Abstract
Objectives
To determine if late preterm (LP) children differ from full term (FT) children in volumes of the cortex, hippocampus, corpus callosum, or amygdala and whether these differences are associated with anxiety symptoms at school-age.
Study design
LP children born between 34 and 36 weeks gestation and FT children born between 39 and 41 weeks gestation from a larger longitudinal cohort had MRI scans at school-age. Brain volumes, cortical surface area and thickness measures were obtained. Anxiety symptoms were assessed using a structured diagnostic interview annually beginning at preschool-age and following the MRI.
Results
LP children (n=21) had a smaller percentage of total, right parietal, and right temporal lobe gray matter volume than FT children (n=87). There were no differences in hippocampal, callosal, or amygdala volumes or cortical thickness. LP children also had a relative decrease in right parietal lobe cortical surface area. LP children had greater anxiety symptoms over all assessments. The relationship between late prematurity and school-age anxiety symptoms was mediated by the relative decrease in right temporal lobe volume.
Conclusion
LP children, comprising 70% of preterm children, are also at increased risk for altered brain development particularly in the right temporal and parietal cortices. Alterations in the right temporal lobe cortical volume may underlie the increased rate of anxiety symptoms among these LP children. These findings suggest that LP delivery may disrupt temporal and parietal cortical development that persists until school-age with the right temporal lobe conferring risk for elevated anxiety symptoms.
Keywords: prematurity, temporal lobe, parietal lobe, cortical surface area
Preterm birth is a major public health problem with well established high risk for adverse medical and developmental outcomes in survivors.(1) These poor outcomes may be mediated by a greater risk for altered brain development associated with prematurity including decreased volumes of gray matter, white matter(2) and in particular regions like the hippocampus(3) and the corpus callosum.(4) These regional alterations have been found during the neonatal period with evidence of persistence into school-age,(5,6) adolescence,(7,8) and adulthood(9,10) especially in those born prior to 34 weeks gestation. Global and regional reductions in gray and white matter volumes have even been found in school-age preterm children that were considered at low risk for neurodevelopmental deficits based on their gestational age and lack of significant medical complications.(11)
Studies of volumetric brain changes in children born preterm, however, have been limited to a focus on very preterm infants, even though LP infants, born between 34 to 36 weeks gestation, comprise approximately 70% of preterm births.(12) LP infants may also be at elevated risk for disrupted brain development. Underscoring the significant brain development that occurs between 34 and 40 weeks, the 34 week brain weighs only 65% of the FT brain, and the cortical volume is only about half that of what it will be at 40 weeks.(13) One study that evaluated neonatal brain volumes of LP infants at term-equivalent age found that they had significantly smaller gray matter volumes and percentage of gray matter volume than term-born infants.(14) Conversely, a study focused on corpus callosum volume, did not find differences in callosal volumes at adolescence between 11 children born LP and 53 FT controls.(15) Given the high prevalence of LP birth, the suggestion of structural differences in LP infants and the steep trajectory of brain development during these final gestational weeks, further investigation of structural brain outcomes in LP children is clearly indicated.
Prematurity is also associated with increased rates of psychiatric disorders including anxiety disorders.(16,17) Indeed, increased rates of anxiety symptoms among those born very preterm (prior to 30-32 weeks gestation) have been reported at both childhood and adulthood(1,16,18), although findings have been mixed.(19) Although there is less evidence in the LP population, available evidence supports an increased rate of psychiatric symptoms in LP children as well, including “emotional”, and anxiety symptoms.(20,21) We have also previously noted increased rates of psychiatric disorders, including a four-fold risk for anxiety disorders, at preschool age among LP children in the cohort to be examined in the analyses presented here.(22) Elevated rates of generalized anxiety disorder and separation anxiety disorders were found in this group. While we found these early childhood anxiety disorders in LP children were mediated by maternal depression, underlying neurodevelopmental differences could confer additional risk.
There has been a burgeoning literature investigating whether altered brain development associated with preterm birth is associated with the increased risk of psychiatric symptoms, with links noted between both global and regional brain differences and childhood psychiatric symptoms(23–25) including anxiety symptoms.(1,26) Given the primary focus of the prior research on children born at earlier gestational ages, it remains unclear whether abnormalities in brain development are also found at an increased rate among LP children. It is also unknown if these brain alterations could underlie an increased risk of anxiety disorders. To address these research gaps, the current study aimed to assess whether children born LP differed from FT children in regions previously associated with preterm birth (hippocampus, corpus callosum) or anxiety (amygdala), in volume of cerebral gray matter or white matter, or in cortical surface area and thickness, and to determine whether any structural differences were related to differences in anxiety symptoms at school age between LP and FT children.
Methods
Data for this analysis was obtained from 108 children born between 34 and 36 weeks gestational age (LP) or between 40 and 41 weeks (FT) from a larger sample enrolled in a 10-year longitudinal study investigating preschool depression (n=306)22. The larger sample was recruited from day cares and preschools around metropolitan St. Louis using a screening checklist to oversample preschoolers with depressive and disruptive symptoms and to include healthy controls. Preschoolers with chronic medical or neurological problems, mental retardation, or autistic spectrum disorders were also excluded from the larger longitudinal study. As previously described, children and their caregivers participated in 3 to 6 comprehensive annual diagnostic and developmental assessments prior to their first neuroimaging session.(27) Participants were screened for standard imaging contraindications. Additionally children with a history of head injury, ischemic insults or stroke or other brain injuries, seizure disorders, history of mechanical ventilation or focal neurological deficits on neurological exam were also excluded. Of the 210 LP (n=40) and FT (n=170) children eligible for the imaging study, 122 had an MRI scan at school-age (6-12 years). Forty-eight children or families refused, 12 cancelled or did not keep appointments repeatedly, 20 had MR imaging contraindications, 2 were deceased and 6 were lost to follow-up/lived out of state. Children also returned for 1 to 3 annual diagnostic assessments after the MRI scan. All study procedures were reviewed and approved by the institutional review board at the Washington University School of Medicine in St Louis. Written informed consent was obtained from parents, and assent was obtained from children.
Children with poor image quality were also excluded (n=14) leaving a final sample of 108 children for inclusion in the analyses that follow.
Preterm Birth
Gestational age (GA) at birth (completed weeks) was reported by the child's primary caregiver. GA groups were categorized as follows: LP (LP; 34-36 weeks), and FT (FT; 40-41 weeks). The focus of this analysis was on the LP population because of our prior findings of increased risk of psychopathology in this group compared to the FT children in this sample.(22)
Anxiety Symptoms
Trained staff conducted annual behavioral/developmental assessments of children and their parents/guardians. Prior to age 8, the Preschool-Age Psychiatric Assessment(28) (PAPA) was administered to assess psychopathology. The PAPA is an interviewer-based diagnostic assessment with empirically established test re-test reliability that covers a broad range of psychiatric symptoms and impairment. The Childhood and Adolescent Psychiatric Assessment (29) (CAPA) was used after age 8 and it also includes a child-report interview. All interviews were audiotaped for quality control and group calibration. In addition to diagnoses, both the PAPA and CAPA provide dimensional counts of symptoms. For this analysis, an anxiety symptom domain score was created for each annual assessment by summing the symptom counts for generalized anxiety disorder (GAD), posttraumatic stress disorder (PTSD), and separation anxiety disorder (SAD). There are 6 possible GAD symptoms, 17 possible PTSD symptoms, and 8 possible SAD symptoms, for a maximum possible anxiety dimensional score of 31. Only symptoms from these anxiety disorders were included as they are assessed both on the PAPA and the CAPA.
Magnetic Resonance Image (MRI) Acquisition
Two 3-dimensional T1-weighted magnetization-prepared rapid gradient echo scans were acquired on a Siemens 3.0-T Tim Trio scanner without sedation (sagittal acquisition; repetition time = 2300 milliseconds; echo time = 3.16 milliseconds; inversion time = 1200 milliseconds; flip angle = 8°; 160 slices; 256 × 256 matrix; field of view = 256 mm; 1.0 mm isotropic voxels; time = 6.3 min per scan). The scan deemed to be of highest quality was used for subsequent processing.
Image Analyses
Total gray and white matter volumes were obtained using FreeSurfer version 5.1, as previously detailed.(30) Briefly, the white and pial surfaces were visually inspected and were regenerated with manual intervention when needed. Total gray matter volumes (cortical and subcortical), white matter volumes, and whole brain volume (total gray matter volume + white matter volume) were obtained. Freesurfer also utilizes an automated labeling system to parcellate the cortex into 34 gyral-based regions of interest (ROIs).(31) This parcellation is applied to the cortical surface using a registration procedure that aligns cortical folding patterns and probabilistically assigns a neuroanatomical label to every point on the cortical surface .(32) ROIs were then summed to provide lobar cortical gray matter volumes, surface areas (defined as the area of the gray matter-white matter junction). Lobar cortical thickness was obtained by weighting the regional thicknesses by their corresponding surface areas. Freesurfer also provided volumes of the amygdala, hippocampus, cerebellum, and corpus callosum.
Pubertal Status
The Tanner staging questionnaire was used to measure children's pubertal status at the time of the scan.(33,34)
Socioeconomic Status
Income to need was used for socioeconomic status (SES) and was defined by total family income divided by the federal poverty level for a family of that size.
Data Analyses
All data analysis was conducted utilizing SPSS (version 21) statistical software. Differences in sociodemographic variables between the LP and FT groups were compared via t-tests and chi-square analyses. Differences in tissue and regional volumes were tested via general linear models, adjusted for age, sex, and pubertal status. Whole brain volume was also entered as a covariate to analyze whether any regional differences were attributable to differences in overall brain volume. To test for mediation, the PROCESS tool for SPSS was used, which is a regression-based approach to test the indirect effect of an independent variable on a dependent variable via a mediator.(35) The significance of the indirect effect was determined via PROCESS by using 10,000 bootstrap resamples to generate 95% confidence intervals (significant when not crossing zero).
Results
Characteristics for the participants are noted in Table 1. LP children were significantly more likely to be white, have a lifetime history of an anxiety disorder, and have mothers with histories of maternal depression. LP children included in the analysis (n=21) did not differ significantly from LP children that were not scanned or that had unusable MRI data (n=19) in birthweight, GA, IQ, adjusted mean anxiety symptoms throughout the assessment periods, preschool-age anxiety symptoms, proportion with a lifetime anxiety diagnosis, income to need, gender, ethnicity, rate of neonatal intensive care unit hospitalization, or rates of maternal major depressive disorder. FT children included in the analysis (n=87) also did not differ significantly from FT children that were not scanned or that did not have usable MRI data (N=82) in these same key variables.
Table 1. Cohort Characteristics.
| Characteristic | Late Preterm (GA 34-36 weeks) (N=21) |
Full Term (GA 40-41 weeks) (N=87) |
|---|---|---|
|
| ||
| Male, % | 61.9 | 51.7 |
|
| ||
| Ethnicity, % | ||
| White | 71.4 | 48.3* |
| Black | 28.6 | 37.9 |
| Other | 15.4 | 13.8 |
|
| ||
| GA at birth, weeks, M (SD) | 35.24 (0.8) | 40.16 (0.4) |
|
| ||
| NICU hospitalization, % | 23.8 | 9.2% |
| Days in NICU, M(SD) | 13.6 (8.8) | 5.6 (5.9) |
|
| ||
| Age at MRI Scan, years, M (SD), | 9.71 (1.27 | 9.99 (1.28) |
| Range | 6-11 | 7-12 |
|
| ||
| Pubertal Status, % | ||
| Prepubertal | 57.1 | 48.8 |
| Early Pubertal | 19.0 | 20.9 |
| Midpubertal | 23.8 | 24.4 |
| Late Pubertal | 0.0 | 5.8 |
|
| ||
| Income to needs ratio M (SD) | 1.98 (1.3) | 2.19 (1.32) |
|
| ||
| IQ, M (SD) | 104.5 (12.7) | 105.6 (16.1) |
|
| ||
| Anxiety Disorder, | ||
| Lifetime, % | 76.2 | 42.5** |
| Current (after MRI), % | 23.5 | 8.4 |
|
| ||
| Number of annual diagnostic assessments, M (SD) | 6.1 (1.2) | 5.8 (1.8) |
|
| ||
| Time between MRI and last diagnostic assessment after MRI, months, M (SD) | 22.1 (11.2) | 20.7 (10.0) |
|
| ||
| History of Maternal MDD, % | 71.4 | 34.5** |
|
| ||
| History of Maternal Anxiety, % | 14.3 | 9.2 |
GA = gestational age, NICU= neonatal intensive care unit
=p<.05,
=p<.01,
=≤ .001
Late Prematurity and Hippocampal, Amygdala and Corpus Callosal Volumes
There were no differences as a function of prematurity in either left (p=0.78) or right (p=0.39) hippocampal volume or left (p=0.14) or right (p=0.22) amygdala volume or total volume of the corpus callosum (p=.10) after adjusting for age, sex, pubertal status, and whole brain volume.
Late Prematurity and Cerebral Gray and White Matter Volumes
LP and FT children did not differ in whole brain volume (p=.45), or total gray (p=.96) or white matter volume (p=.10) after adjusting for age, sex, and pubertal status. After adjusting for whole brain volume in addition to age, sex, and pubertal status, LP children had significantly less relative total gray matter volume (standardized beta = -.137; 95% CI: -.237 to -.037; p=.008). After adjustment for the above mentioned covariates, LP children had on average 1 percent less percent gray matter volume (gray matter volume divided by whole brain volume) than the FT children (LP 62.3% [61.5% - 63.0%]; FT 63.3% [62.8% - 63.8%]; p =.007; bracketed values here and thenceforth give 95% confidence intervals). This decrease in percentage of relative total gray matter (which includes subcortical gray matter) was mainly due to a reduction in the relative percentage of cortical gray matter volume (LP 46.6%, [46.0% to 47.3%]; FT 47.5%, [47.1% to 48.0%]; p =.007). Given our prior report of a relationship between income to need and smaller gray matter volume in the larger study population,(30) we added income to need to the model even though there was not a significant difference in SES between the LP and FT children. Late prematurity remained a significant predictor of relative gray matter volume (standardized beta = -.189 [-.317 to -.062]; p=.004).
Exploratory Analyses of Regional Cortical Volume Differences
Exploratory analyses were conducted to evaluate whether differences in relative gray matter volumes were diffuse or attributable to particular regional volume differences. Freesurfer parcellated regions were combined based on the parcellations of Desikan and colleagues(31) to calculate frontal, temporal, parietal, and occipital lobe gray matter volumes and cingulate gray matter volumes per hemisphere. These volumes were analyzed in MANCOVAs adjusting for age, sex, pubertal status, and whole brain volume for each hemisphere. For the right hemisphere there was an overall main effect of late prematurity (p=.002). Follow-up analyses correcting for multiple comparisons (.05/5 regions = .01) indicated that preterm children had significantly smaller right temporal lobes (adjusted mean 63305 mm3 [61972 – 64638 mm3] vs. adjusted mean 64966 mm3 [61158 – 65775 mm3], respectively; p=.01) and right parietal lobes (adjusted mean 73061 mm3 [71348 – 74774 mm3], vs. adjusted mean 76589 mm3 [75550 – 77628 mm3], respectively; p<.001). Further exploration revealed that the parcellated regions in the temporal lobe with significant differences were the right superior temporal gyrus (p=.036) and the right fusiform gyrus (p=.046), though these relationships did not persist after Bonferroni adjustment for multiple comparisons (alpha =.05/9 parcellated regions=.006). In the right parietal lobe, the right supramarginal gyrus was significantly smaller in the LP children (p<.001), which remained significant after adjustment for multiple comparisons (alpha = .05/5=.01). There was no main effect for late prematurity in left hemisphere regional volumes.
Cortical Thickness, Cortical Surface Area and LP Birth
There was no difference in total cortical surface area (p=.63) nor overall (mean) cortical thickness (p=.19) between FT and LP children after adjustment for age, sex, and pubertal status nor for cortical surface area after additionally adjusting for whole brain volume (p=.57). Given the above noted volume findings, we explored whether regions with volume differences between preterm and term children also differed in cortical surface area or cortical thickness. Regional surface area and thickness in the right parietal and right temporal lobes were analyzed in MANCOVAs adjusting for age, sex, pubertal status, and total surface area and mean thickness respectively. Right parietal surface area was significantly smaller in LP kids compared to term-born children (adjusted mean 17463 mm2 [17190 – 17736 mm2], vs. 17759 mm2 [17625 – 17893 mm2], p=.004). Further analyses demonstrated that the only parcellated region that was significantly decreased in LP children compared to FT children was the right supramarginal gyrus surface area (adjusted mean 3814 mm2 [3592 – 4035 mm2], vs. 4160 mm2 [4025 – 4295 mm2], p=.002, alpha =.01 after Bonferonni correction). Right temporal lobe area trended toward a difference between groups (p=.057), but thickness did not (p=.19). There was also no difference in right parietal lobe thickness (p=.34) between groups.
Late Prematurity and School-Age Anxiety Symptoms
LP children who returned for at least 2 annual assessments (n=38) had higher mean anxiety scores averaged over all assessments than FT children (n=150) after adjusting for age and gender (mean 3.94, SD 12.04 vs. mean 3.01, SD 1.66; beta .568, p=.009). This relationship remained significant when restricted to those who had an available MRI scan for analyses (LP n=21, FT n=87) even after adjusting for age at scan and gender (mean 4.19, SD 1.97 vs. mean 2.90, SD 1.98; beta 1.31, p=.009).
Total and Lobar Gray Matter Volume and Anxiety Symptoms
We investigated whether the differences in total or lobar gray matter volumes (temporal and parietal, since these differed in LP children) between LP and FT children were related to increased anxiety symptoms among LP children. For those children with available assessment data in subsequent study waves following their school-age MRI scan, LP children (n=17) still had higher anxiety scores than FT children (n=83) after adjusting for age at scan and sex (mean 2.43, SD 1.84 vs. mean 1.43, SD 1.44; beta .977, p=.017). Both percent total gray matter and percent right temporal lobe volume were significantly related to their later anxiety symptoms as noted in Table 2, while percent right parietal lobe volume was not (p=.59). When both percent total gray matter and right temporal lobe volume were entered into the same model only percent right temporal lobe volume remained significant (Table 2).
Table 2. Impact of Late Prematurity and Percent Total and Right Temporal Lobe Gray Matter Volume on School Age Anxiety Symptoms.
| Model 1 | Beta (95% CI) | Sig. |
|---|---|---|
| Sex | -.48 (-1.07 to .11) | .108 |
| Age at Scan | -.032 (-.06 to -.01) | .006 |
| % Total Gray Matter Volume | -20.09 (-38.64 to -1.54) | .034 |
| Late Preterm Birth | .78 (-.03 to 1.58) | .059 |
|
| ||
| Model 2 | ||
|
| ||
| Sex | -.56 (-1.13 to .01) | .052 |
| Age at Scan | -.03 (-.05 to -.01) | .003 |
| % Right Temporal Volume | -222.42 (-350.87 to -93.96) | .001 |
| Late Preterm Birth | .64 (-.149 to 1.42) | .111 |
|
| ||
| Model 3 | ||
|
| ||
| Sex | -.56 (-1.13 to .02) | .057 |
| Age At Scan | -.03 (-.06 to -.01) | .004 |
| % Right Temporal Volume | -204.45 (-358.09 to – 50.82) | .010 |
| % Total Gray Matter Volume | -4.62 (-26.04 to 16.81) | .670 |
| Late Preterm Birth | .62 (-.18 to 1.41) | .126 |
This finding raised the question of whether the alteration in the right temporal lobe mediated the relationship between LP birth and subsequent anxiety symptoms assessed ∼ 1.5 years later. We formally tested whether percent right temporal lobe volume mediated the relationship between LP birth and later school age anxiety symptoms. As noted in Figure 2, results yielded a significant indirect effect of LP birth on later anxiety symptoms through percent right temporal lobe volume (beta = -.171, [-.357 to -.048]). The direct effect of preterm group on post MRI anxiety symptoms was no longer significant indicating that the percent right temporal lobe volume mediated the relationship between late prematurity and anxiety symptoms assessed after the MRI scan. We had previously found maternal MDD to mediate preschool age anxiety symptoms.(22) In the current study, LP children at school-age again had higher rates of maternal MDD. Thus, we assessed whether maternal MDD had a similar impact on post scan anxiety symptoms. Maternal MDD was not significantly related to post scan anxiety symptoms (p=.46) after adjusting for age, sex, and LP birth. Percent right temporal lobe volume remained significant (p=.001) when adjusted for maternal MDD.
Figure 2.
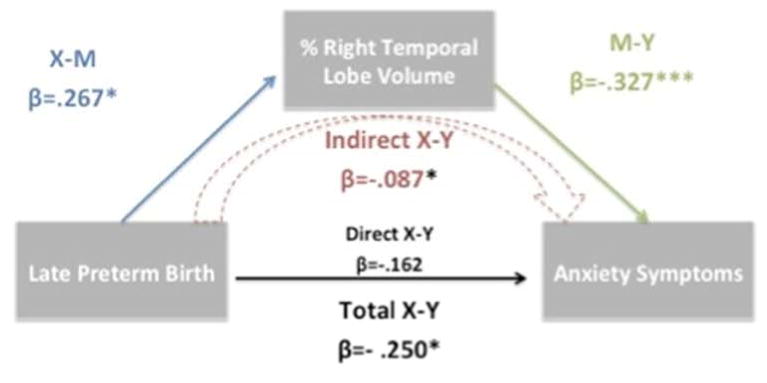
Mediation model of percent right temporal lobe volume mediating the relationship between LP birth and anxiety symptoms. The schematic represents the significant relationship between LP birth and anxiety symptoms (Total X-Y) is indirectly (Indirect X-Y) accounted for by the significant relationships between LP birth and % right temporal lobe volume (X-M) and between % right temporal lobe volume and anxiety symptoms (M-Y).
β= standardized regression coefficient. *= p<.05,***=p<.001
We also investigated whether the anxiety symptoms assessed at study waves before the MRI scan were related to the percent right temporal lobe volume. After adjusting for age, sex, LP birth, and puberty, the average of anxiety symptoms across the annual waves until the time of the scan was significantly related to the percent right temporal lobe volume (p=.02). Given this relationship, we added the mean anxiety symptom score measured until the time of the scan in the model predicting post scan anxiety symptoms. Percent right temporal lobe volume remained a significant predictor of post scan anxiety symptoms (beta -150.48, [-266.01 to -32.94], p =.01). Given the above noted differences in specific regions of the right temporal gyrus (i.e., superior) and right parietal cortex (ie, supramarginal gyrus) in FT and LP children, we also similarly assessed if these regional volumes related to anxiety symptoms. The right superior temporal gyrus volume was not related to pre-scan or post-scan anxiety symptoms in the entire cohort or after adjusting for LP birth. The right supramarginal gyrus was associated with pre-scan anxiety symptoms (p=.04) but this relationship was not significant (p=.25) once LP birth was entered in the model.
Discussion
This study comparing school-age children born LP and FT found that although there were no differences in total brain volume, LP children had a smaller percentage of their brain volumes as gray matter, particularly in the right temporal and parietal lobes. LP children also had a significantly smaller cortical surface area in the right parietal lobe (and nearly so in the right temporal lobe). Additionally, LP children had more anxiety symptoms than the FT children measured at school age, extending the finding noted in this cohort during the preschool period. Notably, the relationship between LP birth and greater anxiety symptoms was mediated by the decreased percentage of gray matter volume in the right temporal lobe. These findings suggest that decreased gray matter volume could be a mechanism by which the risk for increased anxiety is conferred in the LP group.
The finding of smaller percent cortical gray matter volume at school-age in this LP cohort is similar to prior results noted in children born very preterm.(2,7) Further, the study also replicates a result found in another LP cohort scanned at term-equivalent age which also reported a decreased percentage of cerebral volume from gray matter in the LP babies.(14) This study extends those findings by highlighting two broad regions that accounted for the difference in gray matter volume between LP and FT children in this study—the right temporal and right parietal lobes. The temporal lobe has been highlighted as a region very susceptible to the deleterious effects of preterm birth and perhaps the region most vulnerable for LP infants as it is one of the latest maturing regions.(36) Consistent with our volumetric results, among a sample of school age children born between 28 and 41 weeks gestation, longer gestation was associated with greater gray matter density particularly in temporal and parietal regions.(39) LP and FT children also differed in cortical surface area in the temporal lobe and even more prominently in the supramarginal gyrus in the parietal lobe, which has also been noted in other studies of children and adults born at earlier gestational ages.(37,38) We did not, however, find differences in cortical thickness, as we did with cortical surface area, in the regions where we found volume differences. Prior work that has found differences in preterm adolescents cortical thickness have noted this difference to be mostly attributable to children born at earlier gestational ages.(40,41) Others who have investigated cortical thickness in school-age preterm children also found no difference in overall cortical thickness, nor differences in right temporal or right parietal cortices.(42)
This disparity in findings between cortical thickness and cortical surface may be due to disparate developmental trajectories in these measures. Evidence indicates that preterm birth results in delayed cortical maturation,(43) with potential decreases in both cortical surface area and thickness at birth. Additionally, the typical cortical thinning that is rapid during school-age (44) may be delayed in preterm children. Thus, children born FT may be declining in cortical thickness earlier and/or more rapidly than LP resulting in no detectable difference between LP and FT children in cortical thinning at school-age. Cortical surface area, however, has a different trajectory, and continues to increase throughout childhood and adolescence (44). Thus, LP children may have delayed growth in cortical surface area from birth and remain delayed in this measure at school-age. These findings may suggest that the volume differences in this study may have been present since birth; however, prospective studies with imaging at birth are needed to test this hypothesis.
School-age children with a history of LP birth had more symptoms of anxiety disorders than children born FT, extending our previous findings in this cohort reporting preschool outcomes.(22) Importantly, this relationship between LP birth and school-age anxiety symptoms was mediated by their relative decreased volume in the right temporal lobe, suggesting that right temporal lobe volume alterations may be partly responsible for the anxiety symptoms associated with prematurity.
Previous work supports volumetric changes across many areas of cortex in adult anxiety disorders including rostral and dorsal anterior cingulate, insula, superior temporal, supramarginal, angular, and occipital cortices.(45–47) Interestingly, larger volumes have been found in the right superior temporal gyrus of pediatric patients with both GAD and PTSD.(48,49) In the current study and consistent with the regions noted in this prior work, anxiety symptoms were significantly associated with right supramarginal gyrus and right temporal lobe volumes. Notably, however, the association between right supramarginal gyrus volumes and anxiety was completely explained by history of late pre-term birth, whereas the association between right temporal lobe volume and anxiety remained significant when accounting for prematurity. The current study may therefore inform previously reported relationships between regional volumetric differences in supramarginal gyrus and anxiety and suggests that prematurity may be an important covariate in studies relating brain volumes to anxiety symptoms. At the intersection of the right supramarginal gyrus and right temporal lobe lies the right temporal-parietal junction, a component of the ventral attention network (VAN).(50) A recent model proposes specific alterations of the VAN in anxiety disorders,(51), and we previously linked changes in resting state functional connectivity of the VAN to children with a history of depression or anxiety in this same cohort.(52) Of course, VAN alterations are only one of many possible explanations for the volume changes reported in this study.
This study did not detect differences in hippocampus or corpus callosum volumes between LP and FT children. Previous studies of hippocampal volumes in preterm children have been mixed(53,54)(55), with differences generally reported only in investigations of children born younger than 34 weeks gestation. As mentioned above, a previous study comparing LP and FT children also did not detect differences in corpus callosal volumes.(15) Despite increased rates of anxiety disorders in the LP children, we also found no evidence of group differences in amygdala volumes. These negative findings, however, must be considered in the context that volumetric changes in these regions are inconsistently demonstrated in child and adolescent populations.
While this study benefits from the use of longitudinal assessments utilizing semi-structured age-appropriate diagnostic interviews, there are some limitations. Oversampling of children with depressive symptoms, known to be comorbid with anxiety, prohibits inferences about the rates of anxiety among LP in the community. We previously noted, however, increased rates of anxiety disorders in this cohort in LP children without comorbid depression diagnoses.(22) Additionally, this study excluded children with known neurological or major medical disorders. Thus, volumetric differences between more disabled LP children may differ or be more pronounced. Another potential limitation of the study is maternal report of gestational age, although maternal report has demonstrated high sensitivity and specificity for preterm birth (56) suggesting sufficient accuracy of maternal report. Another potential limitation of the study design is that the interval between MRI and psychiatric evaluation was somewhat variable for each child. There was, however, no significant difference in this interval between the LP and FT children. Also, while percent right temporal lobe volume mediated the relationship between LP birth and later school age anxiety symptoms, mediation analysis does not necessarily prove a causal relationship. Finally, future work would benefit from cohorts with larger samples of LP children.
The findings in this study indicate that altered brain development particularly in the right temporal lobe may relate to the increased rate of anxiety symptoms found in LP children at school-age. Such a risk trajectory is plausible given the known function of this region in anxiety related emotion processing. It remains unclear if these altered brain volumes were also present at birth and represent a biomarker of risk for the preschool age anxiety symptoms previously reported in this cohort. The finding of a significant relationship between anxiety symptoms prior to the scan and percent temporal lobe volume is suggestive. Future work that evaluates brain volumes at birth and assesses subsequent development of behavioral inhibition or later preschool anxiety symptoms will be needed to identify these children at the earliest possible time point for intervention. Future work will also need to determine the functional correlates of these structural alterations, which may help guide future treatment development in this population particularly vulnerable to anxiety disorders.
In summary, school-age children born LP had significantly smaller adjusted right temporal lobe and right parietal lobe gray matter than children born FT. The increased rate of anxiety symptoms in LP children persisted into school-age and was mediated by this alteration in right temporal lobe gray matter.
Figure 1.
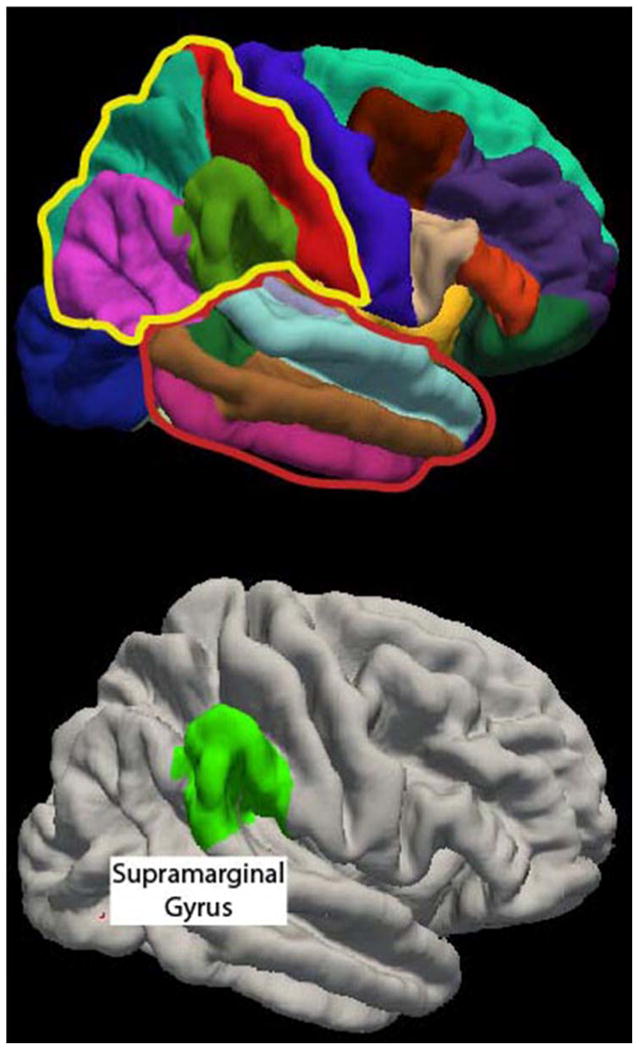
Top, Right hemisphere parcellations of cortical surface regions generated by Freesurfer. The two lobes in which LP children had significantly smaller percentages of cortical gray matter were the right parietal lobe (outlined in yellow) and the right temporal lobe (outlined in red). Bottom, After correcting for multiple comparisons, adjusted volume in the right supramarginal gyrus was significantly smaller in the LP children relative to children born FT.
Acknowledgments
The authors wish to thank the entire EEDP team for assistance in manuscript preparation and the families that participated in this research.
Supported by the National Institute of Mental Health (MH64769 [to J.L.] and RO1 MH090786 [to J.L., D.B., and K.B.]). C.R. was supported by the National Center for Advancing Translational Sciences (UL1 TR000448 sub award KL2 TR000450). C.M. was supported by the National Institute of Mental Health (RO1 MH090786-03S1). J.L. received funding from the Sidney R. Baer Jr. Foundation and the CHADS Coalition, and has served as a consultant for the Food and Drug Administration. D.B. has received consultant compensation from Pfizer, Amgen, and Roche.
Footnotes
The other authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Spittle AJ, Treyvaud K, Doyle LW, Roberts G, Lee KJ, Inder TE, et al. Early emergence of behavior and social-emotional problems in very preterm infants. J Am Acad Child Adolesc Psychiatry. 2009 Sep;48(9):909–18. doi: 10.1097/CHI.0b013e3181af8235. [DOI] [PubMed] [Google Scholar]
- 2.Inder TE, Warfield SK, Wang H, Hüppi PS, Volpe JJ. Abnormal cerebral structure is present at term in premature infants. Pediatrics. 2005 Feb;115(2):286–94. doi: 10.1542/peds.2004-0326. [DOI] [PubMed] [Google Scholar]
- 3.Beauchamp MH, Thompson DK, Howard K, Doyle LW, Egan GF, Inder TE, et al. Preterm infant hippocampal volumes correlate with later working memory deficits. Brain J Neurol. 2008 Nov;131(Pt 11):2986–94. doi: 10.1093/brain/awn227. [DOI] [PubMed] [Google Scholar]
- 4.Thompson DK, Inder TE, Faggian N, Johnston L, Warfield SK, Anderson PJ, et al. Characterization of the corpus callosum in very preterm and full-term infants utilizing MRI. NeuroImage. 2011 Mar 15;55(2):479–90. doi: 10.1016/j.neuroimage.2010.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kesler SR, Ment LR, Vohr B, Pajot SK, Schneider KC, Katz KH, et al. Volumetric analysis of regional cerebral development in preterm children. Pediatr Neurol. 2004 Nov;31(5):318–25. doi: 10.1016/j.pediatrneurol.2004.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peterson BS, Vohr B, Staib LH, Cannistraci CJ, Dolberg A, Schneider KC, et al. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA J Am Med Assoc. 2000 Oct 18;284(15):1939–47. doi: 10.1001/jama.284.15.1939. [DOI] [PubMed] [Google Scholar]
- 7.Nosarti C, Al-Asady MHS, Frangou S, Stewart AL, Rifkin L, Murray RM. Adolescents who were born very preterm have decreased brain volumes. Brain J Neurol. 2002 Jul;125(Pt 7):1616–23. doi: 10.1093/brain/awf157. [DOI] [PubMed] [Google Scholar]
- 8.Isaacs EB, Lucas A, Chong WK, Wood SJ, Johnson CL, Marshall C, et al. Hippocampal volume and everyday memory in children of very low birth weight. Pediatr Res. 2000 Jun;47(6):713–20. doi: 10.1203/00006450-200006000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Lawrence EJ, McGuire PK, Allin M, Walshe M, Giampietro V, Murray RM, et al. The very preterm brain in young adulthood: the neural correlates of verbal paired associate learning. J Pediatr. 2010 Jun;156(6):889–95. doi: 10.1016/j.jpeds.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 10.Nosarti C, Shergill SS, Allin MP, Walshe M, Rifkin L, Murray RM, et al. Neural substrates of letter fluency processing in young adults who were born very preterm: alterations in frontal and striatal regions. NeuroImage. 2009 Oct 1;47(4):1904–13. doi: 10.1016/j.neuroimage.2009.04.041. [DOI] [PubMed] [Google Scholar]
- 11.Soria-Pastor S, Padilla N, Zubiaurre-Elorza L, Ibarretxe-Bilbao N, Botet F, Costas-Moragas C, et al. Decreased regional brain volume and cognitive impairment in preterm children at low risk. Pediatrics. 2009 Dec;124(6):e1161–1170. doi: 10.1542/peds.2009-0244. [DOI] [PubMed] [Google Scholar]
- 12.Martin J, Hamilton B, Ventura S, et al. National vital statistics reports. Hyattsville, MD: National Center for Health Statistics; 2011. Births: Final data for 2009. [PubMed] [Google Scholar]
- 13.Kinney HC. The near-term (late preterm) human brain and risk for periventricular leukomalacia: a review. Semin Perinatol. 2006 Apr;30(2):81–8. doi: 10.1053/j.semperi.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Munakata S, Okada T, Okahashi A, Yoshikawa K, Usukura Y, Makimoto M, et al. Gray matter volumetric MRI differences late-preterm and term infants. Brain Dev. 2013 Jan;35(1):10–6. doi: 10.1016/j.braindev.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 15.Narberhaus A, Segarra D, Caldú X, Giménez M, Pueyo R, Botet F, et al. Corpus callosum and prefrontal functions in adolescents with history of very preterm birth. Neuropsychologia. 2008 Jan 15;46(1):111–6. doi: 10.1016/j.neuropsychologia.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Johnson S, Hollis C, Kochhar P, Hennessy E, Wolke D, Marlow N. Psychiatric disorders in extremely preterm children: longitudinal finding at age 11 years in the EPICure study. J Am Acad Child Adolesc Psychiatry. 2010 May;49(5):453–463.e1. [PubMed] [Google Scholar]
- 17.Scott MN, Taylor HG, Fristad MA, Klein N, Espy KA, Minich N, et al. Behavior Disorders in Extremely Preterm/Extremely Low Birth Weight Children in Kindergarten. J Dev Behav Pediatr JDBP. 2012 Jan 12; doi: 10.1097/DBP.0b013e3182475287. Internet. [cited 2012 Feb 28]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/22245934. [DOI] [PMC free article] [PubMed]
- 18.Burnett AC, Anderson PJ, Cheong J, Doyle LW, Davey CG, Wood SJ. Prevalence of psychiatric diagnoses in preterm and full-term children, adolescents and young adults: a meta-analysis. Psychol Med. 2011 Dec;41(12):2463–74. doi: 10.1017/S003329171100081X. [DOI] [PubMed] [Google Scholar]
- 19.Burnett A, Davey CG, Wood SJ, Wilson-Ching M, Molloy C, Cheong JLY, et al. Extremely preterm birth and adolescent mental health in a geographical cohort born in the 1990s. Psychol Med. 2013 Aug;28:1–12. doi: 10.1017/S0033291713002158. [DOI] [PubMed] [Google Scholar]
- 20.Westrupp EM, Mensah FK, Giallo R, Cooklin A, Nicholson JM. Mental health in low-to-moderate risk preterm, low birth weight, and small for gestational age children at 4 to 5 years: the role of early maternal parenting. J Am Acad Child Adolesc Psychiatry. 2012 Mar;51(3):313–23. doi: 10.1016/j.jaac.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 21.De Jong M, Verhoeven M, van Baar AL. School outcome, cognitive functioning, and behaviour problems in moderate and late preterm children and adults: A review. Semin Fetal Neonatal Med. 2012 Jun;17(3):163–9. doi: 10.1016/j.siny.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 22.Rogers CE, Lenze SN, Luby JL. Late preterm birth, maternal depression, and risk of preschool psychiatric disorders. J Am Acad Child Adolesc Psychiatry. 2013 Mar;52(3):309–18. doi: 10.1016/j.jaac.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skranes J, Vangberg TR, Kulseng S, Indredavik MS, Evensen KAI, Martinussen M, et al. Clinical findings and white matter abnormalities seen on diffusion tensor imaging in adolescents with very low birth weight. Brain J Neurol. 2007 Mar;130(Pt 3):654–66. doi: 10.1093/brain/awm001. [DOI] [PubMed] [Google Scholar]
- 24.Treyvaud K, Ure A, Doyle LW, Lee KJ, Rogers CE, Kidokoro H, et al. Psychiatric outcomes at age seven for very preterm children: rates and predictors. J Child Psychol Psychiatry. 2013 Jul;54(7):772–9. doi: 10.1111/jcpp.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rogers CE, Anderson PJ, Thompson DK, Kidokoro H, Wallendorf M, Treyvaud K, et al. Regional cerebral development at term relates to school-age social-emotional development in very preterm children. J Am Acad Child Adolesc Psychiatry. 2012 Feb;51(2):181–91. doi: 10.1016/j.jaac.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loe IM, Lee ES, Feldman HM. Attention and Internalizing Behaviors in Relation to White Matter in Children Born Preterm. J Dev Behav Pediatr. 2013 Apr;34(3):156–64. doi: 10.1097/DBP.0b013e3182842122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luby JL, Belden AC, Pautsch J, Si X, Spitznagel E. The clinical significance of preschool depression: impairment in functioning and clinical markers of the disorder. J Affect Disord. 2009 Jan;112(1-3):111–9. doi: 10.1016/j.jad.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Egger H, Asher B, Angold A. Center for Developmental Epidemiology, Department of Psychiatry and Behavioral Sciences. The Preschool Age Psychiatric Assessment: Version 1.4. Durham, NC: Duke University Medical Center; 2003. [Google Scholar]
- 29.Angold A, Costello EJ. The Child and Adolescent Psychiatric Assessment (CAPA) J Am Acad Child Adolesc Psychiatry. 2000 Jan;39(1):39–48. doi: 10.1097/00004583-200001000-00015. [DOI] [PubMed] [Google Scholar]
- 30.Luby J, Belden A, Botteron K, Marrus N, Harms MP, Babb C, et al. The effects of poverty on childhood brain development: the mediating effect of caregiving and stressful life events. JAMA Pediatr. 2013 Dec 1;167(12):1135–42. doi: 10.1001/jamapediatrics.2013.3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006 Jul;31(3):968–80. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 32.Fischl B, van der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex N Y N 1991. 2004 Jan;14(1):11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- 33.Carskadon MA, Acebo C. A self-administered rating scale for pubertal development. J Adolesc Health Off Publ Soc Adolesc Med. 1993 May;14(3):190–5. doi: 10.1016/1054-139x(93)90004-9. [DOI] [PubMed] [Google Scholar]
- 34.Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. J Youth Adolesc. 1988 Apr;17(2):117–33. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- 35.Hayes AF. New York: The Guilford Press; 2013. Introduction to mediation, moderation, and conditional process analysis a regression-based approach. Internet. [cited 2013 Sep 8]; Available from: http://search.ebscohost.com/login.aspx?direct=true&scope=site&db=nlebk&db=nlabk&AN;=572976. [Google Scholar]
- 36.Hill J, Inder T, Neil J, Dierker D, Harwell J, Van Essen D. Similar patterns of cortical expansion during human development and evolution. Proc Natl Acad Sci U S A. 2010 Jul 20;107(29):13135–40. doi: 10.1073/pnas.1001229107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lax ID, Duerden EG, Lin SY, Mallar Chakravarty M, Donner EJ, Lerch JP, et al. Neuroanatomical consequences of very preterm birth in middle childhood. Brain Struct Funct. 2013 Mar;218(2):575–85. doi: 10.1007/s00429-012-0417-2. [DOI] [PubMed] [Google Scholar]
- 38.Skranes J, Løhaugen GCC, Martinussen M, Håberg A, Brubakk AM, Dale AM. Cortical surface area and IQ in very-low-birth-weight (VLBW) young adults. Cortex J Devoted Study Nerv Syst Behav. 2013 Sep;49(8):2264–71. doi: 10.1016/j.cortex.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 39.Davis EP, Buss C, Muftuler LT, Head K, Hasso A, Wing DA, et al. Children's Brain Development Benefits from Longer Gestation. Front Psychol. 2011;2:1. doi: 10.3389/fpsyg.2011.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martinussen M, Fischl B, Larsson HB, Skranes J, Kulseng S, Vangberg TR, et al. Cerebral cortex thickness in 15-year-old adolescents with low birth weight measured by an automated MRI-based method. Brain J Neurol. 2005 Nov;128(Pt 11):2588–96. doi: 10.1093/brain/awh610. [DOI] [PubMed] [Google Scholar]
- 41.Nagy Z, Lagercrantz H, Hutton C. Effects of Preterm Birth on Cortical Thickness Measured in Adolescence. Cereb Cortex. 2011 Feb 1;21(2):300–6. doi: 10.1093/cercor/bhq095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zubiaurre-Elorza L, Soria-Pastor S, Junque C, Sala-Llonch R, Segarra D, Bargallo N, et al. Cortical Thickness and Behavior Abnormalities in Children Born Preterm. Draganski B, editor. PLoS ONE. 2012 Jul 30;7(7):e42148. doi: 10.1371/journal.pone.0042148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dean JM, Bennet L, Back SA, McClendon E, Riddle A, Gunn AJ. What brakes the preterm brain? An arresting story. Pediatr Res. 2014 Jan;75(1-2):227–33. doi: 10.1038/pr.2013.189. [DOI] [PubMed] [Google Scholar]
- 44.Schnack HG, van Haren NEM, Brouwer RM, Evans A, Durston S, Boomsma DI, et al. Changes in Thickness and Surface Area of the Human Cortex and Their Relationship with Intelligence. Cereb Cortex. 2014 Jan 9; doi: 10.1093/cercor/bht357. Internet. [cited 2014 Apr 21]; Available from: http://www.cercor.oxfordjournals.org/cgi/doi/10.1093/cercor/bht357. [DOI] [PubMed]
- 45.Talati A, Pantazatos SP, Schneier FR, Weissman MM, Hirsch J. Gray matter abnormalities in social anxiety disorder: primary, replication, and specificity studies. Biol Psychiatry. 2013 Jan 1;73(1):75–84. doi: 10.1016/j.biopsych.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Tol MJ, van der Wee NJA, van den Heuvel OA, Nielen MMA, Demenescu LR, Aleman A, et al. Regional brain volume in depression and anxiety disorders. Arch Gen Psychiatry. 2010 Oct;67(10):1002–11. doi: 10.1001/archgenpsychiatry.2010.121. [DOI] [PubMed] [Google Scholar]
- 47.Asami T, Yamasue H, Hayano F, Nakamura M, Uehara K, Otsuka T, et al. Sexually dimorphic gray matter volume reduction in patients with panic disorder. Psychiatry Res. 2009 Aug 30;173(2):128–34. doi: 10.1016/j.pscychresns.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 48.De Bellis MD, Keshavan MS, Shifflett H, Iyengar S, Dahl RE, Axelson DA, et al. Superior temporal gyrus volumes in pediatric generalized anxiety disorder. Biol Psychiatry. 2002 Apr 1;51(7):553–62. doi: 10.1016/s0006-3223(01)01375-0. [DOI] [PubMed] [Google Scholar]
- 49.De Bellis MD, Keshavan MS, Frustaci K, Shifflett H, Iyengar S, Beers SR, et al. Superior temporal gyrus volumes in maltreated children and adolescents with PTSD. Biol Psychiatry. 2002 Apr 1;51(7):544–52. doi: 10.1016/s0006-3223(01)01374-9. [DOI] [PubMed] [Google Scholar]
- 50.Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008 May 8;58(3):306–24. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sylvester CM, Corbetta M, Raichle ME, Rodebaugh TL, Schlaggar BL, Sheline YI, et al. Functional network dysfunction in anxiety and anxiety disorders. Trends Neurosci. 2012 Sep;35(9):527–35. doi: 10.1016/j.tins.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sylvester CM, Barch DM, Corbetta M, Power JD, Schlaggar BL, Luby JL. Resting state functional connectivity of the ventral attention network in children with a history of depression or anxiety. J Am Acad Child Adolesc Psychiatry. 2013 Dec;52(12):1326–1336.e5. doi: 10.1016/j.jaac.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheong JLY, Anderson PJ, Roberts G, Burnett AC, Lee KJ, Thompson DK, et al. Contribution of brain size to IQ and educational underperformance in extremely preterm adolescents. PloS One. 2013;8(10):e77475. doi: 10.1371/journal.pone.0077475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Kieviet JF, Zoetebier L, van Elburg RM, Vermeulen RJ, Oosterlaan J. Brain development of very preterm and very low-birthweight children in childhood and adolescence: a meta-analysis. Dev Med Child Neurol. 2012 Apr;54(4):313–23. doi: 10.1111/j.1469-8749.2011.04216.x. [DOI] [PubMed] [Google Scholar]
- 55.Gousias IS, Edwards AD, Rutherford MA, Counsell SJ, Hajnal JV, Rueckert D, et al. Magnetic resonance imaging of the newborn brain: manual segmentation of labelled atlases in term-born and preterm infants. NeuroImage. 2012 Sep;62(3):1499–509. doi: 10.1016/j.neuroimage.2012.05.083. [DOI] [PubMed] [Google Scholar]
- 56.Poulsen G, Kurinczuk JJ, Wolke D, Boyle EM, Field D, Alfirevic Z, et al. Accurate reporting of expected delivery date by mothers 9 months after birth. J Clin Epidemiol. 2011 Dec;64(12):1444–50. doi: 10.1016/j.jclinepi.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 57.McCormick MC, Brooks-Gunn J. Concurrent child health status and maternal recall of events in infancy. Pediatrics. 1999 Nov;104(5 Pt 2):1176–81. [PubMed] [Google Scholar]
- 58.Adegboye A, Heitmann B. Accuracy and correlates of maternal recall of birthweight and gestational age. BJOG Int J Obstet Gynaecol. 2008 Jun;115(7):886–93. doi: 10.1111/j.1471-0528.2008.01717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


