Abstract
Growing evidence suggests that early social deprivation impacts the activity of the hypothalamic-pituitary-adrenocortical axis. Early adverse care in the form of institutional or orphanage care provides a human model for early social deprivation. The present study examined changes in diurnal cortisol during the transition to family care in the first two years post-adoption. Children adopted between 15 and 36 months from institutional care were examined four times during their first two years post-adoption (N=58). Comparison groups included same-aged peers reared in their birth families (N=50) and children adopted during their first year from overseas foster care (N=47). Children provided daily cortisol samples at roughly 2, 9, 17, and 25 months post-adoption. Post-institutionalized and post-foster care children exhibited less steep diurnal cortisol compared to non-adopted same-aged peers; these differences did not diminish across the two year period. For post-institutionalized children, lower social care quality in institutions was associated with less steep cortisol slopes. Lastly, shallower diurnal cortisol was a mediator between adoption status and increased behavioral problems two years post-adoption. Consistent with the non-human primate literature, early social deprivation may contribute to early programming of the HPA axis.
Keywords: Cortisol, social deprivation, early adversity, international adoption, problem behavior, early childhood
1. Introduction
There has been a long-standing interest in the impact of early social deprivation on activity of the hypothalamic-pituitary-adrenocortical (HPA) axis. In rodent models, social deprivation induced by prolonged separations of pups from the dam increases the reactivity of the HPA axis through epigenetic modifications in the glucocorticoid gene (Meaney & Szyf, 2005). While rodent models are useful, studies of non-human primates are sometimes more informative because of their greater similarity to humans. In non-human primates, while results are not entirely consistent (Dettmer et al, 2012), early social deprivation appears to down- rather than up-regulate the HPA axis (Meyer et al., 1975; Clarke, 1993; Cirulli et al., 2009). This was confirmed recently in a large study of Rhesus macaques (Hawkley et al., 2012). The results demonstrated that socially depriving infant monkeys (i.e., nursery rearing) resulted in smaller HPA responses to stressors and pharmacological challenges. Notably, HPA activity normalized with increasing social stimulation through early peer experiences. Thus, far from being hyper-active, in rhesus monkeys early social deprivation reduced HPA set point and reactivity.
Children reared in orphanages or similar institutions provide a human model of early social deprivation. Studies of HPA axis activity in these children have yielded mixed results, perhaps because social deprivation is typically confounded with physical deprivation, malnutrition, pathogen exposure and abuse, which may also impact the HPA axis. Despite heterogeneity across institutions, institutional rearing nearly uniformly involves fewer supportive adult-child interactions than most family contexts (Smyke et al., 2007; van IJzendoorn et al., 2011). Toddlers living in an orphanage in Romania were found to exhibit lower morning and slightly elevated evening cortisol levels (Carlson & Earls, 1997); however, preschool-aged children in a Ukrainian institution exhibited normal diurnal rhythms, although those who had been growth-stunted had elevated levels (Dobrova-Krol et al., 2008). The remaining studies have been conducted with children adopted out of institutional care (post-institutionalized, PI). One report on Romanian PI children noted higher cortisol levels over the day, even though children had been in their adoptive families for 6 years or more (Gunnar et al., 2001). All other studies comparing PI to non-adopted children have noted either lower morning cortisol or less marked diurnal rhythms (Gunnar & Vazquez, 2001; Johnson et al., 2011). Similar findings emerged for children in foster care, particularly if they experienced severe early neglect (Bruce et al., 2009). One study followed internationally adopted individuals into adulthood and found that those who were severely neglected prior to adoption had lower morning cortisol and a less marked decrease in cortisol over the day (van der Vegt et al., 2009).
The goals of the present study were threefold: 1) to examine changes in children’s HPA axis activity following adoption, 2) to examine aspects of preadoptive care that may contribute to individual differences in HPA activity, and 3) to examine individual differences in HPA activity in contributing to problem behavior. The majority of studies of the HPA axis in deprived children have either examined them during periods of deprivation or years following removal from these conditions. Little is known about changes in HPA activity as the child transitions from institutional to family care. One study did examine Eastern European children ranging from 7 to 30 months of age at adoption; children’s cortisol was assessed at 1 and 6 months post-adoption and found that the diurnal decrease in cortisol became more robust over that time (Kroupina et al., 2012). However, as that study did not include a sample of non-adopted children we do not know whether all children show increases in the robustness of the rhythm during the same age period. One purpose of this study was to examine changes in diurnal cortisol patterns of PI children over the first two years in the adoptive home and compare them to cortisol trajectories in age-matched non-adopted children. Given the literature on PI children and non-human primates, we expected that the diurnal cortisol slope would be flatter at adoption but become more robust with time spent in the family. However, we expected that the most socially deprived PI children might continue to show a flatter cortisol slope and/or lower morning cortisol even after two years in their new homes.
Children reared in overseas orphanages experience a wide variety of deprivation. In addition to examining change in children’s HPA axis functioning following adoption, the present study sought to examine factors that may contribute to individual differences in any changes that might be observed. The present study examined the role of duration of institutionalization, variations in physical and social care quality, and growth-stunting. Growth-stunting at adoption has been associated with disruptions in diurnal cortisol following adoption. Kroupina and colleagues (2012) noted that it was the growth-stunted children who exhibited the most depressed diurnal rhythm at adoption and the greatest change over time. In addition, Johnson and colleagues (2011) reported that, years after adoption, children who had been growth-stunted at adoption continued to exhibit an altered diurnal cortisol pattern compared to non-adopted children and children who had not been growth-stunted at adoption. Kertes and colleagues (2008) also noted an association between growth stunting and altered HPA activity six or more years after adoption among PI children.
Children adopted from institutions are at heightened risk for numerous behavior problems, including problems with emotion and behavior regulation (Gunnar & van Dulmen, 2007; Colvert et al., 2008; Bos et al., 2011). A third purpose of the present study was to examine whether individual differences in diurnal HPA activity contributed to individual differences in behavioral problems. More specifically, patterns of daily HPA axis activity was examined as a mediator of the relation between early social deprivation and problem behavior. This study focused on children adopted from institutions between 15 and 36 months of age, which is both a common adoption age and late enough that most studies of similarly aged adopted children find a significant percentage of them exhibiting cognitive and emotional difficulties (Zeanah et al., 2011).
In this study we employed several comparison groups. First, we compared PI children to non-adopted children born and raised in families of similarly high socioeconomic status as those who adopt internationally. To disentangle the effects of deprivation due to institutional care from those of broader experiences of deprivation and disruption in early care, we employed a second comparison group of children adopted internationally from foster care. While children adopted from foster care experienced more home-like pre-adoption care, they also experienced several disruptions in early care like the PI children. Some countries largely employ foster care to house wards of the state, while others rely on institutions to care for the same type of children. Whether a child ends up in foster care or institutional care depends primarily on where they live and not on child characteristics (Gunnar et al., 2000). We recruited children who had been adopted by a year of age, a typical adoption age in countries using foster care. The PI group and this second comparison group differed in two key ways. First, the PI children experienced conditions of deprivation for a longer span of early development. Second, deprivation and disruption in the PI group was due uniquely to institutional care. Thus, if their patterns of cortisol production are similar to the PI children, then the effects observed are not specific to institutional care.
2. Method
2.1 Participants
The participants were 66 children who had recently been adopted from institutional care overseas (Post-Institutionalized, PI), 53 children who served as a non-adopted (NA) comparison group reared by parents of similar educations and incomes as parents who adopt internationally and 50 children who served as international adoption comparison group adopted from foster care overseas (Post Foster Care, PFC). Demographic information on the children and their families in the larger longitudinal sample is shown in Table 1. The PFC children were on average 5 months older than the PI and NA children at the start of the study, thus, age was examined as a covariate. Parents provided informed consent at the start of the study and all procedures were approved by the University’s institutional review board. 155 children were included in the present analysis (58 PI, 47 PFC, 50 NA) if they participated in the at-home cortisol collection.
Table 1.
Child and Family Demographics by Group
| PI | PFC | NA | ||
|---|---|---|---|---|
| Total | N | 66 | 50 | 53 |
| Child Sex | N Female | 38 (58%) | 20 (40%) | 27 (51%) |
| Age at Initial Sampling | M (SD) months | 26.4 (5.0) | 32.3 (5.4) | 27.8 (5.8) |
| Child Race | ||||
| African/Black | N | 23 (35%) | -- | 1 (2%) |
| American Indian/Alaska Native | N | 2 (3%) | 9 (18%) | -- |
| Asian | N | 26 (39%) | 38 (76%) | -- |
| White | N | 11 (17%) | -- | 48 (91%) |
| Multiracial | N | 2 (3%) | -- | 4 (7%) |
| Unknown | N | 2 (3%) | 3 (6%) | -- |
| Child Ethnicity | ||||
| Hispanic/Latino | N | 3 (5%) | 12 (24%) | 2 (4%) |
| Region of Origin | ||||
| Africa | N | 22 (33%) | -- | -- |
| Latin America/Caribbean | N | 5 (8%) | 12 (24%) | -- |
| Russian/Eastern Europe | N | 18 (27%) | -- | -- |
| Southeast Asia | N | 21 (32%) | 38 (76%) | -- |
| United States | N | -- | -- | 53 (100%) |
| Primary Caregiver | N Female | 63 (95%) | 44 (88%) | 52 (98%) |
| Single Parent Households | N | 7 (11%) | 1 (2%) | 2 (4%) |
| Household Income Median Range | Median | $75–100K | $75–100K | $75–100K |
| Education Median Level | ||||
| Primary Caregiver | Median | College | College | College |
| Secondary Caregiver | Median | College | College | College |
| Age at Adoption | M (SD) months | 24.7 (5.0) | 9.7 (1.5) | -- |
| Institutional Care Duration | M (SD) months | 18.0 (7.6) | 0.9 (1.0) | -- |
| Foster Care Duration | M (SD) months | 0.3 (1.7) | 7.9 (1.9) | -- |
| Biological Parent Care Duration | M (SD) months | 4.9 (7.4) | 0.1 (0.3) | -- |
Note. Percentages reported in parentheses reflect the percentage within group.
2.2 Procedure and Measures
The children were part of a more extensive examination of recovery from institutional care. PI children were recruited from an adoption medical clinic and through the major adoption agencies in our state. These agencies routinely advise that families visit the clinic soon after arrival. The PFC children were recruited through the International Adoption Project (IAP) Registry maintained by our research group. Letters are mailed every several months to all families who have recently adopted internationally through the major agencies. Interested parents join the registry and provide information about the child’s age at adoption and type of pre-adoption living situation. NA children were recruited from a participant pool maintained by the department through letters sent to all families of live births in the metropolitan area and through website and other advertising.
For PI children the inclusion criteria were age between 15 and 36 months when they came into their adoptive parents’ full time care (18-36 months at recruitment), able to come in for testing within the first 3 months of arrival in the United States, and having been adopted out of an institutional setting (PI children spent an average of 75% of their pre-adoption lives in institutional care). For the PFC children the inclusion criteria were foster care placement and having spent less than 50% of pre-adoption care in any kind of institution. Nearly all PFC children either never experienced institutional care or were in institutions for less than a month. For both the NA and PFC children, inclusion criteria included being between 18 and 36 months at the time of recruitment. Children who were suspected to have been exposed to alcohol prenatally were excluded (6 PI, 1 PFC). Fetal alcohol exposure was determined through photographic screening using the FAS Facial Photographic Analysis Software (Astley & Clarren, 2000). Congenital, and cognitive and endocrine disorders were also exclusion criteria (2 PI). NA comparison children were excluded for atypical development including maltreatment (1 NA) and autism (1 NA). In total there were 8 PI, 2 NA and 1 PFC children excluded. Ns reported above were after exclusion.
2.2.1 Preadoption Care
Information about the child’s preadoption experiences was obtained through parent interviews by a retired adoption social worker. The interviewer guided the parent in creating as complete a timeline as possible regarding where and with whom the child was living from birth onwards. Duration of institutional care was abstracted from the timeline. For the PI parents, if the parent had seen the institution (49/66) the interviewer guided the parent through descriptions of what they had seen. The parent was asked to describe the physical and social care. The interviewer probed for different aspects of physical and social care quality. The physical care construct assessed the quality of the physical environment the child lived in including the cleanliness of the rooms and the children, the quality of clothing relative to room temperature, and availability of toys. The social care construct assessed the quality of caregiver-child relationships including the caregiver-child ratio and quality and type of interaction between caregivers and children (e.g., basic needs care or “assembly line” care vs. individualized social interaction, cuddling, playing). Based on the parent’s descriptions, the interviewer provided 5-point quality ratings (needs poorly met to needs well met) for physical and social care. For reliability, 10 interview scenarios were constructed describing institutional care of various qualities. The interviewer and another adoption social worker read the scenarios and made independent ratings (kappas > .80).
2.2.2 Salivary Cortisol
Parents were trained during laboratory visits to collect saliva according to a standardized protocol. Laboratory visits were timed to the PI children’s arrival in the family, with the initial visit between 1–3 months after arrival (M=1.70 months, SD=.78), and the next at roughly 8, 16, and 24 months post arrival (Session 2: M=8.39, SD=.59; Session 3, M=16.40, SD=.48; and Session 4, M=24.20, SD=.43). Parents were sent home with a saliva collection kit that they returned when completed. Parents were instructed to collect three samples (morning, midday, bedtime) on three days in conjunction with each of the four laboratory sessions (including both weekdays/weekends and consecutive/non-consecutive days). Parents were asked to collect samples on days the child was with the parent and not in daycare or preschool. The saliva collection kit consisted of a daily diary, cotton swabs and Kool-Aid™ crystals, needleless syringes, and a large bottle sealed with a MEMS® 5 TrackCap, 1.5-ml Eppendorf Safe-Lock microtubes, and labels. Cotton dental rolls were used because at the time this study was started, an appropriate synthetic alternative was not yet on the market. The TrackCap data allowed examination of compliance. Parents also recorded the time of each sample. To assess the reliability, parent-reported time data were compared to the TrackCap information. Most parents were quite accurate with the average difference from parent to TrackCap report being 4.9 minutes (SD=12.71). Some families were unable or unwilling to sample at home and some were not able to provide reliable samples. Of 169 participants, 8% were completely missing cortisol data. This resulted in sample size of 155 for analysis (58 PI, 47 PFC, 50 NA).
The parent diaries were examined to determine how long after each session the saliva samples were obtained. Parents were asked to obtain the samples as soon as possible but to avoid days when the child was sick or when unusually arousing events were occurring (e.g., holidays, doctor/dental visits, etc.). At all four collection periods, parents collected saliva on average within 2–3 weeks of the laboratory visit, with 90% of samples collected within 1 month. For the PI children this meant that the first home cortisol data were obtained about 3 months post-entry into the family (M=2.1 months, SD=.85) with the second session home samples being about 9 months post-entry (M=8.8 months, SD=.58) and so on. Parents were asked to collect one sample 30 minutes after the child awoke in the morning and before the child was fed (Morning: M=07:50, SD=25–30 min, Time since waking (TSW) M = 36 minutes, SD = 15 minutes), one sample mid-day but not within 30 minutes following a nap (Midday: M= 12:45 PM, SD= 1 hr; TSW M = 5 hours, 37 minutes, SD = 1 hour, 1 minute) and one sample at bedtime (Bed: M=19:50 PM, SD 45 min; TSW M = 12 hours, 36 minutes, SD = 46 minutes). There were no significant group or collection period differences in the timing of sampling. Time-since-awakening was calculated for each sample and used as a covariate.
Saliva was collected using cotton dental rolls and small amounts of Kool-Aid™ crystals following procedures described in Talge and colleagues (2005) which have been shown to produce reliable cortisol estimates. Once the cotton roll was saturated the parent placed it in a needleless syringe and expressed the saliva into a vial, labeled it with time and date and placed it in a zipped plastic bag in the refrigerator. Once all samples for that collection period were obtained, parents mailed them to the laboratory where they were stored at −20 °C. These procedures do not to affect cortisol data (Clements & Parker, 1998). Annually, samples were mailed to Trier, Germany for assay in duplicate using a time-resolved fluorescence immunoassay (DELFIA). Intra- and inter-assay coefficients of variation were at or less than 6.7% and 8.8%, respectively, and duplicates correlated highly (r=.998, p <.001).
Cortisol values were deleted for days with fevers, children with active infections, children on corticosteroids, and cortisol data diluted to linear portion in combination with high values. For days with known fevers, up to 3 days of cortisol were deleted. Cortisol values were deleted at the individual sample level (1.7% of individual samples deleted) and cortisol means across the three sampling days were calculated using all available data. Values above or below 4 SDs of the mean were then examined and these outlying values were winsorized (6–10 participants with winsorized data across collection periods). The data were then averaged across the 3 days based on all data available for each participant for each time period, resulting in 12 cortisol values (4 Wake, 4 Midday, 4 Bed). Total Ns for cortisol measure are shown in Table 2.
Table 2.
Zero-Order Correlations and Descriptive Statistics
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Correlations for Full Sample | |||||||||||||||||
| 1. S1 Cort Wake | 1.00 | ||||||||||||||||
| 2. S1 Cort Mid | .73*** | 1.00 | |||||||||||||||
| 3. S1 Cort Bed | .72*** | .78*** | 1.00 | ||||||||||||||
| 4. S2 Cort Wake | .16 | .09 | .09 | 1.00 | |||||||||||||
| 5. S2 Cort Mid | .21* | .07 | .09 | .71*** | 1.00 | ||||||||||||
| 6. S2 Cort Bed | .11 | .07 | .06 | .71*** | .81*** | 1.00 | |||||||||||
| 7. S3 Cort Wake | .30*** | .27** | .23** | .06 | .08 | −.01 | 1.00 | ||||||||||
| 8. S3 Cort Mid | .23** | .30*** | .29*** | .07 | .09 | .05 | .75*** | 1.00 | |||||||||
| 9. S3 Cort Bed | .32*** | .39*** | .39*** | .11 | .07 | .03 | .67*** | .71*** | 1.00 | ||||||||
| 10. S4 Cort Wake | .28** | .25** | .23* | .21* | .19* | .16 | .30*** | .45*** | .28** | 1.00 | |||||||
| 11. S4 Cort Mid | .27** | .28** | .26** | .05 | .16 | .17 | .18* | .44*** | .25** | .63*** | 1.00 | ||||||
| 12. S4 Cort Bed | .25** | .17 | .17 | .04 | .22* | .17 | .18* | .36*** | .19* | .63*** | .73*** | 1.00 | |||||
| 13. S4 BITSEA | .00 | −.02 | .02 | −.08 | −.01 | −.03 | −.03 | −.08 | −.08 | −.04 | −.07 | −.03 | 1.00 | ||||
| Correlations for PI Children Only | |||||||||||||||||
| 14. Duration | .08 | .05 | −.06 | −.03 | −.07 | .07 | −.14 | .18 | .17 | −.03 | −1.9 | .13 | .10 | 1.00 | |||
| 15. Height for Age | .10 | .14 | .14 | .14 | .26 | .16 | .20 | .10 | .18 | .20 | .04 | .03 | −.06 | −.18 | 1.00 | ||
| 16. Physical Care | −.09 | −.03 | .07 | .18 | .18 | .13 | .04 | −.02 | −.03 | −.10 | .03 | .13 | −.25* | .25 | .25 | 1.00 | |
| 17. Social Care | .24 | .19 | .20 | .04 | .20 | .14 | .39* | .08 | .01 | .12 | .20 | −.10 | −.13 | −.02 | −.02 | .42** | 1.00 |
|
| |||||||||||||||||
| M | .29 | .12 | .06 | .33 | .14 | .09 | .33 | .14 | .08 | .31 | .12 | .07 | 5.76 | 17.57 | −1.26 | 3.53 | 3.55 |
| SD | .18 | .11 | .10 | .21 | .15 | .16 | .19 | .16 | .13 | .16 | .11 | .12 | 4.78 | 7.81 | 1.16 | .98 | 1.16 |
| N | 151 | 151 | 149 | 143 | 143 | 141 | 132 | 133 | 131 | 130 | 130 | 127 | 134 | 58 | 57 | 46 | 44 |
Note. Correlations among cortisol samples are for the full sample. Correlations with preadoptive care variables are among PI children only. S1 = Session 1, S2 = Session 2, S3 = Session 3, S4 = Session 4. All cortisol variables are winsorized.
p<.05,
p<.01,
p<.001.
2.2.3 Growth Stunting
Height-for-age at adoption was determined from the child’s first medical check-up in this country. Using World Health Organization standards, Z-scores were calculated and those ≤ −2 were considered growth stunted (Johnson et al., 2010). Four percent of PFC and 28% of PI children were stunted at adoption. Height-for-age was also assessed at each testing session. Examining group by sex by testing session, PI children exhibited rebound growth, while Z-scores for the other groups did not change, F(2,136)=48.31, p<.000, η2=.45. By sessions 3 and 4 the PI and PFC children did not differ (Ms about ½ SD below the norm, SDs .99), while both were shorter for age than NA children (LSD post-hoc tests, p<.05) whose mean was at the norm (near 0).
2.2.4 Brief Infant Toddler Social and Emotional Assessment (BITSEA)
The BITSEA (Briggs-Gowan et al., 2002) was examined at the fourth session which was 2 years post-adoption for the PI children. This session was chosen to allow examination of behavior problems after the child had sufficient time to adjust. The BITSEA yields Problem Behavior and Competence Scales. The Problem Behavior Scale correlates highly with the Child Behavior Checklist Externalizing and Internalizing scales (Briggs-Gowan et al., 2002). Only the Problem Behavior Scale was examined in this report; the scale includes 31 items reflecting internalizing, externalizing, emotion dysregulation, and other atypical problems (in present sample, Cronbach α=.72). Examination of more differentiated behavior and emotional problems was planned for when the children were older.
3. Results
3.1 Descriptive Statistics
Descriptive statistics are displayed in Table 2. As expected, cortisol values were higher in the morning hours and decreased across the day. Rank order stability was high within and modest across collection periods. Physical care and social care were significantly correlated. In all subsequent analyses, parent-report of duration of institutionalization was dichotomized at 24 months (19% > 24 months). Previous research has indicated that 2 years may be a critical window for removal from institutional care (Zeanah et al., 2011).
3.2 Data Analytic Plan
Latent growth models were fit to model the diurnal change (wake, midday, bed). To account for the nested structure of the samples (within collection period and individuals), multilevel structural equation modeling (MSEM) was conducted in MPLUS (version 7; Múthen & Múthen, 1998–2012). To account for non-linearity, the latent basis approach was used which allows for flexibility in the shape of the slope parameter by freeing time scores (McArdle & Epstein, 1987). In the present sample, factor loadings for the latent slope variable were constrained to 0 (wake sample), 1 (midday), and the bedtime time score was free-to-vary to capture non-linear change. Full information maximum likelihood estimation with robust standard errors was utilized to estimate missing data. As this method is suitable for non-normal continuous data, raw cortisol values were modeled.
Analyses were conducted in three parts: First, a latent growth model was fit to the whole sample to examine group differences on wake cortisol and diurnal slope. Group was included at the between-subjects level and was dummy-coded with NA children used as the comparison group. Second, a latent growth model was fit within the PI group to examine the association of preadoption conditions and growth-stunting with morning cortisol and diurnal cortisol slopes at the between-subject level. All analyses included time-since-waking as time-varying covariates at the within-subject level. Additionally, daily medication use was included as a time-invariant covariate within collection periods. Medications affecting cortisol were coded using the procedures outlined in Granger and colleagues (2009). Child chronological age, age at adoption, time spent with family, and sex were examined as potential covariates but were not included as these were not significant predictors of morning cortisol or diurnal slope at the between-subjects level. Time-varying child chronological age and time spent with family were also not significant predictors of morning cortisol or diurnal slope at the within-subjects level. Lastly, factor scores for diurnal cortisol were extracted from the full sample analysis and diurnal cortisol was examined as a mediator between adoption and T4 behavioral problems.
3.3 Group Effects on Morning Cortisol and Diurnal Rhythms
Cortisol decreased throughout the day (see Table 3; Model 1). The model provided adequate fit to the data (χ2(24)=77.69, p < .001; χ2/df = 3.24; RMSEA = .06, CFI = .95). The free-to-vary time score on the slope variable (e.g., bedtime, λ=1.031) indicated that the decrease was sharper (97% of all change) from morning to midday samples than from midday to bedtime samples. The effect of collection period was modeled at the within-subjects level to examine the possibility of change in morning cortisol and diurnal slope over the course of the longitudinal study. Counter to expectations, there was no effect of collection period on the intercept or slope. Time-since-waking was a significant covariate of the morning sample only. There were no effects of medication. Collection period, time-since-waking, and medication use accounted for very little variation at the within-subject level (Intercept R2=.002; Slope R2=.002).
Table 3.
Multilevel Latent Growth Model Results examining Predictors of Children’s Morning and Diurnal Cortisol Change.
| Model 1 (N = 155) | Model 2 (N = 57) | ||||
|---|---|---|---|---|---|
|
| |||||
| Unstand Est (SE) | R2 | Unstand Est (SE) | R2 | ||
| Within-Subjects Level | |||||
| Collection Period | → Intercept | .006 (.006) | −.006 (.011) | ||
| → Slope | −.003 (.004) | .002 (.007) | |||
| Medication use | → Intercept | .002 (.015) | −.021 (.022) | ||
| → Slope | .001 (.009) | .017 (.010) t | |||
| TSW Morning | → Cortisol Wake | −.100 (.019)*** | −.127 (.030)*** | ||
| TSW Midday | → Cortisol Midday | .002 (.003) | .004 (.005) | ||
| TSW Evening | → Cortisol Bedtime | −.003 (.002) t | .000 (.002) | ||
| Intercept residual variance | .024 (.005)*** | .019 (.008)* | |||
| Slope residual variance | .007 (.002)*** | .007 (.003)* | |||
| Intercept-Slope covariance | −.010 (.003)*** | −.008 (.004)* | |||
| Within-Subject Intercept R2 | .002 | .012 | |||
| Within-Subject Slope R2 | .002 | .020 | |||
| Between-Subjects Level | |||||
| Intercept | Mean | .378 (.023)*** | .329 (.066)*** | ||
| Variance | .006 (.002)** | .005 (.003)t | |||
| Slope | Mean | −.267 (.026)*** | −.255 (.054)*** | ||
| Variance | .001 (.001)* | .001 (.001) | |||
| Bedtime Time Score | 1.031 (.108)*** | 1.091 (.123)*** | |||
| Intercept-Slope Covariance | −.002 (.001)** | −.002 (.001) | |||
| Predictors in Full Sample Model | |||||
| PI Group | → Intercept | −.026 (.024) | --- | ||
| → Slope | .032 (.015)* | --- | |||
| PFC Group | → Intercept | −.032 (.015) | --- | ||
| → Slope | .037 (.014)** | --- | |||
| Predictors in PI Only Model | |||||
| Physical Care Rating | → Intercept | --- | −.032 (.023) | ||
| → Slope | --- | .011 (.011) | |||
| Social Care Rating | → Intercept | --- | .055 (.017)*** | ||
| → Slope | --- | −.021 (.007) ** | |||
| Growth Stunting at Adoption | → Intercept | --- | −.026 (.040) | ||
| → Slope | --- | .004 (.024) | |||
| Duration of Institutionalization | → Intercept | --- | −.021 (.029) | ||
| → Slope | --- | .032 (.023) | |||
| Between-Subject Intercept R2 | .003 | .506 | |||
| Between-Subject Slope R2 | .170 | .513 | |||
Note. Model 1: Group as a predictor of children’s cortisol among NA, PI, and PFC children; N = 155 children, 552 observations. Model 2: Preadoptive adversity as predictors of PI children’s cortisol; N = 57 PI children, 193 observations. Unstandardized parameter estimates and standard errors (SE) are presented. The within-subject level reflects intra-individual change in children’s cortisol across the four sessions. The between-subject level reflects inter-individual differences between individuals. The intercept factor corresponds to morning cortisol and the slope factor reflects diurnal cortisol change across the day. Bolded values are significant. TSW = Time since waking.
p < .001,
p < .01,
p < .05,
p < .10.
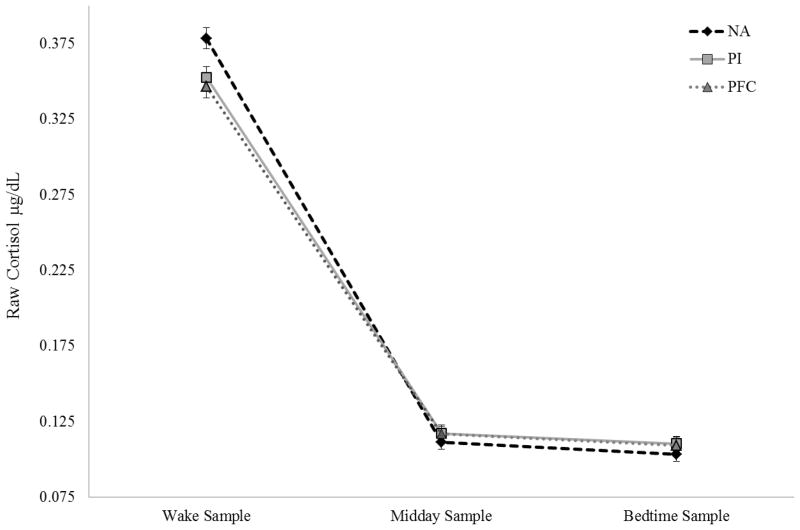
At the between-subject level, group was a significant predictor of diurnal slope. Unexpectedly, both PI children and PFC children had less steep declines in cortisol compared to NA children (See Figure 1); however, the differences in morning cortisol (e.g., latent intercepts) were not significant. Despite these slope differences, both PI and PFC children displayed a typical diurnal rhythm with steady declines across the day. Group accounted for a modest amount of variance in the diurnal slope, but not morning cortisol, at the between-subject level (Intercept R2=.03; Slope R2=.17). The group variable was also re-parameterized to set the PI group as the reference group to examine differences between PI and PFC children. There was no effect of group for the PI-PFC comparison on morning cortisol (β = −.01, SE = .02, ns) or diurnal slopes (β = .01, SE = .01, ns).
Figure 1.
Diurnal Cortisol across the Day for Non-Adopted (NA), Post-Institutionalized (PI), and Post Foster Care (PFC) Children.
3.4 Preadoptive Care and PI Children’s Cortisol
Results for the PI-only analyses (N=57; 1 PI excluded for not having any preadoptive care variables) are presented in Table 3 (Model 2). The model provided adequate fit to the data (χ2(22) = 41.30, p < .01; χ2/df = 1.88; RMSEA = .07, CFI = .95). The free-to-vary time score on slope was comparable to the total sample (e.g., bedtime, λ=1.091). Additionally, the effect of collection period was modeled to examine the unique effect of longitudinal change in morning cortisol and diurnal slope in the PI children. Similar to the full model analysis, there was no collection period effect, indicating no intra-individual change in morning or diurnal cortisol over the first two years post adoption. Collection period, time-since-waking, and medication use accounted for very little variation at the PI within-subject level (Intercept R2=.012; Slope R2=.020).
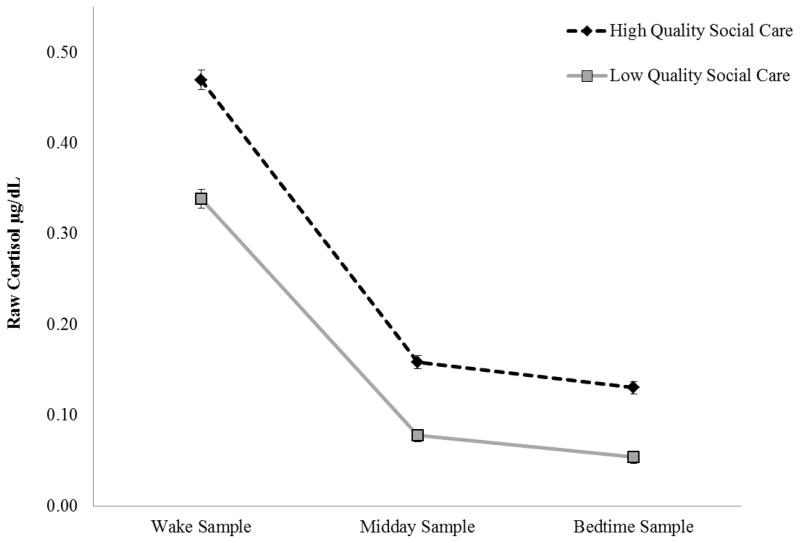
Preadoptive care, duration of institutionalization, and growth stunting at adoption was modeled at the between-subject level. Social care ratings were a significant predictor of both morning and diurnal cortisol. Children receiving better social care (e.g., higher social care ratings) had significantly higher morning cortisol (p <.001) and steeper declines in cortisol across the day (p <.01; See Figure 2). Preadoptive care variables accounted a large portion of the variation in PI children’s cortisol at the between-subjects level (Intercept R2=.51, Slope R2=.51).
Figure 2.
Predicted Diurnal Cortisol across the Day for Post-Institutionalized Children among High and Low Quality Social Care in Institutions. Graphs depict 1 SD above and below the mean of the social care construct as an illustration of high and low quality social care.
3.5 Cortisol as a Mediator between Adoption and Behavioral Problems
Due to non-significant differences in children’s cortisol in the full sample analysis, PI and PFC children were collapsed into one group to examine the effect of adoption. Adoption was associated with increased behavioral problems (β=.19, p<.05; R2=.04). Subsequently, diurnal slope was examined as a mediator between adoption and session four behavioral problems. Because there were no differences in children’s morning cortisol, only diurnal slope was examined as a mediator. Adoption was related to shallower diurnal slopes (β=.63, p<.001, R2=.40) and shallower diurnal slopes were related to increased behavioral problems (β=.21, p<.05, R2=.06). As a test of mediation, bootstrapping was conducted (10000 bootstrap samples); bootstrapping can yield more accurate estimates of the standard errors of the indirect effect (Shrout & Bolger, 2002). The 95% confidence interval did not contain zero (CI: .004, .260), indicating a significant indirect effect (see Figure 3). The effect size for the indirect effect was calculated using the proportion of the maximum indirect effect (Κ2=.12; Preacher & Kelley, 2011).
Figure 3.
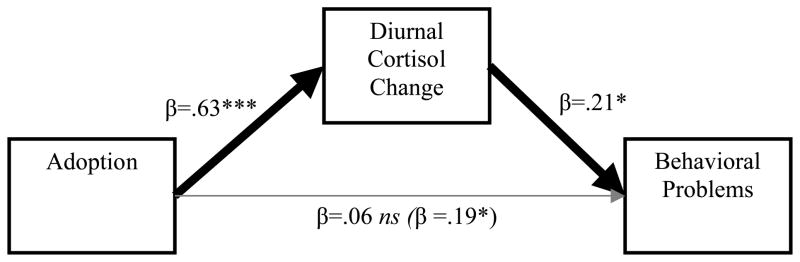
Diurnal Cortisol as a Mediator between Adoption and Behavioral Problems. Standardized path coefficients presented. Values provided in parentheses indicate the path coefficient of the direct effect prior to the addition of the mediator. Cortisol Slope R2=.40. Behavioral Problems R2=.06. *p<.05, ***p<.001.
3.6 Race as a Confounding Factor
Finally, racial differences in children’s diurnal cortisol slope were examined as a potential confounding factor due to differences in racial/ethnic backgrounds between groups. To examine whether race differences between adopted and non-adopted children might account for the findings, first we selected only the children whose parents reported they were Caucasian. Using estimates of the between-subjects slope (e.g., the aggregate slope for all study sessions), we performed a one-way between-subjects analysis of variance. The results were significant, with the Caucasian adopted children (N=10; M= −.24, SD=.02) exhibiting a less marked slope than the Caucasian non-adopted children (N=45; M= −.27, SD=.02; F(1,53)=16.40, p<.001). To examine race as a potential confounding variable within group, a one-way between subjects analysis of variance was conducted. There were no differences in the diurnal cortisol slope among the PI children across different racial backgrounds (F(3,54) = 1.10, ns; Caucasian PI M = −.24, SD = .02, N = 10; Asian PI M = −.23, SD = .02, N = 21; African/Black PI M = −.24, SD = .02, N = 22; Other PI M = −.23, SD = .01, N = 5) nor among the PFC children across different racial backgrounds (F(1,45) = .01, ns; Asians PFC M = −.23, SD = .02, N = 36; Other PFC M = −.23, SD = .01, N = 11). Taken together these results suggest that group differences between adopted and non-adopted children may not be due to race and ethnicity.
4. Discussion
The results of the present study indicated that early deprivation and disruption resulted in a less steep cortisol slope over the daytime hours, and this was especially true for PI children reared in more socially depriving institutional settings. The results also showed that children adopted from overseas foster care homes showed less steep cortisol slopes than the non-adopted children. Thus the factors underlying these effects cannot be restricted to children’s experiences in institutional care. Contrary to expectations, we observed no changes in the pattern of daytime cortisol production from 2 to 25 months post-adoption for the PI children. Finally, daytime cortisol slope mediated the relationship between being an internationally adopted child and the problem behaviors noted by the parents.
The less steep cortisol slope was not due to either a significantly lower morning or higher evening level of cortisol, although this was the direction of the effects producing the slope differences. Other studies of children developing in difficult circumstances have found significantly lower morning levels related to severity of adversity (Bruce et al., 2009; van der Vegt et al., 2009), but overall the most consistent effect has been a reduction in the robustness of the cortisol slope (e.g., diurnal rhythm) over the daytime hours (e.g., Johnson et al., 2011; Zalewski et al., 2012). We do not know whether the slope findings in the present study are a reflection of hypocortisolism, although reductions in cortisol slopes are often interpreted this way (Lumeng et al., 2014). To assess whether the findings of the present study are in line with hypocortisolism, additional assessments of the HPA axis would be necessary including measures of cortisol responsivity to stressors which are not included in the present analysis. The effect might partly reflect a reduction in the cortisol awakening response (CAR) as we did ask the parents to take the morning sample about 30 minutes after the child awoke. The CAR is present as early as infancy (Stalder et al., 2013) and we have previously shown it to be blunted in pre-pubertal children adopted from institutional care (Quevedo et al., 2012). Because collecting saliva samples the moment a child awakens and then again 30 minutes later is a heavy burden on parents, we did not attempt it in this study. However, future work should examine if only the CAR is blunted or whether both wake up and 30 minute cortisol levels are somewhat reduced. Notably, the latter pattern was noted among adults who had been adopted internationally as infants and young children from neglecting circumstances (van der Vegt et al., 2009).
Contrary to expectations, there was no change over time in children’s diurnal cortisol following adoption. Counter to Kroupina and colleagues (2012), we did not see a markedly more blunted cortisol slope soon after adoption that became less pronounced with time. Instead, we found no significant change over the first two years in the home, and indeed, for longer than that if we consider the PFC children. This less steep slope was not a transient finding that went away after the child transitioned into the adoptive home. Of course, we might have missed a more markedly diminished initial slope because, unlike Kroupina et al (2012), we did not sample within the first month post-adoption. Nonetheless, the fact that we observed a statistically more blunted slope over the two years of sampling suggests that it is a relatively enduring condition of the HPA axis in these internationally adopted children, which might indicate early programming of the axis (Meaney et al., 2007). Because the adopted children came from many regions of the world, while the NA children were nearly all Caucasian, race differences were explored as a potential explanation in the findings. However, when we compared only the Caucasian children we still found that the average slope was less steep. Thus racial differences between adopted and non-adopted children are unlikely to explain the effects.
If these data do reflect early programming of the HPA axis, what are the conditions that produced this programming? Within the PI group, we do have evidence that more severe social deprivation may have played a role. Furthermore, we have no evidence that variations in physical deprivation were involved. PI children from institutions with high quality social care may have been more likely to have access to a fairly stable caregiver resulting in a diurnal slope more closely resembling the NA children. Alternatively, access to multiple caregivers that provide sensitive and responsive caregiving may be sufficient to prevent blunting in the diurnal rhythm. Additional research will be necessary to disentangle which particular aspects of high quality social care serve to buffer against a blunting of the HPA activity in the context of social deprivation. The impact of social care quality above and beyond other preadoptive adversity may point to providing better caregiver-child relationships as a promising focus of intervention and prevention efforts in institutional care settings (e.g., McCall et al., 2013). These findings should be viewed cautiously, however, as we did not have trained observers in the institutions scoring the degree of physical and social deprivation, but rather had parents reporting on what they saw, which in some cases was likely limited. The fact that social deprivation in rhesus monkeys appears to produce a reduction in the HPA set point independent of the availability of good nutrition and medical care does provide some support, although species and measurement differences should be noted (Capitanio et al., 2006). Children reared in overseas foster homes experience a more typical social environment than those reared in institutions (Gunnar et al., 2000), nonetheless, they have experienced multiple primary caregivers as they transition from biological family to foster home to adoptive home. In addition, many foster homes have multiple children and may operate more like small institutions. Unfortunately, we do not have the same degree of detail about the foster homes as many families did not get a chance to see the home prior to adoption. Thus we cannot examine whether variations in social stimulation/deprivation or the child’s history of adverse events within these homes may have contributed to the comparable findings for PFC and PI children. What we can note is that the effects are not particular to children adopted from institutional care.
It is also possible that low social care ratings served as a proxy measure for other factors that played a causal role in altering the cortisol daytime rhythm. In other studies, but not this one, growth stunting at adoption has predicted later diurnal cortisol patterns in PI children (Johnson & Gunnar, 2011). While it was surprising that growth at adoption was not related to cortisol patterns in the present study, it might indicate that both slowed growth and social care measures were indices of yet other factors that was part of the causal pathway. It is possible, of course, that nutrition might be involved as it is certainly likely that the non-adopted children had a more nutritious diet than either the PI or PFC children. Indeed, research shows that malnutrition during the first years of life is associated with internalizing and externalizing symptoms during later childhood and adolescence (Galler et al., 2010; 2012). Until we understand the biological pathway to altered patterning of cortisol production in children coming from high-risk early life circumstances, we should think broadly about the conditions that might affect the development of the HPA axis. Nonetheless, the present evidence for social deprivation in the PI group is notable, especially as it corresponds to what was noted for non-human primates reared in the nursery, a situation that is an animal analogue of institutional care.
Although the cortisol slope was less pronounced in the PI and PFC groups, the diurnal slope was still quite apparent. Inspection of the individual data showed that all of the children displayed a decrease in cortisol from morning to evening. In addition, the variance explained by group was generally small, albeit statistically significant. This raises the question of whether the differences were meaningful. The behavior problems data are helpful in this regard. Parents reported significantly more problem behavior for the PI and PFC children and the daytime cortisol slope mediated this finding. This indicates that the children with the least robust cortisol decreases over the day were the ones whom parents described as having the most behavior problems several years post-adoption. There are some limitations to these data. We used a behavior problems screener and as such it does not distinguish between problems in behavior regulation, internalizing issues, and externalizing problems. Because the children were so young, differentiating types of problems would have been difficult. This will be done during assessments planned as the children age. Thus, at this time, we cannot determine whether the cortisol slope findings are more strongly associated with internalizing or externalizing problems. In addition, the BITSEA was designed for infants and toddlers and the children in the present study were somewhat older at the time of the fourth assessment. We chose this instrument purposely; however, because children adopted from conditions of deprivation are commonly delayed developmentally. In addition, the instrument has been validated against instruments that are designed for children up through age five, which does encompass our age group (Briggs-Gowan et al., 2002). A review of the items clearly reveals their relevance for our age group (e.g., “throws tantrums”; “nervous”, “tense and fearful”; “restless, can’t sit still”). Of course, the fact that it was not validated on children of the same age as our participants means that we did not use clinical cut points as they might not be valid; but we argue that the dimensional scores do reflect individual differences in behaviors that are difficult for parents. Despite these limitations, the present findings provide further support for the notion that disruptions in HPA axis functioning may have clinical and developmental relevance for the mechanisms that underlie the emergence of problem behavior.
To summarize, this study adds to the evidence that adverse early life conditions are associated with a less steep cortisol slope for children. Because the effect continued for several years post-adoption, it would seem that it reflects a relatively persistent characteristic of the HPA axis in these children and, thus, perhaps reflects early programming of the axis. Despite frequent reports of early adverse care reducing the cortisol slope, we have no good mechanistic explanation of such findings. Previous explanations for blunted cortisol rhythms or lowered cortisol set points in the context of adversity have argued that there must have been a period of chronic elevation in cortisol that then resulted in a down-regulation of the axis (Fries et al., 2005; Miller et al., 2007). Chronic hypercortisolism has been linked to depressive symptomatology (Stetler & Miller, 2011), thus these HPA axis alterations may have mental health implications. Nevertheless, we have no direct evidence for an earlier period of chronic cortisol elevations. Regardless, there must be mechanisms to explain these findings. These might include receptor numbers, binding affinities, or reduced responsiveness of the adrenal cortex to sympathetic innervation. The fact that nursery-reared monkeys might provide a model for probing the mechanisms involved is important as uncovering the mechanisms will involve procedures that are too invasive for use with healthy young children from community samples (e.g., frequent blood sampling, dexamethasone and CRH/ACTH stimulation tests, administration of receptor agonists/antagonists to probe function). These data and other like it also clearly indicate that we may need to look beyond the early experience rodent models (e.g., Szyf et al., 2005) to understand this infancy and early childhood human phenomenon.
We have already noted some of the limitations of the present study. In addition to those mentioned, this is a convenience sample. Although we attempted to find and contact every family who adopted a child from an institution into a home within driving distance of our university, we likely missed some and some we did contact refused participation. Of those who agreed, not all were able to start soon after adoption, and thus were not included in this analysis. Next, even though parents were remarkably good at following the sampling protocol, collecting all the samples was a challenge for some of them and required several weeks, thus extending the time frame of each collection period. Given the lack of changes in diurnal slopes over time, these imprecisions are less concerning.
Despite the limitations, this is the first longitudinal assessment of daily cortisol production in children as they adapt to their new families. Findings from the present study have implications for understanding the early programing of the HPA axis under conditions of chronic stress. Despite alleviation of conditions of deprivation and disruption, findings from both the PI and PFC groups highlight the potential long-term alterations of the HPA axis due to early life adversity. The evidence it provides of a less steep cortisol slope mirrors previous evidence reported for adults adopted internationally as young children and thus suggests that there may have been an early programming of the HPA axis. This finding and others like it strongly argue that we need to focus on understanding the mechanisms that may have produced this type of down-regulation of the axis.
Highlights.
Less steep diurnal cortisol slopes were found in children adopted from institutional care and overseas foster care compared to non-adopted same aged-peers.
No changes were found in children’s diurnal cortisol in the two years following adoption from overseas institutional care.
Lower quality of social care in institutions was associated with less steep diurnal cortisol slopes.
Children’s diurnal cortisol was a mediator between adoption status and increased behavioral problems two years post-adoption.
Findings highlight the role of early social deprivation in contributing to early programming of the HPA axis.
Acknowledgments
The authors would like to thank the other members of the Minnesota International Adoption Team for their efforts in collecting these data: Shanna Mliner, Kristin Frenn, Meg Bale, Bao Moua, and Sarah Stellern. We would also like to thank the parents and children without whom this study would not have been possible. This work was supported by grants R01 MH080905 and P50 MH078105 from the National Institute of Mental Health awarded to Megan Gunnar. Support was provided to Kalsea Koss by a National Institute of Mental Health training grant (T32 MH015755) during the preparation of this article.
Role of the Funding Source
Study sponsors provided financial support but did not have a role in the data collection, analysis, or interpretation. The decision to submit the manuscript for publication was the sole decision of the authors.
Footnotes
Conflict of Interest
All authors report no conflicts of interest.
Contributors
Kalsea Koss conducted the statistical analysis, contributed to the preparation of the manuscript, and the preparation/cleaning of the cortisol data. Camelia Hostinar contributed to the preparation of the manuscript and the preparation/cleaning of the cortisol data. Bonny Donzella contributed to the study design, data collection, and the preparation/cleaning of the cortisol data. Megan Gunnar is the Principal Investigator of the project and contributed to the study design and preparation of the manuscript. All authors have contributed to and approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kalsea J. Koss, Institute of Child Development, University of Minnesota, Minneapolis, MN 55455 USA
Camelia E. Hostinar, Institute for Policy Research, Northwestern University, Evanston, IL 60208 USA
Bonny Donzella, Institute of Child Development, University of Minnesota, Minneapolis, MN 55455 USA.
Megan R. Gunnar, Institute of Child Development, University of Minnesota, Minneapolis, MN 55455 USA
References
- Astley S, Clarren S. Diagnosing the full spectrum of fetal alcohol exposed individuals: introducing the 4-digit diagnostic code. Alcohol Alcohol. 2000;35:400–410. doi: 10.1093/alcalc/35.4.400. [DOI] [PubMed] [Google Scholar]
- Bos K, Zeanah CH, Fox NA, Drury SS, McLaughlin KA, Nelson CA. Psychiatric outcomes in young children with a history of institutionalization. Harv Rev Psychiatry. 2011;19:15–24. doi: 10.3109/10673229.2011.549773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs-Gowan MJ, Carter AS, Irwin JR, Wachtel K, Cicchetti DV. The brief infant-toddler social and emotional assessment: screening for social-emotional problems and delays in competence. J Pediatr Psychol. 2002;29:143–155. doi: 10.1093/jpepsy/jsh017. [DOI] [PubMed] [Google Scholar]
- Bruce J, Fisher PA, Pears KC, Levine S. Morning cortisol levels in preschool-aged foster children: differential effects of maltreatment type. Dev Psychobiol. 2009;51:14–23. doi: 10.1002/dev.20333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitanio JP, Mendoza SP, Mason WA, Maninger N. Rearing environment and hypothalamic-pituitary-adrenal regulation in young rhesus monkeys (Macaca mulatta) Dev Psychobiol. 2006;46:318–330. doi: 10.1002/dev.20067. [DOI] [PubMed] [Google Scholar]
- Carlson M, Earls F. Psychological and neuroendocrinological sequelae of early social deprivation in institutionalized children in Romania. Ann NY Acad Sci. 1997;807:419–428. doi: 10.1111/j.1749-6632.1997.tb51936.x. [DOI] [PubMed] [Google Scholar]
- Cirulli F, Francia N, Branchi I, Antonucci MT, Aloe L, Suomi SJ, Alleva E. Changes in plasma levels of BDNF and NGF reveal a gender-selective vulnerability to early adversity in rhesus macaques. Psychoneuroendocrinology. 2009;34:172–180. doi: 10.1016/j.psyneuen.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke AS. Social rearing effects on HPA axis activity over early development and in response to stress in rhesus monkeys. Dev Psychobiol. 1993;26:433–446. doi: 10.1002/dev.420260802. [DOI] [PubMed] [Google Scholar]
- Clements AD, Parker RC. The relationship between salivary cortisol concentrations in frozen versus mailed samples. Psychoneuroendocrinology. 1998;23:613–616. doi: 10.1016/s0306-4530(98)00031-6. [DOI] [PubMed] [Google Scholar]
- Colvert E, Rutter M, Beckett C, Castle J, Groothues C, Hawkins A, Kreppner J, O’Connor TG, Stevens S, Sonuga-Barke EJ. Emotional difficulties in early adolescence following severe early deprivation: findings from the English and Romanian Adoptees study. Dev Psychopathol. 2008;20:547–567. doi: 10.1017/S0954579408000278. [DOI] [PubMed] [Google Scholar]
- Dettmer AM, Novak MA, Suomi SJ, Meyer JS. Physiological and behavioral adaptation to relocation stress in differentially reared rhesus monkeys: hair cortisol as a biomarker for anxiety-related responses. Psychoneuroendocrinology. 2012;37:191–199. doi: 10.1016/j.psyneuen.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrova-Krol NA, van Ijzendoorn MH, Bakermans-Kranenburg MJ, Cyr C, Juffer F. Physical growth delays and stress dysregulation in stunted and non-stunted Ukrainian institution-reared children. Infant Behav Dev. 2008;31:539–553. doi: 10.1016/j.infbeh.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Fries E, Hesse J, Hellhammer J, Hellhammer DH. A new view on hypocortisolism. Psychoneuroendocrinology. 2005;30:1010–1016. doi: 10.1016/j.psyneuen.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Galler JR, Bryce CP, Waber D, Hock RS, Exner N, Eaglesfield GD, Fitzmaurice G, Harrison R. Early childhood malnutrition predicts depressive symptoms at ages 11–17. J Child Psychol Psychiatry. 2010;51:789–798. doi: 10.1111/j.1469-7610.2010.02208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galler JR, Bryce CP, Waber DP, Hock RS, Harrison R, Eaglesfield GD, Fitzmaurice G. Infant malnutrition predicts conduct problems in adolescents. Nutr Neurosci. 2012;15:186–192. doi: 10.1179/1476830512Y.0000000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger DA, Hibel LC, Fortunato CK, Kapelewski CH. Medication effects on salivary cortisol: tactics and strategy to minimize impact in behavioral and developmental science. Psychoneuroendocrinology. 2009;34:1437–1448. doi: 10.1016/j.psyneuen.2009.06.017. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Bruce J, Grotevant HD. International adoption of institutionally reared children: research and policy. Dev Psychopathol. 2000;12:677–693. doi: 10.1017/s0954579400004077. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Morison SJ, Chisholm K, Schuder M. Salivary cortisol levels in children adopted from Romanian orphanages. Dev Psychopathol. 2001;13:611–628. doi: 10.1017/s095457940100311x. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, van Dulmen M. Behavior problems in post-institutionalized internationally-adopted children. Dev Psychopathol. 2007;19:129–148. doi: 10.1017/S0954579407070071. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez D. Low cortisol and a flattening of expected daytime rhythm: potential indices of risk in human development. Dev Psychopathol. 2001;13:515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Hawkley LC, Cole SW, Capitanio JP, Norman GJ, Cacioppo JT. Effects of social isolation on glucocorticoid regulation in social mammals. Horm Behav. 2012;62:314–323. doi: 10.1016/j.yhbeh.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AE, Bruce J, Tarullo AR, Gunnar MR. Growth delay as an index of allostatic load in young children: predictions to disinhibited social approach and diurnal cortisol activity. Dev Psychopathol. 2011;23:859–871. doi: 10.1017/S0954579411000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DE, Gunnar MR. Growth failure in In stitutionalized children. Children without permanent parents: research, practice, and policy. In: McCall RB, van IJzendoorn MH, Juffer F, Groark CJ, Groza VK, editors. Monogr Soc Res Child Dev. 2011. pp. 92–126. [Google Scholar]
- Johnson DE, Guthrie D, Smyke AT, Koga SF, Fox NA, Zeanah CH, Nelson CA. Growth and associations between auxology, caregiving environment, and cognition in socially deprived Romanian children randomized to foster vs ongoing institutional care. Arch Pediatr Adolesc Med. 2010;164:507–516. doi: 10.1001/archpediatrics.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroupina MG, Fuglestad AJ, Iverson SL, Himes JH, Mason PW, Gunnar MR, Miller BS, Petryk A, Johnson DE. Adoption as an intervention for institutionally reared children: HPA functioning and developmental status. Infant Behav Dev. 2012;35:829–837. doi: 10.1016/j.infbeh.2012.07.011. [DOI] [PubMed] [Google Scholar]
- Kertes DA, Gunnar MR, Madsen NJ, Long J. Early deprivation and home basal cortisol levels: a study of internationally-adopted children. Dev Psychopathol. 2008;20:473–491. doi: 10.1017/S0954579408000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loman MM, Wiik KL, Frenn KA, Pollak SD, Gunnar MR. Post-institutionalized children’s development: growth, cognitive, and language outcomes. J Dev Behav Pediatr. 2009;30:426–434. doi: 10.1097/DBP.0b013e3181b1fd08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng JC, Miller A, Peterson KE, Kaciroti N, Sturza J, Rosenblum K, Vazquez DM. Diurnal cortisol pattern, eating behaviors and overweight in low-income preschool-aged children. Appetite. 2014;73:65–72. doi: 10.1016/j.appet.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle JJ, Epstein D. Latent growth curves within developmental structural equation models. Child Dev. 1987;58:110–133. [PubMed] [Google Scholar]
- McCall RB, Groark CJ, Fish L, Muhamedrahimov RJ, Palmov OI, Nikiforova NV. Maintaining a social-emotional intervention and its benefits for institutionalized children. Child Dev. 2013;84:1734–1749. doi: 10.1111/cdev.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ, Szyf M, Seckl JR. Epigenetic mechanisms of perinatal programming of hypothalamic-pituitary-adrenal function and health. Trends Mol Med. 2007;13:269–277. doi: 10.1016/j.molmed.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Szyf M. Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome. Dialogues Clin Neurosci. 2005;7:103–123. doi: 10.31887/DCNS.2005.7.2/mmeaney. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JS, Novak MA, Bowman RE, Harlow HF. Behavioral and hormonal effects of attachment object separation in surrogate-peer-reared and mother-reared infant rhesus monkeys. Dev Psychobiol. 1975;8:425–435. doi: 10.1002/dev.420080507. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User’s Guide. 7. Los Angeles, CA: Muthén & Muthén; 1998–2012. [Google Scholar]
- Preacher KJ, Kelley K. Effect sizes for mediation models: quantitative strategies for communicating indirect effects. Psychol Methods. 2011;16:93–115. doi: 10.1037/a0022658. [DOI] [PubMed] [Google Scholar]
- Quevedo K, Johnson A, Loman M, Lafavor T, Gunnar MR. The confluence of early deprivation and puberty on the cortisol awakening response: A study of post-institutionalized children. Int J Behav Dev. 2012;36:19–28. doi: 10.1177/0165025411406860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrout PE, Bolger N. Mediation in experimental and nonexperimental studies: new procedures and recommendations. Psychol Methods. 2002;7:422–445. [PubMed] [Google Scholar]
- Smyke AT, Koga SF, Johnson DE, Fox NA, Marshall PJ, Nelson CA, Zeanah CH. The caregiving context in institution-reared and family-reared infants and toddlers in Romania. J Child Psychol Psychiatry. 2007;48:210–218. doi: 10.1111/j.1469-7610.2006.01694.x. [DOI] [PubMed] [Google Scholar]
- Stalder T, Baumler D, Miller R, Alexander N, Kliegel M, Kirschbaum C. The cortisol awakening response in infants: ontogeny and association with development related variables. Psychoneuroendocrinology. 2013;38:552–559. doi: 10.1016/j.psyneuen.2012.07.015. [DOI] [PubMed] [Google Scholar]
- Stetler C, Miller GE. Depression and hypothalamic-pituitary-adrenal activation: a quantitative summary of four decades of research. Psychosom Med. 2011;73:114–126. doi: 10.1097/PSY.0b013e31820ad12b. [DOI] [PubMed] [Google Scholar]
- Szyf M, Weaver IC, Champagne FA, Diorio J, Meaney MJ. Maternal programming of steroid receptor expression and phenotype through DNA methylation in the rat. Front Neuroendocrinol. 2005;26:139–162. doi: 10.1016/j.yfrne.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Talge NM, Donzella B, Kryzer E, Gierens A, Gunnar MR. It’s not that bad: Error introduced by oral stimulants in salivary cortisol research. Dev Psychbiol. 2005;47:369–376. doi: 10.1002/dev.20097. [DOI] [PubMed] [Google Scholar]
- van der Vegt EJ, van der Ende J, Kirschbaum C, Verhulst FC, Tiemeier H. Early neglect and abuse predict diurnal cortisol patterns in adults: a study of international adoptees. Psychoneuoendocrinology. 2009;34:660–669. doi: 10.1016/j.psyneuen.2008.11.004. [DOI] [PubMed] [Google Scholar]
- van IJzendoorn MH, Palacios J, Sonuga-Barke EJS, Gunnar MR, Vorria P, McCall RB, LeMare L, Bakersmans-Kranenburg MJ, Dobrova-Krol NA, Juffer F. Children in institutional care: delayed development and resilience. Children without permanent parents: research, practice, and policy. In: McCall RB, van IJzendoorn MH, Juffer F, Groark CJ, Groza VK, editors. Monog Soc Res Child Dev. 2011. pp. 8–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalewski M, Lengua LJ, Kiff CJ, Fisher PA. Understanding the relation of low income to HPA-axis functioning in preschool children: cumulative family risk and parenting as pathways to disruptions in cortisol. Child Psychiatry Hum Dev. 2012;43:924–942. doi: 10.1007/s10578-012-0304-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeanah CH, Gunnar MR, McCall RB, Kreppner JM, Fox NA. Sensitive periods. Children without permanent parents: research, practice, and policy. In: McCall RB, van IJzendoorn MH, Juffer F, Groark CJ, Groza VK, editors. Monog Soc Res Child Dev. 2011. pp. 147–162. [DOI] [PMC free article] [PubMed] [Google Scholar]