0.03b | 30.1 ± 0.02a | 30 ± 0.05a | 29.3 ± 0.02a |
| a | 33.3 ± 0.91 | ND | 6.9 ± 0.02a | 7.1 ± 0.02a | 8.2 ± 0.04b | 8.6 ± 0.03b | 9.2 ± 0.04b |
| b | 17.6 ± 0.57 | ND | 6.2 ± 0.05a | 7 ± 0.04a | 8. ± 0.04b | 9.7 ± 0.05c | 10.1 ± 0.04c |
| a/b | 1.89 | ND | 1.12a | 1.01a | 1.01a | 0.89a | 0.90a |
| Sensory quality2 | |||||||
| Colour | ND | ND | 7.2 ± 0.04c | 7.5 ± 0.05c | 7.8 ± 0.05b | 8.3 ± 0.04a | 8.2 ± 0.05a |
| Flavour | ND | ND | 8.5 ± 0.05a | 8.5 ± 0.04a | 8.3 ± 0.05a | 8.5 ± 0.03a | 7.2 ± 0.04b |
| Taste | ND | ND | 8.2 ± 0.03b | 8.3 ± 0.05a | 8.4 ± 0.04a | 8.5 ± 0.05a | 6.8 ± 0.05c |
| Overall Quality | ND | ND | 8.2 ± 0.04b | 8.2 ± 0.05b | 8.4 ± 0.05a | 8.5 ± 0.04a | 6.8 ± 0.05c |
Mean ± S.D (n = 3); 1 g of pink guava pulp and tomato puree used for the preparation of 100 g of beverage, Means in the rows with the same superscript are not significantly different (p 0.05) 2Mean ± S.D (n = 10) ; L = Lightness ; a = Redness ; b = Yellowness ; a/b = ratio of redness and yellowness ; ND = Not Done ; A,B,C,D,E are different ratio of guava and tomato puree
Blending of guava and papaya pulps for beverage reportedly improved the nutritional quality. Guava and papaya blended in the proportion of 70:30 was found to be highly acceptable for consistency and flavor. Blended guava beverage contained ascorbic acid of 24.7 mg/100 g and carotene of 303.7 μg/100 g which was acceptable after 6 months storage at room temperature (Tiwari 2000). Probiotic dairy beverages with acceptable flavors were reportedly developed with fermented buffalo milk. The fermented milk beverages fortified with carrot and pumpkin were found to be a good source of betacarotene, where as addition of tomato increased lycopene levels and addition of strawberries, black mulberries and red grapes increased anthocyanin levels (Salem et al. 2006).
Quality characteristics of lycopene fortified guava beverage formulations
Guava beverage formulations (A,B,C & D) fortified with lycopene showed increase of lycopene content from 760 μg/100 g to 2010 μg/100 g with increase in tomato puree. Ascorbic acid content of the beverage formulations decreased from 19.58 mg/100 g to 10.67 mg/100 g (Table 2). The decrease of ascorbic acid can be explained by the substitution of guava pulp by tomato puree in the beverage formulations.
Color analysis of the lycopene fortified guava beverages indicated that ‘a’ and ‘b’ values increased and ‘L’ values decreased with increase in tomato puree concentration showing the increase in redness of the lycopene fortified guava beverage. Sensory analysis of the beverage confirmed increase in color scores with increase in concentration of lycopene. Flavor and overall quality of the fortified guava beverage formulations added with more than 6 % tomato puree decreased significantly. Therefore, fortified beverage formulation (D) containing 14 g of guava pulp and 6 g of tomato puree per 100 g of beverage was selected for storage studies (Table 2). Guava fortified beverage formulation contained significant amount of lycopene (1880 μg/100 g).
Storage studies of the lycopene fortified guava beverage
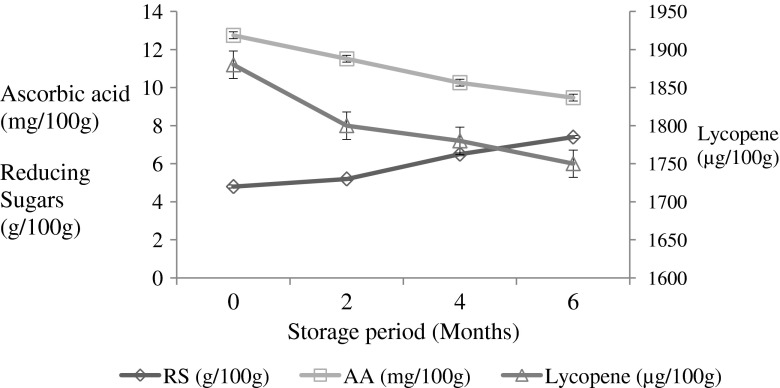
Fortified guava beverage was stored at room temperature (25 ± 5 °C) and analysed periodically for the composition and sensory quality. No significant changes were found in total soluble solids, titrable acidity and pH during storage. Reducing sugars of the beverage increased with increase in storage period, which could be due to the inversion of sucrose at acidic pH and storage at room temperature (Fig. 1). Reducing sugars, acidity increased and pH, total sugar, ascorbic acid and beta carotene decreased in jackfruit beverage with 10 % pulp, 18°B and 0.25 % acidity during storage at room temperature (Krishnaveni et al. 2001). Increase in reducing sugar content of untreated guava pulp and guava pulp treated with pectinolytic enzymes was also reported by Isabella et al. (1995). The increase of reducing sugars was associated with the hydrolysis of non-reducing sugars, which was very high in acidic medium at high temperature (Hernandez and Villegas 1986). Ascorbic acid content of the beverage formulation decreased by 25.72 % during the storage of 6 months (Fig. 1) which could be due to the oxidation of ascorbic acid. Kalra et al. (1987) reported that loss of ascorbic acid was 35–40 % in guava beverage stored at 20–36 °C for 12 months. The higher loss could be due to the long period of storage and temperature.
Fig. 1.
Reducing sugars, ascorbic acid and lycopene contents of fortified guava beverage during storage at room temperature, Observations are Mean ± S.D (n = 3)
Fortification of lycopene from tomato puree resulted in significant increase in the lycopene content of pink guava beverage and also improved the color and appearance of the fortified beverage. The beneficial effects of lycopene have been widely reported by different workers. The protective effect of carotenoids in the diet as photoprotectants against UV light induced erythema were also reported earlier. The photoprotective effects of synthetic lycopene were compared with tomato extract (Lyc-o-Mato R) and a beverage with lyco-o-Mato (Lyc-o-Guard-drink) by feeding volunteers with 10 mg lycopene per day. Significant increase in serum levels of phytofluene and phytoene occurred in the groups fed with the above formulations (Aust et al. 2005). Both these carotenoids exhibit absorption maxima at UV wavelengths, which explain the photoprotective effect of these compounds. These reports indicate the therapeutic value of the lycopene in beverages. Lycopene content of the guava beverage formulation decreased by 6.9 % during the storage of 6 months (Fig. 1). Lycopene is stable against light and temperature as compared to other pigments which explains the lower loss during storage. Sensory scores for color and flavor decreased during storage. Similarly, taste and overall quality decreased slightly. The marginal decrease of sensory quality could be due to the effect of light and temperature of storage. Sensory analysis found that the beverage was acceptable during the storage of 6 months. It was reported earlier that litchi ready to serve beverage prepared from litchi juice concentrate stored for 6 months was acceptable with slight reduction in color, flavor and overall quality (Vijayanand et al. 2010). It is evident from the present study that pink guava beverage could be fortified with tomato puree for increasing the lycopene content and sensory quality of beverage. Guava fortified beverage developed, contained lycopene pigment as a source of natural color which improved the nutritional and sensory quality of the beverage.
Conclusion
Pink guava pulp had total soluble solids of 9.1°brix and is found to be a good source of ascorbic acid content (96.41 mg /100 g). Lycopene, the red carotenoid was present in significant quantities in the pink guava pulp (6900 μg/100 g). Guava beverage formulation fortified with tomato puree was developed for enhancing the organoleptic and nutritional quality of the beverage. Tomato puree could be used as a natural source of lycopene for fortification of guava beverage. Fortified guava beverage formulation had a lycopene content of 1880 μg/100 g, ascorbic acid content of 12.75 mg/100 g and total sugar content of 10.35 g/100 g. Fortified beverage was a good source of nutrients apart from its sensory quality. Physico chemical quality characteristics of the beverage indicated that the beverage was acceptable during 6 months of storage at room temperature. Fortification of lycopene significantly improved the color and appearance of the guava beverage, which is in accordance with the growing consumer preference for natural additives in processed foods.
References
- AOAC (2000) Official methods of analysis. 17th edition. Association of Official analytical chemists USA. DC
- Askar A, Treptow H (1993) Quality assurance in tropical fruit processing. Springer - Verlag Berlin Heidelberg Germany 10–13
- Aust O, Stahl W, Sies H, Tronnier H, Heinrich U. Supplementation with tomato based products increases lycopene, phytofluene and phytoene levels in human serum and protects against UV-light induced erythema. Int J Vita Nutri Research. 2005;75:54–60. doi: 10.1024/0300-9831.75.1.54. [DOI] [PubMed] [Google Scholar]
- Floribeth V, Lastreto C. A study of the production of clarified banana juice using pectinolytic enzymes. J Food Technol. 1981;16:115–125. [Google Scholar]
- Harnanan SW, Bains GS, Singh KK. Studies on processing of pink and white fleshed guava varieties for pulp. Punjab Hort J. 1980;20:179–189. [Google Scholar]
- Hernandez TM, Villegas MJ. Effect of freezing storage on the quality of some tropical fruit pulps- preliminary study. Technology Chemistry. 1986;7:33–37. [Google Scholar]
- Isabella MB, Geraldo AM, Raimundo WF. Physical-chemical changes during extraction and clarification of guava juice. J Food Chem. 1995;54:383–386. doi: 10.1016/0308-8146(95)00066-R. [DOI] [Google Scholar]
- Kalra SK, Tandon DK, Lohani HC. Prevention of discoloration in guava beverage during storage. Indian Fd Pakr. 1987;41:21–25. [Google Scholar]
- Khan A, Singh H, Krishna B, Bhatia AK. Carotene enriched beverages. Indian Fd Pakr. 1988;42:27–29. [Google Scholar]
- Krishnaveni A, Manimegalai G, Saravanakumar R. Storage stability of jackfruit (Artocarpus heterophyllus) RTS beverage. J Food Sci Technol. 2001;38:601–602. [Google Scholar]
- Maruyama C, Imamura K, Oshima S, Suzukawa M, Egami S, Tonomoto M, Baba N, Harada M, Ayaori M, Inakuma IT. Effects of tomato supplementation on plasma and lipoprotein carotenoids concentrations and the susceptibility of low density lipoprotein to oxidative modification. J Nutr Sci Vitaminol. 2001;47:213–221. doi: 10.3177/jnsv.47.213. [DOI] [PubMed] [Google Scholar]
- Mercadante AZ, Steck A, Pfander H. Carotenoids from guava (Psidium guajava L): isolation and structure elucidation. J Agr Food Chem. 1999;47:145–151. doi: 10.1021/jf980405r. [DOI] [PubMed] [Google Scholar]
- Porrini M, Riso P, Brusamolino A, Berti C, Guarnieri S, Visioli F. Daily intake of tomato drink affects carotenoids plasma and lymphocyte concentrations and improves cellular antioxidant protection. Brit J Nutr. 2005;93(1):93–99. doi: 10.1079/BJN20041315. [DOI] [PubMed] [Google Scholar]
- Ranganna S. Hand book of analysis and quality control for fruits and vegetables. 2. New Delhi: Mc Graw Hill; 1995. [Google Scholar]
- Salem AS, Gafour WA, Eassaw EAY. Probiotic milk beverage fortified with antioxidants as functional ingredients. Egypt J Dairy Sci. 2006;34:23–32. [Google Scholar]
- Sandhu KS, Bhatia BS. Physico-chemical changes during preparations of fruit juice concentrate. J Food Sci Technol. 1985;22(3):202–206. [Google Scholar]
- Steel RGD, Torrie JH. Principles and procedures of statistics. New York: Mc Graw – Hill; 1980. [Google Scholar]
- Tiwari RB. Studies on blending of guava and papaya pulp for RTS beverage. Ind Food Pack. 2000;54:68–72. [Google Scholar]
- Vijayanand P, Kulkarni SG, Prathibha GV. Effect of pectinase treatment and concentration of litchi juice on quality characteristics of litchi juice. J Food Sci Technol. 2010;47(2):235–239. doi: 10.1007/s13197-010-0023-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villadsen H. Going natural. Soft drinks Int. 1999;6:28–29. [Google Scholar]