Abstract
Pomegranate peels of Ganesh variety were subjected to extraction using different solvents viz. water, methanol and ethanol either alone or in combination with water. The extraction yield, antioxidant activity (DPPH and ABTS inhibition) and total phenolic contents were evaluated. Highest yield was obtained from 50 % ethanol: 50 % water (16.3 ± 1.99 %). The DPPH and ABTS inhibition activity was found to be the highest for methanol and 70 % ethanol: 30 % water extract (79.5 ± 6.5; 94.6 ± 6.10), respectively. The phenolic content was the highest in the aqueous extract (438.3 ± 14.15). The antibacterial activity of peel extracts was tested against four bacterial strains, Staphylococcus aureus, Enterobacter aerogenes, Salmonella typhi and Klebsiella pneumoniae and the extracts demonstrated remarkable antibacterial activities against all the tested bacterial strains. The 70 % ethanol: 30 % water and 100 % water extract had a higher antioxidant activity and phenolic content and has the potential for nutraceutical application.
Keywords: Antibacterial activity, Antioxidants, Extraction, Phenols, Pomegranate Peel
Introduction
Pomegranate (Punica granatum L.) is native to the Mediterranean region and has been used extensively in the folk medicine of Indian subcontinent and many other countries. The world pomegranate production amounts to approximately 1,500,000 tons (FAO 2012). The pomegranate peel is considered as an agro-waste but it can be a potential source of antioxidants, phenols, flavanoids and also possesses antibacterial and antifungal activity. The peels (pericarp, rind or hull) amounts to approximately 60 % of the weight of the pomegranate fruit (Lansky and Newman 2007). The peel of pomegranate possesses higher total phenolic content and antioxidant activity than the pulp (Li et al. 2006). Elfalleh et al. (2012) reported that pomegranate peel contains higher antioxidant activity when compared with flower, leaf and seed.
The pomegranate peel is reported to have several health benefits due to the presence of various tannins, flavonoids, alkaloids and organic acids. Gallagyldilacton, gallic acid, granatin B showed anti-inflammatory activity (Tanaka et al. 1990; Satomi et al. 1993; Amakura et al. 2000). Likewise, Tannins possessing antioxidant activities are punicalin, punicalagin, pedunculagin, gallic acid and casuarinin (Tanaka et al. 1986; Satomi et al. 1993; Gil et al. 2000). Apart from that, various flavonoids like catechin, epicatechin, epigallocatechin-3-gallate, flavan-3-ol, kaempferol, kaempferolerol-3-O-glucoside, kaempferol-3-O-rhamnoglycoside, luteolin, luteolin 7-O-glucoside, Naringin, pelargonidin, prodelphindin, quercetin and rutin have been also found in peel extracts of pomegranate which shows antibacterial, antiviral, antioxidant, anti-inflammatory and anti-neoplastic bioactivities (Nawwar et al. 1994; Artik 1998; Plumb et al. 2003; Lansky and Newman 2007).
In addition to antioxidant activity, the peel extract also has antimutagenic properties as well as beneficial effects on the cardiovascular diseases (Cook and Samman 1996). During the past decade, considerable efforts have been made to extract and identify pomegranate bioactive compounds (Gil et al. 2000; Kulkarni and Aradhya 2005; Mousavinejad et al. 2009; Qu et al. 2010; Zhang et al. 2011). Pomegranate peel extracts exhibited marked antioxidant capacity in several studies using unsafe solvents such as methanol and a mixture of methanol, acetone, ethyl acetate and water (Ghasemian et al. 2006).
The present study was conducted with the objective to investigate the most effective solvent either alone or in combination for extracting the potent antioxidant, phenolics and antibacterial compounds from pomegranate peel.
Materials and methods
Materials
Pomegranates of Ganesh variety were procured from the local market of Varanasi, Uttar Pradesh, India. All the chemicals (viz, Folin-Ciocalteu reagent, ethanol and methanol) were of analytical grade and were procured from Merck, Mumbai, India. Tannic acid standard, 2, 2-diphenyl-1-picrylhydrazine (DPPH) and 2, 2-azinobis-3-ethyl-benzothiazoline-6-sulfonic acid (ABTS) were procured from Sigma Chemical Company (St. Louis, MO, USA).
Preparation of samples
Pomegranate peels were separated and washed with tap water and subjected to drying in vacuum oven (Sonar, Model No. VORP 5030, Associated Scientific Technologies, New Delhi, India) at 50 °C upto dryness under a vacuum of 700 mm Hg. The dried peels were ground with pestle and mortar to coarse powder of approximately 1 mm size and stored in a incubator at 4 °C. To prepare samples, 20 g of ground pomegranate peel were separately soaked in 100 ml solvents. The extract was prepared in 6 types of solvents i.e. ethanol, methanol, water, 30 % ethanol: 70 % water, 50 % ethanol: 50 % water and 70 % ethanol: 30 % water. The samples were incubated at 37 °C for 24 h in a shaking incubator (Labtech, Model No. LSI-3016R, Daihan Labtech India Pvt. Ltd., Hyderabad, India) with 200 rpm. After this, the samples were filtered with Whatman no. 1 filter paper and filtrate was stored in the incubator at 4 °C. This extraction procedure was repeated three times to extract maximum components from pomegranate peel. The pooled extract was used for the analysis of phenolics, antioxidant and antibacterial activities.
Yield of extract
All the extracts were dried in a hot air oven (Perfit India, Model No. 992/10, Ambala, India) at 105 °C for 12 h. The yields of the extracts were based on dry weight and reported in percent yield.
DPPH radical scavenging activity
Antiradical activity against DPPH was determined by following the method of Nishino et al. (2000) with slight modifications in preparation of samples and reaction volume. Stock solutions (1 mg/ml) of the peel extracts were prepared in ethanol. DPPH (80 μg/ml) solution was prepared in absolute ethanol and 2.0 ml of this solution was added to 1 ml of extract solution. A positive blank was prepared in the same manner except that respective solvent was added instead of extract. After 30 min of incubation at 37 °C, absorbance of the solution was recorded by using ultraviolet (UV)-1800 spectrophotometer (Shimadzu, Kyoto, Japan) at 517 nm against ethanol. The inhibition of the DPPH radical by the sample was calculated according to the following formula:
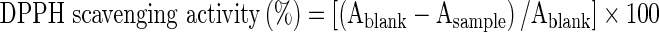 |
Where, Ablank is the absorbance value of the control reaction and Asample is the absorbance value of the extract.
ABTS radical scavenging assay
The ABTS radical scavenging assay was done according to the method of Re et al. (1999) with slight modifications. The ABTS radicals were generated by the oxidation of ABTS with ammonium persulphate. The ABTS radical cation solution was obtained as follows: Five ml of ABTS (7 mM) was mixed with 88 μl of ammonium persulphate (140 mM) and then incubated in the dark at 25 °C for 12–16 h. The working solution was prepared by diluting the previous solution with phosphate buffered saline (pH 7.2 PBS) until the absorbance at 734 nm was 0.70 ± 0.02. Then, 100 μl of each sample was mixed with 3 ml of the ABTS working solution and the change in absorbance was observed at 734 nm for 10 min at 25 °C. The ABTS radical scavenging capacity of the sample was calculated by the following formula:
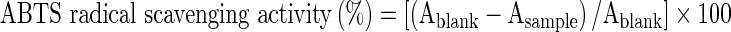 |
where, Ablank is the absorbance value of the control reaction (containing all reagents except the extract) and Asample is the absorbance value of the extract.
Assay for total phenols
Total phenolic constituents of extracts were determined by methods involving Folin-Ciocalteu reagent and tannic acid standard according to the European Pharmacopoeia (Druckerei 2002). Solutions of each extract (50 μl; 1 mg/ml) were taken individually in test tubes and final volume was made upto 0.5 ml with distilled water. To this solution, 2.5 ml of 10-fold diluted Folin-Ciocalteu reagent was added, and the flask was thoroughly shaken. After 1 min, 2.0 ml of 7.5 % Na2CO3 solution was added and the mixtures were allowed to stand for 30 min at 25 °C with intermittent shaking. Absorbance was taken at 760 nm. The same procedure was repeated for all the standard tannic acid solutions, and a standard curve was obtained (5–50 mg). Total phenols of the extract, as tannic acid equivalent, were determined by using the absorbance of the extract measured at 760 nm. All the tests were carried out in triplicate and phenolic contents as tannic acid equivalents were reported.
Assessment of antibacterial activity
In this study, four bacterial strains and agar well diffusion method were used to determine the antibacterial activity of the pomegranate peel water extracts. The agar cultures of Staphylococcus aureus, Enterobacter aerogenes, Salmonella typhi and Klebsiella pneumoniae was prepared to assess the pomegranate peel extracts inhibitory effects. Fifty ml of Muller Hinton agar were measured into an Erlenmeyer flask; four flasks were prepared for each sample and examined. The flasks including agar medium were sterilized in an autoclave at 121 °C for 15 min. For antibacterial tests, bacterial cultures were grown at 35 °C for 22 h by inoculation in Muller Hinton agar. Petri dishes with 10 ml of Muller Hinton agar were prepared, previously inoculated with 100 μl of the culture suspension (1 %, containing 106–107 cfu/ml). The wells (5.0 mm in diameter) were cut from the agar under sterile conditions and 1.0 g of lyophilised extract was dissolved in 6 ml of deionised water (1:6 w/v) and 10 μl added into the wells of agar plates directly and same volume of deionised water was used as control. The inoculated plates were incubated for 24 h at 35 °C. At the end of the incubation period, inhibition zones formed on the medium and the diameter of the inhibition zone was measured and recorded as mean diameter (mm). The measurements were done basically from the edge of the zone to the edge of the well.
Statistical analysis
All the data were expressed as mean ± standard error of mean and was calculated from three independent experiments. One-way analysis of variance (ANOVA) was applied by using the Systat software to measure the test for significance as described by Snedecor and Cochran (1989).
Results and discussion
Ganesh variety of pomegranate is a famous variety of India. It is characterized with yellowish red rind. Pomegranates of Ganesh variety have soft seeds with pink-red colour arils. In the present study, the antioxidants were extracted from pomegranate peel by using different solvents and their antioxidant and antibacterial potential were investigated.
Extraction yield
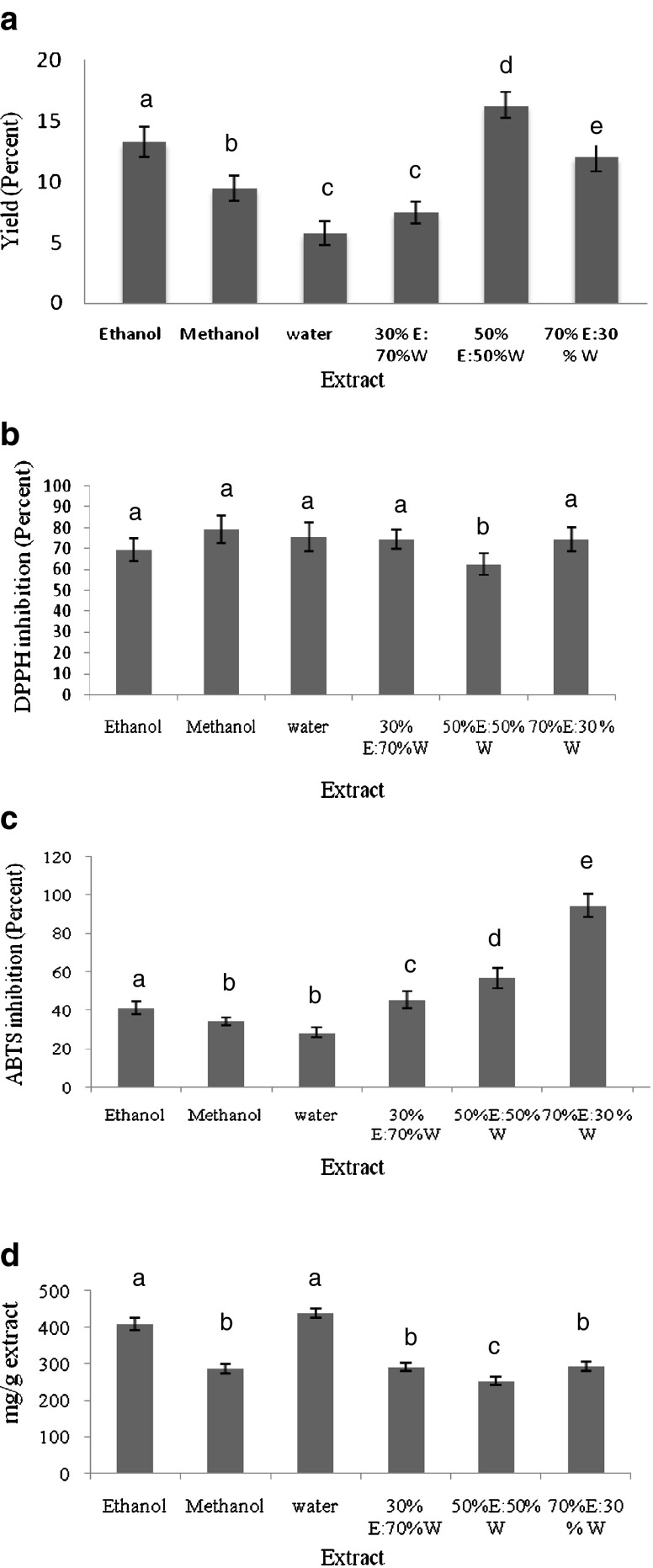
Selection of solvent is an important step for obtaining extracts with acceptable yields and strong antioxidant activity. The yields of extract from different solvents were obtained in the order 50 % ethanol: 50 % water > 30 % ethanol: 70 % water > methanol > ethanol > 70 % ethanol: 30 % water > water. The highest (16.28 %) and the lowest yields (5.74 %) were obtained from 50 % ethanol: 50 % water and water, respectively (Fig. 1a).
Fig. 1.
Yield (a); 2, 2-diphenyl-1-picrylhydrazine (DPPH) inhibition (b); 2, 2-azinobis-3-ethylbenzothiazoline- 6-sulfonic acid (ABTS) inhibition (c) and phenol content (d) (mg/g) in pomegranate peel extract in different solvents; n = 3. Bars with different letters are significantly different (p < 0.05). 30E/70 W- 30 % Ethanol: 70 % water; 50E/50 W- 50 % Ethanol: 50 % water; 70E/30 W- 70 % Ethanol: 30 % water
DPPH radical scavenging activity of various extracts
DPPH is a purple-coloured stable free radical with an absorption band at 517 nm. It is reduced to 2, 2-diphenyl-1-picrylhydrazine (yellow coloured) by accepting an electron or hydrogen radical from an antioxidant (Soares et al. 1997). This radical is sensitive to detect low concentrations of antioxidant compounds present in different extracts (Hseu et al. 2008). In the present study, pomegranate peel extracts showed a significant variability in their inhibitory activity against DPPH radical. Amongst the tested solvents, the highest radical scavenging activity was detected in the following order: methanol > water > 30 % ethanol: 70 % water > 70 % ethanol: 30 % water > ethanol > 50 % ethanol: 50 % water (Fig. 1b). It was found that 50 % ethanol: 50 % water extract showed significantly lower (p < 0.05) antioxidant activity as compared to the other solvents. All the solvents showed almost similar antioxidant potential of pomegranate extract. Water as an environmental friendly solvent has been found to be very effective for antioxidant extraction from pomegranate marc in previous studies (Singh et al. 2002; Qu et al. 2009). Methanol extract of the pomegranate peel showed the highest antioxidant activity among all the extracts and it was selected for testing of its effect on lipid peroxidation, hydroxyl radical scavenging activity, and human low-density lipoprotein (LDL) oxidation (Jayaprakasha and Rao 2000). In one of the other studies reported, the methanol extract of the peels showed 83 and 81 % antioxidant activity at 50 ppm using the β-carotene-linoleate and DPPH model systems, respectively (Chidambara et al. 2002).
ABTS radical scavenging activity of various extracts
The ABTS radical cation decolourization test is another technique usually used to investigate the antioxidant activity. Decrease in colour indicates reduction of ABTS radical (Adedapo et al. 2008). As shown in Fig. 1c, all the extracts reduced the absorbance at 734 nm, and the concentration of the extracts was directly proportional to the reduction in absorbance. The 70 % ethanol: 30 % water extract showed the highest ABTS radical scavenging activity. The pomegranate peel extracts showed the ABTS radical scavenging activities in the order of 70 % water: 30 % ethanol extract > 50 % water: 50 % ethanol > 70 % ethanol:30 % water > ethanol > methanol > water. The results are in agreement with Li et al. (2006) in which the peel extracted with mixture of solvents exhibited higher antioxidant activity than the individual solvent extraction. Elfalleh et al. (2012) reported that methanol is a better antioxidant extraction solvent as compared to water.
Total phenolic content
Several studies have revealed that phenolic contents in plants are associated with their antioxidant activities, probably due to their redox properties, which allows them to act as reducing agents, hydrogen donors, and singlet oxygen quenchers (Chang et al. 2011). The phenolic content of various extracts is shown in Fig. 1d. The total phenolic contents varied from 297.5 to 435 mg tannic acid equivalents/g pomegranate peel extract. The highest value of total phenolic compounds was detected in the water extracts whereas the lowest content was obtained in the 30 % water: 70 % ethanol extracts. The phenolic content of water and ethanol extract was not significantly different but significantly higher (p < 0.05) than other solvents. These findings demonstrate the influence of the solvents on the extractability of phenolics. Findings of this study suggested that the nature of the solvent exerts a great influence on phenolic extraction capacity of the plant (Akowuah et al. 2005; Turkmen et al. 2006).
Antibacterial activity of various extracts
The tannin - rich ellagitannins and phenolic acids of Punica granatum have antibacterial activity (Prashanth et al. 2001; Supayang et al. 2005). In the current study (Table 1), the aqueous, methanolic, ethanolic, 30 % ethanol:70 % water, 50 % ethanol:50 % water and 70 % ethanol:30 % water showed zone of inhibition of 22.6 ± 0.31 mm, 24.5 ± 0.53 mm, 20.0 ± 0.31 mm, 23.3 ± 0.43 mm, 22.9 ± 0.24 mm and 22.9 ± 0.44 mm, respectively against Staphylococcus aureus. For Enterobacter aerogenes, the zones of inhibition for aqueous, methanolic, ethanolic, 30 % ethanol:70 % water, 50 % ethanol:50 % water and 70 % ethanol:30 % water were 18.4 ± 0.53 mm, 18.2 ± 0.41 mm, 17.3 ± 0.44 mm, 17.9 ± 0.45 mm, 21.9 ± 0.41 mm and 19.3 ± 0.71 mm, respectively.
Table 1.
Zone of inhibition exhibited by pomegranate peel extract against pathogenic strains
| Bacterial Sps. | Zone of inhibition in millimeters | |||||
|---|---|---|---|---|---|---|
| Methanol | Ethanol | Water | 30E/70 W | 50E/50 W | 70E/30 W | |
| Staphylococcus aureus | 24.5 ± 0.53a | 20.3 ± 0.31a | 22.6 ± 0.31a | 23.3 ± 0.43a | 22.9 ± 0.24a | 22.9 ± 0.44a |
| Enterobacter aerogenes | 18.2 ± 0.41a | 17.3 ± 0.44a | 18.4 ± 0.53a | 17.9 ± 0.45a | 21.9 ± 0.41a | 19.3 ± 0.71a |
| Salmonella typhi | 18.3 ± 0.31a | 15.1 ± 0.20a | 28.0 ± 0.41b | 16.1 ± 0.21a | 22.3. ± 0.31a | 24.3 ± 0.34b |
| Klebsiella pneumoniae | 11.3 ± 0.34a | 12.4 ± 0.44a | 11.2 ± 0.23a | 11.6 ± 0.31a | 12.3 ± 0.25a | 14.3 ± 0.51a |
n = 3
Values with different superscripts in rows are significantly different (p < 0.05)
30E/70 W- 30 % Ethanol: 70 % water; 50E/50 W- 50 % Ethanol: 50 % water; 70E/30 W- 70 % Ethanol: 30 % water
For Salmonella typhi, the zone of inhibition was 28.0 ± 0.41 mm, 18.3 ± 0.31 mm, 15.1 ± 0.20 mm, 16.1 ± 0.21 mm, 22.3 ± 0.31 mm and 24.3 ± 0.34 mm for aqueous, methanolic, ethanolic, 30 % ethanol:70 % water, 50 % ethanol:50 % water and 70 % ethanol:30 % water. For Klebsiella pneumonia, the zone of inhibition was found to be 11.2 ± 0.23 mm, 11.3 ± 0.34 mm, 12.4 ± 0.44 mm, 11.6 ± 0.31 mm, 12.3 ± 0.25 mm and 14.3 ± 0.51 mm for aqueous, methanolic, ethanolic, 30 % ethanol:70 % water, 50 % ethanol:50 % water and 70 % ethanol:30 % water, respectively.
The antibacterial activity of peels of Punica granatum may be indicative of the presence of metabolic toxins or broad spectrum antimicrobial compounds that act against both gram + ve and gram –ve bacteria. The maximum antibacterial activity of different extracts against different bacterial strains was found in the order S. aureus > S. typhi > E. aerogenes > K. pneumoniae. P. granatum contains large amount of tannins (25 %) and antibacterial activity may be indicative of the presence of secondary metabolites. Earlier studies have also been done on the antibacterial activity of pomegranate peels and different results were revealed by different researchers. Diameter of inhibition zone (DIZ) against S. aureus and E. coli was found to be comparatively less than the other bacterial strains (Khan and Hanee 2011). Melendez and Capriles (2006) tested the antimicrobial properties of a number of tropical plants from Puerto Rico using the disc diffusion method against E. coli and S. aureus. They demonstrated that pomegranate extract produced inhibition zone sizes of 11 and 20 mm, for E. coli and S. aureus, respectively. Al-Zoreky (2009) reported the antibacterial activity of pomegranate peel extract against Listeria monocytogenes and Salmonella enteritidis.
Conclusions
In the present study, different extracts of pomegranate peel were screened for their total yield, antioxidant, phenolic content and antibacterial potential. The highest yield was obtained from 50 % ethanol: 50 % water. Higher antioxidant activity and phenolic content were found in 100 % water and 70 % ethanol: 30 % water. The antibacterial activity was also found significant against various pathogenic strains. The maximum antibacterial activity was found against the S. aureus and minimum activity was against K. pneumoniae. These extracts can be used for incorporation in various food products such as dairy foods, beverages and chocolate etc. to formulate nutraceutical and functional foods as these extracts could be considered as safer than other organic solvents.
References
- Adedapo AA, Jimoh FO, Koduru S, Masika PJ, Afolayan AJ. Evaluation of the medicinal potentials of the methanol extracts of the leaves and stems of Halleria lucida. Bioresource Technol. 2008;99:4158–4163. doi: 10.1016/j.biortech.2007.08.066. [DOI] [PubMed] [Google Scholar]
- Akowuah GA, Ismail Z, Norhayati I, Sadikun A. The effects of different extraction solvents of varying polarities on polyphenols of Orthosiphon stamineus and evaluation of the free radical-scavenging activity. Food Chem. 2005;93:311–317. doi: 10.1016/j.foodchem.2004.09.028. [DOI] [Google Scholar]
- Al-Zoreky NS. Antimicrobial activity of Pomegranate (Punica granatum L.) fruit peels. Int J Food Microbiol. 2009;134:244–248. doi: 10.1016/j.ijfoodmicro.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Amakura Y, Okada M, Tsuji S, Tonogai Y. High performance liquid chromatographic determination with photodiode array detection of ellagic acid in fresh and processed fruits. J Chromat. 2000;896:87–93. doi: 10.1016/S0021-9673(00)00414-3. [DOI] [PubMed] [Google Scholar]
- Artik N. Determination of phenolic compounds in pomegranate juice by using HPLC. Fruit Process. 1998;8:492–499. [Google Scholar]
- Chang LW, Juang LJ, Wang MY, Tai HM, Hang WJ, Chen YJ, Huang MH (2011) Antioxidant and antityrosinase activity of mulberry (Morus alba L.) twigs and root bark. Food Chem Texicol 49:785–790 [DOI] [PubMed]
- Chidambara MKN, Jayaprakasha GK, Singh RP. Studies on antioxidant activity of pomegranate (Punica granatum) peel extract using in vivo models. J Agri Food Chem. 2002;50:4791–4795. doi: 10.1021/jf0255735. [DOI] [PubMed] [Google Scholar]
- Cook NC, Samman S. Flavonoids-chemistry metabolism, cardioprotective effects and dietary sources. J Nutr Biochem. 1996;7:66–76. doi: 10.1016/0955-2863(95)00168-9. [DOI] [Google Scholar]
- Druckerei CH. European Pharmacopoeia. 4. Nordlingen: Beck; 2002. p. 187. [Google Scholar]
- Elfalleh W, Hannachi H, Tlili N, Yahia Y, Nasri N, Ferchichi A. Total phenolic contents and antioxidant activities of pomegranate peel, seed, leaf and flower. J Med Plants Res. 2012;6:4724–4730. doi: 10.5897/JMPR11.995. [DOI] [Google Scholar]
- FAO (2012) Statistical database. Food and Agriculture Organization of the United Nations, Codex Alimentarius Commission: Tunis, Tunesia. http://www.fao.org. (Accessed May 23, 2012)
- Ghasemian A, Mehrabian S, Majd A. Peel extracts of two Iranian cultivars of pomegranate (Punica granatum) have antioxidant and antimutagenic activities. Pakistan J Biol Sci. 2006;9:1402–1405. doi: 10.3923/pjbs.2006.1402.1405. [DOI] [Google Scholar]
- Gil MI, Tomas-Barberan FA, Hess-Pierce B, Holcroft DM, Kader AA. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J Agric Food Chem. 2000;48:4581–4589. doi: 10.1021/jf000404a. [DOI] [PubMed] [Google Scholar]
- Hseu YC, Chang WH, Chen CS, Liao JW, Huang CJ, Lu FJ. Antioxidant activities of Toona sinensis leaves extracts using different antioxidant models. Food Chem Toxicol. 2008;46:105–114. doi: 10.1016/j.fct.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Jayaprakasha GK, Rao JL (2000) Phenolic constituents from lichen Parmotrema stuppeum (Nyl.) Hale and their antioxidant activity. Z Naturforsch 55:1018–1022 [DOI] [PubMed]
- Khan AJ, Hanee S. Antibacterial properties of Punica granatum peels. Int J Applied Biol Pharmaceutical Technol. 2011;2:23–27. [Google Scholar]
- Kulkarni AP, Aradhya SM. Chemical changes and antioxidant activity in pomegranate arils during fruit development. Food Chem. 2005;93:319–324. doi: 10.1016/j.foodchem.2004.09.029. [DOI] [Google Scholar]
- Lansky EP, Newman RA. Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. J Ethnopharmacol. 2007;109:177–206. doi: 10.1016/j.jep.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Li Y, Guo C, Yang J, Wei J, Xu J, Cheng S. Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chem. 2006;96:254–260. doi: 10.1016/j.foodchem.2005.02.033. [DOI] [Google Scholar]
- Melendez PA, Capriles VA. Antibacterial properties of tropical plants from Puerto Rico. Phytomedicine. 2006;13:272–276. doi: 10.1016/j.phymed.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Mousavinejad G, Emam-Djomeh Z, Rezaei K, Khodaparast MHH. Identification and quantification of phenolic compounds and their effects on antioxidant activity in pomegranate juices of eight Iranian cultivars. Food Chem. 2009;115:1274–1278. doi: 10.1016/j.foodchem.2009.01.044. [DOI] [Google Scholar]
- Nawwar MAM, Hussein SAM, Mefort I. NMR spectral analysis of polyphenols from Punica granatum. Phytochem. 1994;36:793–798. doi: 10.1016/S0031-9422(00)89820-9. [DOI] [Google Scholar]
- Nishino T, Shibahara-Sone H, Kikuchi-Hayakawa H, Lshikawa F. Transit of radical scavenging activity of milk products prepared by Millard reaction and Lactobacillus casei strain shirota fermentation through the Hamster intestine. J Dairy Sci. 2000;83:915–922. doi: 10.3168/jds.S0022-0302(00)74954-X. [DOI] [PubMed] [Google Scholar]
- Plumb GW, De PS, Santos BC. Antioxidant properties of gallaocatechins and prodelphindins from pomegranate peels. Redox Rep. 2003;7:41–46. doi: 10.1179/135100002125000172. [DOI] [PubMed] [Google Scholar]
- Prashanth D, Asha M, Amit A. Antibacterial activity of Punica granatum. Fitoterapia. 2001;72:171–173. doi: 10.1016/S0367-326X(00)00270-7. [DOI] [PubMed] [Google Scholar]
- Qu WJ, Pan ZL, Zhang RH, Ma HL, Chen XG, Zhu BN, Wang ZB, Atungulu GG (2009) Integrated extraction and anaerobic digestion process for recovery of nutraceuticals and biogas from pomegranate marc. Trans ASABE 52:1997–2006
- Qu W, Pan Z, Ma H. Extraction modeling and activities of antioxidants from pomegranate marc. J Food Engg. 2010;99:16–23. doi: 10.1016/j.jfoodeng.2010.01.020. [DOI] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorisation assay. Free Radical Biol Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Satomi H, Umemura K, Ueno A, Hatano T, Okuda T, Noro T. Carbonic anhydrase inhibitors from the pericarps of Punica granatum L. Biol Pharma Bull. 1993;16:787–790. doi: 10.1248/bpb.16.787. [DOI] [PubMed] [Google Scholar]
- Singh RP, Murthy KNC, Jayaprakasha GK (2002) Studies on the antioxidant activity of pomegranate (Punica granatum) peel and seed extract using in vitro models. J Agric Food Chem 50:81–86 [DOI] [PubMed]
- Snedecor GW, Cochran WG. Statistical methods. 8. Ames: Iowa State University Press; 1989. [Google Scholar]
- Soares JR, Dinis TC, Cunha AP, Almeida LM. Antioxidant activity of some extracts of Thymus zygis. Free Radical Res. 1997;26:469–478. doi: 10.3109/10715769709084484. [DOI] [PubMed] [Google Scholar]
- Supayang PV, Treechada S, Surasak L, Thanomjit S, Tetsuya I, Takeshi H (2005) Inhibitory effects of active compounds from Punica granatum pericarp on verocytotoxin production by enterohemorrhagic Escherichia coli O157:H7. J Health Sci 51:590–596
- Tanaka T, Nonaka G, Nishioka I. Tannin and related compounds. XI. Revision of the structure of punicalin and punicalagin, and isolation and characterization of 2-O- galloylpunicalin from the bark of Punica grantum L. Chem Pharma Bull. 1986;34:650–655. doi: 10.1248/cpb.34.650. [DOI] [Google Scholar]
- Tanaka T, Nonaka G, Nishioka I. Tannin and related compounds. C. Reaction of dehydrohexahydroxydiphenic acid esters with bases, and its structure to the application determination of pomegranate tannins, granatins a and b. Chem Pharma Bull. 1990;38:9424–9428. [Google Scholar]
- Turkmen N, Sari F, Velioglu YS (2006) Effect of extraction solvents on concentration and antioxidant activity of black and black mate polyphenols determined by ferrous tartrate and Folin-Ciocalteu methods. Food Chem 99:838–841
- Zhang L, Fu Q, Zhang Y. Composition of anthocyanins in pomegranate flowers and their antioxidant activity. Food Chem. 2011;127:1444–1449. doi: 10.1016/j.foodchem.2011.01.077. [DOI] [Google Scholar]