Abstract
Bacoside rich juice (BRJ) was developed using date syrup as base. BRJ was evaluated for physicochemical, sensory attributes and its effect on physical endurance. Overall acceptability of BRJ and date syrup juice (DSJ) was good according to hedonic scale/ratings. Twenty four adult male Wistar rats were divided into 4 groups (n = 6). Sedentary (Group I) and control (Group II) group rats were allowed to drink water whereas DSJ and BRJ group rats were provided free access to drink DSJ (Group III) and BRJ (Group IV) for 14 days and were subjected to weight-loaded forced swim test (WFST) for every alternate day in order to evaluate the physical endurance. Both BRJ and DSJ group rats swimming efficiency was improved by 3 and 2 folds respectively in comparison with control group on day- 15. Improved physical endurance in BRJ group is due to reduced malondialdehyde levels in brain, liver and muscle tissues by 16.50 %, 17.88 % and 30.20 %, respectively, compared to DSJ group (p < 0.01). In addition, administration of BRJ significantly protected the hepatic and muscle glycogen levels and reduced the levels of lactic acid in comparison to DSJ group. Hence, the present study clearly indicates that BRJ is an effective anti-fatigue drink ameliorates the various impairments associated with physical endurance.
Keywords: Bacoside date syrup juice, Anti-fatigue drink, Weight loaded forced swim test (WFST), Physical endurance
Introduction
Phytomedicine obtained from herbal sources are in great demand as they are able to alleviate various infectious diseases, physical and cognitive disorders and there by provide outstanding contribution to modern therapeutics. Bacopa monniera is a member of Scrophulariaceae, hold great promise for the improvement of cognitive and endurance enhancing function. It is a perennial prostrate or creeping, juicy, succulent, glabrous annual herb, found throughout India in wet, damp and marshy areas (Satyavati et al. 1976). It is commonly known as Brahmi. It is an important constituent of the ayurvedic medica and classified into medhyarasayana, a drug known to improve memory and intellect (Satyavati et al. 1976). Extensive investigations indicated that the cognition facilitating effect of B. monniera was due to the presence of active saponins, bacoside A. (Singh and Dhawan 1992). These active principles, apart from facilitating learning and memory in normal rats, it has several other health benefits such as anti-stress (Chowdhuri et al. 2002), anti-inflammatory (Channa et al. 2006), anxiolytic (Ernst 2006), vasodilator (Channa et al. 2003) and anti-fatigue properties (Anand et al. 2012).
Date fruits have therapeutic properties and high nutritive value with a good amount of carbohydrates, minerals and vitamins (Karagul et al. 2004). Date contains invert sugars which are better utilized as source of energy and also controls the glycemic index and lipid profile in diabetic patients (Miller et al. 2002, 2003). In addition dates have antioxidant, anti-mutagenic and anti-tumor activity (Ishurd and Kennedy 2005; Vayalil 2002; Mansouri et al. 2005), Date extract treats various diseases in folk remedies (Duke 1992) and also act as immune-stimulant in women after child birth (Puri et al. 2000). Dates are being consumed in modern cultures for its pleasant flavor, odour and its use for flavoring foods, beverages and medication (Vayalil 2002).
Fatigue is the inability to sustain maximum muscular contraction at a given work load over a period of time (Fitts 1994; Enoka and Duchateau 2008). During exercise there will be excessive generation of free radicals in body tissues which leads to disruption of muscle contractile function there by decrease the physical performance (Mohanraj et al. 1998; Wright et al. 2005; Clanton 2007). Free radicals generated beyond physiological limits are found to reduce muscle force production by altering calcium ion sensitivity and thus contribute to fatigue (Reid et al. 1992; Westerblad and Allen 2003; Barker and Traber 2007; Bruton et al. 2008). It has been demonstrated that exercise increases free radical formation thus causes oxidative damage to cell membranes.
It leads to depletion of energy generating molecules like ATP, glucose, glycogen etc., and accumulation of metabolic end products like lactic acid etc., Thus fatigue causes various disorders in relation to bio-regulatory, autonomic nervous, endocrine and immune systems (Maes et al. 1998).
In our earlier studies we have demonstrated the physical endurance capacity of bacoside rich extract in rats (Anand et al. 2012). Since, the bacoside rich extract is bitter in taste. It was blended with date syrup as base for sensory attributes and enhancing the nutritive and nutracuetical value. The present study was undertaken to investigate the effect of developed date syrup based bacoside enriched juice for its physical endurance by forced swimming tests in rats.
Materials and methods
Chemicals
Thiobarbituric acid, trichloro acetic acid, ferric chloride, dinitro salicylic acid, p-hydroxydiphenyl, copper suphate, 1, 1 diphenyl-2-picrylhydrazyl were purchased from Himedia, India and hydrochloric acid, glycine, sodium hydroxide, potassium hydroxide, calcium hydroxide, sodium sulphate, sodium dodecyl sulphate, disodium hydrogen orthophosphate and sodium dihydrogen orthophosphate were purchased from Merck, Germany. Bacoside A standard was purchased from M/S Natural remedies Pvt, Ltd, Bangaluru, India. Acetonitril, methanol was purchased from Qualigens, India, Ltd, Mumbai, India.
Lion Date syrup, (Lion Dates Impex Pvt. Ltd, Chennai) was purchased from the local market, Mysore, India.
Isolation and estimation of bacoside
Bacoside rich extract from Bacopa monniera was prepared from the standard procedure (Singh et al. 1988). The amount of bacosides present is quantified by using a Jasco LC-NetII/ADC HPLC system equipped with a HiQ Sil C18HS (150 × 4.6 mm, 5 μm particle size) column, photodiode array detector (PDA), an PU 1580 pump (Jasco, USA) and a Rheodyne injector with 20 μL loop. The mobile phase consisted of 0.2 % phosphoric acid and acetonitrile (65:35 v/v). The pH of the mobile phase was adjusted to 3.0 with 5 M NaOH. The flow rate and total run time were 1.0 mL/min and 40 min, respectively. All peaks were integrated at the wavelength of 205 nm. They were initially assigned by comparing retention times with standards, and confirmed with characteristic spectra obtained from the PDA (Watoo et al. 2007).
DPPH radical scavenging activity
The antioxidant activity of bacoside rich extract was checked on the basis of 1, 1 diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging activity. DPPH assay was performed as per the method described by Eberhardt et al. (2000). DPPH (500 μl, 0.5 mM in methanol) solution was mixed with different amounts of sample and volume was made to 3.5 ml with methanol. The mixture was incubated in dark for 45 min at room temperature. Absorbance was recorded at 515 nm in a spectrophotometer. Butylated hydroxyl anisole (BHA) was used as standard antioxidant compound. A positive control was prepared by mixing 3 ml methanol and 0.5 ml of DPPH solution. Sample blanks were prepared in methanol without DPPH solution to eliminate the absorbance of crude extracts. Methanol was used as blank. The DPPH radical scavenging activity percentage was calculated by using the formula as given below:
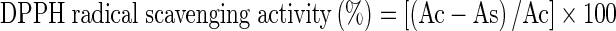 |
Where Ac is the absorbance of positive control solution and As is the absorbance of test solution. IC50 value, the concentration of sample or extract required to scavenge 50 % of the DPPH free radicals in the mixture, was calculated using a linear regression equation derived from the graph of % DPPH scavenging activity and sample concentration.
Preparation of date syrup based juice
Based on the amount and activity of bacosides, juice was prepared with a combination of bacoside (0.075 %), date syrup (10 %) with sugar (2.5 %) and mint leaves in order to enhance its taste and flavor.
Physico-chemical analysis of bacoside rich juice
The developed BRJ was analyzed for moisture, pH, acidity, according to standard methods (AOAC 2000). Total soluble solids (°Brix) were determined by means of Abbe refractometer (Milton Roy, USA).
Sensory analysis
Sensory evaluation of the BRJ was carried out to access the acceptability of the product by 20 trained panelists comprised of scientists of the Defence Food Research Laboratory, Mysore. Sensory parameters evaluated were: color, taste, appearance, flavour and over all acceptability (OAA) using a score scale of 1 to 9 where 1 indicates extreme dislike and 9 indicates extreme like (Larmond 1979).
Animals and feeding
Animal studies were conducted according to the Institute Animal Ethical Committee regulations approved by the Committee for the Purpose of the Control and Supervision of Experiments on Animals (CPCSEA). Twenty four male albino rats of Wistar strain weighing 120–140 g were selected from the stock colony, Defence Food Research Laboratory, Mysore, India, housed individually in stainless steel cages in a temperature controlled room (25 ± 2 °C) and was maintained in a 12 h light/dark cycle. The rats were then divided into the following four groups: (a) sedentary (water) (Group I), (b) control (water) (Group II), (c) DSJ supplemented (Group III) (d) BRJ supplemented (Group IV) groups. Rats were fed with a standard commercial pellet diet (Sri Venkateswara Enterprises, Bangalore, India). Fluid intake and body weight were monitored daily.
Weight-loaded forced swim test (WFST)
The weight loaded forced swim test (WFST) was performed with some modifications (Jung et al. 2007). The rats of the BRJ, DSJ and control groups were allowed to swim with constant load (tagged to the tail base) corresponding to 7.5 % of their body weight until exhaustion. The swimming exercise was carried out in a small tank with 30 cm deep, water maintained at 25 ± 2 °C. Exhaustion was determined by observing the loss of coordinated movements and failure to return to the surface within 10 s (Wang et al. 2006) and was repeated every alternate day for a period of 15 days. Animals were sacrificed under mild anesthesia immediately after the last exercise. Blood was collected from the heart using a heparinized syringe into centrifuge tubes. Separated plasma and tissue samples viz., brain, liver and gastrocnemius muscle were collected and stored at −80 °C until further analysis.
Determination of thiobarbituric acid-reactive substances (TBARS)
TBARS as malondialdehyde was analyzed by Buege and Aust method (1978) with slight modifications. Brain, liver and muscle tissues (100 mg) were homogenized in 2 ml of phosphate buffer (pH 7.0). TCA (10 %), 0.5 ml and 2 ml of TBA mixture were added to tissue homogenate (0.5 ml). The TBA mixture contained TBA (0.35 %), SDS (0.2 %), FeCl3 (0.05 mM) and BHT in glycine–HCl buffer (100 mM, pH 3.6). The above reaction mixture was kept in boiling water bath for 30 min and then allowed to cool. The mixture was centrifuged at 8,000 rpm for 10 min and the absorbance was measured at 532 nm. The MDA content was calculated using molar extinction co-efficient 1.56 × 105/mol/cm (Girotti and Deziel 1983).
Determination of glycogen
Liver and muscle tissues (0.5 g) were digested with 2 ml of KOH (30 %) and boiled in a water bath for 30 min with occasional shaking and then allowed to cool at room temperature. Saturated Na2SO4 solution was added to the mixture and stirred well. Glycogen was precipitated by adding 5 ml of ice cold ethanol to the sample mixture and centrifuged at 8,000 rpm for 15 min. 1 ml of HCl (1.2 N) was added to the diluted residue (1: 1 v/v) and kept on boiling water bath for 2 h, and then allowed to cool and further neutralized with 0.5 M NaOH. The DNS method was followed to determine the hydrolyzed product of tissue glycogen (Miller 1972).
Determination of lactic acid
The lactic acid content was measured by the method of Sawhney and Singh (2005). Tissue samples were homogenized in phosphate buffer (100 mM, pH 7.2) and deproteinized with TCA (10 %). These deproteinized samples were centrifuged at 5,000 rpm for 15 min. To the supernatant, 1 ml of copper sulphate solution (20 %) and 1 g of Ca (OH)2 power was added and kept for 30 min in room temperature with intermittent shaking. After incubation, all the samples were centrifuged at 5,000 rpm for 15 min. To 1 ml of supernatant, 50 μl of copper suphate solution (4 %) and 6 ml of H2SO4 (conc.) was added and kept in a boiling water bath for 5 min and allowed to cool. 100 μl of p-hydroxydiphenyl reagent was added to the above sample mixture and incubated at 37 °C for 30 min. The absorbance was measured at 560 nm.
Statistical analysis
Data are expressed as mean ± standard deviation. Data were analyzed using Student’s t-test. Differences at p < 0.05 were considered to be significant.
Results and discussion
Quantification of bacosides by HPLC analysis
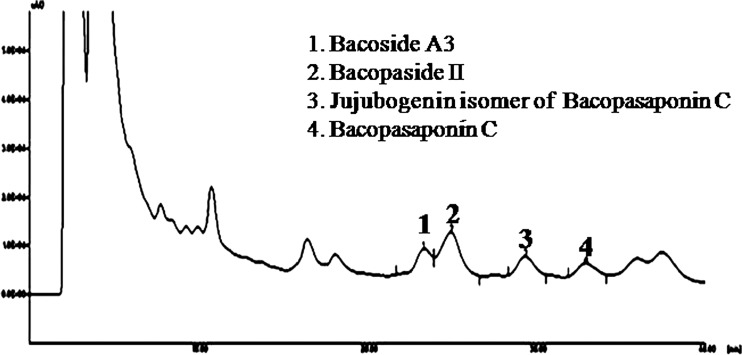
Four major saponins were detected and quantified. The percentage of Bacoside A3, Bacopaside II, jujubogenin isomer of bacopasaponin C, bacopasaponin C is found to be 3.54 ± 0.04, 7.46 ± 0.05, 3.52 ± 0.12, 1.82 ± 0.01 respectively (Fig. 1).
Fig. 1.
Chromatogram of HPLC analysis of bacoside rich extract
DPPH radical scavenging activity of Bacopa extract
Antioxidant activity of bacoside rich extract was determined based on DPPH radical scavenging activity. The reduction capability of DPPH radicals was determined by the decrease in its absorbance at 515 nm induced by antioxidants. The IC50 value of bacoside rich extract was found to be 247.97 μg/ml.
Physicochemical analysis of bacoside rich juice
The moisture content of BRJ was 90.04 % with total solids 9.96 %. The Total Soluble Solids (ºBrix) of the sample was 11 %. Total titratable acidity of sample was found to be 0.096 % as citric acid equivalents of juice with pH 5.5. Based on sensory evaluation colour of the juice scored 7.4 ± 0.25, consistency scored 7.4 ± 0.36, flavor scored 7.4 ± 0.28, taste scored 7.3 ± 0.27 and the overall acceptability (OAA) of the product was good (7.3 ± 0.32). In the present study, Date syrup was used to mask the bitter taste and color given by the bacoside rich extract and thereby enhancing the sensory attributes as well as neutracuetical value of the drink.
Effect of BRJ on swimming performance
The potential of date syrup based bacoside juice was evaluated for its physical endurance enhancing property by preventing exercise-induced oxidative damage to muscle fiber. The forced swim test is perhaps one of the most commonly used animal models for evaluating the physical endurance properties of novel compounds (Jung et al. 2004). Control, DSJ and BRJ group rats were subjected to WFST on every alternative from day- 3 onwards. The swimming time gradually increased from day- 5 onwards in BRJ supplemented rats and day- 7 onwards in DSJ supplemented rats. The maximum swimming time was recorded on day- 15 in both the groups. Swimming time of BRJ supplemented group was significantly higher than DSJ supplemented group (p < 0.001) (Table 1). In the present study, the endurance exercise data (Table 1) showed that the DSJ group rats swimming performance was increased by 2 fold on day-15. This improved swimming performance is due to presence of rich carbohydrate source in the drink. But supplementation of BRJ further increased the swimming time to 3 fold more (p < 0.001), which was better than DSJ supplemented group. Bacoside rich extract is found to enhance physical endurance by protecting mitochondria, muscle fibers from the oxidative damage. It also improves enzymatic and non-enzymatic antioxidant status (Anand et al. 2012). This antioxidant property of bacoside with added energy and minerals improves physical performance. Bacoside A is mainly working on the enhancement of cognitive performance. In our earlier studies we have reported that bacoside rich extract with antioxidants enhances the physical endurance (Anand et al. 2012). Further studies is warranted to prove physical endurance of bacoside A mixture alone. Studies have shown that dietary supplementation with specific antioxidants will be beneficial for enhancing the antioxidant status thus elevate physical endurance (Ji 1995). The anti-oxidative activities of antioxidant compounds against exercise-induced oxidative damage include reducing the production of ROS, inhibiting lipid peroxidation and enhancing antioxidant defenses such as increasing the production of antioxidant enzymes and endogenous antioxidants. Antioxidants may be involved in glycogen metabolism so as to meet the energy requirements of working skeletal muscles and may act by terminating the chain reaction of lipid peroxidation so as to maintain the morphological stability of mitochondria in spinal motor neurons (Yu et al. 2006). In our previous study, we have clearly shown that bacoside rich extract reduced the exercise-induced oxidative damage by inhibiting lipid peroxidation and enhancing the antioxidant defense by elevating the activities of antioxidant enzymes (Anand et al. 2012).
Table 1.
Effect of BRJ on swimming time in minutes
| Day–3 | Day–5 | Day–7 | Day–9 | Day–11 | Day–13 | Day–15 | |
|---|---|---|---|---|---|---|---|
| Control | 5.0 ± 0.50 | 5.1 ± 1.07 | 5.3 ± 1.37 | 7.2 ± 1.10 | 7.3 ± 1.15 | 7.3 ± 1.27 | 7.5 ± 1.16 |
| DSJ | 5.0 ± 1.00 | 5.2 ± 1.26 | 6.0 ± 2.13 | 9.1 ± 1.18 | 12.1 ± 2.34* | 15.2 ± 2.58* | 22.5 ± 4.08** |
| BRJ | 5.0 ± 1.03 | 7.0 ± 2.18 | 9.1 ± 1.27 | 15.5 ± 1.15# | 21.2 ± 2.17## | 27.1 ± 2.09### | 33.3 ± 1.56### |
Data express the mean ± S.D. for 6 rats. Control rat fed with distilled water, DSJ rat fed with Date Syrup Juice, BRJ rat fed with bacoside rich juice for 14 days. Data express the mean ± S.D, n = 6 in each group, *p < 0.05 and **p < 0.01 vs. sedentary. # p < 0.05, ## p < 0.01 and ### p < 0.001 vs. DSJ
Effect of BRJ on lipid peroxidation, glycogen and lactic acid levels
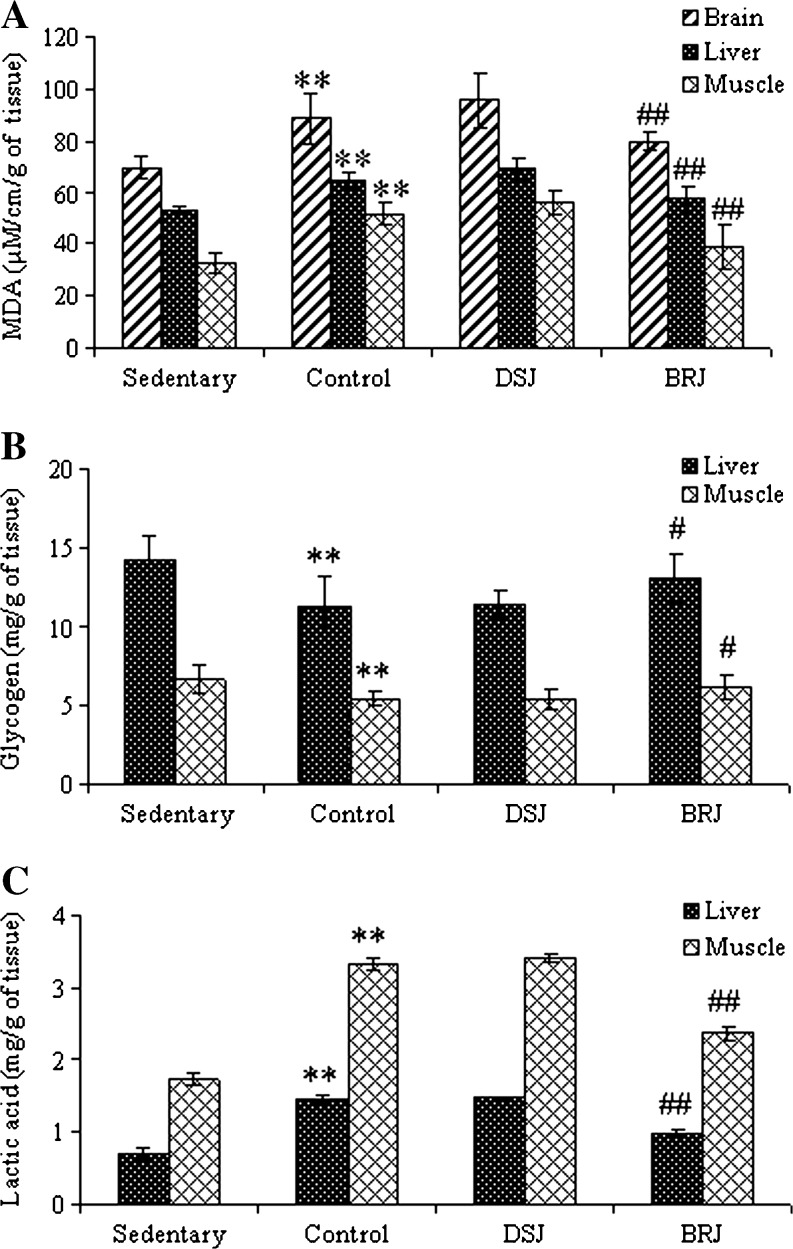
Swimming exercise significantly increased MDA level in muscle, liver and brain in control group compared to that of sedentary group. However, no significant change was observed in DSJ supplemented group and control group. The BRJ supplementation has significantly decreased the MDA level in muscle (30.2 %), liver (17.88 %) and brain (16.5 %), compared with that of DSJ group (p < 0.01) (Fig. 2). Thiobarbituric acid reactive substances/MDA is one of the sensitive biomarker of oxidative stress. Mitochondrial membrane and other biological membrane consist of glycophospholipids, fatty acids and other essential lipids, are damaged by the oxidation process resulting in the release of lipid peroxidation end products such as MDA. Most studies suggest that endurance exercise causes an increase in MDA levels (Urso and Clarkson 2003; Anand et al. 2012). In present study, BRJ supplementation effectively inhibited lipid peroxidation in brain, liver and muscle tissues, thus enhances physical efficiency by reducing oxidative stress. However a marginal increase in lipid peroxidation products was observed in DSJ supplemented group than control group. This could be due to increased swimming time (twice than control group) that may release more free radicals which could not be efficiently neutralized in DSJ supplemented group. Lactic acid (LA) levels in liver and muscle tissues were significantly increased by exhaustive swimming in the control and DSJ group compared to that of sedentary group (p < 0.01) (Fig. 2). However there was no significant change observed between DSJ supplemented and control group. The BRJ supplemented animals had lowest lactic acid levels in both liver and muscle tissues when compared with DSJ group (p < 0.01). Exercise reduced the levels of glycogen in control and DSJ groups, however in BRJ supplementation liver and muscle glycogen content was higher (p < 0.05) than the DSJ supplemented group. Anaerobic breakdown of glycogen/glucose leads to an intracellular accumulation of lactic acid, a strong acid, dissociates into lactate and H+. The increase in H+ (i.e. reduced pH or acidosis) is the classic cause of skeletal muscle fatigue and may be considered as one of the biomarkers for judging the degree of fatigue (Wang et al. 2006; Yu et al. 2008). The present results also showed that there was a significant decrease in the concentration of lactic acid in the BRJ treated group compared with the control and DSJ group in liver and muscle (p < 0.01) (Fig. 2). Reduced levels of lactic acid in the BRJ treated group support the physical endurance property. However a marginal increase in lactic acid level was observed in DSJ supplemented group then control group. This could be due to increased swimming time (twice than control group) that may produce more lactic acid, an end product of anaerobic glucose metabolism. Glycogen is an important source of energy during exercise. The increase in glycogen stores in the liver is an advantage to enhance the physical endurance (Yu et al. 2008). Depletion of liver glycogen is an important factor during exercise fatigue and may lead to hypoglycemia impairing nervous function (Dohm et al. 1983). Energy for exercise is derived initially from the breakdown of glycogen in muscle, after strenuous exercise may be depleted and at later stages the energy will be derived from hepatic glycogen (Suh et al. 2007). It has been reported that depletion of muscle and liver glycogen in endurance exercises (Jung et al. 2004). In the present study, the results showed that the control rats utilized more glycogen than the sedentary rats in liver and muscle. In BRJ supplemented group higher levels of glycogen were observed (14.35 % and 15.60 % in liver and muscle) when compared with the DSJ group, indicating better utilization of available energy.
Fig. 2.
a Effect of BRJ on Thiobarbituric acid reactive oxygen species (TBARS) in brain, liver and muscle. b Glycogen level in liver and muscle. c Lactic acid level in liver and muscle in rats. (a) Sedentary (b) Control (c) DSJ = rat fed with Date Syrup Juice, (d) BRJ = rat fed with bacoside rich juice for 14 days. Data express the mean ± S.D, n = 6 in each group, *p < 0.05 and **p < 0.01 vs. sedentary. # p < 0.05 and ## p < 0.01 vs. DSJ
Conclusion
In the present study we have clearly shown that bacoside enriched date syrup juice was very well accepted and significantly enhanced the swimming performance by ameliorating the various impairments associated with physical fatigue in rats and therefore could form a good supplement to enhance physical performance in sports personal and/or in persons exposed to strenuous exercise.
References
- Anand T, Phani Kumar G, Pandareesh MD, Swamy MSL, Khanum F, Bawa AS. Effect of bacoside extract from Bacopa monniera on physical fatigue induced by forced swimming. Phytother Res. 2012;26:587–593. doi: 10.1002/ptr.3611. [DOI] [PubMed] [Google Scholar]
- AOAC . Official methods of analysis. 17. Washington: Association of Official Analytical Chemists; 2000. [Google Scholar]
- Barker T, Traber MG. From animals to humans: evidence linking oxidative stress as a causative factor in muscle atrophy. J Physiol. 2007;583:421–422. doi: 10.1113/jphysiol.2007.139378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruton JD, Place N, Yamada T, Silva JP, Andrade FH, Dahlstedt AJ, Zhang S-J, Katz A, Larsson NG, Westerblad H. Reactive oxygen species and fatigue-induced prolonged low-frequency force depression in skeletal muscle fibres of rats, mice and SOD2 over expressing mice. J Physiol. 2008;586:175–184. doi: 10.1113/jphysiol.2007.147470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:301–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- Channa S, Dar A, Yaqoob M, Anjum S, Sultani Z, Rahman A. Bronchovasodilatory activity of fractions and pure constituents isolated from Bacopa monniera. J Ethnopharmacol. 2003;86:27–35. doi: 10.1016/S0378-8741(03)00013-8. [DOI] [PubMed] [Google Scholar]
- Channa S, Dar A, Anjum S, Yaqoob M, Rahman A. Anti-inflammatory activity of Bacopa monniera in rodents. J Ethnopharmacol. 2006;104:286–289. doi: 10.1016/j.jep.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Chowdhuri DK, Parmar D, Kakkar P, Shukla R, Seth PK, Srimal RC. Antistress effects of bacosides of Bacopa monnieri: modulation of HSP70 expression, superoxide dismutase and cytochrome P450 activity in rat brain. Phytother Res. 2002;16:639–645. doi: 10.1002/ptr.1023. [DOI] [PubMed] [Google Scholar]
- Clanton TL. Hypoxia-induced reactive oxygen species formation in skeletal muscle. J Appl Physiol. 2007;102:2379–2388. doi: 10.1152/japplphysiol.01298.2006. [DOI] [PubMed] [Google Scholar]
- Dohm GL, Tapscott EB, Barakat HA, Kasperek GJ. Influence of fasting on glycogen depletion in rats during exercise. J Appl Physiol. 1983;55:830–833. doi: 10.1152/jappl.1983.55.3.830. [DOI] [PubMed] [Google Scholar]
- Duke JA. Handbook of phytochemical of GRAS herbs and other economic plants. Boca Raton: CRC Press; 1992. [Google Scholar]
- Eberhardt MV, Lee CY, Liu RH. Antioxidant activity of fresh apple. Nature. 2000;405:903–904. doi: 10.1038/35016151. [DOI] [PubMed] [Google Scholar]
- Enoka RM, Duchateau J. Muscle fatigue: what, why and how it influences muscle function. J Physiol. 2008;586:11–23. doi: 10.1113/jphysiol.2007.139477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst E. Herbal remedies for anxiety—a systematic review of controlled clinical trials. Phytomedicine. 2006;13:205–208. doi: 10.1016/j.phymed.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Fitts RH. Cellular mechanisms of muscle fatigue. Physiol Rev. 1994;74:49–94. doi: 10.1152/physrev.1994.74.1.49. [DOI] [PubMed] [Google Scholar]
- Girotti AW, Deziel MR. Photodynamic action of protoporphyrin on resealed erythrocyte membranes: mechanism of release of trapped markers. Adv Exp Med Biol. 1983;160:213–225. doi: 10.1007/978-1-4684-4406-3_19. [DOI] [PubMed] [Google Scholar]
- Ishurd O, Kennedy JF. The anti-cancer activity of polysaccharide prepared from Libyan dates (Phoenix dactylifera L.) Carbohydr Polym. 2005;59:531–535. doi: 10.1016/j.carbpol.2004.11.004. [DOI] [Google Scholar]
- Ji LL. Exercise and oxidative stress: role of the cellular antioxidant systems. Exerc Sport Sci Rev. 1995;23:135–166. doi: 10.1249/00003677-199500230-00007. [DOI] [PubMed] [Google Scholar]
- Jung K, Kim I, Han D. Effect of medicinal plant extracts on forced swimming capacity in mice. J Ethnopharmacol. 2004;93:75–81. doi: 10.1016/j.jep.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Jung CH, Jung H, Shin YC. Eleutherococcus senticosus extract attenuates LPS-induced iNOS expression through the inhibition of Akt and JNK pathways in murine macrophage. J Ethnopharmacol. 2007;113:183–187. doi: 10.1016/j.jep.2007.05.023. [DOI] [PubMed] [Google Scholar]
- Karagul Y, Wilson C, White H. Formulation and processing of yoghurt. J Dairy Sci. 2004;87:543–550. doi: 10.3168/jds.S0022-0302(04)73195-1. [DOI] [PubMed] [Google Scholar]
- Larmond E (1979) Laboratory method of sensory evaluation of food. Publication No.1637, Dept. of Agriculture Ottawa, Canada, pp 33–37 & 57
- Maes M, Song C, Lin A. The effects of psychological stress on humans: increased production of pro-inflammatory cytokines and a Th1-like response in stress-induced anxiety. Cytokine. 1998;10:313–318. doi: 10.1006/cyto.1997.0290. [DOI] [PubMed] [Google Scholar]
- Mansouri A, Embared G, Kokkalou E, Kefalas P. Phenolic profile and antioxidant activity of the Algerian ripe date palm fruit (Phoenix dactylifera) Food Chem. 2005;89:411–420. doi: 10.1016/j.foodchem.2004.02.051. [DOI] [Google Scholar]
- Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugars. Anal Chem. 1972;31:426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- Miller CJ, Dunn EV, Hashim IB. Glycemic index of 3 varieties of dates. Saudi Med J. 2002;23:536–538. [PubMed] [Google Scholar]
- Miller CJ, Dunn EV, Hashim IB. The glycemic index of dates and date/yoghurt mixed meals. Are dates the candy that grows on trees? Eur J Clin Nutr. 2003;57:427–430. doi: 10.1038/sj.ejcn.1601565. [DOI] [PubMed] [Google Scholar]
- Mohanraj P, Merola AJ, Wright VP, Clanton TL. Antioxidants protect rat diaphragmatic muscle function under hypoxic conditions. J Appl Physiol. 1998;84:1960–1966. doi: 10.1152/jappl.1998.84.6.1960. [DOI] [PubMed] [Google Scholar]
- Puri A, Sahai R, Singh KL, Saxena RP, Tandon JS, Saxena KC. Immunostimulant activity of dry fruits and plants materials used in Indian traditional medical system for mother after child birth and invalids. J Ethnopharmacol. 2000;71:89–92. doi: 10.1016/S0378-8741(99)00181-6. [DOI] [PubMed] [Google Scholar]
- Reid MB, Haack KE, Franchek KM, Valberg PA, Kobzik L, West MS. Reactive oxygen in skeletal muscle I. Intracellular oxidant kinetics and fatigue in vitro. J Appl Physiol. 1992;73:1797–1804. doi: 10.1152/jappl.1992.73.5.1797. [DOI] [PubMed] [Google Scholar]
- Satyavati GV, Raina MK, Sharma M. Medicinal plants of India. New Delhi: ICRM; 1976. pp. 118–122. [Google Scholar]
- Sawhney SK, Singh R. In introductory practical biochemistry. New Delhi: Narosa Publishing House Pvt. Ltd; 2005. [Google Scholar]
- Singh HK, Dhawan BN. Drugs affecting learning, memory. New Delhi: Wiley Eastern; 1992. pp. 189–207. [Google Scholar]
- Singh HK, Rastogi RP, Srima RC, Dhawan BN. Effects of bacosides A and B on avoidance response in rats. Phytother Res. 1988;2:70–75. doi: 10.1002/ptr.2650020205. [DOI] [Google Scholar]
- Suh SH, Paik IY, Jacobs K. Regulation of blood glucose homeostasis during prolonged exercise. Mol Cell. 2007;23:272–279. [PubMed] [Google Scholar]
- Urso ML, Clarkson PM. Oxidative stress, exercise, and antioxidant supplementation. Toxicol. 2003;189:41–54. doi: 10.1016/S0300-483X(03)00151-3. [DOI] [PubMed] [Google Scholar]
- Vayalil PK. Antioxidant and antimutagenic properties of aqueous extract of date fruit (Phoenix dactylifera L. Arecaceae) J Agric Food Chem. 2002;50:610–617. doi: 10.1021/jf010716t. [DOI] [PubMed] [Google Scholar]
- Wang JJ, Shieh MJ, Kuo SL, Lee CL, Pan TM. Effect of red mold rice on anti-fatigue and exercise-related changes in lipid peroxidation in endurance exercise. Appl Microbiol Biotechnol. 2006;70:247–253. doi: 10.1007/s00253-005-0051-5. [DOI] [PubMed] [Google Scholar]
- Watoo P, Sakchal W, Kanchalee J, Ubrapron P, Hiroyuki T, Karnkanok I. Determination of sopanin glycosides in Bacopa monnieri by reverced phase high performance liquid chromatography. Thai Pharm Health Sci J. 2007;2:26–32. [Google Scholar]
- Westerblad H, Allen DG. Cellular mechanism of skeletal muscle fatigue. Adv Exp Med Biol. 2003;538:563–570. doi: 10.1007/978-1-4419-9029-7_50. [DOI] [PubMed] [Google Scholar]
- Wright VP, Klawitter PF, Iscru DF, Merola AJ, Clanton TL. Superoxide scavengers augment contractile but not energetic responses to hypoxia in rat diaphragm. J Appl Physiol. 2005;98:1753–1760. doi: 10.1152/japplphysiol.01022.2004. [DOI] [PubMed] [Google Scholar]
- Yu F, Lu S, Feng S, McGuire PM, Li R, Wang R. Protective effects of polysaccharide from Euphorbia kansui (Euphorbiaceae) on the swimming exercise-induced oxidative stress in mice. Can J Physiol Pharmacol. 2006;84:1071–1079. doi: 10.1139/y06-052. [DOI] [PubMed] [Google Scholar]
- Yu B, Lu ZX, Bie XM, Lu FX, Huang XQ. Scavenging and anti-fatigue activity of fermented defatted soybean peptides. Eur Food Res Technol. 2008;226:415–421. doi: 10.1007/s00217-006-0552-1. [DOI] [Google Scholar]