Abstract
Turmeric (Curcuma longa L) rhizome extracts were evaluated for their antidiabetic, antihypertensive and antioxidant potentials. α-Glucosidase (0.4 μg/mL) and α-amylase (0.4 μg/mL) inhibitory potential of turmeric ethyl acetate extract was significantly higher than those of the reference drug acarbose (17.1 μg/mL and 290.6 μg/mL respectively). Protein glycation inhibitory potential of ethyl acetate extract was 800 times higher than that of ascorbic acid. High potential of ethyl acetate extract to scavenge free radicals and to reduce LDL oxidation and cellular oxidative stress was also revealed. The positive correlation obtained between the free radical scavenging capacity of the extracts and their antiglycation potential further confirmed the role of antioxidants in controlling glycation reactions. Ethyl acetate extract was also found as effective in reducing hypertension by inhibiting angiotensin converting enzyme (ACE). Antidiabetic, ACE inhibitory and antioxidant capacities of the extracts were in the order of their curcumin contents.
Keywords: Curcuma longa L, Glucosidase, Angiotensin, Antiglycation, LDL oxidation and antioxidant
Introduction
Diabetes is the most common endocrine disorder characterized by hyperglycemia and long term complications affecting eyes, kidneys and blood vessels. Elevated postprandial hyperglycemia (PHg) is one of the risk factors inherent to diabetes mellitus. Glucosidase inhibitors by retarding the enzymatic hydrolysis of starch at intestine play major role in managing PHg (Gin and Rigalleau 2000). Non-enzymatic protein glycation by the reducing sugars such as glucose and fructose in presence of ROS lead to a training of compounds collectively called advanced glycation end products (AGEs). AGEs are well known contributors to the pathophysiology of aging and diabetic chronic complications including retinopathy, neuropathy, nephropathy and vasculopathy (Singh et al. 2001). Serum levels of AGEs reflect the severity of these complications and therapeutic interventions aimed at reducing AGEs can inhibit or delay the progression. Thus design and discovery of inhibitors of AGEs formation can also offer a promising therapeutic approach for the prevention of diabetic and related disorders (Singh et al. 2001). Hypertension is about twice as common in diabetics as in non-diabetics. In addition to being the leading attributable risk factor for death throughout the world, hypertension results in substantial illness due to its effect on several target organs, including the brain, eyes, heart, arteries, and kidneys (Nathan 1993). Hypertension is a multifactorial disease and is also considered as one of the long-term complication of diabetes mellitus. Hypertension is a major risk factor for both large and small vessel disease, contributing to accelerated atherogenesis and progression of diabetic nephropathy and retinopathy (Barnett 1994). Controlling the raised blood pressure in diabetics is beneficial in slowing the progression of nephropathy and retinopathy. Angiotensin converting enzyme (ACE) increases blood pressure by converting the inactive angiotensin-I to the potent vasoconstrictor angiotensin II. ACE is a multifunctional enzyme which also catalyses the degradation of a vasodilating peptide, bradykinin. Therefore, inhibition of ACE activity is considered to be a useful therapeutic approach in the treatment of high blood pressure. Clinical trials have shown the beneficial effects of ACE inhibitors in delaying the progression of diabetic renal disease (Kshirsagar et al. 2000). Diabetes has also been shown to be associated with increased free radical activity and oxidative stress (Greismacher et al. 1995). It is well known that dietary antioxidants (AOs) play a major role in the protection of animal tissues from oxidative stress mediated degenerative diseases. Epidemiological and biological studies have provided various lines of evidences that free radical induced peroxidation of low density lipoprotein (LDL) critically contribute to the risk of human atherosclerosis (Witztum and Steinberg 1991).
Adverse side effects associated with the synthetic antihypertensive and antidiabetic drugs generate great deal of attention towards the development of such drugs of natural origin. Turmeric (Curcuma longa L.) rhizome, one of the oldest and most widely used spices of the world, is well documented for its antidiabetic and antihypertensive activities in Indian traditional medicine ‘Ayurveda’ (Chattopadhyay et al. 2004). It’s anti- inflammatory, anticancer and AO roles have been established in modern studies (Anupama et al. 2011; Braga et al. 2003; Chattopadhyay et al. 2004; Jayaprakasha et al. 2005; Singh et al. 2010). The authors recently reported glucosidase inhibitory potential of turmeric peptides (Lekshmi et al. 2011). Curcuminoids namely curcumin (C-1), demethoxycurcumin (C-2) and bis-demethoxycurcumin (C-3) have been reported as the major bioactive compounds in turmeric rhizome (Chattopadhyay et al. 2004; Jayaprakasha et al. 2005). In this study, turmeric extracts were measured for their glucosidase and AGEs formation inhibitory capacities to evaluate their antidiabetic potential. AO potential of the extracts were also confirmed as their ability to reduce cellular oxidative stress and to inhibit LDL oxidation. Since hypertension contributes to diabetes related disorders, ACE inhibitory capacity of the extracts was also evaluated. The active extract was profiled for their composition to explore the role of phenolics and curcuminoids in these bioactivities.
Materials and methods
Plant material
Authenticated samples of fresh rhizomes of turmeric were obtained from Nedumangad, Thiruvananthapuram (India). The voucher specimens were deposited in the Herbarium of the Institute. The rhizomes were dried at 50 °C in an air oven, powdered and 500 g was extracted sequentially with 1 L each of hexane, ethyl acetate, methanol, 70 % methanol and water respectively at room temperature. The extracts were evaporated to dryness at <50 °C under vacuum. Sample stock solution in methanol was used for in vitro antioxidant studies. DMSO solutions of the extracts were used for all other biological assays.
Chemicals and reagents
ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid), acarbose, ACE, α-amylase from Aspergillus oryzae, ascorbic acid, bovine serum albumin (BSA), captopril, catechin, curcumin, 2′,7′-deoxyribose, dichlorofluorescin diacetate (DCFH-DA), DMEM media, DPPH (2,2′-diphenyl-1-picrylhydrazin), fetal calf serum, Folin-Ciocalteu (FC) reagent, gallic acid, glucose, α-glucosidase from Baker’s Yeast, hippuryl-histidyl-leucine (HHL), LDL, nitrotetrazolium blue chloride (NBT), paranitrophenyl glucosidase (pNPG), pencillin, phophate buffered saline (PBS), phenazinemethosulphate (PMS), quercetin, soluble starch, streptomycin, thiobarbituric acid (TBA), tricholoroactetic acid (TCA) and trolox were purchased from Sigma Aldrich (St. Louis, MO, USA). 3, 5 dinitrosalicylic acid (DNSA), EDTA, NADH and tris HCl were purchased from Sisco Research Laboratory Ltd, India. All other chemicals and reagents used were of analytical grade.
Cell-culture and treatment
C2C12 purchased from National Centre for Cell Sciences, Pune, India was cultured in DMEM medium supplemented with 10 % Fetal calf serum, 100 U/mL penicillin and 100 μg/mL streptomycin. Cultures were maintained at 37 °C in 5 % CO2 incubator. When the cells were about to cover 80 % of the flask area, they were disrupted and seeded on 24 well plates. After attaining ~70–80 % confluency, the cells were rinsed twice with PBS and changed with medium containing extracts at different concentrations. After 24 h incubation, the cells were washed twice with PBS and 50 μM H2O2 was maintained in individual well for 1 h at 37 °C. These cells were detached by trypsin to assay in flow cytometry.
Total phenolics
Aliquots of the extracts in methanol (1 mg/mL) were oxidized with 0.5 mL of FC reagent and the reaction was neutralized with the addition of 1 mL 20 % Sodium carbonate (Na2CO3). The mixture was incubated at room temperature for 90 min. and the absorbance of the resulting solution at 760 nm was measured spectrophotometrically. Calibration plot was drawn with gallic acid and total phenolic content was expressed in gallic acid equivalents (GAE) (Sato et al. 1996).
HPTLC quantification of curcuminoids
Chromatography was performed on a pre-activated (110 °C) silica gel HPTLC plate (60F, 254, 20 × 20 cm. Merck, Mumbai, India). Samples and C-1 were applied to the plate as 6 mm wide bands with an automatic TLC applicator, Linomat IV, with N2 flow (Camag, Muttenz, Switzerland), 10 mm from the bottom of the plate at a delivery speed of the syringe 10 μL/s. The plates were developed using a Camag twin-trough glass tank which was pre-saturated with the mobile phase chloroform-methanol (95:5) for 1 h and each plate was developed to a height of about 8 cm. The TLC runs were made under laboratory conditions of 25 ± 5 °C and 50 % relative humidity. After development, the plate was removed and dried and spots were visualized in UV light. Then the plates were scanned in Camag TLC Scanner-3, equipped with CATS −4 software under the following conditions: slit width 6 × 0.4 mm, wavelength 366 nm, absorption/reflection detection mode. Peaks corresponding to C-2 and C-3 were identified from their spectral and retention data reported by Pathania et al. (2006). Amount of C-1, C-2 and C-3 in the extracts was quantified from the calibration curves plotted with C-1.
Antidiabetic assays
α-Amylase inhibition
The inhibition assay was performed according to the method of Apostolidis et al. (2007). with some modifications. 10 μL of extract/acarbose (10–100 μg) in DMSO and 20 μL of 0.02M sodium phosphate buffer (PH 6.9 with 0.006M NaCl) containing α-amylase solution (0.5 mg/mL) were incubated at 25 °C for 10 min. After pre-incubation, 25 μL of 1 % starch solution in 0.02M sodium phosphate buffer was added to each tube at timed intervals. The reaction mixtures were then incubated at 25 °C for 10 min. The reaction was stopped with 50 μL of DNSA reagent. The tubes were then incubated in boiling water for 5 min and cooled to room temperature. The reaction mixture was then diluted with 500 μL water and absorbance at 540 nm was measured using a multimode plate reader (Synergy 4, Biotek, USA). Enzyme inhibition data were expressed as IC50 values.
α-Glucosidase inhibition
This inhibition assay was performed according to the method of Apostolidis et al. (2007). α-Glucosidase (20 μL, 1 U/mL) was premixed with 25–500 μg of extract/acarbose and made up to 500 μL with 50 mM phosphate buffer at PH 6.8. Then it was incubated for 5 min at 37 °C. 1 mM pNPG (200 μL) in 50 mM of phosphate buffer was added to initiate the reaction and the mixture was further incubated for 20 min at 37 °C. The reaction was terminated by the addition of 500 μL of 1M sodium carbonate and the final volume was made up to 1.5 mL with water. α-Glucosidase activity of the mixtures was determined by measuring the quantity of nitrophenol released from pNPG. The absorbance of the mixtures at 405 nm was measured using a multimode plate reader (Synergy 4, Biotek, USA). The concentration of extract required to inhibit 50 % of α-glucosidase under the assay conditions was defined as the IC50 value.
Antiglycation activity
Antiglycation assay was performed according to the methods reported by Matsuura and colleagues with slight modification (Matsuura et al. 2002). In all experiments, the final reaction volume was 1 mL. BSA (1 mg/mL final concentration) was incubated with glucose (500 mM final concentration) in presence of extracts, vitamin C or PBS as control buffer at specified concentration. The reaction was allowed to proceed at 60 °C for 24 h, and thereafter, stopped by adding 10 μL of 100 % (w/v) trichloroacetic acid (TCA). The mixture was kept at 4 °C for 10 min and subjected to centrifugation at 10,000 g. The precipitate was redissolved with alkaline PBS (pH 10) and immediately quantitated for the relative amount of glycated BSA based on fluorescence intensity by multimode plate reader. The excitation and emission wavelengths used were at 370 nm and 440 nm, respectively.
ACE inhibition assay
The assay was performed as per the method described by Cushman and Cheung (1971). with some modifications. A 100 μL amount of substrate (HHL dissolved in a pH 8.3 buffer with 0.03M NaCl) and 150 μL of ACE dissolved in glycerol at 50 % were added to 15 μL of water. The reaction solution was incubated at 37 °C for 1 h. ACE activity was arrested by the addition of 250 μL of 0.5N HCl. Ethyl acetate (1 mL) was added to the mixture shaken well, and centrifuged at 3,000 g for 10 min. A 750 μL amount of the organic layer was taken and dried out at 95 °C for 10 min. The residue was redissolved in 1 mL water and absorbance at 228 nm was measured. The reaction blank was prepared in the same way indicated above, changing the order in which the reagents were added, i.e., by adding the HCl before adding the enzyme. The inhibition capacities of the samples were expressed in terms of their IC50 values.
AO assays
Radical scavenging capacity
DPPH radical scavenging activity was measured according to the method of Brand-Williams et al. (1995). ABTS radical cation scavenging assay was carried out by the method of Re et al. (1999) and expressed as trolox equivalent AO capacity (TEAC). Super oxide radical scavenging activity of extracts was evaluated using non-enzymatic phenazine methosulfate- nicotinamide adenine dinucleotide (PMS-NADH) system (Qui et al. 2006). The method reported by Paya et al. (1992) was used to measure the hydroxyl radicals scavenging capacity of the extracts. DPPH, super oxide and hydroxyl radical scavenging capacities of the extracts/compounds were expressed as IC50 values.
Cellular oxidative stress inhibition
Cytoprotective effect against the oxidative stress induced by H2O2 was measured by determining intracellular content of ROS. Intracellular ROS levels were measured employing DCFH-DA. DCFH-DA is cleaved intracellularly by non-specific esterase and turn to high fluorescent 2,7-dichlorofluorescein (DCF) upon oxidation by ROS, which were analyzed with FACS Aria II (BD Bioscience, San Jose, USA). C2C12 cells pretreated with ethyl acetate extract of turmeric were incubated with DCFH-DA at 37 °C for 1 h and then read in FACS Aria II.
LDL oxidation inhibition
Malondialdehyde (MDA) formed by the oxidation of LDL was measured by reaction with TBA according to the method of Kotamballi et al. (2002) with slight modification. Various concentrations (0.2–10 μg/ml) of turmeric extracts were taken in test tubes; 50 μg/mL LDL and 50 μL copper sulphate was added and the volume was made up to 1.5 mL with phosphate buffer (pH 7.4) and incubated at 37 °C. Aliquots of 0.5 mL from each tube were drawn at 2 h intervals and 250 μL of TBA (1 % in 50 mM NaOH) was added followed by 250 μL of TCA (2.8 %). Samples were incubated at 95 °C for 45 min. Tubes were cooled to room temperature and a pink chromogen was extracted by centrifugation. TBA-reactive species in the pink chromogen was detected by fluorescence at 515 nm excitation and 553 nm emission using a multiplate reader (Synergy-4, Biotek, USA). Data expressed in terms of MDA equivalent, which was estimated by comparison with the standard ascorbic acid upon oxidation. From the amount of MDA, the percentage inhibition was calculated using the formula,
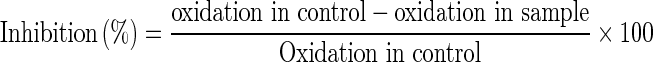 |
Statistical analysis
Results were reported as mean ± standard deviation of 5 trials. Significant differences between means were determined by ANOVA followed by Turkey’s pair wise comparison test at a level of p < 0.05. Analysis was performed using Microsoft Office Excel 2003.
Results and discussion
Antidiabetic potential
Glucosidase inhibition
Elevated PHg is one of the risk factors associated with diabetes. Glucosidase inhibitors play a major role in managing PHg in diabetic patients. In this study turmeric extracts were screened for their inhibitory activity against α-glucosidase and α-amylase enzymes and the results are given in Fig. 1. Ethyl acetate, methanol and water extracts inhibited α-glucosidase activity in dose dependent manner with IC50 values 0.4, 3.1 and 12.6 μg/mL respectively (Fig. 1a). IC50 values obtained for α-amylase inhibitory potential of ethyl acetate, methanol and water extracts were respectively 71.6, 90.3 and 498.3 μg/mL (Fig. 1b). Under the experimental conditions, the standard glucosidase inhibiting drug acarbose inhibited α-glucosidase and α-amylase enzymes with IC50 values 17.1 and 290.6 μg/mL respectively. Ethyl acetate extract had the highest α-glucosidase and α-amylase inhibitory potential among the extracts. Glucose inhibitory potential of both ethyl acetate and methanol extracts was significantly (p < 0.05) higher than those of acarbose
Fig. 1.
Biochemical changes in turmeric rhizome extracts as function of concentrations in different solvents (mean ± SD, n = 3) [a: α-glucosidase inhibitory potential, b: α-amylase inhibitory potential, c: antiglycation activity, d: cellular oxidation stress reduction activity, e: LDL oxidation inhibitory potential, f: ACE inhibitory potential (EtOAc Ex ethyl acetate extract; MeOH Ex methanol extract; Water Ex water extract)]
Antiglycation effects
The oxidation process is believed to play an important role in the etiology and prevalence of diabetes. In diabetic patients hyperglycemia coupled with increased concentration of reactive oxygen species leads to Maillard or glycation reactions. AGEs thus formed from protein and carbohydrates in presence of reactive oxygen species play major role in occurrence and prevalence of diabetes and related disorders (Singh et al. 2001). The role of AGEs in occurrence of degenerative diseases and aging is well established. Agents those inhibit or retard AGEs formation are believed to have therapeutic potential in diabetic patients (Singh et al. 2001). In this study turmeric extracts were evaluated for their ability to retard glycation reaction between albumin and glucose (Fig. 1c). Turmeric extracts except hexane extract were potent to inhibit the glycation reaction. Similar to glucosidase inhibition, in antiglycation activity also ethyl acetate (IC50 = 0.09 μg/mL) showed the highest potential followed by methanol (IC50 = 1.48 μg/mL) and water (IC50 = 10.42 μg/mL) extracts. Antiglycation potential of turmeric ethyl acetate extract was about 800 times higher than ascorbic acid.
Antioxidant activity
Radical scavenging capacity
Radical scavenging capacities of turmeric extracts and curcumin are given in Table 1. DPPH and ABTS are two important chemical probes used for evaluating the radical scavenging capacities of plant extracts. Ethyl acetate extract (11.4 μg/mL) was found to exhibit the highest DPPH radical scavenging capacity followed by methanol, water and hexane extracts. Turmeric extracts except hexane extract effectively scavenged ABTS radicals. The order of reactivity of the extracts was similar to that of DPPH. Hydroxyl and super oxide radicals are formed in living system as results of many physiological reactions. Hydroxyl radicals are highly potent oxidants that react with almost all biomolecules found in living systems. Ethyl acetate extract showed a potent hydroxyl radical scavenging capacity followed by methanol and water extracts. Super oxide radical as a free radical as well as a precursor for many reactive oxygen species is known to be harmful to cellular components. Super oxide radical scavenging capacity of extracts also followed an activity order of ethyl acetate, methanol, water and hexane. Among the turmeric extracts studied ethyl acetate extract showed the highest DPPH (11.4 μg/mL), ABTS (7.78 μg/mL), hydroxyl (1.2 μg/mL) and super oxide (58.6 μg/mL) radical scavenging capacity.
Table 1.
Radical scavenging capacities of turmeric rhizome extracts (mean ± SD, n = 5)
| Solvent/compounds | IC50 $ (μg/mL) | TEAC* (nM) | ||
|---|---|---|---|---|
| DPPH# | Super Oxide | Hydroxyl | ||
| Hexane | 297.3 ± 14.1f | NS | 533.0 ± 27.6 e | NS |
| Ethyl acetate | 11.4 ± 0.81 c | 59.1 ± 3.81 c | 13.1 ± 1.92 b | 7.80 ± 0.25 c |
| Methanol | 53.7 ± 0.82 d | 80.3 ± 5.13 d | 23.2 ± 1.43 c | 2.40 ± 0.33 b |
| Water | 77.6 ± 1.15 e | 181.0 ± 9.72 e | 93.0 ± 8.41 d | 0.60 ± 0.14 a |
| Curcumin | 6.90 ± 0.43 b | 37.2 ± 4.17 b | 12.8 ± 1.96 b | 11.6 ± 0.91 d |
| Reference compound | 3.20 ± 0.21 a (gallic acid) | 23.9 ± 2.14 a (catechin) | 8.90 ± 0.75 a (catechin) | – |
Different letters in the same column indicate significant difference between values (n = 5, Turkey P < 0.05)
NS Not significant (<0.01)
$IC50: Concentration required to inhibit 50 % of the activity
#DPPH: 2,2-diphenyl-1-picrylhydrazyl
*TEAC: ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) radical scavenging capacity in trolox equivalence (nanomoles)
Cellular oxidative stress reduction potential
The ability of the extracts to decrease the oxidative stress in cells was evaluated. For this, intracellular oxidative stress induced by H2O2 in C2C12 cells was monitored by flow cytometer using DCFH-DA as a probe. The concentration of fluorescent DCF formed from non-florescent DCFH by the action of cellular esterase and subsequent oxidation by various oxidants indicates intracellular oxidation stress. The oxidative stress reduction potential of turmeric extracts is given Fig. 1d. The capacity of 10, 50 and 100 μg/mL extracts to reduce the oxidative stress was measured and compared with that of 25 μg/mL of ascorbic acid. Ethyl acetate, methanol and water extracts showed cellular oxidative stress reduction potential in dose dependent manner. Ethyl acetate extract was more potent than methanol and water extracts. Reduction capacity of 100 μg/mL of ethyl acetate was comparable to that of 25 μg/mL of ascorbic acid. In the studied concentration range hexane extract did not show a measurable reduction potential.
Inhibition of human LDL oxidation
The free radical induced peroxidation of LDL critically contributes to the risk of human atherosclerosis. Oxidized LDL gets deposited in artery wall and lead to atherosclerosis and related disorders. AOs are believed to play a major role in preventing the oxidation of LDL. In this study turmeric ethyl acetate, methanol and water extracts were found as effective in retarding the oxidation of LDL in dose dependent manner (Fig. 1e). Interestingly ethyl acetate extract (IC50 = 0.6 μg/mL) was 40 times more effective than ascorbic acid (IC50 = 24.5 μg/mL) in inhibiting LDL oxidation. LDL oxidation inhibitory potential of turmeric extracts was in correlation with their radical scavenging and cellular oxidation stress reduction potentials. The results were in agreement with the previous report on the LDL oxidation capacity of turmeric fractions (Ramírez-Tortosa et al. 1999).
ACE inhibitory potential
The antihypertensive action of turmeric extracts was evaluated in terms of their ACE inhibitory capacity. ACE inhibitory potential of extracts was quantified by monitoring the formation of hippuric acid by the action of ACE on HHL. The dose response curves for the activity of extracts and captopril are given in Fig. 1f. Ethyl acetate (IC50 = 0.06 μg/mL), methanol (IC50 = 0.19 μg/mL) and water (IC50 = 0.38 μg/mL) extracts were more potent in inhibiting ACE activity than the reference captopril (IC50 = 6.28 μg/mL). Similar to AO and antidiabetic potentials, ethyl acetate extract had the highest ACE inhibitory capacity followed by methanol and water extracts. The values indicated that turmeric extracts could be a good source of natural antihypertensive agents.
Composition of extracts
Phenolic and curcuminoid contents in the extracts are given in Table 2. Hexane, ethyl acetate, methanol and water extracts of turmeric respectively had 98, 624, 297 and 231 mg/g of phenolics. Since curcuminoids are reported as the major bioactive principles in turmeric rhizome (Pathania et al. 2006; Singh et al. 2010), composition of curcuminoids in terms of C-1, C-2 and C-3 in the extracts was estimated by HPTLC analysis (Table 2). Curcuminoids content in the extracts followed a decreasing order of ethyl acetate (607.3 mg/g), methanol (184.0 mg/g), hexane (34.1 mg/g) and water (25.1 mg/g). C-1 was found as the prominent curcuminoids in all the extracts followed by C-2 and C-3. Number of reports describing curcuminoid composition of turmeric extracts is available (Gupta et al. 1999; Pathania et al. 2006; Wichitnithad et al. 2009). Since the composition largely depends on the variety and maturity of rhizomes and extraction conditions, the present results were not attempted to compare with the available reports. The positive correlation obtained between curcuminoids content and AO, antidiabetic and ACE inhibitory capacities of the extracts further confirmed their role in bioactivities of turmeric rhizome.
Table 2.
Phenolic and curcuminoid composition of turmeric rhizome extracts (mg/g) (mean ± SD, n = 5)
| Solvent | Phenolics | Curcumin | Demethoxycurcumin | Bis-demethoxycurcumin | Total Curcuminoids |
|---|---|---|---|---|---|
| Hexane | 98.4 ± 4.39 | 27.2 ± 1.72 | 5.10 ± 0.34 | 1.70 ± 0.14 | 34.0 |
| Ethyl acetate | 624.5 ± 10.2 | 343.2 ± 12.4 | 192.0 ± 8.83 | 72.1 ± 3.92 | 607.3 |
| Methanol | 297.5 ± 24.4 | 101.2 ± 9.14 | 55.3 ± 1.51 | 27.6 ± 1.73 | 184.1 |
| Water | 231.4 ± 21.4 | 10.1 ± 0.92 | 8.70 ± 0.24 | 6.30 ± 0.44 | 25.1 |
High antidiabetic efficacy of turmeric rhizome was revealed in this study. Glucosidase inhibitory potential showed by turmeric ethyl acetate extract was significantly (p ≤ 5) higher than those of the reference drug acarbose. Glucosidase inhibitors are reported to delay carbohydrate digestion and prolong overall carbohydrate digestion time causing a reduction in the rate of glucose absorption and consequently blunting the postprandial plasma glucose rise in diabetic patients (Gin and Rigalleau 2000). The high antiglycation potential of turmeric rhizome revealed in this study further confirmed its importance as an antidiabetic agent. The positive correlation between glucosidase and AGEs formation inhibitory potential of the extracts and their curcuminoid contents indicated curcuminoids as the major antidiabetic principles in the rhizome. Curcumin has been shown to decrease blood glucose levels in diabetic animals (Nishiyama et al. 2005; Murugan and Pari 2007). Turmeric extracts were also found as effective in inhibiting ACE activity. Inhibition of ACE mediated conversion of angiotensin I to angiotensin II is considered as an effective therapeutic hypertension control approach. ACE inhibitors can also reduce the vascular and renal damages in diabetic patients (Kshirsagar et al. 2000). The potential of turmeric rhizome as antidiabetic and antihypertensive agents as revealed by this study rationalized its traditional usage in antidiabetic and antihypertensive formulations.
Increased levels of reactive oxygen species are seen in diabetic patients (Greismacher et al. 1995). Hyperglycemia coupled with oxidative stress favors glycation reactions and subsequently contributes to the diabetic complications and other degenerative diseases. Many studies have demonstrated AO potential of turmeric rhizomes (Naito et al. 2002; Ramírez-Tortosa et al. 1999; Braga et al. 2003; Singh et al. 2010). In this study, AO potential of turmeric was further confirmed by free radical scavenging, cellular oxidative stress reduction and LDL oxidation inhibition studies. Antidiabetic capacities of the extracts were in correlation with their AO capacities. Ethyl acetate extract showed the highest AO and antidiabetic potentials. Oxidation is believed to have a major role in AGEs formation. The intermediate carbonyl compound formed by the oxidation of Amadori product reacts with lysine and arginine residues to form protein cross links and AGEs (Voziyan et al. 2003). Several antioxidative and metal chelating compounds have been shown to prevent AGEs formation (Yamaguchi et al. 2000; Kim and Kim 2003). The positive correlation obtained between the antiglycation potential and AO potential of turmeric extracts in this study also indicated the role of AOs in preventing AGEs formation. The antiglycation agents such as ALT-711 and arbutin have also been reported to improve skin hydration and elasticity (Jedsadayanmata 2005; Vasan et al. 2003). AGEs mediated cross-linking of collagen is considered to contribute to age related skin stiffening from the loss of collagen elasticity (Dyer et al. 1993). The high antiglycation activity shown by the turmeric extracts also rationalized the traditional usage of turmeric paste as a tropical skin softener.
Conclusion
Turmeric rhizome extracts showed high potential to inhibit glucosidase activities and glycation reactions. The extracts were also effective in scavenging free radicals and inhibiting LDL and cellular oxidations and ACE activity. The high antidiabetic, AO and antihypertensive capacities of turmeric rhizome revealed in this research highlighted its potential to serve as a source for preventive and therapeutic agents for the management of diabetes and related disorders.
Acknowledgments
We gratefully acknowledge the financial support provided by Council of Scientific and Industrial Research, India.
References
- Annapurna A, Suhasin G, Akondi RB, Prakash GJ, Reddy CS. Anti-cancer activity of curcuma longa linn. (Turmeric) J Pharm Res. 2011;4:1274–1276. [Google Scholar]
- Apostolidis E, Kwon YI, Shetty K. Inhibitory potential of herb, fruit, and fungal-enriched cheese against key enzymes linked to type 2 diabetes and hypertension. Inno Food Sci Emerg Tech. 2007;8:46–54. doi: 10.1016/j.ifset.2006.06.001. [DOI] [Google Scholar]
- Barnett AH. Diabetes and hypertension. Br Med Bull. 1994;50:397–407. doi: 10.1093/oxfordjournals.bmb.a072899. [DOI] [PubMed] [Google Scholar]
- Braga MEM, Leal PF, Carvalho JE, Meireles MAA. Comparison of yield, composition and antioxidant activity of turmeric (Curcuma longa L.) extracts obtained using various techniques. J Agric Food Chem. 2003;51:6604–6611. doi: 10.1021/jf0345550. [DOI] [PubMed] [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. Lebensm Wiss Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Chattopadhyay I, Biswas K, Bandyopadhyay U, Banerjee KR. Turmeric and curcumin: biological actions and medicinal applications. Curr Sci. 2004;87:44–53. [Google Scholar]
- Cushman DW, Cheung HS. Spectrophotometric assay and properties of the angiotensin-converting enzyme of rabbit lung. Biochem Pharmacol. 1971;20:1637–1648. doi: 10.1016/0006-2952(71)90292-9. [DOI] [PubMed] [Google Scholar]
- Dyer DG, Dunn JA, Thorpe SR, Bailie KE, Lyons TJ, Mc Cance DR, Baynes JW. Accumulation of Maillard reaction products in skin collagen in diabetes and aging. J Clin Invest. 1993;91:2463–2469. doi: 10.1172/JCI116481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gin H, Rigalleau V. Post-prandial hyperglycemia and diabetes. Diab Metab. 2000;26:265–272. [PubMed] [Google Scholar]
- Greismacher A, Kindhauser M, Andert SE, Anderty ME, Schreiner W, Toma C, Mueller MD. Enhanced serum levels of thiobarbituric-acid-reactive substances in diabetes mellitus. Am J Med. 1995;98:469–474. doi: 10.1016/S0002-9343(99)80347-7. [DOI] [PubMed] [Google Scholar]
- Gupta AP, Gupta MM, Kumar S. Simultaneous determination of curcuminoids in curcuma samples using high performance thin layer chromatography. J Liq Chrom Rel Tech. 1999;22:1561–1569. doi: 10.1081/JLC-100101751. [DOI] [Google Scholar]
- Jayaprakasha GK, Rao LJM, Sakariah KK. Chemistry and biological activities of C. longa. Trend Food Sci Technol. 2005;16:533–548. doi: 10.1016/j.tifs.2005.08.006. [DOI] [Google Scholar]
- Jedsadayanmata A. In vitro antiglycation activity of arbutin. Naresuan Univ J. 2005;13:35–41. [Google Scholar]
- Kim HY, Kim K. Protein glycation inhibitory and antioxidative activities of some plant extracts in vitro. J Agri Food Chem. 2003;2003(51):1586–1591. doi: 10.1021/jf020850t. [DOI] [PubMed] [Google Scholar]
- Kotamballi N, Chidambara M, Ravendra PS, Jayaprakasha GK. Antioxidant activities of grape (Vitis vinifera) pomace extracts. J Agric Food Chem. 2002;50:5909–5914. doi: 10.1021/jf0257042. [DOI] [PubMed] [Google Scholar]
- Kshirsagar AV, Joy MS, Hogan SL, Falk RJ, Colindres RE. Effect of ACE inhibitors in diabetic and nondiabetic chronic renal disease: a systematic overview of randomized placebocontrolled trials. Am J Kidney Dis. 2000;35:695–707. doi: 10.1016/S0272-6386(00)70018-7. [DOI] [PubMed] [Google Scholar]
- Lekshmi PC, Arimboor R, Raghu KG, Nirmala MA (2011) Turmerin, the antioxidant protein from turmeric (Curcuma longa) exhibits antihyperglycemic effects. Nat Prod Res. doi:10.1080/14786419.2011.589386 [DOI] [PubMed]
- Matsuura N, Aradate T, Sasaki C, et al. Screening system for the Maillard reaction inhibitor from natural products extacts. J Health Sci. 2002;48:520–526. doi: 10.1248/jhs.48.520. [DOI] [Google Scholar]
- Murugan P, Pari L. Influence of tetrahydrocurcumin on erythrocyte membrane bound enzymes and antioxidant status in experimental type 2 diabetic rats. J Ethnopharmacol. 2007;113:479–486. doi: 10.1016/j.jep.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Naito M, Wu X, Nomura H, Kodama M, Kato Y, Kato Y, Osawa T. The protective effects of tetrahydrocurcumin on oxidative stress in cholesterol-fed rabbits. J Atheroscler Thromb. 2002;9:243–250. doi: 10.5551/jat.9.243. [DOI] [PubMed] [Google Scholar]
- Nathan DM. Long term complications of diabetes mellitus. N Engl J Med. 1993;328:1676–1685. doi: 10.1056/NEJM199306103282306. [DOI] [PubMed] [Google Scholar]
- Nishiyama T, Mae T, Kishida H, et al. Curcuminoids and sesquiterpenoids in turmeric (Curcuma longa L.) suppress an increase in blood glucose level in type 2 diabetic KK-Ay mice. J Agric Food Chem. 2005;53:959–963. doi: 10.1021/jf0483873. [DOI] [PubMed] [Google Scholar]
- Pathania V, Gupta AP, Singh B. Improved HPTLC method for determination of curcuminoids from Curcuma longa. J Liq Chromatogr Rel Tech. 2006;29:877–887. doi: 10.1080/10826070500531417. [DOI] [Google Scholar]
- Paya M, Halliwell B, Hoult JRS. Interactions of a series of coumarins with reactive oxygen species. Scavenging of superoxide, hypochlorous acid and hydroxyl radicals. Biochem Pharmacol. 1992;44:205–214. doi: 10.1016/0006-2952(92)90002-Z. [DOI] [PubMed] [Google Scholar]
- Qui H, Zhan Q, Zhao T, Hu R, Zhan K, Li Z. Invitro antioxidant activity of acetylated and benzoylated derivatives of polysaccharide extracted from Ulva pertusa (chlorophyta) Bioorg Med Chem Lett. 2006;16:2441–2445. doi: 10.1016/j.bmcl.2006.01.076. [DOI] [PubMed] [Google Scholar]
- Ramírez-Tortosa MC, Mesa MD, Aguilera MC, Quiles JL, Baro L, Ramirez-Tortosa CL, Martinez-Victoria E, Gil A. Oral administration of a turmeric extract inhibits LDL oxidation and has hypocholesterolemic effects in rabbits with experimental atherosclerosis. Atherosclerosis. 1999;147:371–378. doi: 10.1016/S0021-9150(99)00207-5. [DOI] [PubMed] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Rad Biol Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Sato M, Ramarathnam N, Suzuki Y, Ohkuho T, Takeuchi M, Ochi H. Varietal differences in the phenolic content and superoxide potential of wines from different sources. J Agric Food Chem. 1996;44:37–41. doi: 10.1021/jf950190a. [DOI] [Google Scholar]
- Singh R, Barden A, Mori T, Beilin L. Advanced glycation end-products: a review. Diabetologia. 2001;44:129–146. doi: 10.1007/s001250051591. [DOI] [PubMed] [Google Scholar]
- Singh G, Kapoor IPS, Pratibha S, Heluani CSD, Lampasona MPD, Catalan CAN. Comparative study of chemical composition and antioxidant activity of fresh and dry rhizomes of turmeric (Curcuma longa Linn) Food Chem Toxicol. 2010;48:1026–1031. doi: 10.1016/j.fct.2010.01.015. [DOI] [PubMed] [Google Scholar]
- Vasan S, Foiles P, Founds H. Therapeutic potential of breakers of advanced glycation end product-protein cross links. Arch Biochem Biophys. 2003;419:89–96. doi: 10.1016/j.abb.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Voziyan PA, Khalifah RG, Thibaudeau C, et al. Modification of proteins in vitro by physiological levels of glucose. J Biol Chem. 2003;278:46616–46624. doi: 10.1074/jbc.M307155200. [DOI] [PubMed] [Google Scholar]
- Wichitnithad W, Jongaroonngamsang N, Pummangura S, Rojsitthisak P. A simple isocratic HPLC method for the simultaneous determination of curcuminoids in commercial turmeric extracts. Phytochem Anal. 2009;20:314–319. doi: 10.1002/pca.1129. [DOI] [PubMed] [Google Scholar]
- Witztum JL, Steinberg D. Role of oxidized low density lipoprotein in atherogenesis. J Clin Invest. 1991;88:1785–1792. doi: 10.1172/JCI115499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi F, Ariga T, Yoshimura Y, Nakazawa H. Antioxidative and antiglycation activity of garcinol from Garcinia indica fruit rind. J Agri Food Chem. 2000;48:180–185. doi: 10.1021/jf990845y. [DOI] [PubMed] [Google Scholar]



