Abstract
Anthracnose, a postharvest disease caused by the fungus Colletotrichum capsici is the most devastating disease of bell pepper that causes great economic losses especially in tropical climates. Therefore, the objective of this study was to evaluate the antifungal properties of chitosan (low molecular weight from crab shell, Mw: 50 kDa and 75–85 % deacetylated) against anthracnose by inducing defense-related enzymes. The concentrations of 0, 0.5, 1.0, 1.5 and 2.0 % chitosan were used to control the fungus in vitro and postharvest. There was a reduction in C. capsici mycelial growth and the highest chitosan concentration (2.0 %) reduced the growth by 70 % after 7 days incubation. In germination test, the concentration of 1.5 and 2.0 % chitosan reduced spore germination in C. capsici between 80 % and 84 %, respectively. In postharvest trial the concentration of 1.5 % decreased the anthracnose severity in pepper fruit by approximately 76 % after 28 days of storage (10 ± 1 °C; 80 % RH). For enzymatic activities, the concentration of 1.5 and 2.0 % chitosan increased the polyphenol oxidase (PPO), peroxidase (POD) and total phenolics in inoculated bell pepper during storage. Based on these results, the chitosan presents antifungal properties against C. capsici, as well as potential to induce resistance on bell pepper.
Keywords: Capsicum annuum, Chitosan, Colletotrichum capsici, Polyphenol oxidase, Peroxidase, Total phenolics
Introduction
Bell pepper (Capsicum annuum) is an important cash crop that is grown throughout tropical, sub-tropical and temperate regions (Pickersgill 1997). It is susceptible to a range of postharvest diseases, which lower financial returns and may pose health hazards to consumers (Freeman et al. 1998). Among others, anthracnose caused by Colletotrichum capsici is a major postharvest disease which causes massive economic losses in bell pepper (Than et al. 2008). Being latent infection, fungal spores infect immature fruits in the field but symptoms on the fruits peel appeared after ripening (Than et al. 2008). Thus, any potential control measure which can effectively delay symptoms of anthracnose infection would have an important role in extending the shelf life of bell pepper fruits during storage.
Synthetic fungicides constitute the primary means of controlling postharvest diseases in fruits and vegetables (Bautista-Baños et al. 2006). However, increasing concerns of health hazards and accumulation of toxic residues in the ecosystem has demanded the development of alternative strategies for crop protection (Terry and Joyce 2004; Faoro et al. 2008). In addition, over usage of synthetic fungicides has facilitated the development of fungicide resistance among some pathogenic populations (Rhouma et al. 2009). Therefore, in the absence of anthracnose resistant cultivars, biopesticides, such as chitosan, offer a more sustainable approach for the control of postharvest diseases in fruits and vegetables (Bautista-Baños et al. 2006; Ali et al. 2010; Maqbool et al. 2010).
Chitosan is a sea waste compound composed of glucosamine, 2-amino-2 deoxy–β-D–glucose and it is the most naturally abundant polysaccharide after cellulose (Muzzarelli 1986). It has been demonstrated that application of chitosan coatings can control several fungal diseases in plants such as table grapes (Romanazzi et al. 2006), delay the ripening mechanisms in banana fruits (Maqbool et al. 2010) and induce resistance against many postharvest pathogens (Wilson et al.1994; Benhamou 1996). It has been hypothesised that the interaction of chitosan with negatively charged molecules on the fungal cell surface causes leakage of proteinaceous compounds (Leuba and Stossel 1986). It is also believed that the interaction between chitosan and fungi leads to the inhibition of mRNA and protein synthesis (Hadwiger 1999). However, the precise physiological mechanisms by which chitosan perturbs fungal growth are still not fully understood.
Several researchers have shown that chitosan in the form of postharvest sprays was very effective against Botrytis cinerea of tomato (Badawy and Rabea 2009) and bell pepper (El Ghaouth et al. 1997). However, the mechanism of action of chitosan against these postharvest pathogens in not clear yet. Therefore, the present study was designed to investigate the antifungal activity of chitosan against C. capsici with the main emphasis to observe the mechanism of action of chitosan by eliciting the defense-related enzymes in bell pepper fruits. It is anticipated that the results of this research will contribute to the development of procedures for using chitosan as a biopesticides on a commercial scale to control anthracnose in bell pepper.
Materials and methods
Materials
Green bell peppers (Capsicum annum, cv. ‘Meno’) (Average weight: 160 g) were collected from a local farm in Cameron Highlands, Malaysia, and sorted on the basis of size and absence of physical injuries. For this study, the commercial product called chitosan (low molecular weight from crab shell, Mw: 50 kDa and 75–85 % deacetylated, Sigma-Aldrich Co. LLC., St. Louis, Messouri, USA) was used.
C. capsici isolate and culture conditions
To isolate C. capsici from infected bell pepper, fruits were placed in humid chamber at room temperature (25 °C and 60 % RH). A small portion of symptomatic tissues was placed on petri dishes containing Potato Dextrose Agar (PDA) and incubated at 25 °C. Once mycelial growth was observed, the colonies were re-isolated on fresh PDA dishes to obtain pure cultures. The isolates obtained were then identified based on their morphological and cultural characters. Re-isolations were carried out continuously on PDA slants to maintain inoculum.
Preparation of PDA and chitosan solutions
To prepare PDA media, 39 g of PDA was dissolved in 1,000 ml purified water and stirred continuously until it was mixed completely. After mixing thoroughly, the media was placed in an autoclave for sterilization at 121 °C and 15 psi pressure for 15 min. Chitosan solution was prepared by dissolving 2.0 g of chitosan powder in 100 ml purified water containing 0.5 ml (v/v) of acetic acid. The solution was heated and agitated constantly for 24 h using hotplate magnetic stirrer (Model: LMS-HTS-1003, Bunkyo-Ku, Tokyo, Japan) at 40 °C. The solution was sterilized as above for 15 min and then pH was adjusted to 5.6 with 1 N NaOH. This stock solution was used to obtain different concentrations of chitosan (0.5, 1.0, 1.5, 2.0 %, w/v). Both the sterilized PDA and chitosan solutions were mixed together in an equal quantity (1:1) under laminar air flow cabinet and then poured into the petri dishes for further use.
Determination of antifungal activity of chitosan in vitro
The in vitro antifungal activity of chitosan was performed and the effect of chitosan concentrations on the inhibition in radial mycelial growth and conidial germination of C. capsici on PDA was observed using the poison food technique. An agar disk (6 mm diameter) from a pure culture of C. capsici was placed in the center of a PDA dish containing chitosan at 0.5, 1.0, 1.5 and 2.0 % (w/v) concentrations. Control plates only contained 0.05 % acetic acid and PDA medium. Four replications of 20 dishes for each replicate were used in each treatment. Petri dishes were incubated at 25 °C and 60 % RH and daily mycelial growth measurements were taken until the fungus reached the edge of the control dish.
The in vitro spore germination inhibition test was carried out by the cavity slide technique and the percentage inhibition in germination was calculated by the method given by Cronin et al. (1996). A 40 μl aliquot of each of the chitosan concentrations was pipetted on a cavity slide. Then 10 μl of a freshly harvested conidial suspension of C. gloeosporioides (adjusted to 104 conidia ml−1 using a haemacytometer) were pipetted into the cavity and the slide covered with a cover slip and kept in the dark for 7 h at 25 °C. Cavity slides containing deionized water were served as the control. After incubation, the conidia were killed by adding 10 μl 2.0 % sodium azide and observed for conidial germination under a light microscope at 40× magnification. The number of germinated conidia was counted in 10 microscopic fields of 100 conidia in 20 replicated plates and presented as percent inhibition. A conidium was considered germinated if its germ tube was longer than the conidium itself (Cronin et al. 1996).
Determination of antifungal activity of chitosan in vivo
The effect of chitosan on growth of C. capsici was evaluated on bell pepper fruits. For in vivo studies, bell pepper fruits were washed with sodium hypochlorite (0.05 %), rinsed with purified water and air-dried at room temperature (25 °C). Chitosan at 0.5, 1.0, 1.5 and 2.0 % (w/v) were used for in vivo experiments and solutions were prepared with Tween 80 (1.0 %) as an emulsifier. The bell pepper fruits were dipped in spore suspension (105 spores ml−1) of C. capsici for 2–3 min and allowed to completely dry at room temperature (25 °C). After drying, fruits were individually dipped for 2–3 min in chitosan solutions (0.5, 1.0, 1.5 or 2.0 %) and then kept at ambient (25 °C) for drying. The control fruits were dipped in spore suspension, air dried and dipped in purified water for 2–3 min. After treatment, the fruits were placed in cardboard boxes and stored (10 ± 1 °C, 80 % RH) for 28 days. The effect of chitosan treatments on disease incidence and disease severity was recorded weekly for 28 days. Each treatment was conducted in four replicates and each replicate contained 40 fruits. Disease incidence data were expressed as the percentage of fruits showing anthracnose symptoms out of the total number of fruits in each treatment, while disease severity was scored following the scale (1 = 0 % of fruits surface with symptomatic lesions; 2 = 1–25 %; 3 = 26–50 %; 4 = 51–75 %; and 5 = 76–100 %) (Sivakumar et al. 2000).
Determination of polyphenol oxidase, peroxidase and total phenolic activities
For enzymes assay, fruits were dipped in spore suspension (105 spores ml−1) for 2–3 min and then treated with chitosan concentrations as described above. Fruits dipped in spore suspension but not treated with chitosan served as the control. After chitosan treatment, fruits were stored at 10 ± 1 °C and 80 % RH. Determinations for polyphenol oxidase (PPO) and peroxidase (POD) were conducted every 5 days over a period of 20 days. Each treatment consisted of four replicates and replicated samples were taken from different fruits.
A crude enzyme extract was prepared by the method described by Liu et al. (2007) with minor modifications. Fruit samples (2 g) from a mixture of six fruits in each treatment were homogenized on ice using mortar and pestle in 10 ml of 100 mM sodium phosphate buffer (pH 6.4) containing 0.2 g of polyvinyl polypyrrolidone. The homogenized samples were centrifuged at 12,000 × g for 30 min at 4 °C and the supernatant used for determining the enzyme activity.
PPO activity was determined by measuring the conversion of catechol to quinine mediated by PPO (Duangmal and Apenten 1999). Crude enzyme extract (0.1 ml) was added to 3 ml of catechol substrate (500 mM in 100 mM sodium phosphate buffer, pH 6.4) and the increase in absorbance was measured at 398 nm using spectrophotometer (Model: Biochrom Libra S12; Biochrom Ltd., Cambridge, UK). The PPO activity was expressed as unit (U) mg−1 protein, where one unit is expressed as the increase in rate of absorbance per mass of protein, per minute (Liu et al.2007).
POD activity was determined according to Chen et al. (2000) with some modifications. Crude extract (0.1 ml) was mixed with 2 ml of guaiacol (8 mM in 100 mM sodium phosphate buffer pH 6.4) and incubated for 30 min at 30 °C. Then, 1 ml of hydrogen peroxide (24 mM) was added. The peroxidase activity was determined by measuring the conversion of guaiacol to tetraguaiacol and measuring the absorbance at 460 nm using the same spectrophotometer. The protein content was measured according to Bradford (1976) with bovine serum albumin (BSA) as standard.
The total phenolic contents of fruits were estimated by the method of Zieslin and Ben-Zaken (1993). Tissue sample (2 g) from a mixture of six fruits in each treatment was homogenized with 10 ml of 80 % methanol and agitated for 15 min at 70 °C. The crude enzyme extract (1 ml) was mixed with 5 ml of purified water and 250 μl of Folin-Ciocalteau’s reagent and incubated for 3–5 min at room temperature. Saturated Na2CO3 (1 ml) was added to the reaction mixture, incubated for 1 h at 25 °C and the absorbance at 725 nm was measured with gallic acid as the standard. The total phenolic contents were expressed as OD725 g−1 fresh weight.
Experimental design
All the experiments were conducted using completely randomised design (CRD) with four replicates. The data were subjected to analysis of variance (ANOVA) using MSTAT-C program (Crop and Soil Science Department, Michigan State University, Version 1.3) and means were separated using Least Significant Difference (LSD) test at (P < 0.05). The entire experiments were repeated twice and data were pooled before analysis.
Results and discussion
Antifungal activity of chitosan in vitro and in vivo
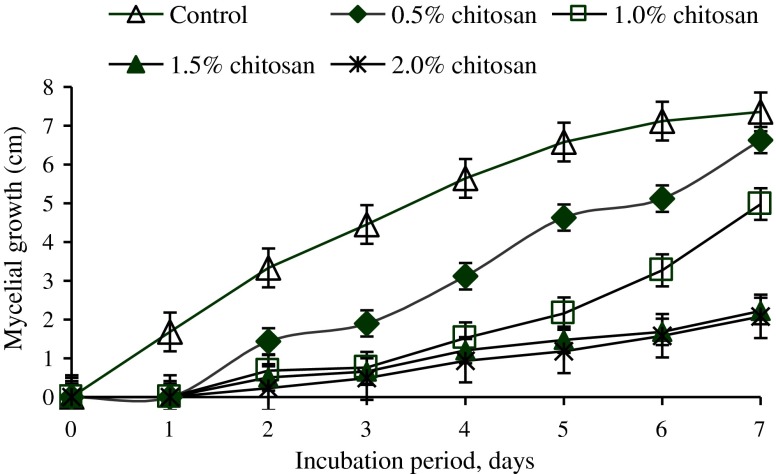
Mycelial growth of C. capsici was significantly (P < 0.05) reduced by chitosan treatments as compared to control during the incubation period of 7 days (Fig. 1). A complete inhibition in mycelial radial growth was not observed with any of the concentrations tested. The antifungal activity of chitosan against C. capsici increased as the concentrations of chitosan increased. The maximum inhibition in mycelial growth was observed with 2.0 % chitosan but that was not significantly different from 1.5 % chitosan concentration. Radial growth in the control dishes was almost five times bigger in size than in the 1.5 % and 2.0 % chitosan treatments by the end of the incubation period.
Fig. 1.
Effect of various concentrations of chitosan on mycelial growth of Colletotrichum capsici during 7 days of incubation period. (n = 4)
Results from the in vitro mycelial growth study confirmed the efficacy of chitosan to inhibit the radial mycelial growth of C. capsici. Chitosan activity was detected at all concentrations and the growth of C. capsici was reduced as the chitosan concentration increased but none of the treatment gave complete inhibition. Similar results were obtained in a previous study by El Ghaouth et al. (1992b) where they found that chitosan at 6 mg ml−1 did not completely inhibit the radial mycelial growth of B. cinerea and Rhizopus stolonifer. Krol (2005) also found that chitosan poorly inhibited the mycelial growth of Phomopsis viticola sacc. but effectively inhibited the spore germination of the pathogen and protected the grapevine from infection.
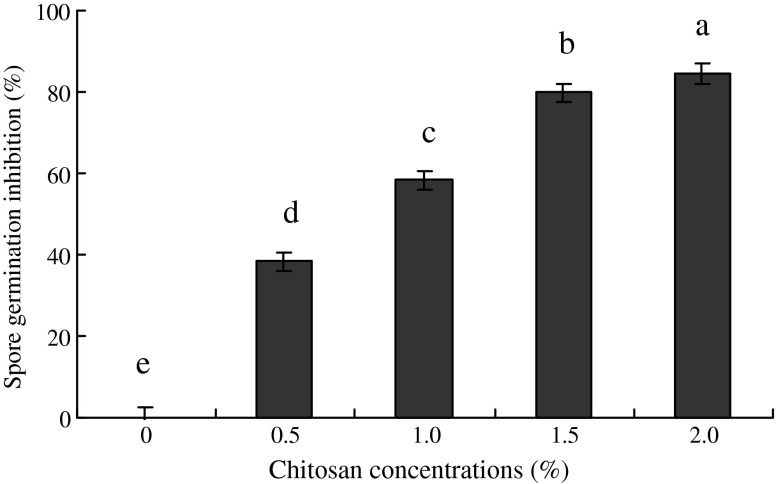
The chitosan treatments significantly (P < 0.05) inhibited the spore germination as compared to control after keeping in dark for 7 h (Fig. 2). Chitosan concentration of 1.5 and 2.0 % proved the best among all other treatments and inhibited the spore germination up to 80 and 84 % followed by 0.5 and 1.0 % (38 and 58 %), respectively.
Fig. 2.
Effect of various concentrations of chitosan on spore germination inhibition (%) of Colletotrichum capsici. (n = 4)
Earlier studies carried out by El Ghaouth et al. (1992b) showed that 1.5 % chitosan induced morphological differences in R. stolonifer mycelium (e.g. excessive branching) but such changes were not observed with B. cinerea, Alternaria alternata or C. gloeosporioides. Allan and Hadwiger (1979) suggested that chitosan within the cell wall of some fungi rendered those strains more resistant to externally amended chitosan.
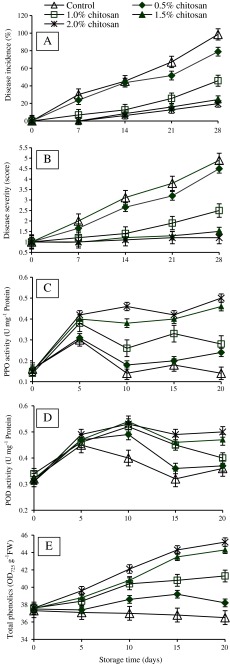
There were significant (P < 0.05) differences in antifungal effects of chitosan among the treatments when tested on bell pepper fruits. The highest antifungal effects were found in those bell pepper fruits treated with 1.5 and 2.0 % chitosan concentrations followed by 1.0 and 0.5 %. However, in control fruits disease incidence and disease severity increased with the time and reached up to 100 % and score 5 respectively, after 28 days of storage (Fig. 3a, b). The anthracnose symptoms appeared on control fruits during the first week of storage and after 28 days most of the bell pepper fruits were spoiled by severe disease. While different concentrations of chitosan not only delayed the onset of anthracnose but also maintained the freshness of bell pepper fruits during first 2 weeks of storage and later on showed only minor symptoms after 28 days of storage. Therefore, by the end of storage period only minor disease incidence (24 and 19 %) and disease severity score (1.5 and 1.2) were observed in fruits treated with 1.5 and 2.0 % chitosan concentrations, respectively. Similar findings have been reported previously by Muñoz et al. (2009) for anthracnose in tomatoes. They noted a significant reduction in the lesion diameter on tomato fruits treated with 1.0 % chitosan, therefore suggesting that significant disease incidence can be attained at lower concentration of chitosan instead of using higher concentration (2.5 %). El Ghaouth et al. (1992a) also showed that 10 mg ml−1 and 15 mg ml−1 chitosan significantly reduced the decay caused by B. cinerea and R. stolonifer in strawberry fruits, but there was no further reduction in the disease using higher concentrations of chitosan. The results presented in this study were also consistent with the findings of Bautista-Baños et al. (2003) who stated that 1.5 % chitosan could control anthracnose caused by C. gloeosporioides in papaya.
Fig. 3.
Effect of various concentrations of chitosan on (a) Disease incidence (%), (b) Disease severity (score), (c) Polyphenol oxidase (PPO) activity, (d) Peroxidase (POD) activity and (e) Total phenolics in inoculated bell pepper during storage (10 °C, 80 % relative humidity) (n = 4). Disease severity: 1 = 0 % of fruits surface rotten; 2 = 1–25 %; 3 = 26–50 %; 4 = 51–75 % and 5 = 76–100 %
Elicitation of enzymes and total Phenolic contents
Activity of PPO enzyme was significantly (P < 0.05) higher in treated fruits compared to the control (Fig. 3c). PPO activity increased in all bell pepper fruits after 5 days of storage but on 10th day of storage there was a significant decrease observed in control, 0.5 and 1.0 % chitosan treatments. However, in 1.5 and 2.0 % chitosan treated fruits the PPO activity continued to be enhanced and reached the maximum of 0.46 and 0.50 U mg−1 protein after 20 days of storage, respectively.
POD activity increased initially after 5 days of storage and remained constant until day 10 in 0.5, 1.0 1.5 and 2.0 % chitosan treatments but in control there was a continuous decreased of POD activity over the storage period (Fig. 3d). At the end of storage period, 1.5 and 2.0 % chitosan coated fruits maintained a higher POD activity compared to the control. It was found that POD activity in 2.0 % chitosan coated fruits was 1.46 times higher than control fruits.
Total phenolic contents increased significantly (P < 0.05) in all the chitosan treated fruits compared to the control with time of storage (Fig. 3e). The maximum increase in phenolic compounds was observed in 2.0 % chitosan treated fruits however, there was no significant difference between 1.5 and 2.0 % chitosan concentration in terms of phenolic contents.
El Ghaouth et al. (1997) found that it was possible for chitosan coating to act as a barrier, limiting the penetration of the fungus, or as a stimulus to induce host defense responses such as activation of defense related enzymes (Bautista-Baños et al. 2006). Chitosan coating also stimulated structural defense reactions such as the formation of hemispherical protuberances along host cell walls, cell wall thickening and occlusion of many intercellular spaces with a fibrillar material in bell pepper (El Ghaouth et al. 1997).
The induction of defense reactions in plants is highly correlated with enzymatic activities which then participate in the first line of defense and inhibit the pathogen (Liu et al.2007). In present study, higher activities of PPO, POD and total phenolics were observed in chitosan coated fruits. It has been suggested that PPO oxidizes polyphenols to quinines (antimicrobial compounds), restricting pathogenic growth and causing lignification in plant cells (El Ghaouth et al. 1997). POD participates in the cell wall reinforcement process, such as oxidation of phenols, suberisation and lignification of host plant cells (Than et al. 2008), producing structural barriers that limit the activities of the pathogen. In the present study, it was found that all the chitosan treatments significantly increased the total phenolic content in bell pepper fruits. Several studies have proven the potential of chitosan to induce defense related enzymes in fresh fruits. In a recent study by our group it was observed that chitosan not only reduced the severity of disease in papaya fruits inoculated with C. gloeosporioides but also enhanced the activities of defense related enzymes such as POD, chitinase, β-1,3-glucanase and total phenolics at 1.5 and 2.0 % chitosan concentrations (Ali et al. 2012). Similarly, Liu et al. (2007) also revealed that the activities of PPO, POD and total phenolics increased significantly in tomato fruits when treated with different concentrations of chitosan. Chitosan’s potential to induce defense related enzymes was also evaluated by Rappussi et al. (2009) in Valencia oranges where chitosan increased the activity of PPO enzyme within 24 h of incubation. It was suggested that this could be an explanation for the reduction of black spots, since these enzymes are involved in the defense response against pathogens. Chitosan eliciting POD activity was also studied by Ben-Shalom et al. (2003) in cucumber plants, which resulted in the induction of resistance against B. cinerea. Similarly, in a recent study by Meng et al. (2010) it was observed that chitosan inhibited two kinds of fungal pathogens Alternaria kikuchiana Tanaka and Physalospora piricola Nose of pear fruits in storage and also increased the activity of POD in treated pear fruits. From the results in the present study suggested that changes involved in the PPO, POD and total phenolic activities may have a role in enhancing disease resistance in chitosan treated fruits.
Conclusion
Based on these results, chitosan, a natural substance which is biodegradable and non-toxic not only directly inhibited the fungus C. capsici but also reduced the severity of disease and induced defense responses in bell pepper, could become a promising substance to control postharvest diseases. However, further studies are required to confirm the optimal concentration of chitosan to control preharvest anthracnose of bell pepper caused by C. capsici and other Colletotrichum species.
Acknowledgments
The authors thank the Ministry of Agriculture (MOA) Malaysia, for providing financial support under the project (05-02-12-SF0031).
References
- Ali A, Mahmud TMM, Sijam K, Siddiqui Y. Potential of chitosan coating in delaying the postharvest anthracnose (Colletotrichum gloeosporioides Penz.) of Eksotika II papaya. Int J Food Sci Technol. 2010;45:2134–2140. doi: 10.1111/j.1365-2621.2010.02389.x. [DOI] [Google Scholar]
- Ali A, Mahmud TMM, Siddiqui Y. Control of anthracnose by chitosan through stimulation of defence-related enzymes in Eksotika II papaya (Carica papaya L.) fruit. J Biol Life Sci. 2012;3:114–126. [Google Scholar]
- Allan CR, Hadwiger LA. The fungicidal effect of chitosan on fungi of varying cell wall composition. Exp Mycol. 1979;3:285–287. doi: 10.1016/S0147-5975(79)80054-7. [DOI] [Google Scholar]
- Badawy MEI, Rabea EI. Potential of the biopolymer chitosan with different molecular weights to control postharvest gray mold of tomato fruit. Postharvest Biol Technol. 2009;51:110–117. doi: 10.1016/j.postharvbio.2008.05.018. [DOI] [Google Scholar]
- Bautista-Baños S, Hernández-López M, Bosquez-Molina E, Wilson CL. Effect of chitosan and plant extracts on growth of Colletotrichum gloeosporioides, anthracnose levels and quality of papaya fruit. Crop Prot. 2003;22:1087–1092. doi: 10.1016/S0261-2194(03)00117-0. [DOI] [Google Scholar]
- Bautista-Baños S, Hernandez-Lauzardo AN, Velazquez-del Valle MG, Hernández-López M, Ait Barka E, Bosquez-Molina E, Wilson CL. Review: chitosan as a potential natural compound to control pre and postharvest diseases of horticultural commodities. Crop Prot. 2006;25:108–118. doi: 10.1016/j.cropro.2005.03.010. [DOI] [Google Scholar]
- Benhamou N. Elicitor-induced plant defense pathways. Trends Plant Sci. 1996;1:233–240. doi: 10.1016/1360-1385(96)86901-9. [DOI] [Google Scholar]
- Ben-Shalom N, Ardi R, Pinto R, Aki C, Fallik E. Controlling gray mould caused by Botrytis cinerea in cucumber plants by means of chitosan. Crop Prot. 2003;22:285–290. doi: 10.1016/S0261-2194(02)00149-7. [DOI] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chen C, Belanger RR, Benhamou N, Paulitz TC. Defense enzymes induced in cucumber roots by treatments with plant growth promoting rhizobacteria (PGPR) and Pythium aphanidermatum. Physiol Mol Plant Pathol. 2000;56:13–23. doi: 10.1006/pmpp.1999.0243. [DOI] [Google Scholar]
- Cronin MJ, Yohalem DS, Harris RF, Andrews JH. Putative mechanism and dynamics of inhibition of apple scab pathogen Venturia inaequalis by compost extracts. Soil Biol Biochem. 1996;28:1241–1249. doi: 10.1016/0038-0717(96)00131-9. [DOI] [Google Scholar]
- Duangmal K, Apenten RKO. A comparative study of polyphenoloxidases from taro (Colocasia esculenta) and potato (Solanum tuberosum var. Romano) Food Chem. 1999;64:351–359. doi: 10.1016/S0308-8146(98)00127-7. [DOI] [Google Scholar]
- El Ghaouth A, Arul J, Grenier J, Asselin A. Antifungal activity of chitosan on two postharvest pathogens of strawberry fruits. Phytopathology. 1992;82:398–402. doi: 10.1094/Phyto-82-398. [DOI] [Google Scholar]
- El Ghaouth A, Arul J, Asselin A, Benhamou N. Antifungal activity of chitosan on postharvest pathogens: induction of morphological and cytological alterations in Rhizopus stolonifer. Mycol Res. 1992;96:769–779. doi: 10.1016/S0953-7562(09)80447-4. [DOI] [Google Scholar]
- El Ghaouth A, Arul J, Wilson C, Benhamou N. Biochemical and cytochemical aspects of the interaction of chitosan and Botrytis cinerea in bell pepper fruit. Postharvest Biol Technol. 1997;12:183–194. doi: 10.1016/S0925-5214(97)00056-2. [DOI] [Google Scholar]
- Faoro F, Maffi D, Cantu D, Iriti M. Chemical-induced resistance against powdery mildew in barley: the effects of chitosan and benzothiadiazole. BioControl. 2008;53:387–401. doi: 10.1007/s10526-007-9091-3. [DOI] [Google Scholar]
- Freeman S, Katan T, Shabi E. Characterization of Colletotrichum species responsible for anthracnose diseases of various fruits. Plant Dis. 1998;82:596–605. doi: 10.1094/PDIS.1998.82.6.596. [DOI] [PubMed] [Google Scholar]
- Hadwiger LA. Host-parasite interactions: elicitation of defense response in plants with chitosan. In: Jolles P, Muzzarelli RAA, editors. Chitin and chitinases. Basel: Birkhauser Verlag; 1999. pp. 185–200. [DOI] [PubMed] [Google Scholar]
- Krol E. Influence of some chemicals on the viability of Phomopsis viticola sacc. spores. J Plant Prot Res. 2005;45:195–203. [Google Scholar]
- Leuba JL, Stossel P. Chitosan and other polyamines: anti-fungal activity and interaction with biological membranes. In: Muzzarelli RAA, Jeuniaux C, Gooday GW, editors. Chitin in nature and technology. New York: Plenum Press; 1986. pp. 215–222. [Google Scholar]
- Liu J, Tian S, Meng X, Xu Y. Effect of chitosan of postharvest diseases and physiological response of tomato fruits. Postharvest Biol Technol. 2007;44:300–306. doi: 10.1016/j.postharvbio.2006.12.019. [DOI] [Google Scholar]
- Maqbool M, Ali A, Ramachandran S, Smith DR, Alderson PG. Control of postharvest anthracnose of banana using a new edible composite coating. Crop Prot. 2010;29:1136–1141. doi: 10.1016/j.cropro.2010.06.005. [DOI] [Google Scholar]
- Meng X, Yang L, Kennedy JF, Tian S. Effects of chitosan and oligochitosan on growth of two fungal pathogens and physiological properties in pear fruit. Carbohydr Polym. 2010;81:70–75. doi: 10.1016/j.carbpol.2010.01.057. [DOI] [Google Scholar]
- Muñoz Z, Moret A, Garcés S. Assessment of chitosan for inhibition of Colletotrichum sp. on tomatoes and grapes. Crop Prot. 2009;28:36–40. doi: 10.1016/j.cropro.2008.08.015. [DOI] [Google Scholar]
- Muzzarelli RAA. Filmogenic properties of chitin/chitosan. In: Muzzarelli RAA, Peter MG, editors. Chitin in nature and technology. New York: Plenum Press; 1986. pp. 475–489. [Google Scholar]
- Pickersgill B. Genetic resources and breeding of Capsicum spp. Euphytica. 1997;96:129–133. doi: 10.1023/A:1002913228101. [DOI] [Google Scholar]
- Rappussi MCC, Pascholati SF, Benato EA, Cia P. Chitosan reduces infection by Guignardia citricarpa in postharvest ‘Valencia’ oranges. Brazilian Arch Biol Technol. 2009;52:513–521. doi: 10.1590/S1516-89132009000300001. [DOI] [Google Scholar]
- Rhouma A, Ben Daoud H, Ghanmi S, Ben Salah H, Romdhane M, Demak M. Antimicrobial activities of leaf extracts of Pistacia and Schinus species against some plant pathogenic fungi and bacteria. J Plant Pathol. 2009;91:339–345. [Google Scholar]
- Romanazzi G, Gabler FM, Smilanick JL. Preharvest chitosan and postharvest UV irradiation treatments suppress gray mold of table grapes. Plant Dis. 2006;90:445–450. doi: 10.1094/PD-90-0445. [DOI] [PubMed] [Google Scholar]
- Sivakumar D, Wilson Wijeratnam RS, Wijesundera RLC, Marikar FMT, Abeyesekere M. Antagonistic effect of Trichoderma harzianum on postharvest pathogen of rambutan (Nephelium lappaceum) Phytoparasitica. 2000;28:240–247. doi: 10.1007/BF02981802. [DOI] [Google Scholar]
- Terry LA, Joyce DC. Elicitors of induced disease resistance in postharvest horticultural crops: a brief review. Postharvest Biol Technol. 2004;32:1–13. doi: 10.1016/j.postharvbio.2003.09.016. [DOI] [Google Scholar]
- Than PP, Jeewon R, Hyde KD, Pongsupasamit S, Mongkolporn O, Taylor PWJ. Characterization and pathogenicity of Colletotrichum species associated with anthracnose on chilli (Capsicum spp.) in Thailand. Plant Pathol. 2008;57:562–572. doi: 10.1111/j.1365-3059.2007.01782.x. [DOI] [Google Scholar]
- Wilson CL, El Ghaouth A, Chaluts E, Droby S, Stevens C, Lu JL, Khan V, Arul J. Potential of induced resistance to control postharvest diseases of fruits and vegetables. Plant Dis. 1994;78:837–844. doi: 10.1094/PD-78-0837. [DOI] [Google Scholar]
- Zieslin N, Ben-Zaken R. Peroxidase activity and presence of phenolic substances in peduncles of rose flowers. Plant Physiol Biochem. 1993;31:333–339. [Google Scholar]