Abstract
Yam tsukuneimo tuber mucilage tororo hydrolysates were prepared by autolysis and three different peptic enzymes. Except for pepsin hydrolysate, tororo was perfectly digested. Each hydrolysate for 100 mg/ml significantly prolonged the induction period of auto-oxidation of linoleic acid, which was similar to 5 mM ascorbic acid. These hydrolysates also possessed high scavenging activities such as superoxide anion radicals, hydroxyl radicals, and DPPH radicals. Moreover, high antihypertensive activities were detected in these hydrolysates except for autolysate, which were similar to various fermented foods such as miso, natto, sake, cheese, and so on. Present findings suggest that yam tsukuneimo tuber mucilage tororo may be useful for preventing diseases associated with reactive oxygen species and blood pressure in the body system and it can fully absorb the useful components from it to digest using the gastrointestinal enzymes.
Keywords: Yam tsukuneimo tuber mucilage tororo, Enzymatic hydrolysis, Antioxidant activity, Free radical scavenging activity, Antihypertensive activity
Introduction
Yams, the tubers of Dioscorea genus are major and important staple food crops in tropical (Omonigho and Ikenebomeh 2000) and subtropical regions, annual world production being around 20 million tones (Ozo et al. 1984). The global distribution of yam species varies greatly from Africa to Asian regions in genotype, wild, and cultivated species: for example, wild ones (jinenjyo), cultivated ones (nagaimo, ichyoimo, and tsukuneimo) in Japan. Yam tubers have been used as a health food as well as herbal medicinal ingredients in traditional Chinese medicines (Liu et al. 1995). These tubers are found to be rich in essential dietary nutrients (Bhandari et al. 2003). Numerous studies have shown that yams are sources of diverse nutrients and non-nutrient molecules, many of that display bioactive properties. In the recent years, several beneficial properties of yam tubers were demonstrated in many papers. Many of the physiological functions of yams are as follows: anti-diabetic, anti-neoplastic (Hu et al. 1996; Hu and Yao 2002), anti-hypercholesterolemia (Ma et al. 2002), anti-osteoporotic (Yin et al. 2003), anti-microbial (Atindehou et al. 2002), anti-acetaminophen-induced hepatotoxicity and nephrotoxicity (Lee et al. 2002), and anti-oxidative activity and modification of serum lipid levels in humans (Araghiniknam et al. 1996).
Antioxidants in food materials have recently attracted because many reports have shown that the oxidative stress is closely related to the aging process of the cells and acts as a trigger to various diseases including cancer (Indu and Nirmala 2011; Kitts and Weiler 2003; Chen et al. 2012). Free radical-mediated reactive oxygen species and lipid peroxidation have gained considerable attention nowadays (Manso et al. 2008; Seema et al. 2008; Nasirullah et al. 2009). Reactive oxygen species were thought to create oxidative stress thereby causing various degenerative diseases, such as atherosclerosis, rheumatoid arthritis, diabetes mellitus, and Alzheimer’s diseases (Butterfield et al. 2002; Marijana et al. 2011; Sachindra et al. 2010). Autoxidation occurring in food materials are also induced by reactive oxygen species because lipid peroxidation is kept on by a free radical mechanisms (Je et al. 2007; Rekha and Vijayalakshmi 2010). Lipid peroxidation contributes to the subsequent development of unpleasant off-flavours, dark colours, and poor texture, and may also generate potentially toxic end product (Thiansilkul et al. 2007). Beneficial effects of antioxidants on promoting health are believed to be achieved though several possible mechanisms, such as directly reacting with and quenching free radicals, chelating transition metals, reducing peroxides, and stimulating the antioxidative defense enzyme system (Zhou and Yu 2004; Sarala et al. 2012). The trend to view many foods not only as substance but also as medicine, so-called functional foods, is increasing (Krishan et al. 2011; Ramakrishnan et al. 2010). In addition, naturally derived antioxidants are demanded by consumers in the food industries. In recent year, protein hydrolysates have been reported to exhibit antioxidative activity (Je et al. 2005; Klompong et al. 2007; Qian et al. 2011). From these reasons, the objective of the present study was to provide antioxidative and antihypertensive data of autolysate and enzymatic hydrolysates from yam tsukuneimo tubers mucilage tororo to apply in food processing and biomedical fields. Such information also may be useful to food technologists for the appropriate exploitation as source of a specific functional compound.
Materials and methods
Materials
Fresh yam tsukuneimo (Dioscorea opposita Thunb.) tubers from gifu, Japan were obtained from a local wholesale market, Aichi, Japan and used in this study. This yam is called as "iseimo" in this part of the country. Pepsin from porcine stomach mucosa (EC 3.4.23.1; 1:10000, 630 units/mg), trypsin from porcine pancreas crystallized (EC 3.4.21.4; 4,500 USP units/mg), papain (EC 3.4.22.2; digestive powder; 1:350), ascorbic acid, α-tocopherol, nitroblue tetrazolium salt, xanthine, 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,2′-azobis(2-amidinopropane) dihydrochloride (AAPH), 2-deoxy-D-ribose, linoleic acid, angiotensin I-converting enzyme from bovine lung (1U), hippuryl-L-histidyl-L-leucine as substrate peptide, and ethyl acetate for spectrochemical analysis grade were purchased from Wako Chemicals Co. Ltd. (Osaka, Japan). Xanthine oxidase from butter milk (XOD; 0.27 U/mg powder) was obtained from Oriental yeast Co., Ltd. (Tokyo, Japan). All other regents were of analytical grade.
Preparation of autolysate and enzymatic hydrolysates from yam tsukuneimo tuber mucilage tororo
The tsukuneimo tuber was washed, cleaned with water, peeled, and immediately ground using a grater. The yam tsukuneimo as grated yam tororo was added and suspended in an equal volume of distilled water and incubated with shaking at 37 °C for 1 day. After centrifugation at 50,000 × g at 20 °C for 1 h, the supernatants were collected and then freeze-dried (autolysate).
The tororo was added and suspended in an equal volume of distilled water, and pH of the suspension was adjusted at 2.0 using HCl. The digestion was started by adding 1.0 % pepsin (w/w) at 37 °C for 1 day, and the hydrolysis was stopped by boiling for 10 min to inactivate the enzyme. The hydrolysate was centrifuged at 50,000 × g at 20 °C for 1 h to remove the residue. The supernatants were collected, adjusted pH at 7.0, and then freeze-dried (pepsin hydrolysate).
The tororo was added and suspended in an equal volume of distilled water, and pH of the suspension was adjusted at 7.6 using NaOH. It was digested with 1.0 % trypsin (w/w) at 37 °C for 1 day. After digestion, the hydrolysate was boiled for 10 min and centrifuged at 50,000 × g at 20 °C for 1 h. The supernatants were collected and then freeze-dried (trypsin hydrolysate).
The tororo was added and suspended in an equal volume of distilled water and pH of the suspension was adjusted at 7.0 using NaOH. After the digestion with 1.0 % papain (w/w) at 37 °C for 1 day, the hydrolysis was stopped by boiling for 10 min. The hydrolysate was centrifuged at 50,000 × g at 20 °C for 1 h and the supernatants were collected and then freeze-dried (papain hydrolysate).
The freeze-dried powders were used as the sample solution (1, 10, and 100 mg/ml H2O) for the following tests.
The contents of protein and total phenolic compounds
The protein concentration was measured by the method of Lowry et al. (1951) using bovine serum albumin (BSA) as standard. The contents of total phenloic compounds were measured by the Folin-Ciocalteu colorimetric method using chlorogenic acid as standard and the absorbance was measured at 760 nm (Slinkard and Singleton 1977).
SDS-polyacrylamide gel electrophoresis (SDS-PAGE)
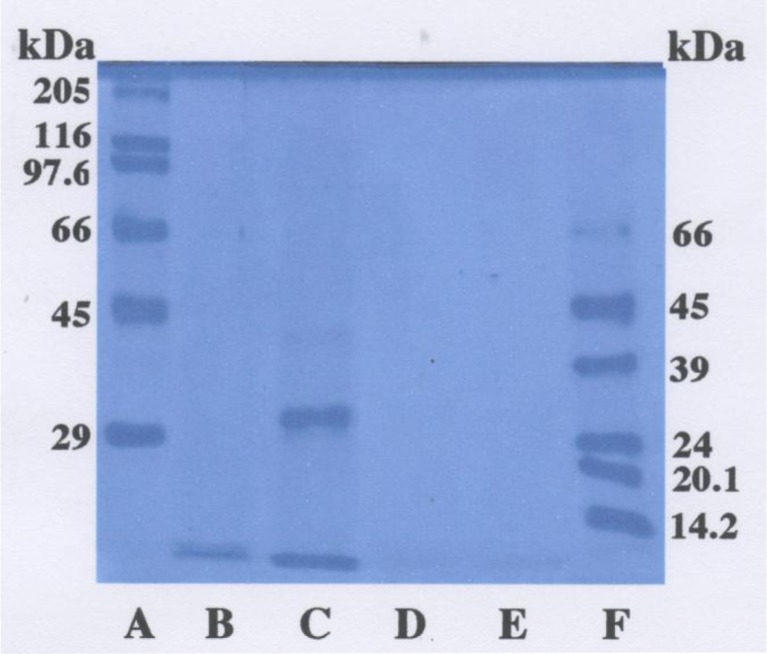
SDS-PAGE was performed to direct confirm the digestion by autolysate and hydrolysis by the method of Laemmli (1970) using 10 % gel. Standards were used as following proteins: myosin (205 kDa), β-galactosidase (116 kDa), phosphorylase (97.4 kDa), bovine serum albumin (66 kDa), ovalbumin (45 kDa), glyceraldehyde-3-phosphate dehydrogenase (36 kDa), carbonic anhydrase (29 kDa), bovine pancreas trypsinogen (24 kDa), soybean trypsin inhibitor (20.1 kDa), and bovine milk α-lactoalbumin (14.2 kDa). After electrophoresis, the gel was stained with Coomassie Brilliant Blue R-250 (Fluka Fine Chemical Co., Ltd., Tokyo, Japan) and then destained with 25 % ethanol and 7.5 % acetic acid.
Antioxidative activity
Antioxidative activities of autolysate and enzymatic hydrolysates from tororo were measured in a linoleic acid oxidation system described by Nagai and Nagashima (2006). A 0.083 ml of sample solution and 0.208 ml of 0.2 M sodium phosphate buffer (pH 7.0) were mixed with 0.208 ml of 2.5 % (w/v) linoleic acid in ethanol. The preoxidation was initiated by the addition of 20.8 μl of 0.1 M AAPH and carried out at 37 °C for 200 min in the dark. The degree of oxidation was measured according to the thiocyanate method for measuring peroxides by reading the absorbance at 500 nm after colouring with FeCl2 and ammonium thiocyanate. Ascorbic acid (1 and 5 mM) and α-tocopherol (1 mM) were used as positive control. Distilled water was used as negative control.
Effect of superoxide anion radical
Effect of superoxide anion radical was evaluated as described by Nagai and Nagashima (2006). This system contained 0.48 ml of 0.05 M sodium carbonate buffer (pH 10.5), 0.02 ml of 0.15 % of BSA, 0.02 ml of 3 mM EDTA, 0.02 ml of 0.75 mM NBT, 0.02 ml of 3 mM xanthine, and 0.02 ml of sample solution. After preincubation at 25 °C for 10 min, the reaction was started by adding 6 mU XOD and carried out at 25 °C for 20 min. The reaction was stopped by adding 0.02 ml of 6 mM CuCl. The solution was centrifuged at 12,000 rpm for 5 min, and the absorbance of the reaction mixture was measured at 560 nm and the inhibition rate was calculated by measuring the amount of formazan that was reduced from NBT by superoxide. Ascorbic acid (1 and 5 mM) and α-tocopherol (1 mM) were used as positive control. Distilled water was used as negative control.
Effect of hydroxyl radical
The effect of hydroxyl radical in autolysate and enzymatic hydrolysates from tororo was assayed using the deoxyribose method (Nagai and Nagashima 2006). The reaction mixture contained 0.45 ml of 0.2 M sodium phosphate buffer (pH 7.0), 0.15 ml of 10 mM 2-deoxy-D-ribose, 0.15 ml of 10 mM FeSO4-EDTA, 0.525 ml of distilled water, and 0.075 ml of sample solution in an Eppendorf tube. The reaction was started by the addition of 0.15 ml of 10 mM H2O2. After incubation at 37 °C for 4 h, the reaction was stopped by adding 0.75 ml of 1.0 % (w/v) of 2-thiobarbituric acid in 50 mM NaOH and 0.75 ml of 2.8 % (w/v) trichloroacetic acid. The solution was boiled for 10 min, and then cooled in water. The solution was centrifuged at 12,000 rpm for 5 min, and the absorbance of the supernatants was measured at 520 nm. Hydroxyl radical scavenging activity was evaluated as the inhibition rate of 2-deoxy-D-ribose oxidation by hydroxyl radicals. Ascorbic acid (1 and 5 mM) and α-tocopherol (1 mM) were used as positive control. Distilled water was used as negative control.
Effect of DPPH radical
The effect of DPPH radical was measured as described by Nagai and Nagashima (2006). The assay mixtute contained 0.03 ml of 1.0 mM of DPPH radical solution in ethanol, 0.24 ml of 99 % of ethanol, and 0.03 ml of sample solution. The mixture was rapidly mixed and scavenging capacity was measured by monitoring the decrease in absorbance at 517 nm. Ascorbic acid (1 and 5 mM) and α-tocopherol (1 mM) were used as positive control. Distilled water was used as negative control.
ACE inhibitory activity
The ACE inhibitory activities of autolysate and enzymatic hydrolysates from tororo were performed as described by Nagai and Nagashima (2006). Twenty five microliters of sample solution and 75 μl of 0.1 M sodium borate buffer (pH 8.3) containing 5.83 mM hippuryl-L-histidyl-L-leucine as substrate and 1.0 M NaCl in an Eppendorf tube were preincubated at 37 °C for 5 min. The mixture was incubated with 25 μl of 0.1 M sodium borate buffer (pH 8.3) containing 1 mU ACE and 1.0 M NaCl at 37 °C for 60 min. By adding 125 μl of 1.0 M HCl the reaction was stopped. The resulting hippuric acid was extracted with 750 μl of ethyl acetate by violently mixing for 15 s. After centrifugation at 6,000 rpm for 3 min, 500 μl of the upper layer was transported into the other tube and evaporated at 80 °C for 2 h. The hippuric acid was dissolved in 500 μl of distilled water, and then the absorbance was measured at 228 nm. The IC50 value was defined as the concentration of the sample required to inhibit 50 % of the ACE activity.
Statistical analysis
Each assay was repeated 3 times independently and the results were reported as means ± standard deviation (SD).
Results and discussion
Preparation of autolysate and enzymatic hydrolysates from tsukuneimo tuber mucilage tororo
The autolysate and enzymatic hydrolysates from yam tsukuneimo tuber mucilage tororo were prepared by autolysis and digestion using three kinds of enzymes such as pepsin, trypsin, and papain. As a result, the yields of freeze-dried powders were as follows: 4.0 % (autolysate), 5.0 % (pepsin), 4.5 % (trypsin), and 5.0 % (papain) on the basis of fresh tuber, respectively (Table 1). The protein contents in lyophilized powder ranged from 36.3 to 64.9 μg/mg on the basis of dry matter. The contents of total phenolic components of these hydrolysates were as follows: 6.4 μg/mg (autolysate), 15.3 μg/mg (pepsin hydrolysate), 11.2 μg/mg (trypsin hydrolysate), 7.4 μg/mg (papain hydrolysate) on the basis of dry matter, respectively. Nagai et al. (2007) prepared autolysate and enzymatic hydrolysate from nagaimo (D. opposita Thunb.) tubers by autolysis and the digestion using three enzymes such as pepsin, trypsin, and papain. As a result, the yields of these hydrolysates were about one half of those of hydrolysates from yam tsukuneimo tubers: it was due to the lower contents of proteins in nagaimo tubers (2.2 g/100 g edible portion) in comparison with the contents in tsukuneimo ones (4.5 g/100 g edible portion) (Anonymous 2012). Next, SDS-PAGE was performed to easily confirm the digestion by autolysis and hydrolysis using these enzymes. Except for pepsin hydrolysate, the proteins in yam tsukuneimo tuber mucilage tororo was perfectly digested in order to not detect the protein band by staining with Coomassie Brilliant Blue R-250 (Fig. 1). It is well-known that dioscorin, the major tuber storage protein of yam species, accounts for about 90 % of extractable water-soluble proteins in yam species tuber (Hou et al. 1999). The dioscorin shows about 32 kDa protein band under reducing condition in SDS-PAGE. In the present investigation, it suggests that the proteins in yam tsukuneimo tuber mucilage tororo, in particular dioscorin, were completely digested by autolysis and hydrolysis using trypsin and papain, although the protein was not perfectly digested by pepsin.
Table 1.
The yields and the contents of proteins and total phenolic components of autolysate and enzymatic hydrolysates from yam tsukuneimo tuber mucilage tororo
| Sample species | Yield (%) | Protein | Total phenols |
|---|---|---|---|
| (μg/mg sample powder) | |||
| Autolysate | 4.0 ± 0.07 | 36.7 ± 1.79 | 6.4 ± 0.08 |
| Pepsin hydrolysate | 5.0 ± 0.08 | 64.9 ± 2.21 | 15.3 ± 1.50 |
| Trypsin hydrolysate | 4.5 ± 0.07 | 60.9 ± 2.03 | 11.2 ± 1.34 |
| Papain hydrolysate | 5.0 ± 0.08 | 36.3 ± 1.86 | 7.4 ± 0.09 |
Each value represents mean ± SD, n = 3
Fig. 1.
SDS-polyacrylamide gel electrophoresis of molecular weight markers, autolysate and enzymatic hydrolysates from yam tsukuneimo tuber mucilage tororo. A high molecular weight markers, B autolysate, C pepsin hydrolysate, D trypsin hydrolysate, E papain hydrolysate, and F low molecular weight markers
Functional properties of autolysate and enzymatic hydrolysates from yam tsukuneimo tuber mucilage tororo
Several toxic by-products of lipid peroxidation can damage biomolecules including DNA, although these biomolecules are away from the site of their generation (Box and Maccubbin 1997). In vitro, the antioxidative activity in a linoleic acid oxidation system was investigated to evaluate the effects at the initiation stage of lipid peroxidation. Activities increased with increasing concentration of the sample species, although activity of each sample species decreased with the passage of time (Table 2). The auto-oxidation of linoleic acid of 1 mg/ml hydrolysates was accompanied by rapidly increase of peroxide value. The inhibitory effect for 10 mg/ml hydrolysates was moderate, which was higher than that of 1 mM ascorbic acid or lower than that of 5 mM ascorbic acid. On the other hand, hydrolysates for 100 mg/ml showed extremely high activities, which it was higher than that of 5 mM ascorbic acid but did not amount to that of 1 mM of a lipid-soluble natural antioxidant α-tocopherol (Table 2). It suggests that autolysate and hydrolysates from yam tsukuneimo tuber mucilage tororo possessed the highest activities to prevent an linoleic acid peroxidation as well as the report by Nagai et al. (2007).
Table 2.
Antioxidative activities of autolysate and enzymatic hydrolysates from yam tsukuneimo tuber mucilage tororo
| Absorbance at 500 nm | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | ||||||||||||||||
| Time (min) | A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | CN |
| 50 | 0.096 ± 0.010 | 0.030 ± 0.002 | 0.021 ± 0.002 | 0.064 ± 0.005 | 0.052 ± 0.004 | 0.000 | 0.078 ± 0.007 | 0.047 ± 0.003 | 0.006 | 0.066 ± 0.005 | 0.053 ± 0.003 | 0.027 ± 0.002 | 0.022 ± 0.001 | 0.016 ± 0.001 | 0.006 | 0.379 ± 0.008 |
| 100 | 0.315 ± 0.023 | 0.066 ± 0.005 | 0.049 ± 0.003 | 0.221 ± 0.018 | 0.119 ± 0.006 | 0.051 ± 0.004 | 0.312 ± 0.022 | 0.104 ± 0.006 | 0.091 ± 0.009 | 0.280 ± 0.014 | 0.184 ± 0.007 | 0.082 ± 0.005 | 0.135 ± 0.006 | 0.032 ± 0.003 | 0.025 ± 0.001 | 0.715 ± 0.025 |
| 200 | 0.634 ± 0.024 | 0.223 ± 0.012 | 0.090 ± 0.009 | 0.587 ± 0.030 | 0.259 ± 0.012 | 0.083 ± 0.008 | 0.748 ± 0.024 | 0.272 ± 0.013 | 0.082 ± 0.008 | 0.662 ± 0.019 | 0.361 ± 0.025 | 0.115 ± 0.003 | 0.469 ± 0.027 | 0.090 ± 0.008 | 0.028 ± 0.002 | 1.406 ± 0.041 |
(A) 1 mg/ml autolysate; (B) 10 mg/ml autolysate; (C) 100 mg/ml autolysate; (D) 1 mg/ml pepsin hydrolysate; (E) 10 mg/ml pepsin hydrolysate; (F) 100 mg/ml pepsin hydrolysate; (G) 1 mg/ml trypsin hydrolysate; (H) 10 mg/ml trypsin hydrolysate; (I) 100 mg/ml trypsin hydrolysate; (J) 1 mg/ml papain hydrolysate; (K) 10 mg/ml papain hydrolysate; (L) 100 mg/ml papain hydrolysate; (M) 1 mM ascorbic acid; (N) 5 mM ascorbic acid; (O) 1 mM α-tocopherol; (CN) control. Each value represents mean ± SD, n = 3
Superoxide anion radical is known to be very harmful to cellular components as a precursor of the more reactive oxygen species, which contribute to tissue damage and cause various diseases, and thus research of the scavenging activity of this radical is important (Kanatt et al. 2007). The effects of autolysate and enzymatic hydrolysates from yam tsukuneimo tuber mucilage tororo against superoxide anion radical were investigated using xanthine/xanthine oxidase system. Pepsin and trypsin hydrolysates for 1 mg/ml exhibited lower activities than 1 mM ascorbic acid (Table 3). The activities of autolysate and papain hydrolysate were not detected in the same condition at all. The hydrolysates for 10 mg/ml showed lower activities than that of 1 mM α-tocopherol. The scavenging effects by autolysate and pepsin and trypsin hydrolysates for 100 mg/ml were found to be about 60-82 %, which was significantly higher than that of 1 mM α-tocopherol. On the contrary, papain hydrolysate for 100 mg/ml completely scavenged this radical (Table 3): the activity was the highest among these sample species tested in this study.
Table 3.
Superoxide anion radicals, hydroxyl radicals, and DPPH radicals scavenging activities of autolysate and enzymatic hydrolysates from yam tsukuneimo tuber mucilage tororo
| Sample | Scavenging activity (%) | ||
|---|---|---|---|
| Superoxide anion radical | Hydroxyl radical | DPPH radical | |
| A | 0.0 | 68.6 ± 4.38 | 1.5 ± 0.01 |
| B | 23.6 ± 0.43 | 78.9 ± 5.93 | 29.7 ± 0.76 |
| C | 60.2 ± 4.01 | 90.4 ± 5.25 | 75.2 ± 4.77 |
| D | 10.0 ± 0.18 | 81.4 ± 6.11 | 2.4 ± 0.01 |
| E | 10.5 ± 0.20 | 82.8 ± 6.25 | 18.6 ± 0.38 |
| F | 82.2 ± 5.95 | 91.2 ± 5.86 | 61.7 ± 4.03 |
| G | 10.0 ± 0.15 | 78.4 ± 5.09 | 2.7 ± 0.01 |
| H | 41.6 ± 2.98 | 88.7 ± 5.17 | 55.0 ± 4.17 |
| I | 56.0 ± 4.36 | 91.2 ± 5.50 | 87.1 ± 5.04 |
| J | 0.0 | 85.2 ± 5.81 | 6.9 ± 0.05 |
| K | 31.9 ± 2.19 | 88.0 ± 5.76 | 23.2 ± 0.55 |
| L | 98.5 ± 3.54 | 91.6 ± 5.92 | 70.2 ± 4.89 |
| M | 14.7 ± 0.20 | 13.2 ± 0.21 | 3.1 ± 0.04* |
| N | 89.9 ± 5.31 | 17.6 ± 0.71 | 34.1 ± 2.01** |
| O | 52.6 ± 4.18 | 67.6 ± 4.34 | 87.6 ± 2.75 |
(A) 1 mg/ml autolysate; (B) 10 mg/ml autolysate; (C) 100 mg/ml autolysate; (D) 1 mg/ml pepsin hydrolysate; (E) 10 mg/ml pepsin hydrolysate; (F) 100 mg/ml pepsin hydrolysate; (G) 1 mg/ml trypsin hydrolysate; (H) 10 mg/ml trypsin hydrolysate; (I) 100 mg/ml trypsin hydrolysate; (J) 1 mg/ml papain hydrolysate; (K) 10 mg/ml papain hydrolysate; (L) 100 mg/ml papain hydrolysate; (M) 0.1 mM ascorbic acid; (N) 5 mM ascorbic acid; (O) 1mM α-tocopherol. *0.1 mM ascorbic acid; **1 mM ascorbic acid. Each value represents mean ± SD, n = 3
The chemical activity of hydroxyl radical is the strongest among reactive oxygen species. It easily induces severe damage to DNA leading to carcinogenesis, mutagenesis and cytotoxicity (Ardestani and Yazdanparast 2007). Therefore, the removal of hydroxyl radical is probably one of the most effective defenses of a living body against various diseases. The effects of autolysate and enzymatic hydrolysates from yam tsukuneimo tuber mucilage tororo against hydroxyl radical were evaluated and hydroxyl radical scavenging activities were measured using the Fenton reaction system. Each sample exhibited high scavenging activity (Table 3). The activities of only 1 mg/ml hydrolysates showed about 69-85 %, which were much higher than that of 1 mM α-tocopherol. The activities tended to increase with an increasing degree of the sample concentration. The hydrolysates for 100 mg/ml had strong hydroxyl radical scavenging activities, which could be attributed to the combined effects of donation of hydrogen atoms and scavenging of active oxygen.
DPPH is a stable free radical with a characteristic absorption, which decreases significantly on exposure to proton radical scavengers (Singh et al. 2009; Kosanic et al. 2011; Suma and Urooj 2012). DPPH radical scavenging activities of autolysate and enzymatic hydrolysates from yam tsukuneimo tuber mucilage tororo were measured. These activities tended to increase with an increasing degree of the sample concentration (Table 3). Trypsin hydrolysate for 10 mg/ml showed moderate activity about 55 % among these hydrolysates. Moreover, the hydrolysates for 100 mg/ml showed strong scavenging activities (about 62-87 %) on DPPH radicals, which was the same activities as 1 mM α-tocopherol.
ACE inhibitory activities of autolysate and enzymatic hydrolysates from yam tsukuneimo tuber mucilage tororo were investigated and the results are indicated as IC50 value. As a result, the activities of these hydrolysates were as follows: 0.21 mg/ml (pepsin hydrolysate), 0.10 mg/ml (trypsin hydrolysate), and 0.10 mg/ml (papain hydrolysate), respectively (Table 4). On the other hand, the activity in autolysate was not detected at all. It is well known that various fermented foods exhibited strong ACE inhibitory activities [miso (2.38-5.35 mg/ml); natto (0.19 mg/ml); sake (3.77-6.85 mg/ml); cheese (0.16-0.27 mg/ml); mirin (85.6 mg/ml), and fish sauce (1.35-3.15 mg/ml), respectively] (Okamoto et al. 1995). Present findings suggest that yam tsukuneimo tuber mucilage tororo may be useful for preventing diseases associated with reactive oxygen species and blood pressure in the body system and it can fully absorb the useful components from it to digest using the gastrointestinal enzymes.
Table 4.
ACE inhibitory activities of autolysate and enzymatic hydrolysates from yam tsukuneimo tuber mucilage tororo
| Sample species | IC50 (mg sample powder/ml) | IC50 (μg/mg sample powder) |
|---|---|---|
| Pepsin hydrolysate | 0.21 ± 0.00 | 5.64 ± 0.07 |
| Trypsin hydrolysate | 0.10 ± 0.00 | 1.67 ± 0.02 |
| Papain hydrolysate | 0.10 ± 0.00 | 2.78 ± 0.03 |
The activity could not detected in autolysate from yam tsukuneimo tuber mucilage tororo. Each value represents mean ± SD, n = 3
In general, yam tsukuneimo tubers contain main ingredients: water (66.7 %), proteins (4.5 %), lipids (0.2 %), carbohydrates (27.1 %), and ash (1.5 %), respectively (Anonymous 2012). This value, particularly in proteins, is about twice as much as that of nagaimo. It is said that the ratio of dioscorin, the storage protein of yam tubers, in extractable water-soluble protein of yam species accounted for about 90 %. It was suggested that most of the proteins (or peptides) were obtained by autolysis and enzymatic hydrolysis from yam tsukuneimo tuber mucilage tororo were derived from dioscorin. In the present study, it became clear that the proteins (or peptides) contain in yam tsukuneimo tuber mucilage tororo exhibited high antioxidative activitiy, scavenging activity against reactive oxygen species such as superoxide anion radicals, hydroxyl radicals, and DPPH radicals, and ACE inhibitory activity. In more recent paper, Hou et al. (2002) reported the high antioxidative activities in yam (D. batatas) tuber mucilage in a series of in vitro tests, including anti-lipid peroxidation and anti-human low density lipoprotein peroxidation tests and scavenging test using electron paramagnetic resonance spectrometer. Although the ACE inhibitory activities of food-derived peptides were weaker than those of commercial drugs, it can be obtained powerful activity to purify the functional substances from crude peptides. Functional foods represent an important, innovate, and rapidly growing part of the overall food market.
Conclusion
Enzyme is a useful tool to improve the functions in food materials. Therefore, the proteins contained in yam tsukuneimo tubers may be convertible by autolysis and enzymatic hydrolysis to useful products with physiological functions such as antioxidative activity, scavenging activity against reactive oxygen species, and antihypertensive activity. In food industry, this result demonstrates that proteins in yam tsukuneimo tubers may be useful to produce functional food products protecting oxidation and regulating blood pressure in the body.
Acknowledgment
We gratefully acknowledge Mrs. Junko Nagai and Mrs. Kayo Nagai for supplying yam tsukuneimo samples for the study.
References
- Anonymous (2012) In: Kagawa Y (ed) Standard tables of food composition in Japan 2010. Kagawa Education Institute of Nutrition, Tokyo
- Araghiniknam M, Chung S, Nelson-White T, Eskelson C, Watson RR. Antioxidative activity of dioscorea and dehydroepiandrosterone (DHEA) in older humans. Life Sci. 1996;59:147–157. doi: 10.1016/0024-3205(96)00396-7. [DOI] [PubMed] [Google Scholar]
- Ardestani A, Yazdanparast R. Antioxidant and free radical scavenging potential of Achillea santolina extracts. Food Chem. 2007;104:21–29. doi: 10.1016/j.foodchem.2006.10.066. [DOI] [Google Scholar]
- Atindehou KK, Kone M, Terreaux C, Traore D, Hostettmann K, Dosso M. Evaluation of the antimicrobial potential of medicinal plants from the lvory Coast. Phytother Res. 2002;16:497–502. doi: 10.1002/ptr.970. [DOI] [PubMed] [Google Scholar]
- Bhandari MR, Kasai T, Kawabata J. Nutritional evaluation of wild edible yam (Dioscorea spp.) tuber of Nepal. Food Chem. 2003;82:619–623. doi: 10.1016/S0308-8146(03)00019-0. [DOI] [Google Scholar]
- Box HC, Maccubbin AE. Lipid peroxidation and DNA damage. Nutrition. 1997;13:920–921. doi: 10.1016/S0899-9007(97)00260-8. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Castegna A, Pocernich CB, Drake J, Scapagnini G, Calabrese V. Nutritional approaches to combat oxidative stress in Alzheimer’s diseases. J Nutr Biochem. 2002;13:444–461. doi: 10.1016/S0955-2863(02)00205-X. [DOI] [PubMed] [Google Scholar]
- Chen H, Zhang Y, Lu X, Qu Z. Comparative studies on the physicochemical and antioxidant properties of different tea extracts. J Food Sci Technol. 2012;49:356–361. doi: 10.1007/s13197-011-0291-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou WC, Liu JS, Chen HJ, Chen TE, Chang CF, Lin YH. Dioscorin, the major tuber storage protein of yam (Dioscorea batatas Decne), with carbonic anhydrase and trypsin inhibitor activities. J Agric Food Chem. 1999;47:2168–2172. doi: 10.1021/jf980738o. [DOI] [PubMed] [Google Scholar]
- Hou WC, Hsu FL, Lee MH. Yam (Dioscorea batatas) tuber mucilage exhibited antioxidant activities in vitro. Plant Med. 2002;68:1072–1076. doi: 10.1055/s-2002-36356. [DOI] [PubMed] [Google Scholar]
- Hu K, Dong A, Yao XS, Kobayashi H, Iwasaki S. Antineoplastic agents. I. Three spirostanol glycosides from rhizomes of Dioscorea collettii var. hypoglauca. Plant Med. 1996;62:573–575. doi: 10.1055/s-2006-957978. [DOI] [PubMed] [Google Scholar]
- Hu K, Yao X. Protodioscin (NSC-698 796): its spectrum of cytotoxicity against 60 human cancer cell lines in an anticancer drug screen panel. Planta Med. 2002;68:297–301. doi: 10.1055/s-2002-26743. [DOI] [PubMed] [Google Scholar]
- Indu S, A Nirmala M (2011) Effects of temerature and solvent on antioxidant properties of curry leaf (Murraya koenigii L.). J Food Sci Technol 48:366–370 [DOI] [PMC free article] [PubMed]
- Je JY, Park PJ, Kim SK. Antioxidant activity of a peptide isolated from Alaska Pollack (Theragra chalcogramma) frame protein hydrolysate. Food Res Int. 2005;38:45–50. doi: 10.1016/j.foodres.2004.07.005. [DOI] [Google Scholar]
- Je JY, Qian ZJ, Byun HG, Kim SK. Purification and characterization of an antioxidant peptide obtained from tuna backbone protein by enzymatic hydrolysis. Process Biochem. 2007;42:840–846. doi: 10.1016/j.procbio.2007.02.006. [DOI] [Google Scholar]
- Kanatt SR, Chander R, Sharma A. Antioxidant potential of mint (Mentha spicata L.) in radiation-processed lamb meat. Food Chem. 2007;100:451–458. doi: 10.1016/j.foodchem.2005.09.066. [DOI] [Google Scholar]
- Kitts DD, Weiler K. Bioactive proteins and peptides from food sources. Applications of bioprocess used in isolation and recovery. Curr Pharm Design. 2003;9:1309–1323. doi: 10.2174/1381612033454883. [DOI] [PubMed] [Google Scholar]
- Klompong V, Benjakul S, Kantachote D, Shahidi F. Antioxidative activity and functional properties of protein hydrolysate of yellow stripe trevally (Selaroides leptolepis) as influenced by the degree of hydrolysis and enzyme type. Food Chem. 2007;102:1317–1327. doi: 10.1016/j.foodchem.2006.07.016. [DOI] [Google Scholar]
- Kosanic M, Rankovic B, Vukojevic J. Antioxidative properties of some lichen species. J Food Sci Technol. 2011;48:584–590. doi: 10.1007/s13197-010-0174-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishan DS, Kathrin S, Bronwen S, Laurie M. Antioxidant capacity, polyphenolics and pigments of broccoli-cheese powder blends. J Food Sci Technol. 2011;48:510–514. doi: 10.1007/s13197-010-0211-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee SC, Tsai CC, Chen JC, Lin JG, Lin CC, Hu ML, Lu S. Effects of Chinese yam of hepato-nephrotoxicity of acetaminophen in rats. Acta Pharmacol Sin. 2002;23:503–508. [PubMed] [Google Scholar]
- Liu SY, Wang JU, Shyu YT, Song LM. Studies of yams (Dioscorea spp.) in Taiwan. J Chinese Med. 1995;6:111–126. [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Ma HY, Zhao ZT, Wang LJ, Wang Y, Zhou QL, Wang BX. Comparative study on anti-hypercholesterolemia activity of diosgenin and total saponin of Dioscorea panthaica. Zhongguo Zhong Yao Za Zhi. 2002;27:528–531. [PubMed] [Google Scholar]
- Manso MA, Miguel M, Even J, Hernández R, Aleixandre A, López-Fandino R. Effect of the long-term intake of an egg white hydrolysate on the oxidative status and blood lipid profile of spontaneously hypertensive rats. Food Chem. 2008;109:361–367. doi: 10.1016/j.foodchem.2007.12.049. [DOI] [PubMed] [Google Scholar]
- Marijana K, Branislav R, Jelena V. Antioxidant properties of some lichen species. J Food Sci Technol. 2011;48:584–590. doi: 10.1007/s13197-010-0174-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai T, Nagashima T (2006) Functional properties of dioscorin, a soluble viscous protein from Japanese yam (Dioscorea opposita Thunb.) tuber mucilage tororo. Z Natureforsh 61c:792–798 [DOI] [PubMed]
- Nagai T, Suzuki N, Nagashima T. Autolysate and enzymatic hydrolysates from yam (Dioscorea opposita Thunb.) tuber mucilage tororo have antioxidant and angiotensin I-converting enzyme inhibitory activities. J Food Agric Environ. 2007;5:39–43. [Google Scholar]
- Nasirullah, Jeyarani T, Rakshitha D. Isolation and antioxidant efficacy of nutraceutical concentrates from sesame and flax seed oils. J Food Sci Technol. 2009;46:66–69. [Google Scholar]
- Okamoto A, Hanagata H, Matsumoto E, Kawamura Y, Koizumi Y, Yanagida F. Angiotensin I converting enzyme inhibitory activities of various fermented foods. Biosci Biotech Biochem. 1995;59:1147–1149. doi: 10.1271/bbb.59.1147. [DOI] [PubMed] [Google Scholar]
- Omonigho SE, Ikenebomeh MJ. Effect of temperature treatment on the chemical composition of pounded white yam during storage. Food Chem. 2000;71:215–220. doi: 10.1016/S0308-8146(00)00158-8. [DOI] [Google Scholar]
- Ozo ON, Caygill JC, Coursey DG. Phenolics of five yam (Dioscorea) species. Phytochemistry. 1984;23:329–331. doi: 10.1016/S0031-9422(00)80327-1. [DOI] [Google Scholar]
- Qian S, Huixing S, Yongkang L. Antioxidant activity of hydrolysates and peptide fractions derived from porcine hemoglobin. J Food Sci Technol. 2011;48:53–60. doi: 10.1007/s13197-010-0128-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan K, Narayanan P, Vasudevan V, Muthukumaran G, Antony U. Nutrient composition of cultivated stevia leaves and the influence of polyphenols and plant pigments on sensory and antioxidant properties of leaf extracts. J Food Sci Technol. 2010;47:27–33. doi: 10.1007/s13197-010-0011-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekha CR, Vijayalakshmi G. Influence of natural coagulants on isoflavones and antioxidant activity of tofu. J Food Sci Technol. 2010;47:387–393. doi: 10.1007/s13197-010-0064-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachindra NM, Airanthi MKWA, Hosokawa M, Miyashita K. Radical scavenging and singlet oxygen quenching activity of extracts from Indian seaweeds. J Food Sci Technol. 2010;47:94–99. doi: 10.1007/s13197-010-0022-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarala M, Velu V, Anandharamakrishnan C, Singh RP. Spray drying of Tinospora cordifolia leaf and stem extract and evaluation of antioxidative activity. J Food Sci Technol. 2012;49:119–122. doi: 10.1007/s13197-011-0364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seema MN, Jayaprakasha GK, Singh RP. Antioxidant activity of custard apple (Annona squamosa) peel and seed extracts. J Food Sci Technol. 2008;45:349–352. [Google Scholar]
- Singh UAK, Singh S, Rai M. Total phenolics content and freeradical scavenging activity of brassica vegetables. J Food Sci Technol. 2009;46:595–597. [Google Scholar]
- Slinkard K, Singleton VL. Total phenol analysis. Am J Enol Viticult. 1977;28:49–55. [Google Scholar]
- Suma PF, Urooj A. Antioxidative activity of extracts from foxtail millet (Setaria italica) J Food Sci Technol. 2012;49:500–504. doi: 10.1007/s13197-011-0300-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiansilkul Y, Benjakul S, Shahidi F. Antioxidative activity of protein hydrolysate from round scad muscle using Alcalase and Flavourzyme. J Food Biochem. 2007;31:266–287. doi: 10.1111/j.1745-4514.2007.00111.x. [DOI] [Google Scholar]
- Yin J, Kouda K, Tezuka K, Tran QL, Miyahara T, Chen Y, Kadota S. Steroidal glycosides from the Rhizomes of Dioscorea spongiosa. J Nat Prod. 2003;66:646–650. doi: 10.1021/np0205957. [DOI] [PubMed] [Google Scholar]
- Zhou K, Yu L. Effects of extraction solvent on wheat bran antioxidant activity estimation. Lebensm Wiss Technol. 2004;37:717–721. doi: 10.1016/j.lwt.2004.02.008. [DOI] [Google Scholar]