Abstract
In our present paper, the effect of water activity and processing conditions in reconstituted potato chips was considered as a model to investigate the changes of acrylamide (AA) and 5-hydroxymethylfurfural (HMF). The results suggested that the formation of AA and HMF was highly correlated with frying temperature and time. Water activity could also influence the formation of AA and HMF. Meanwhile, the formation of HMF has significant correlation with the formation of AA in reconstituted potato chips. A typical exponential growth curve was observed by plotting AA levels vs HMF content which were all determined under different heating condition: 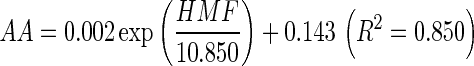 . The model could be used as a tool for estimating the formation of AA when the content of HMF was known.
. The model could be used as a tool for estimating the formation of AA when the content of HMF was known.
Keywords: Acrylamide (AA), 5-hydroxymethylfurfural (HMF), Water activity, Reconstituted potato chips
Introduction
In April 2002, researchers in Swedish National Food Administration (SNFA) and Stockholm University announced that carbohydrate-rich foods which were heated or fried at high temperature contained relatively high level of AA, such as fried potato products. These investigations cause considerable concern since AA has been known to be neurotoxic, genotoxic and carcinogenic compound in animal, and is classified as a probable human carcinogen (IARC 1994) by IARC. Nowadays, numerous research groups study into the possible approaches of the formation of AA in foods. These studies showed that an important route to form AA is the Maillard reaction which is favored by high temperature (Mottram et al. 2002; Stadler et al. 2002; Yaylayan et al. 2003; Zyzak et al. 2003). HMF is also a typical compound formed during the advanced step in the Maillard reaction. The Maillard reaction is a cascade of highly complex chemical reaction. The initial stages of the Maillard reaction involve the condensation of the amino compound with the carbonyl group that affords an N-glucosyl derivative to the reacting amino acid. N-glucosyl derivative rearranges to form the Amadori rearrangement product. The subsequent degradation of the Amadori product is dependent on the pH of the system. When pH value was 7 or below, it undergoes mainly 1,2-enolisation with the formation of furfural (when pentoses are involved) or HMF (when hexoses are involved) (Martins et al. 2001). It has been shown that HMF is cytotoxic at high concentrations, irritating to eyes, upper respiratory tract, skin and mucous membranes (Capuano and Fogliano 2011). But other studies such as the research of Severin et al. (2010), suggested that HMF did not pose a serious health risk. The toxicity of HMF to human body is still a matter to be discussed. HMF can exist in many foods, except fresh, untreated foods. But when the food is processed, HMF concentration in foods can vary largely, sometimes exceeds 1 g kg−1 in certain dried fruits and caramel products (Capuano and Fogliano 2011; Jha et al. 2012). HMF is considered to be an excellent indicator of carbohydrate-containing foods’ quality deterioration that due to excessive heating or storage for a period of time (Ait-Ameur et al. 2006).
In recent studies, some important transient intermediates, such as 3-aminopropionamide (3-APA), methylglyoxal (MG), and HMF, were found to participate the formation of AA in Maillard model systems and in food matrix (Granvogl et al. 2004; Granvogl and Schieberle 2006; Yuan et al. 2008a, 2008b; Ye et al. 2011; Gökmen et al. 2012). In the study of Gökmen et al. (2012), they found that in the Maillard model systems HMF is a potent carbonyl for accelerating AA formation during heating by the method of in-depth high resolution mass spectrometry and kinetics analysis.
Water plays an important role in the formation of AA and HMF. Bassama et al. (2011) reported that in plantain system, increasing initial water activity clearly resulted in decreasing AA formation. But Mestdagh et al. (2006) reported that AA content was rather dependent upon the moisture content than upon the water activity in the high-moisture potato powder model system. Clearly, more researches are needed to get a better understanding about the role of water in AA formation in different foods. Ait-Ameur et al. (2007) studied that HMF was very sensitive to the water activity in cookies systems. The formation of 1 mol HMF needed the releasing of 3 mol of water. HMF started to accumulate while water activity was between 0.5 and 0.7. The formation of HMF content also depended on the temperature. Relationship between HMF content and temperature followed a first order kinetic, the results also highly dependent on the type of sugar. Below 250 °C, sucrose produced less HMF than glucose and fructose, but the inverse result was observed at 300 °C.
Current observations and our previous studies stimulated our interest in the study of AA and HMF formation in reconstituted potato chips systems, and the role of water activity in AA and HMF formation. For this purpose, the main objective of this work was to investigate the effect of processing conditions on the formation of AA and HMF in reconstituted potato chips. And the relationship between HMF and AA formation and the effect of water activity on the AA and HMF formation during the heating treatment were also investigated in the present study.
Materials and methods
Reagents
All solvents used were of HPLC grade and other chemicals were of analytical grade. AA (>99.5 %) and [13C3]-AA (99 % isotopic purity) were purchased from Sigma-Aldrich (St. Louis, Mo., U.S.A.) and Cambridge Isotope Laboratories (Andover, MA, USA), respectively. 5-hydroxymethylfurfural (HMF) were obtained from Fluka Biochemika Company (Beijing, China). Other chemicals were obtained from Beijing Dingguo Changsheng BioTechnology Co., Ltd. (Beijing, China). HPLC-grade methanol was purchased from Fisher Scientific International Inc (Beijing, China). SPE cartridges Oasis MCX (3 ml, 60 mg) were supplied by Waters (Milford, MA, USA).
Stock solutions of 1 mg kg−1 for AA and [13C3]-AA were prepared in methanol. The stock solution was diluted with water to give a series of standard solutions (1.0, 2.0, 5.0, 8.0, 10.0, 15.0, 20.0 μg kg−1). The 90 μg L−1 of [13C3]-AA was used as the internal standard in quantification.
Preparation of reconstituted potato chips by frying heating
Potato starch, flour and water were mixed in a ratio of 3:1:2.5, and sealed with a plastic wrap. The mixture were left to rise for 20 min and tableted to a patch with a small pressing machine (Beverly Electric Co., Ltd. Yongkang, China), and cut into the same dimensions of chips (3.0 cm × 3.0 cm × 2.0 mm). The chips were dried in a drier at 50 °C for 30 min. Thirty chips were put into a heating basket, which was large enough to enable free movement of the chips in the frying oil. And the chips were fried at 170 ± 1 °C for 4 min, 180 ± 1 °C for 3 min and 190 ± 1 °C for 2 min in a 5 L of fresh palm oil in an electrical fryer (HH-S, Ronghua Instrument Co., Ltd., Jintan, Zhejiang). The potato chips processed by frying at different temperatures had the same final moisture conditions of 2.0–2.3 g water 100 g−1 (wet basis). Then the chips were drained over a wire screen for 5 min. The chips were cooled to room temperature and homogenized in a blender (HR 2094, Philips Instruments Co., Ltd., Zhuhai, China) for further analysis.
Analysis AA by HPLC-MS/MS
The sample preparation was performed according to the method of Liu et al. (2008). A portion of 1 g of sample was transferred to a 50 mL centrifuge tube. Then, 1 mL [13C3]-AA solution of 900 μg L−1 and 9 mL water were added into the tube. The mixture was incubated on a horizontal shaker at 25 °C for 20 min, then 10 mL of acetonitrile, 4 g of anhydrous magnesium sulfate and 0.5 g of sodium chloride were added consecutively. The tube was sealed and shaken vigorously for 1 min immediately, and then centrifuged at 6,000 × g for 5 min at 4 °C. The salt combination induced the separation of water and acetonitrile layers and forced the majority of AA into the acetonitrile layer. Three layers were obtained as follows, an acetonitrile layer containing AA at the top, the matrix layer in the middle and the water layer with the excessive salts in the bottom. The acetonitrile solution (9 mL) was transferred into a glass test tube and evaporated to dryness under a stream of nitrogen in a water bath at 40 °C. The residues on the wall of glass tube were re-dissolved in 0.5 mL of water under vortexing, so some highly lipophilic co-extractives were excluded again. Aqueous extract was filtered through a 0.45 μm syringe filter for further clean-up by Oasis MCX SPE cartridge. The re-dissolved extract (0.5 mL) passed through the SPE cartridge which was conditioned consecutively with 2 mL of methanol and 2 mL of water, and the effluent was collected. Subsequently, the AA retained on the SPE column was eluted by 0.5 mL water. The initial effluent and this wash were combined and filtered through a 0.22 μm syringe filter for LC-MS/MS analysis.
The analysis of AA in potato chips was performed by an Alliance 2695 Separation Module (Waters, Milford, MA, USA) coupled to a Micromass Quattro Micro triple-quadrupole mass spectrometer (Micromass, Manchester, UK) with MassLynx software. The final tested solution (20.0 μL) was injected onto a reversed ODS-C18 column (250 × 4.6 mm, 5 μm, Hypersil, Thermo, USA) maintained at 30 °C. The elution mode was isocratic using a mixture of 10 % acetonitrile and 90 % water containing 0.1 % formic acid as mobile phase at a flow rate of 0.4 mL min−1. AA was detected by MS/MS using electrospray ionization in the positive ion mode. The multiple reaction monitoring (MRM) of degradation patterns m/z 72 → 55 for AA and m/z 75 → 58 for [13C3]-AA was used for quantification of AA, respectively. The optimized MS instrument parameters obtained by the tuning were as follows: capillary voltage, 1 kV; cone voltage, 20 V; source temperature, 110 °C; desolvation temperature, 400 °C; desolvation gas flow, 600 L h−1; cone gas flow, 50 L h−1; argon collision gas pressure was 2 × 10−3 mbar for MS/MS, and the collision energy for each transition was 13 eV in MRM mode. In the MRM transitions, the dwell and inter scan times were 0.4 and 0.1 s, respectively. Each determination was performed in triplicate.
The performance of the analysis method for AA was evaluated by the limit of detection (LOD) and limit of quantification (LOQ). The values of LOD and LOQ in potato chips were 10 and 25 μg kg−1, respectively.
Analysis of HMF by HPLC
Two grams of potato chips were weighted accurately, 18 mL of distilled water and 5 mL of n-hexane were added. The mixture was mixed vigorously for 2 min immediately, and then centrifuged at 6,000 × g for 5 min at 4 °C. The water layer was taken and filtered through a 0.45 μm filter (Millipore Corporation, Bedford, MA, USA) and used for HPLC analysis.
HMF were analyzed using an LC-2010A HPLC system (Shimadzu Co., Japan). Twenty microlitres of sample were injected onto a C18 column (250× 4.6 mm, 5 μm, Shimadzu, Japan), and eluted isocratically at 30 °C with 10 % methanol in water at a flow rate of 1.0 mL min−1. The detection wavelength was 283 nm. HMF was quantified by external calibration, in which the concentrations of HMF standard solution ranged from 0.10 to 20 mg L−1.
The performance of the analysis method for HMF was evaluated by the limit of detection (LOD) and limit of quantification (LOQ). The values of LOD and LOQ for HMF in reconstituted potato chips were 0.05 and 0.2 mg kg−1, respectively.
Detection of water activity
The water activity of the reconstituted potato chips was analyzed directly with a water activity indicator (Hygrolab 2-SET 40, Rontronic Instrument Corp., Swissland).
Statistical analysis
Statistical analysis was performed using SPSS 11.5 software (Chicago, USA). The significance of difference was calculated by one-way ANOVA test, and the results with p < 0.05 were considered to be statistically significant. Graphs were drawn with OriginPro 7.5 software (OriginLab Corporation, Northampton, MA, USA). All the data were expressed as mean ± standard deviation (SD). Standard deviation (SD) was calculated from three independent experiments.
Results and discussion
AA contents of reconstituted potato chips during frying heating
AA was not detected in untreated reconstituted potato chips. AA concentration was highly affected by frying times and temperatures. With the increasing of treatment time and temperature, AA contents were increased accordingly (Fig. 1a). The highest AA content was found when reconstituted potato chips were frying at 190 °C for 2 min. After 2 min of frying treatment at 190 °C, the AA content was increased to 2.586 ± 0.156 mg kg−1. When reconstituted potato chips were frying at 170 °C and 180 °C, AA content became low, reaching 2.037 ± 0.318 mg kg−1 after 3 min at 180 °C and 0.835 ± 0.217 mg kg−1 after 4 min at 170 °C, respectively. At each temperature, AA content followed a first order kinetic (Fig. 1a). The rate constants and the activation energy were calculated and kinetic data were summarized in Table 1. With the increasing of frying temperature, the rate constant was increased accordingly. The results promoted that the temperature could increase the reaction rate in reconstituted potato chips. It was the same kinetic model used for AA formation in potato matrices. Franke et al. (2009) also used a first order kinetic model to consider the formation reaction as well as elimination of AA during heat treatment with the temperature range from 115 °C to 173 °C. While through vacuum frying of potato chips, the influence of time on AA formation was also described by first order kinetics, because it increased exponentially with time at all temperatures (Granda and Moreira 2005). The activation energy for AA formation in reconstituted potato chips was calculated as 139.1 kJ mol−1, using the Arrhenius equation. Lukac et al. (2007) calculated that the activation energy for the AA formation during the roasting of almonds was 123 kJ mol−1, while Pedreschi et al. (2007) reported that the activation energy of AA formation was around 176.6 kJ mol−1 when the potato slices were frying with a ratio of 3.0 (g water/g dry solid).
Fig. 1.
Water activity, acryalmide (AA) and 5-hydroxymethylfurfural (HMF) contents during frying of reconstituted potato chips at different temperatures (n = 3), (a) Acrylamide (AA) (mg kg−1) contents during frying of reconstituted potato chips at different temperature (n = 3); (b) Water activity contents during frying of reconstituted potato chips at different temperature (n = 3); (c) 5-hydroxymethylfurfural (HMF) (mg kg−1) contents during frying of reconstituted potato chips at different temperature (n = 3)
Table 1.
Kinetic parameters for the formation of acryalmide (AA) and 5-hydroxymethylfurfural (HMF) in reconstituted potato chips as function of different temperatures
| Compounds | Rate constant | R 2 | |
|---|---|---|---|
| Acrylamide (AA) | k 170 o C (min−1) | 0.079a | 0.983 |
| k 180 o C (min−1) | 0.214b | 0.812 | |
| k 190 o C (min−1) | 0.405c | 0.991 | |
| Ea (KJ mol−1) | 139.1a | ||
| 5-hydroxymethylfurfural (HMF) | k 170 o C (min−1) | 7.860a | 0.868 |
| k 180 o C (min−1) | 11.598a | 1 | |
| k 190 o C (min−1) | 20.015b | 0.992 | |
| Ea (KJ mol−1) | 81.0b | ||
For each line of AA and HMF, the k and Ea values with different letters are significantly different (P < 0.05). AA, acrylamide; HMF, 5-hydroxymethylfurfural
Water activity of reconstituted potato chips during frying heating and its effect on AA formation
Water activity during frying of reconstituted potato chips was reported in Fig. 1b, following a decreased trend. When the reconstituted potato chips were fried at 170 °C for 4 min, water activity dropped from 0.728 ± 0.012 to 0.360 ± 0.011. The decreasing rate was 50.5 min−1. While fried at 180 °C for 3 min and 190 °C for 2 min, the decreasing rate of water activity was 52.7 and 53.0 min−1, respectively. Water activity decreased very rapidly till to a residual water activity which was about 0.340–0.360. As expected, the rate of water loss in the evaporative process depended on frying temperature. The higher the temperature, the faster the rate is.
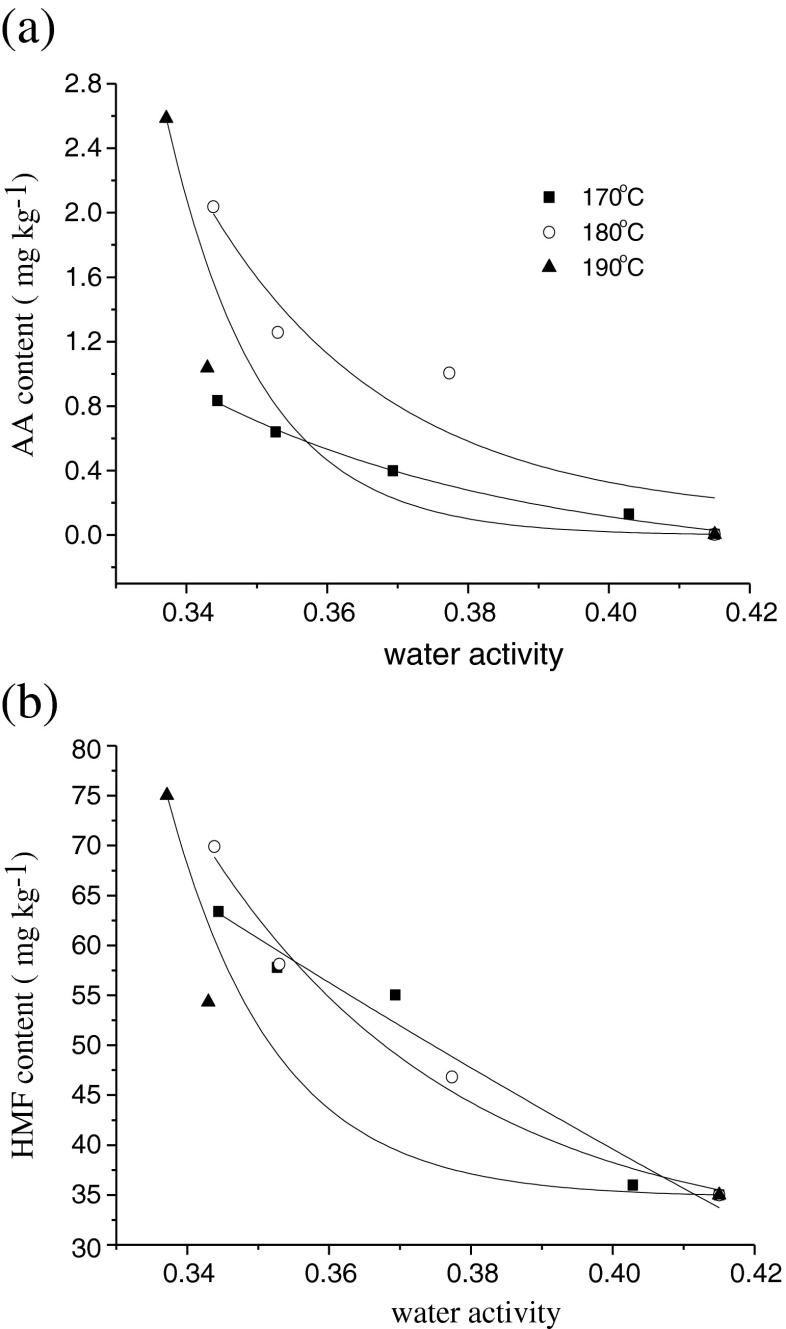
Water plays a complex role in AA formation and elimination. Water activity reflects the degree of moisture-binding in the food. Only free moisture could participate in the Maillard reaction. Theoretically, water activity should play an important role in the formation of AA by influencing the Maillard reaction. Many studies confirmed that water activity is a key factor to consider in the Maillard reaction (Fabien et al. 2004; Gertz et al. 2004; Taubert et al. 2004). Optimal rates of Maillard browning at intermediate water activity are reported (Biedermann et al. 2002; Jung et al. 2003; Levine and Sean 2009). At lower water activity levels, the molecular mobility or solubility is hindered. At higher levels, the reaction rates decrease because of a dilution effect of the reactants. Because water is produced during the Maillard reaction, the law of mass action plays an inhibiting role, as well at high water activity (Mestdagh et al. 2006). In our present study, the AA formation was highly correlated with the water activity in reconstituted potato chips, especially when the reconstituted potato chips were fried at 170 °C, the results could be seen in Fig. 2a. With the decrease of water activity, the formation of AA increased accordingly. At each temperature, the relationship between AA formation and water activity also followed a first order kinetic. The rate constants were calculated and kinetic data were summarized in Table 2. According to the data, the experimental results agreed to the theory. Amrein et al. (2006) found that moisture content has a strong influence on the activation energy of AA formation. With the decreasing of moisture content, there is a corresponding increase of the activation energy for AA formation and most AA is formed on the last phase of the frying process. Pedreschi et al. (2007) also reported the Arrhenius activation energy increases alongside with decreasing chip moisture content. De Vleeschouwer et al. (2007) calculated that the rate constant for AA formation varied only slightly with the initial water activity of the model system, the elimination rate constant showed a clear minimum around a water activity of 0.82.
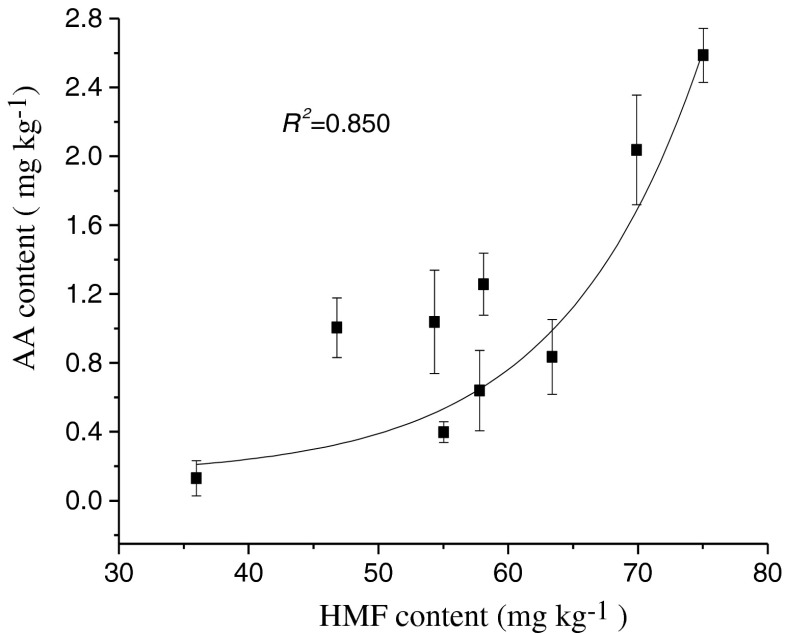
Fig. 2.
Acryalmide (AA) and 5-hydroxymethylfurfural (HMF) contents during frying of reconstituted potato chips at different temperatures as function of water activity (n = 3), (a) Acryalmide (AA) (mg kg−1) contents during frying of reconstituted potato chips at different temperatures as function of water activity (n = 3); (b) 5-hydroxymethylfurfural (HMF) (mg kg−1) contents during frying of reconstituted potato chips at different temperatures as function of water activity (n = 3)
Table 2.
Kinetic parameters for the formation of acryalmide (AA) and 5-hydroxymethylfurfural (HMF) in reconstituted potato chips as function of water activity
| Compounds | Rate constant | R 2 | |
|---|---|---|---|
| Acrylamide (AA) | k 170 o C (min−1) | 21.626a | 0.991 |
| k 180 o C (min−1) | 37.994b | 0.889 | |
| k 190 o C (min−1) | 7.496c | 0.682 | |
| 5-hydroxymethylfurfural (HMF) | k 170 o C (min−1) | 2.314a | 0.946 |
| k 180 o C (min−1) | 27.949b | 0.967 | |
| k 190 o C (min−1) | 66.357c | 0.895 | |
For each line of AA and HMF, the k values with different letters are significantly different (P < 0.05). AA, acrylamide; HMF, 5-hydroxymethylfurfural
HMF contents in reconstituted potato chips during frying heating
HMF is another harmful compound formed in the Maillard reaction. The formation of HMF was also studied in our study (Fig. 1c). HMF content was highly affected by frying times and temperatures, it increased while increasing the frying time at each temperature as well as increasing temperature. The highest amount of HMF was found when the reconstituted potato chips were treated with frying at 190 °C for 2 min, and the HMF content was 75.03 mg kg−1. When the reconstituted potato chips were fried at 170 °C and 180 °C, HMF content were lower, reaching 63.39 mg kg−1 after 4 min at 170 °C and 69.89 mg kg−1 after 3 min at 180 °C. The higher temperature could promote the formation and elimination of HMF in the frying reconstituted potato chips. At each temperature, HMF formation followed a zero order kinetic (Fig. 1c), and the rate constant and activation energy were calculated and kinetic data were summarized in Table 1. The kinetic rate constant values increased when increasing heat treatment temperatures. The activation energy of HMF formation was calculated by Arrhenius equation was 81.0 kJ mol−1. Capuano et al. (2008) calculated the activation energy as 13.3 kJ mol−1 in bread crisps system while Ait-Ameur et al. (2006) reported that the activation energy of HMF formation in cookies system was 10.63 kJ mol−1. In our study, the activation energy of HMF formation was much higher than the other food matrixes, the difference may due to many factors, such as the moisture content or water activity, the composition and structure of food systems. Bates et al. (1998) reported that as the water content of food is decreased, the activation energy increases. In our study, we found that HMF formation is highly correlated with water activity (Fig. 2b). With the decrease of water activity, HMF formation increased accordingly. At each temperature, HMF formation and water activity followed a first order kinetic, and the rate constant was showed in Table 2. When water activity was lower than 0.38, the formation of HMF was increased significantly. Gökmen et al. (2008) found in cookies system that decreasing moisture caused increasing of HMF formation in cookies, but 0.40 appeared to be the highest critical point to the water activity. Ait-Ameur et al. (2006) pointed out that the water activity was a fundamental parameter in HMF production. Since the formation of one mole of HMF from one mole of hexose needs the release of three moles of water, the presence of too much water in the early stages of the baking process might have inhibited the reaction (Gökmen et al. 2007).
Analysis of relationship between HMF and AA in reconstituted potato chips
In our present study, we also studied the relationship between HMF and AA formation in reconstituted potato chips under different conditions, and the relationship was shown in Fig. 3. A non-linear exponential growth curve prepared by plotting AA levels vs HMF content formed under different heating condition was observed: 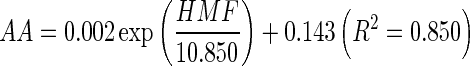 . HMF was a furanic compound which formed as an intermediate in the Maillard Reaction. AA formation and HMF content were highly correlated in our study. With the increase of heating time, the Maillard reaction was accelerated, and the formation of AA and HMF was increased with heating time. The degraded reactive carbonyls products of the sugars from the Maillard reaction, HMF, as one of the main hexose dehydration products, has a lower melting point that makes it thermodynamically more favorable to form amine condensation products during heating (Gökmen et al. 2012), thus stimulate the formation of AA. Morales and Arribas-Lorenzo (2008) reported that for AA and HMF, an induction period was observed after which the formation of HMF and AA followed the same upward trend reaching values of about 90 mg kg−1 and 60 μg kg−1 for HMF and AA, respectively in churros systems. In the study of Capuano et al. (2009), high correlation was found between AA and HMF ranging from R2 = 0.90 for rye formulation at 180 °C to R2 = 0.98 for whole-wheat formulation at 160 °C.
. HMF was a furanic compound which formed as an intermediate in the Maillard Reaction. AA formation and HMF content were highly correlated in our study. With the increase of heating time, the Maillard reaction was accelerated, and the formation of AA and HMF was increased with heating time. The degraded reactive carbonyls products of the sugars from the Maillard reaction, HMF, as one of the main hexose dehydration products, has a lower melting point that makes it thermodynamically more favorable to form amine condensation products during heating (Gökmen et al. 2012), thus stimulate the formation of AA. Morales and Arribas-Lorenzo (2008) reported that for AA and HMF, an induction period was observed after which the formation of HMF and AA followed the same upward trend reaching values of about 90 mg kg−1 and 60 μg kg−1 for HMF and AA, respectively in churros systems. In the study of Capuano et al. (2009), high correlation was found between AA and HMF ranging from R2 = 0.90 for rye formulation at 180 °C to R2 = 0.98 for whole-wheat formulation at 160 °C.
Fig. 3.
The relationship between 5-hydroxymethylfurfural (HMF) content and acryalmide (AA) concentration in reconstituted potato chips at different temperatures. Each observation is a mean ± SD of three replicate experiments
Conclusion
In conclusion, the effects of water activity and frying conditions on the formation of AA and HMF were studied in reconstituted potato chips system. In this respect, the results suggest that the formation of AA and HMF were highly correlated with water activity, and the formation of HMF has significant correlation with the formation of AA in reconstituted potato chips. A typical exponential growth curve was observed by plotting AA levels vs HMF content which were all determined under different heating condition: 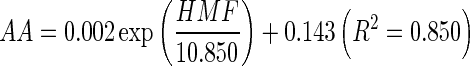 .
.
Acknowledgements
This work was supported by the Fund of “National Natural Science Fundation (31000750)”, the National Basic Research Program of China (“973” Program, 2012CB720805), China Postdoctoral Science Foundation Special Funded Project (201104527), and Fund for Distinguished Young Scholars of Heping Campus of Jilin University (4305050102Q9). Accordingly, the authors gratefully acknowledge the fund supports.
Abbreviations
- AA
Acrylamide
- HMF
5-hydroxymethylfurfural
- SD
Standard deviation
References
- Ait-Ameur L, Trystram G, Birlouez-Aragon I. Accumulation of 5-hydroxymethyl-2-furfural in cookies during the backing process: validation of an extraction method. Food Chem. 2006;98:790–796. doi: 10.1016/j.foodchem.2005.07.038. [DOI] [Google Scholar]
- Ait-Ameur L, Mathieu O, Lalanne V, Trystram G, Birlouez-Aragon I. Comparison of the effects of sucrose and hexose on furfural formation and browning in cookies baked at different temperatures. Food Chem. 2007;101:1407–1416. doi: 10.1016/j.foodchem.2006.03.049. [DOI] [Google Scholar]
- Amrein TM, Andres L, Manzardo GGG, Amadò R. Investigations on the promoting effect of ammonium hydrogencarbonate on the formation of acrylamide in model systems. J Agric Food Chem. 2006;54:10253–10261. doi: 10.1021/jf0625860. [DOI] [PubMed] [Google Scholar]
- Bassama J, Brat P, Bohuon P, Hocine B, Boulanger R, Günata Z. Acrylamide kinetic in plantain during heating process: precursors and the effect of water activity. Food Res Int. 2011;44:1452–1458. doi: 10.1016/j.foodres.2011.03.018. [DOI] [Google Scholar]
- Bates L, Ames JM, MacDougall DB, Taylor PC. Laboratory reaction cell to model Maillard color development in a starch-glucose-lysine system. J Food Sci. 1998;63:991–996. doi: 10.1111/j.1365-2621.1998.tb15840.x. [DOI] [Google Scholar]
- Biedermann M, Noti A, Biedermann-Brem S, Mozetti V, Grob K. Experiments on acrylamide formation and possibilities to decrease the potential of acrylamide formation in potatoes. Mitt Lebensm Hyg. 2002;93:668–687. [Google Scholar]
- Capuano E, Fogliano V. Acrylamide and 5-hydroxymethylfurfural (HMF): a review on metabolism, toxicity, occurrence in food and mitigation strategies. LWT. 2011;44:793–810. doi: 10.1016/j.lwt.2010.11.002. [DOI] [Google Scholar]
- Capuano E, Ferrigno A, Acapa I, Ait-Mueur L, Fogliano V. Characterization of the Maillard reaction in bread crisps. Eur Food Res Tech. 2008;228:311–319. doi: 10.1007/s00217-008-0936-5. [DOI] [Google Scholar]
- Capuano E, Ferrigno A, Acampa I, Serpen A, Açar ÖÇ, Gökmen V, Fogliano V. Effect of flour type on Maillard reaction and acrylamide formation during toasting of bread crisp model systems and mitigation strategies. Food Res Int. 2009;42:1295–1302. doi: 10.1016/j.foodres.2009.03.018. [DOI] [Google Scholar]
- De Vleeschouwer K, Van der Plancken I, Van Loey A, Hendrickx ME. Kinetics of acrylamide formation/elimination reactions as affected by water activity. Biotechnol Prog. 2007;23:722–728. doi: 10.1021/bp060389f. [DOI] [PubMed] [Google Scholar]
- Fabien R, Gilles V, Philippe P, Francoise S, Maria-Isabelle A, Isabells B, Imre B. Acrylamide formation from asparagine under low-moisture maillard reaction conditions. 1. Physical and chemical aspects in crvstalline model systems. J Agric Food Chem. 2004;52:6837–6842. doi: 10.1021/jf0492464. [DOI] [PubMed] [Google Scholar]
- Franke K, Strijowski U, Reimerder EH. Kinetics of acrylamide formation in potato powder. J Food Eng. 2009;90:135–140. doi: 10.1016/j.jfoodeng.2008.06.015. [DOI] [Google Scholar]
- Gertz C, Klostermann S, Kochhar SP. Deep frying: the role of water from food being fried and acrylamide formation. Oleagineux Corps Gras Lipides. 2004;10:297–303. doi: 10.1051/ocl.2003.0297. [DOI] [Google Scholar]
- Gökmen V, Açar ÖÇ, Köksel H, Acar J. Effects of dough formula and baking conditions on acrylamide and hydroxymethylfurfural formatin in cookies. Food Chem. 2007;104:1136–1142. doi: 10.1016/j.foodchem.2007.01.008. [DOI] [Google Scholar]
- Gökmen V, Açar ÖÇ, Serpen A, Morales FJ. Effect of leavening agents and sugars on the formation of hydroxymethylfurfural in cookies during baking. Eur Food Res Tech. 2008;220:1031–1037. doi: 10.1007/s00217-007-0628-6. [DOI] [Google Scholar]
- Gökmen V, Kocadağli T, Göncüoğlu N, Ataç Mogol B. Model studies on the role of 5-hydroxymethyl-2-furfural in acrylamide formation from asparagine. Food Chem. 2012;132:168–174. doi: 10.1016/j.foodchem.2011.10.048. [DOI] [PubMed] [Google Scholar]
- Granda C, Moreira RG. Kinetics of acrylamide formation during traditional and vacuum frying of potato chips. J Food Process Eng. 2005;28:478–493. doi: 10.1111/j.1745-4530.2005.034.x. [DOI] [Google Scholar]
- Granvogl M, Schieberle P. Thermally generated 3-aminopropionamide as a transient intermediate in the formation of acrylamide. J Agric Food Chem. 2006;54:5933–5938. doi: 10.1021/jf061150h. [DOI] [PubMed] [Google Scholar]
- Granvogl M, Jezussek M, Koehler P, Schieberle P. Quantitation of 3-aminopropionamide in potatoes—a minor butpotent precursor in acrylamide formation. J Agric Food Chem. 2004;52:4751–4757. doi: 10.1021/jf049581s. [DOI] [PubMed] [Google Scholar]
- IARC (1994) IARC Monographs on the evaluation of carcinogenic risks to humans, WHO, Some industrial chemicals, acrylamide, 60:389–433 [PMC free article] [PubMed]
- Jha A, Kumar A, Jain P, Om H, Singh R, Bunkar DS (2012) Physico-chemical and sensory changes during the storage of lal peda. J Food Sci Tech. doi:10.1007/s13197-012-0613-3 [DOI] [PMC free article] [PubMed]
- Jung MY, Choi DS, Ju JW. A novel technique for limitation of acrylamide formation in fried and baked corn chips and in French fries. J Food Sci. 2003;68:1287–1290. doi: 10.1111/j.1365-2621.2003.tb09641.x. [DOI] [Google Scholar]
- Levine RA, Sean MR. Determining the effect of calcium cations on acrylamide formation in cooked wheat products using a model system. J Agric Food Chem. 2009;57:6823–6829. doi: 10.1021/jf901120m. [DOI] [PubMed] [Google Scholar]
- Liu J, Zhao GH, Yuan Y, Chen F, Hu XS. Quantitative analysis of acrylamide in tea by liquid chromatography coupled with electrospray ionization tandem mass spectrometry. Food Chem. 2008;108:760–767. doi: 10.1016/j.foodchem.2007.11.015. [DOI] [PubMed] [Google Scholar]
- Lukac H, Amrein TM, Perren R, Coude-Petit B, Amadò R, Escher F. Influence of roasting conditions on the acrylamide content and the color of roasted almonds. J Food Sci. 2007;72:C033–C038. doi: 10.1111/j.1750-3841.2006.00206.x. [DOI] [PubMed] [Google Scholar]
- Martins SIFS, Jongen WMF, van Boekel MAJS. A review of Maillard reaction in food and implications to kinetic modeling. Trends Food Sci Tech. 2001;11:364–373. doi: 10.1016/S0924-2244(01)00022-X. [DOI] [Google Scholar]
- Mestdagh F, De Meulenaer B, Cucu T, Van Peteghem C. Role of water upon the formation of acrylamide in a potato model system. J Agric Food Chem. 2006;54:9092–9098. doi: 10.1021/jf061652v. [DOI] [PubMed] [Google Scholar]
- Morales FJ, Arribas-Lorenzo G. The formation of potentially harmful reconstituteds in churros, a Spanish fried-dough pastry, as influenced by deep frying conditions. Food Chem. 2008;109:421–425. doi: 10.1016/j.foodchem.2007.12.042. [DOI] [PubMed] [Google Scholar]
- Mottram DS, Wedzicha BI, Dodson AT. Acrylamide is formed in the Maillard reaction. Nature. 2002;419:448–449. doi: 10.1038/419448a. [DOI] [PubMed] [Google Scholar]
- Pedreschi F, Bustos O, Mery D, Moyano P, Kaack K, Granby K. Color kinetics and acrylamide formation in NaCl soaked potato chips. J Food Eng. 2007;79:989–997. doi: 10.1016/j.jfoodeng.2006.03.020. [DOI] [Google Scholar]
- Severin I, Dumont C, Jondeau-Cabaton A, Graillot V, Chagnon MC. Genotoxic activities of the food contaminant 5-hydroxymethylfurfural using different in vitro bioassays. Toxicol Lett. 2010;192:189–194. doi: 10.1016/j.toxlet.2009.10.022. [DOI] [PubMed] [Google Scholar]
- Stadler RH, Blank I, Varga N, Verzegnassi L, Grigorov M, Studer A, Riediker S, Schilter B. Acrylamide from Maillard reaction products. Nature. 2002;419:445–450. doi: 10.1038/419449a. [DOI] [PubMed] [Google Scholar]
- Taubert D, Harlfinger S, Henkes L, Berkels R, Schömig E. Influence of processing parameters on acrylamide formation during frying of potatoes. J Agric Food Chem. 2004;52:2735–2739. doi: 10.1021/jf035417d. [DOI] [PubMed] [Google Scholar]
- Yaylayan VA, Wnorowski A, Locas PC. Why asparagine needs carbohydrate to generate acrylamide. J Agric Food Chem. 2003;51:1753–1757. doi: 10.1021/jf0261506. [DOI] [PubMed] [Google Scholar]
- Ye HQ, Miao YT, Zhao CC, Yuan Y. Acrylamide and methylglyoxal formation in potato chips by microwaving and frying heating. Int Food Sci Tech. 2011;46:1921–1926. doi: 10.1111/j.1365-2621.2011.02702.x. [DOI] [Google Scholar]
- Yuan Y, Zhao GH, Chen F, Liu J, Wu JH, Hu XS. Correlation of methylglyoxal with acrylamide formation in fructose/asparagine Maillard reaction model system. Food Chem. 2008;108:885–890. doi: 10.1016/j.foodchem.2007.11.054. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Zhao GH, Hu XS, Wu JH, Liu J, Chen F. High correlation of methylglyoxal on acrylamide formation in glucose/ asparagine Maillard reaction model. Eur Food Res Technol. 2008;226:1301–1307. doi: 10.1007/s00217-007-0658-0. [DOI] [PubMed] [Google Scholar]
- Zyzak DV, Sanders RA, Stojanovic M, Tallmadge DH, Eberhart BL, Ewald DK, Gruber DC, Morsch TR, Strothers MA, Rizzi GP, Villagran MD. Acrylamide formation mechanism in heated foods. J Agric Food Chem. 2003;51:4782–4787. doi: 10.1021/jf034180i. [DOI] [PubMed] [Google Scholar]