Abstract
Typhoid (enteric fever) remains a major cause of morbidity and mortality worldwide, causing over 21 million new infections annually, with the majority of deaths occurring in young children. As typhoid fever-causing Salmonella have no known environmental reservoir, the chronic, asymptomatic carrier state is thought to be a key feature of continued maintenance of the bacterium within human populations. In spite of the importance of this disease to public health, our understanding of the molecular mechanisms that catalyze carriage, as well as our ability to reliably identify and treat the Salmonella carrier state, have only recently begun to advance.
Keywords: Salmonella, typhoid fever, chronic carrier, epidemiology, diagnostics
Salmonella spp. and human disease
Organisms belonging to Salmonella enterica sub-species I are estimated to cause over 93 million new infections annually [1], and are responsible for a variety of clinical manifestations in humans. The primary Salmonella-induced diseases in humans are gastroenteritis (caused by non-typhoidal Salmonella; NTS) and typhoid fever (caused by S. Typhi and the various S. Paratyphi pathovars). Infections with S. Typhi are responsible for approximately 21 million new cases of typhoid each year, globally [2]. Annual mortality from typhoid is estimated to be >190,000 and has increased by 39% between 1990 and 2010 [3]. Although rarely encountered in western countries, typhoid is not a conquered disease; a recent analysis of global mortality data revealed that, in highly endemic regions such as Southeast Asia and sub-Saharan Africa, the relative years of life lost to typhoid ranked similarly to those lost to breast cancer, prostate cancer, and leukemia in North America [3].
Acute Salmonella infections
NTS infection is characterized by acute enterocolitis accompanied by inflammatory diarrhea. These disease symptoms are thought to be driven by a rapid and efficient innate immune response to Salmonella surface-bound pathogen-associated molecular patterns (PAMPs), such as lipopolysaccharide (LPS) and flagellin, following localized bacterial invasion of the gastrointestinal (GI) mucosa [4]. Resultant interleukin-8 (IL-8)-mediated neutrophil recruitment and generation of reactive oxygen and nitrogen species, activity of antimicrobial peptides, and efficient phagocytic killing are thought to be critical for limiting NTS infections at an early stage [5].
Conversely, infections by invasive serovars such as S. Typhi and S. Paratyphi A rarely result in diarrhea, with the majority of patients experiencing fever, headache and malaise within 6–30 days of bacterial ingestion [6]. In vitro studies have shown that the Vi antigen (Vi ag) capsule of S. Typhi (Box 1) facilitates escape of early, innate detection and aids subsequent systemic dissemination [7, 8]. Additional factors specific to typhoid-related strains (e.g. typhoid toxin [9]) also likely play a role in systemic disease. Salmonella spp. that cause typhoidal illness are able to survive and replicate within host cells, particularly phagocytes, transiting within these cells to common distal sites of acute infection including the liver, spleen, and bone marrow [5].
Box 1. Vi antigen.
The Vi antigen (Vi ag) is a surface-associated capsular polysaccharide produced by S. Typhi, S. Dublin and S. Paratyphi C. Although not required for GI colonization, the Vi ag capsule is thought to enhance systemic virulence by: (i) increasing bacterial resistance to complement and phagocytic killing [78], and (ii) interfering with the generation of efficient primary and secondary immune responses by reducing pathogen-associated molecular pattern (PAMP) expression and/or exposure on the bacterial surface [8]. The Vi ag-encoding viaB locus is located within the SPI-7 pathogenicity island and is under the control of RcsB-RcsC and OmpR-EnvZ two-component regulatory systems [8, 79]. Activation of the viaB locus results in Vi ag production and concurrent repression of the flagellar master regulator flhDC by the TviA regulatory protein [80].
Vi ag expression can be lost following laboratory passage, and Vi ag genes have been found to be inactivated in some patient isolates. The Vi ag structure is composed of repeating units of (1–4)-2-deoxy-2-N-acetyl galacturonic acid [81], and the immunogenicity of Vi ag is largely a function of O-acetylation at the C-3 position. Although clinical reports have shown that naturally occurring anti-Vi titers do not necessarily correlate with protection from subsequent infection [82], vaccines generating anti-Vi antibodies do provide significant protection against typhoid [83]. Currently available Vi polysaccharide vaccines provide up to 70% protection in adults for approximately 2 years while newer Vi-conjugate vaccines demonstrate up to 90% protection, although protection is more limited in children under 2 years of age [83, 84]. While not 100% efficacious, prophylactic vaccination following natural disasters, in which the water supply was compromised, has been shown to mitigate typhoid outbreaks [85]. New formulations of Vi-conjugate vaccines, in which Vi ag polysaccharide is conjugated to a carrier protein (such as Pseudomonas aeruginosa exoprotein A (rEPA), diphtheria toxoid (DT), an inactivated form of diphtheria toxin (CRM-197) and even modified fruit pectin) have shown significant promise and are being targeted for use in young children [83, 86, 87].
What is Salmonella chronic carriage?
Following the resolution of disease, approximately 2–5% of typhoid patients fail to fully clear the infection within one year of recovery, instead progressing to a state of carriage [10]. The basic requirements for establishment of long-term extraintestinal infection are likely to involve successful breach of the intestinal epithelial barrier, evasion of early innate immune-mediated killing, and localization to a permissive niche. The permissive niche in humans is primarily the biliary tract and gallbladder [11]. In order to induce gallbladder chronic carriage, organisms must enter the biliary tract either via a descending route after systemic infection, or an ascending route di rectly from the small intestine. In the ascending route, the bacteria would enter the biliary system via the sphincter of Oddi, which if malfunctioning due to surgical intervention or pathology, may fail as a mechanical barrier. However, the more likely route is direct transfer into the gallbladder from the liver during the systemic phase of typhoid fever. Normally, Kupffer cells in the liver prevent toxic metabolites and bacteria from entering the hepatobiliary system and the continuous flushing action of bile and the bacteriostatic effect of bile salts keep the biliary tract relatively sterile. The failure of these, or other gallbladder functions, in addition to the organism’s ability to bypass these systems, likely induces and helps to maintain long-term carriage.
Gallbladder colonization and fecal shedding form a central dogma for the transmission and persistence of typhoid fever. Chronic carriers intermittently shed the bacteria for a prolonged, ill-defined period of time in the local environment and thus may spread the disease in the community and maintain a reservoir of infection [11]. Although the precise role of chronic carriers in disease transmission remains unclear, these asymptomatic carriers presumably act as reservoirs for a diverse range of S. Typhi strains and may act as a breeding ground for new genotypes [12]. Research conducted in hospitalized patients in Nepal undergoing gallbladder removal has shown that in addition to S. Typhi, S. Paratyphi A can also effectively colonize and persist in the gallbladder [13]. NTS strains have also recently been implicated in persistent human infections (Box 2). In this review we will discuss the current understanding and recent progress in the areas of epidemiology, diagnosis and resolution of persistent Salmonella infections.
Box 2. NTS carriage.
It has long been known that some enteropathogens (including non-typhoidal Salmonella) can continue to be excreted asymptomatically in stool for many weeks following resolution of an initial diarrheal episode [88]. Although asymptomatic carriage of NTS is thought to occur infrequently (0.15% in healthy adults, 3.9% in children [89]) clinical reports indicate that NTS may have the potential for longer-term human carriage. As with S. Typhi, there is a distinct delay between the resolution of disease symptoms and clearance of NTS from the body, and reports of extended NTS shedding by convalescent patients are common (<28 days to >55 days [90, 91]). Interestingly, administration of antibiotics does not facilitate clearance of infection in these patients [92], and may actually increase duration of asymptomatic shedding [93].
While reported most commonly in the context of patients with impaired immunity (transplant, elderly, HIV+, malaria co-infection) there are numerous, well-documented cases of otherwise healthy carriers of NTS [94]. These reports provide a clinical picture of NTS carriage that shares several features with our current understanding of typhoid carriage, including: intermittent periods of active shedding marked by months-long intervals of culture negativity, shedding that is associated with transmission to close contacts, recalcitrance to treatment and eradication and a high (60%) co-incidence with gallstones [89, 94]
The possibility for long-term human carriage of NTS is particularly important in sub-Saharan Africa, where recent reports have indicated the emergence of invasive NTS (iNTS) exhibiting numerous characteristics classically associated with typhoidal serovars. For instance, phylogenetic analysis based on whole-genome sequences of such iNTS isolates has indicated genomic degradation representing potential host-adaptation [95]. Clinical findings have implied long-term, relapsing infection reminiscent of carriage, and epidemiology studies indicate direct human-to-human transmission [96]. Hepatobiliary pathology is a sequela of acute NTS disease [97, 98], and abnormalities of the biliary tree have been implicated as a predisposing factor to development of iNTS in children [91], implying that bacterial colonization of this organ may be a factor in invasive NTS as well.
The mechanisms of Salmonella persistence in the gallbladder
Evidence in support of gallbladder and gallstone biofilm carriage of S. Typhi
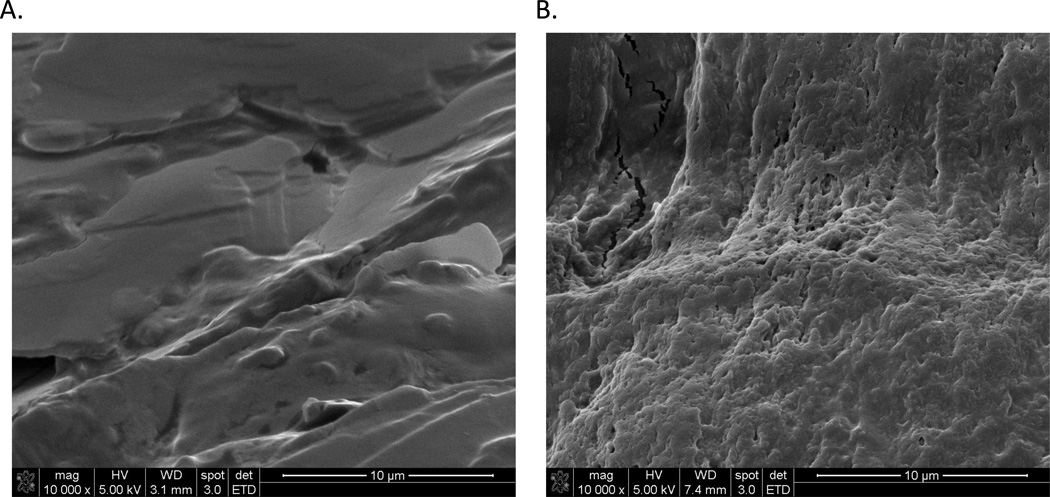
Although further studies are needed to understand the molecular mechanisms and epidemiological factors inducing the carrier state, there are numerous clinical indicators linking persistent Salmonella carriage to the human gallbladder. Gallbladder pathology is evident in 60% of patients at early stages of disease [14], indicating bacterial colonization of the gallbladder even during acute infection. In chronic carriage, there is a positive association with the presence of gallstones, which have been reported in up to 90% of S. Typhi carriers [15]. In patients from endemic regions undergoing cholecystectomy, 3.5% of gallbladder tissues harbored the bacterium [13] while electron micrographic observation of gallstones retrieved from such individuals demonstrate bacterial biofilm formation on the gallstone surface (Figure 1). Gallstones are present in 5–10% of the human population and gallstone disease is quite widespread [16]. Although identification of non-salmonellae bacteria in gallbladder tissues of cholecystectomy patients is not uncommon [17], gallstones of such patients infected with other Enterobacteriaceae are not similarly covered in a bacterial biofilm [18]. In vitro studies further demonstrate the inability of many bacteria that cause acute gallbladder infections to form a biofilm on gallstones under conditions in which salmonellae form biofilms [19]. Animal studies employing a newly developed murine model of chronic salmonellosis revealed that mice with gallstones exhibited an increased amount and duration of bacterial shedding. In agreement with observations of human S. Typhi carrier gallstones, microscopic examination of infected human and murine gallstones revealed dense bacterial biofilms on gallstone surfaces [18, 20].
Figure 1.
Biofilm formation on gallstones of typhoid carriers. Gallstones from Nepalese patients without (A) and with (B) S. Typhi chronic infections were imaged by scanning electron microscopy (SEM). After removal from the patient, the gallstones were air dried in a laminar flow hood for 24 hrs. They were then fixed in 2.5% glutaraldehyde (0.1M Phosphate buffer, 0.1M sucrose, pH 7.4) for 24 hrs., washed 3 times with water and air dried prior to SEM. Note the extensive biofilm of bacteria in image B with a visible extracellular matrix that encases and protects the bacteria.
Salmonella are adapted to gallbladder colonization
Salmonellae appear to be well-adapted to survive in the gallbladder and both clinical and murine data strongly suggest that biofilm formation is involved in Salmonella gallbladder persistence. Biofilm formation is thought to be a patho-adaptive response permitting bacterial aggregation, surface adherence, and antimicrobial resistance through the elaboration of a thick, protective extracellular matrix. Laboratory studies have shown that Salmonella respond rapidly in vitro to growth in gallbladder mimicking conditions, increasing biofilm formation and antimicrobial resistance in the presence of bile [19], and altering expression of fimbrial and outer membrane proteins in response to growth in bile and on cholesterol substrates [21, 22]. Recent clinical evidence supports the notion of ongoing bacterial adaptation to the gallbladder environment; with clinical isolates from gallbladder carriers exhibiting a common genetic profile distinct from those of isolates infecting other sites of the body [23], potentially implying a specific S. Typhi genotype associated with preferential infection of gallbladder. Phenotypic differences (e.g. carbon utilization, biofilm forming ability, and motility) among strains isolated from stool versus systemic sites may imply unique gene expression patterns facilitating adaptation to different anatomic locations [24]. Such adaptation can be modeled in vitro as well through bacterial cultivation in gallbladder-mimicking conditions, such as growth on gallbladder epithelial cells or cholesterol-coated surfaces or gallstones in the presence of bile [25, 26].
Recent work has suggested that the gallbladder epithelium may also be a possible niche for bacterial persistence. Salmonella exhibit rapid surface association and intracellular invasion of gallbladder epithelial cells, and can form biofilms on the epithelial surface. Gallbladder epithelium invasion and sloughing can be observed during acute infection in a murine model of typhoid fever [27], with bacterial invasion of and replication within gallbladder tissues evident for >2 months after infection [28]. Invasion of the gallbladder epithelium can mediate further damage to the organ and may permit bacterial escape from the harsh gallbladder environment [27]. Infected epithelial cells may proceed to re-initiate active gastrointestinal infection through a process of extrusion wherein bacterially infected epithelial cells are expelled from the epithelial layer by neighboring cells [29, 30]. Both models of persistent gallbladder infection (epithelial invasion/periodic extrusion and gallstone biofilm formation) likely involve a relatively small foci of bacteria associated with ongoing, low-level gallbladder damage and inflammation with intermittent re-seeding of the GI tract with viable bacteria.
Although the shedding of viable bacteria is of primary concern for the community, this process of ongoing inflammation is significant for the individual, as evidence supporting a link between persistent infectious inflammation and neoplastic transformations has accumulated in recent years. Indeed, epidemiologic studies have shown that long-term bacterial persistence within the gallbladder results in a dramatically increased likelihood of gallbladder carcinoma [31]. Gallbladder cancer (GBC) is the 5th most common gastrointestinal malignancy and is considered to be the most aggressive type of biliary cancer. Population-level studies show that risk factors for GBC are similar to those for S. Typhi carriage and include female gender, >50 years of age, and residence in regions such as the Gangetic belt of India [31]. While gallbladder abnormalities and gallstones are associated with an increased risk of GBC, it has been reported that chronic S. Typhi carriage is associated with as much as 12-fold increased risk of GBC development in these individuals. While the precise mechanisms remain unclear, chronic irritation or inflammation of the gallbladder epithelium may be an important feature of malignant transformation in GBC. Gallstones are known to increase damage to the epithelium, and it has also been proposed that bacterial or parasitic infections may alter the characteristics of bile, potentially producing cytotoxic byproducts [32]. The clinical association between S. Typhi infection and malignancy is supported by studies employing a murine model of long-term gallbladder carriage of Salmonella in which histopathological analysis of gallbladder tissue revealed significant pathology from the presence of gallstones alone, but pre-malignant transformations were present only in the gallbladders of mice with chronic Salmonella infections [30].
Epidemiology and treatment of chronic carriage
The epidemiological risk factors for becoming a persistent carrier have not been extensively investigated, primarily because this is a challenging population to prospectively identify. Studies conducted in endemic regions have shown an S. Typhi chronic carriage rate of 2 – 5% and indicated that it may be possible to prospectively detect these individuals through their abnormally high anti-Vi antibody titers [33, 34]. Epidemiological studies are complicated by the fact that the majority of chronic carriers in endemic settings are asymptomatic [35], and up to 25% have no clinical history of typhoid [36]. Additionally, potential risk factors may be confounded by the fraction of the population with gallbladder abnormalities [37]. For instance, the risk of becoming a chronic carrier following an acute infection increases with age, is greater for women than for men, and is particularly associated with cholelithiasis and cholecystitis [10, 38].
During the course of infection with invasive Salmonella, carriage may be split into three different periods: convalescent, temporary, and chronic. Convalescent carriers shed the bacilli in feces for three weeks to three months post-infection. Temporary carriers shed the bacilli for between three and twelve months, and chronic carriers shed the bacilli for more than one year [36]. The precise importance of short-term, convalescent fecal carriers versus long-term, chronic carriers in the dynamics of transmission in endemic regions has not been fully evaluated [39]. The majority of typhoid infections do not result in chronic infections but resolve after a period of convalescence, which may be accompanied by up to three months of bacterial shedding. Therefore, ongoing transmission of disease in endemic regions is likely to be driven largely by these recently-infected patients [40]. This scenario is supported by epidemiological studies that have shown disease transmission to close contacts within the household primarily from convalescent carriers rather than chronically infected individuals [13, 41]. Further, improved genetic analysis employing haplotyping of S. Typhi isolates and whole genome sequencing in highly endemic regions such as Vietnam and Nepal also found that acute typhoid is generally associated with a random distribution of organisms, rather than the same genotypes in close proximity [42]. Mathematical modeling of transmission dynamics has supported a scenario in which the contribution of carriers to overall disease burden is difficult to discern in the context of the high overall number of transmission events occurring in epidemics and highly endemic regions [39]. In such models, however, the role of carrier-transmitted infections becomes progressively more important as the overall incidence of disease decreases, becoming critical for the maintenance of a stable, endemic infection within the community. This scenario is supported by population data from regions where implementation of effective public health interventions to prevent and treat typhoid have resulted in dramatic reduction- but not elimination- of the disease [43]. Furthermore, in these regions, chronic asymptomatic carriers play an important role in continued disease transmission, with the majority of outbreaks being foodborne and associated with asymptomatic chronic carriers employed as food preparers or handlers [44]. Thus, as our ability to control overall disease burden improves, the role of chronic carriers will become increasingly important, making the identification and treatment of chronic carriers a key step in the eventual elimination of typhoid from a population.
Various antimicrobials have been used to treat chronic carriers; however, no treatment is completely effective in the resolution of chronic colonization of the gallbladder. Prolonged treatment courses with oral amoxicillin and ampicillin combined in some instances with probenecid or oral trimethorpim-sulfamethozaole have been used in the past, but did not always lead to successful eradication and are no longer recommended [45–47]. Fluoroquinolones appear to be more effective and better tolerated than amoxicillin; they are now the drug of choice for treatment of humans with chronic carriage [48]. A study of 28-day course of norfloxacin (400 mg twice daily) in 23 carriers demonstrated a cure rate of 86% in individuals with normal gallbladders and 75% in those with gallstones [49]. Smaller studies of ciprofloxacin (500 mg or 750 mg orally twice daily) for 14 to 28 days demonstrated cure rates of 90–93% [48]. However, the increasing development of multidrug resistant (MDR, defined as resistance to ampicillin, chloramphenicol, and trimethoprim) strains, decreasing susceptibility to fluoroquinolones, and development of beta-lactam resistance complicate treatment regimens of carriers. A study from Nepal found no MDR strains among the Salmonella isolated from chronic carriers [13]; whereas, in India, 19.4% of the S. Typhi isolates from chronic carriers were MDR; ceftriaxone resistance was also noted in 17% of the isolates [50]. Failure to effectively treat carriers with antimicrobials may be dependent on resistant organisms being selected in the gallbladder or the protective effect of biofilm formation. As carriage isolates are hypothesized to have been in the gallbladder for some time, the latter seems more likely and may be dependent on either slow or incomplete penetration of the antimicrobial into the biofilm, the biofilm altering the micro-environment for the antimicrobial, or a bacterial sub-population that forms a highly protected phenotypic state similar to spore formation [51]. In the presence of severe cholelithiasis, antimicrobial therapy in combination with removal of the gallbladder (cholecystectomy) may be required [36], as biofilm infections usually persist until the colonized surface is surgically removed from the body. Cholecystectomy alone raises the cure rate to 85% [52], but importantly, does not guarantee elimination of the carrier state [53]. Additional foci of infection can persist in the biliary tree, mesenteric lymph nodes, or the liver [54].
The rationale for the need of better diagnostics and preventive strategies
The introduction of public health principles such as sanitary infrastructure, pasteurization of milk, and water supply chlorination has led to the virtual elimination of typhoid from most developed countries in the 20th century [55]. It is anticipated that a similar shift will occur if these advances are made in currently endemic regions. In addition, in the short and intermediate term, a number of typhoid conjugate vaccines are in various stages of development and licensure. While there have been major public health efforts aimed at vaccination, and there is still hope for new, highly efficacious and safe vaccines, the most widely distributed form of the typhoid vaccine elicits protective immunity in only 50–70% of patients. The absence of a vaccine that is approved for use in young children (Ty21a ≥ 5yrs, Vi polysaccharide ≥ 2yrs) leaves a pivotal portion of the population unprotected. Importantly, a number of typhoid conjugate vaccines (Box 1) are in late stage development, and these vaccines show excellent promise even in the youngest children. As these better vaccines are incorporated into typhoid fever control programs, and as broader public health advances are made, the importance of carriers in these areas will likely increase.
In search of diagnostic markers for Salmonella carriage
What are the current methods of detecting Salmonella carriers?
Detection of carriers poses a difficult challenge since up to 25% of these individuals do not recall a history of typhoid [36] and currently available diagnostic assays are limited. As shedding of the organisms is intermittent and sometimes occurs in low levels [56], methods to detect the organisms in feces have had significant limitations and are not amenable to large-scale screening. Standard practice has been to detect typhoid carriage through analysis of serial stool and urine samples, which is logistically difficult to perform at a population level and is associated with low sensitivity [56]. Cultures of duodenal specimens obtained by post-pyloric duodenal tube aspiration or string device have slightly better sensitivity, but limited public health utility [57, 58]. Recovery of S. Typhi or S. Paratyphi A in bile, gallbladder stones, or tissue of afebrile individuals undergoing elective cholecystectomy is considered a gold standard of diagnosis, but this approach also has minimal public health utility because of its invasive nature. Molecular methods have also been employed to increase sensitivity, including fluorescent antibody techniques on fecal smears, but sensitivity has not surpassed culture [59]. Polymerase chain reaction (PCR) amplification for S. Typhi specific genes (e.g. fimbrial gene staA or fliC) have performed well in detecting organisms in bile or gallbladder specimens [60], but current PCR methods have been less successful in detecting organisms in stool [61].
A serologic marker for detection of carriers is more practical, but there are drawbacks to the currently available methods. The Widal test, developed in 1896, detects antibodies against Salmonella LPS O antigen and flagellar H antigen; however, the Widal test is unable to differentiate carriers from individuals with a history of prior infection [62]. Various other antigens have been used to assist with identifying carriers [63]; perhaps most importantly to date have been those directed at the Vi ag that comprises the capsule of S. Typhi. In 1934, Arthur Felix identified the Vi ag and showed that antibody responses to Vi disappeared from the majority of patients recovering from acute typhoid fever, but that anti-Vi ag responses persisted in the blood of chronic carriers [64]. Since then, various modified diagnostic techniques targeting Vi ag have been developed to increase the specificity and sensitivity of the test [65, 66]. Despite improvements in specificity, there remains ambiguity associated with the use of the test in endemic settings. There is high prevalence of elevated levels of anti-Vi antibodies in apparently healthy individuals in typhoid endemic areas, despite the fact that prevalence of culture-confirmed carriers remains low [67]. This may suggest that anti-Vi ag responses may be present either in chronic carriers or repetitively infected individuals in endemic zones. The detection of anti-Vi ag antibody, however, may remain useful in outbreak investigations in non-endemic areas [44].
New diagnostic strategies for carriers
In order to break the transmission cycle of S. Typhi and S. Paratyphi A, new strategies to identify chronic carriers that are specific, sensitive, and cost-effective are needed. Ideally, such approaches would be minimally or non-invasive, and could be deployed broadly to identify the few asymptomatic chronic carriers in a population. Serology-based assays targeting antigens expressed during acute infection have not proven to be effective. However, identification of antibody responses to antigens uniquely expressed by S. Typhi during the carrier state could lead to a useful diagnostic assay. In pursuit of this, an immunoscreening technique called in vivo-induced antigen technology was used to identify humorally immunogenic bacterial antigens expressed uniquely in individuals with S. Typhi biliary carriage [34]. Thirteen S. Typhi antigens were identified that were immunoreactive in carriers, but not in endemic zone healthy controls (Table 1). This included YncE (STY1479), an uncharacterized protein with ATP and DNA-binding domains. Further immunologic characterization of YncE demonstrated that IgG serum responses against YncE are elevated in S. Typhi carriers, but not in individuals at the acute or convalescent phase of disease, and not in healthy endemic zone controls [34]. Further analysis into the diagnostic po tential for carrier detection using YncE and the other antigens identified in this analysis are ongoing, as are the potential roles of these carrier-specific proteins in establishing and maintaining gallbladder carriage.
Table 1.
Immunoreactive S. Typhi proteins in carriers identified via In vivo-induced antigen technology
|
S. Typhi gene |
S. Typhimurium gene |
Function/details |
|---|---|---|
| corC (STY0712) | ybeX (STM0667) | Magnesium and cobalt efflux protein; magnesium and cobalt transporter [34] |
| artB (STY1364) | Upregulates chemokines, cytokines and adhesion molecules in human macrophage, colonic epithelial, and brain microvascular endothelial cell lines [34] | |
| yncE (STY1479) | Unknown, ATP and DNA-binding domains [70] | |
| sirA (STY2155) | uvrY (STM1947) | Response regulator; post-transcriptional regulatory system regulate the expression of both the SPI-1 and SPI-2 genes (Salmonella pathogenicity islands); transcription regulator; cytoplasmic protein [71] |
| STY2386 | Putative lipoprotein [72] | |
| pduG (STY2248) | pduG (STM2043) | Propanediol degradation; pdu operon co-regulated with cob operon appears to encode for cobalamin adenosyl transferase activity [73] |
| STY2386 | STM2156A | Putative lipoprotein [34] |
| yejE (STY2454) | yejE (STM2218) | Confers resistance to antimicrobial peptides and contributes to virulence; ABC-type dipeptide/oligopeptide/nickel transport system permease component; upregulated in macrophages [74] |
| xapB (STY2657) | xapB (STM2421) | Xanthosine permease; proton nucleoside symporter that can transport 6-oxo-purine ribonucleosides, adenosine, cytidine, uridine, and thymidine; biosynthesis [75] |
| purH (STY3709) | purH (STM4176) | Purine biosynthesis [76] |
| repE (HCM1.137) | On plasmid; replication initiation protein [77] | |
| HCM1.213c | Unknown, on plasmid [34] | |
| HCM2.0043 | Unknown, on plasmid [34] | |
| HCM2.0069c | Unknown, on plasmid [34] |
Another promising strategy is the identification of other host-associated peripheral blood biomarkers specific to the chronic carrier state. A study evaluating the transcriptional profile of acutely infected typhoid fever patients over time demonstrated that the transcriptional profile of the majority of typhoid fever patients appeared to revert to that of healthy controls within 9 months of acute infection. However, there was a small group whose transcriptional profile at 9 months remained similar to that of acutely convalescing patients. Although S. Typhi was not detected in the single stool sample obtained in the late phase from these individuals, such data may support the possibility that host biomarkers beyond antibodies may be used to identify carriers [68]. Similarly, another study comparing the proteome in the blood of chronic carriers, patients with acute typhoid fever, and healthy individuals identified changes in serum proteins that may be indicative of the chronic carrier state [69]. These and related studies suggest that development of an assay(s) to identify carriers is theoretically possible, although for maximal public health utility, any such assay(s) would need to be minimally or non363 invasive, inexpensive, practical, and easily applied in the field among target populations to identify a relatively few asymptomatic individuals.
Concluding remarks
Asymptomatic, chronic gallbladder carriers of S. Typhi have been recognized for over a century, the most famous of these being Typhoid Mary, who was the subject of one of the first true epidemiological investigations of an infectious disease. What has been lacking are studies to address how this bacterium uniquely establishes a persistent infection in this organ and how we can better identify, diagnose, and treat these carriers. These issues need to be solved, as carriers represent a reservoir for the continued spread of infection of this human-restricted pathogen (Box 3). Recent molecular and patient-based studies have advanced the field, and provide a foundational basis for a greater understanding of this chronic human illness.
Box 3. Outstanding questions.
Is there a critical level of typhoid endemicity at which chronic carriers account for the majority of new transmission?
Can we identify a sensitive and minimally invasive way to detect asymptomatic chronic carriers?
Can we develop improved regimens to treat asymptomatic carriers?
What is the role that carriers of S. Paratyphi A play in transmission and/or maintenance of (para)typhoid fever in a population?
What are the ethical considerations of carrier surveillance?
What is the primary mechanism of gallbladder carriage: biofilms on gallstones, biofilms on the gallbladder epithelium, or invasion of the gallbladder epithelium?
What are the bacterial and host responses that allow development and maintenance of asymptomatic chronic gallbladder carriage?
What is the role of the Vi ag and other extracellular matrix components in biofilm formation and establishment of gallbladder carriage?
Highlights.
Salmonella enterica serovar Typhi and Paratyphi A can cause chronic, asymptomatic infection, persisting primarily in the gallbladder.
Salmonella persists in biofilms on gallstones and the gallbladder epithelium.
Identification of carriers is difficult as shedding is intermittent and current diagnostics are not reliably accurate.
New treatment options are needed due to antibiotic resistance and gallbladder removal being a poor option in endemic regions.
Acknowledgements
This work was supported by grants from the National Institutes of Health, including the National Institute of Allergy and Infectious Diseases (AI100023, AI106878, AI058935 [E.T.R.]; AI066208 [J.S.G.]) and Career Development Award K08 AI089721 [R.C.C.]. S.B is a Sir Henry Dale Fellow, jointly funded by the Wellcome Trust and the Royal Society (100087/Z/12/Z).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Majowicz SE, et al. The global burden of nontyphoidal Salmonella gastroenteritis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2010;50:882–889. doi: 10.1086/650733. [DOI] [PubMed] [Google Scholar]
- 2.Crump JA, et al. The global burden of typhoid fever. Bulletin of the World Health Organization. 2004;82:346–353. [PMC free article] [PubMed] [Google Scholar]
- 3.Lozano R, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hallstrom K, McCormick BA. Salmonella interaction with and passage through the intestinal mucosa: through the lens of the organism. Frontiers in microbiology. 2011;2:88. doi: 10.3389/fmicb.2011.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dougan G, et al. Immunity to salmonellosis. Immunological reviews. 2011;240:196–210. doi: 10.1111/j.1600-065X.2010.00999.x. [DOI] [PubMed] [Google Scholar]
- 6.Hornick RB, et al. Typhoid fever: pathogenesis and immunologic control. The New England journal of medicine. 1970;283:686–691. doi: 10.1056/NEJM197009242831306. [DOI] [PubMed] [Google Scholar]
- 7.Tran QT, et al. The Salmonella enterica serotype Typhi Vi capsular antigen is expressed after the bacterium enters the ileal mucosa. Infection and immunity. 2010;78:527–535. doi: 10.1128/IAI.00972-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atif SM, et al. Salmonella enterica serovar Typhi impairs CD4 T cell responses by reducing antigen availability. Infection and immunity. 2014 doi: 10.1128/IAI.00020-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song J, et al. Structure and function of the Salmonella Typhi chimaeric A(2)B(5) typhoid toxin. Nature. 2013;499:350–354. doi: 10.1038/nature12377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levine MM, et al. Precise estimation of the numbers of chronic carriers of Salmonella typhi in Santiago, Chile, an endemic area. The Journal of infectious diseases. 1982;146:724–726. doi: 10.1093/infdis/146.6.724. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez-Escobedo G, et al. Chronic and acute infection of the gall bladder by Salmonella Typhi: understanding the carrier state. Nature reviews. Microbiology. 2011;9:9–14. doi: 10.1038/nrmicro2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roumagnac P, et al. Evolutionary history of Salmonella Typhi. Science. 2006;314:1301–1304. doi: 10.1126/science.1134933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dongol S, et al. The microbiological and clinical characteristics of invasive Salmonella in gallbladders from cholecystectomy patients in Kathmandu, Nepal. PloS one. 2012;7:e47342. doi: 10.1371/journal.pone.0047342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mateen MA, et al. Ultrasound in the diagnosis of typhoid fever. Indian journal of pediatrics. 2006;73:681–685. doi: 10.1007/BF02898444. [DOI] [PubMed] [Google Scholar]
- 15.Schioler H, et al. Biliary calculi in chronic Salmonella carriers and healthy controls: a controlled study. Scandinavian journal of infectious diseases. 1983;15:17–19. doi: 10.3109/inf.1983.15.issue-1.04. [DOI] [PubMed] [Google Scholar]
- 16.Enochsson L, et al. The Swedish Registry of Gallstone Surgery and Endoscopic Retrograde Cholangiopancreatography (GallRiks): A nationwide registry for quality assurance of gallstone surgery. JAMA surgery. 2013;148:471–478. doi: 10.1001/jamasurg.2013.1221. [DOI] [PubMed] [Google Scholar]
- 17.Levay B, et al. [The frequency of bacteria in human gallstones] Magyar sebeszet. 2013;66:353–356. doi: 10.1556/MaSeb.66.2013.6.8. [DOI] [PubMed] [Google Scholar]
- 18.Crawford RW, et al. Gallstones play a significant role in Salmonella spp. gallbladder colonization and carriage. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:4353–4358. doi: 10.1073/pnas.1000862107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prouty AM, et al. Biofilm formation and interaction with the surfaces of gallstones by Salmonella spp. Infection and immunity. 2002;70:2640–2649. doi: 10.1128/IAI.70.5.2640-2649.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marshall JM, et al. Visualization of extracellular matrix components within sectioned Salmonella biofilms on the surface of human gallstones. PloS one. 2014;9:e89243. doi: 10.1371/journal.pone.0089243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzalez-Escobedo G, Gunn JS. Identification of Salmonella enterica serovar Typhimurium genes regulated during biofilm formation on cholesterol gallstone surfaces. Infection and immunity. 2013;81:3770–3780. doi: 10.1128/IAI.00647-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antunes LC, et al. Repression of Salmonella enterica phoP expression by small molecules from physiological bile. Journal of bacteriology. 2012;194:2286–2296. doi: 10.1128/JB.00104-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hatta M, et al. Multi-locus variable-number tandem repeat profiling of Salmonella enterica serovar Typhi isolates from blood cultures and gallbladder specimens from Makassar, South-Sulawesi, Indonesia. PloS one. 2011;6:e24983. doi: 10.1371/journal.pone.0024983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalai Chelvam K, et al. Variations in motility and biofilm formation of Salmonella enterica serovar Typhi. Gut pathogens. 2014;6:2. doi: 10.1186/1757-4749-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gunn JS. Mechanisms of bacterial resistance and response to bile. Microbes and infection / Institut Pasteur. 2000;2:907–913. doi: 10.1016/s1286-4579(00)00392-0. [DOI] [PubMed] [Google Scholar]
- 26.Hernandez SB, et al. Adaptation and preadaptation of Salmonella enterica to Bile. PLoS genetics. 2012;8:e1002459. doi: 10.1371/journal.pgen.1002459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Menendez A, et al. Salmonella infection of gallbladder epithelial cells drives local inflammation and injury in a model of acute typhoid fever. The Journal of infectious diseases. 2009;200:1703–1713. doi: 10.1086/646608. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez-Escobedo G, Gunn JS. Gallbladder epithelium as a niche for chronic Salmonella carriage. Infection and immunity. 2013;81:2920–2930. doi: 10.1128/IAI.00258-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knodler LA, et al. Dissemination of invasive Salmonella via bacterial-induced extrusion of mucosal epithelia. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:17733–17738. doi: 10.1073/pnas.1006098107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gonzalez-Escobedo G, et al. Histopathological analysis of Salmonella chronic carriage in the mouse hepatopancreatobiliary system. PloS one. 2013;8:e84058. doi: 10.1371/journal.pone.0084058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wernberg JA, Lucarelli DD. Gallbladder Cancer. The Surgical clinics of North America. 2014;94:343–360. doi: 10.1016/j.suc.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 32.Stinton LM, Shaffer EA. Epidemiology of gallbladder disease: cholelithiasis and cancer. Gut and liver. 2012;6:172–187. doi: 10.5009/gnl.2012.6.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nolan CM, et al. Vi serology in the detection of typhoid carriers. Lancet. 1981;1:583–585. doi: 10.1016/s0140-6736(81)92033-x. [DOI] [PubMed] [Google Scholar]
- 34.Charles RC, et al. Identification of immunogenic Salmonella enterica serotype Typhi antigens expressed in chronic biliary carriers of S. Typhi in Kathmandu, Nepal. PLoS neglected tropical diseases. 2013;7:e2335. doi: 10.1371/journal.pntd.0002335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mortimer PP. Mr N the milker, and Dr Koch's concept of the healthy carrier. Lancet. 1999;353:1354–1356. doi: 10.1016/S0140-6736(98)11315-6. [DOI] [PubMed] [Google Scholar]
- 36.Parry CM, et al. Typhoid fever. The New England journal of medicine. 2002;347:1770–1782. doi: 10.1056/NEJMra020201. [DOI] [PubMed] [Google Scholar]
- 37.Yap KP, et al. Genome sequence and comparative pathogenomics analysis of a Salmonella enterica serovar Typhi strain associated with a typhoid carrier in Malaysia. Journal of bacteriology. 2012;194:5970–5971. doi: 10.1128/JB.01416-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vaishnavi C, et al. Epidemiology of typhoid carriers among blood donors and patients with biliary, gastrointestinal and other related diseases. Microbiology and immunology. 2005;49:107–112. doi: 10.1111/j.1348-0421.2005.tb03709.x. [DOI] [PubMed] [Google Scholar]
- 39.Saul A, et al. Stochastic simulation of endemic Salmonella enterica serovar Typhi: the importance of long lasting immunity and the carrier state. PloS one. 2013;8:e74097. doi: 10.1371/journal.pone.0074097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parry CM. The treatment of multidrug-resistant and nalidixic acid-resistant typhoid fever in Viet Nam. Trans R Soc Trop Med Hyg. 2004;98:413–422. doi: 10.1016/j.trstmh.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 41.Karkey A, et al. Differential epidemiology of Salmonella Typhi and Paratyphi A in Kathmandu, Nepal: a matched case control investigation in a highly endemic enteric fever setting. PLoS neglected tropical diseases. 2013;7:e2391. doi: 10.1371/journal.pntd.0002391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baker S, et al. Combined high-resolution genotyping and geospatial analysis reveals modes of endemic urban typhoid fever transmission. Open biology. 2011;1:110008. doi: 10.1098/rsob.110008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keddy KH, et al. Molecular epidemiological investigation of a typhoid fever outbreak in South Africa, 2005: the relationship to a previous epidemic in 1993. Epidemiology and infection. 2011;139:1239–1245. doi: 10.1017/S0950268810002207. [DOI] [PubMed] [Google Scholar]
- 44.Olsen SJ, et al. Outbreaks of typhoid fever in the United States, 1960–99. Epidemiology and infection. 2003;130:13–21. doi: 10.1017/s0950268802007598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Phillips WE. Treatment of chronic typhoid carriers with ampicillin. JAMA : the journal of the American Medical Association. 1971;217:913–915. [PubMed] [Google Scholar]
- 46.Nolan CM, White PC., Jr Treatment of typhoid carriers with amoxicillin. Correlates of successful therapy. JAMA : the journal of the American Medical Association. 1978;239:2352–2354. doi: 10.1001/jama.239.22.2352. [DOI] [PubMed] [Google Scholar]
- 47.Brodie J, et al. Effect of trimethoprim-sulphamethoxazole on typhoid and Salmonella carriers. British medical journal. 1970;3:318–319. doi: 10.1136/bmj.3.5718.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zavala Trujillo I, et al. Fluoroquinolones in the treatment of typhoid fever and the carrier state. European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology. 1991;10:334–341. doi: 10.1007/BF01967008. [DOI] [PubMed] [Google Scholar]
- 49.Gotuzzo E, et al. Use of norfloxacin to treat chronic typhoid carriers 10.1093/infdis/157.6.1221. Journal of Infectious Diseases. 1988;157:1221–1225. doi: 10.1093/infdis/157.6.1221. [DOI] [PubMed] [Google Scholar]
- 50.Pratap CB, et al. Drug resistance in Salmonella enterica serotype Typhi isolated from chronic typhoid carriers. International journal of antimicrobial agents. 2012;40:279–280. doi: 10.1016/j.ijantimicag.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 51.Costerton JW, et al. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 52.Main RG. Treatment of the chronic alimentary enteric carrier. British medical journal. 1961;1:328–333. doi: 10.1136/bmj.1.5222.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ristori C, et al. Persistence of the Salmonella typhi-paratyphi carrier state after gallbladder removal. Bulletin of the Pan American Health Organization. 1982;16:361–366. [PubMed] [Google Scholar]
- 54.Nath G, et al. Isolation of Salmonella Typhi from apparently healthy liver. Infection, genetics and evolution : journal of molecular epidemiology and evolutionary genetics in infectious diseases. 2011;11:2103–2105. doi: 10.1016/j.meegid.2011.08.029. [DOI] [PubMed] [Google Scholar]
- 55.Hardy A. Scientific strategy and ad hoc response: the problem of typhoid in America and England, C. 1910–50. Journal of the history of medicine and allied sciences. 2014;69:3–37. doi: 10.1093/jhmas/jrs005. [DOI] [PubMed] [Google Scholar]
- 56.Bokkenheuser V. Detection of typhoid carriers. American journal of public health and the nation's health. 1964;54:477–486. doi: 10.2105/ajph.54.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gilman RH, et al. IDENTIFICATION OF GALLBLADDER TYPHOID CARRIERS BY A STRING DEVICE. The Lancet. 1979;313:795–796. doi: 10.1016/s0140-6736(79)91316-3. [DOI] [PubMed] [Google Scholar]
- 58.Merselis JG, Jr, et al. Quantitative bacteriology of the typhoid carrier state. The American journal of tropical medicine and hygiene. 1964;13:425–429. doi: 10.4269/ajtmh.1964.13.425. [DOI] [PubMed] [Google Scholar]
- 59.Thomason BM, McWhorter AC. Rapid detection of typhoid carriers by means of fluorescent antibody techniques. Bulletin of the World Health Organization. 1965;33:681–685. [PMC free article] [PubMed] [Google Scholar]
- 60.Pratap CB, et al. Targeting of putative fimbrial gene for detection of S. Typhi in typhoid fever and chronic typhoid carriers by nested PCR. Journal of infection in developing countries. 2013;7:520–527. doi: 10.3855/jidc.2561. [DOI] [PubMed] [Google Scholar]
- 61.Kumar G, et al. Use of urine with nested PCR targeting the flagellin gene ( fliC ) for diagnosis of typhoid fever. Journal of clinical microbiology. 2012;50:1964–1967. doi: 10.1128/JCM.00031-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Olopoenia LA, King AL. Widal agglutination test-- 100 years later: still plagued by controversy. Postgraduate medical journal. 2000;76:80–84. doi: 10.1136/pmj.76.892.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chau PY, et al. Crossed immunoelectrophoretic analysis of anti-Salmonella typhi antibodies in sera of typhoid patients and carriers: demonstration of the presence of typhoid-specific antibodies to a non-O, non-H, non-Vi antigen. Infection and immunity. 1984;43:1110–1113. doi: 10.1128/iai.43.3.1110-1113.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Felix A. DETECTION OF CHRONIC TYPHOID CARRIERS BY AGGLUTINATION TESTS. The Lancet. 1938;232:3. [Google Scholar]
- 65.Lanata CF, et al. Vi serology in detection of chronic Salmonella typhi carriers in an endemic area. Lancet. 1983;2:441–443. doi: 10.1016/s0140-6736(83)90401-4. [DOI] [PubMed] [Google Scholar]
- 66.Losonsky GA, et al. Development and evaluation of an enzyme-linked immunosorbent assay for serum Vi antibodies for detection of chronic Salmonella typhi carriers. Journal of clinical microbiology. 1987;25:2266–2269. doi: 10.1128/jcm.25.12.2266-2269.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gupta A, et al. Evaluation of community-based serologic screening for identification of chronic Salmonella Typhi carriers in Vietnam. International journal of infectious diseases : IJID. 2006;10:309–314. doi: 10.1016/j.ijid.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 68.Thompson LJ, et al. Transcriptional response in the peripheral blood of patients infected with Salmonella enterica serovar Typhi. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:22433–22438. doi: 10.1073/pnas.0912386106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kumar A, et al. Proteomics-based identification of plasma proteins and their association with the host-pathogen interaction in chronic typhoid carriers. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases. 2014;19:59–66. doi: 10.1016/j.ijid.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kagawa W, et al. Structural basis for the DNA-binding activity of the bacterial beta-propeller protein YncE. Acta crystallographica. Section D, Biological crystallography. 2011;67:1045–1053. doi: 10.1107/S0907444911045033. [DOI] [PubMed] [Google Scholar]
- 71.Teplitski M, et al. Contribution of the SirA regulon to biofilm formation in Salmonella enterica serovar Typhimurium. Microbiology. 2006;152:3411–3424. doi: 10.1099/mic.0.29118-0. [DOI] [PubMed] [Google Scholar]
- 72.Teplitski M, et al. Pathways leading from BarA/SirA to motility and virulence gene expression in Salmonella. Journal of bacteriology. 2003;185:7257–7265. doi: 10.1128/JB.185.24.7257-7265.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bobik TA, et al. The propanediol utilization (pdu) operon of Salmonella enterica serovar Typhimurium LT2 includes genes necessary for formation of polyhedral organelles involved in coenzyme B(12)-dependent 1, 2-propanediol degradation. Journal of bacteriology. 1999;181:5967–5975. doi: 10.1128/jb.181.19.5967-5975.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Qimron U, et al. Identification of Salmonella Typhimurium genes responsible for interference with peptide presentation on MHC class I molecules: Deltayej Salmonella mutants induce superior CD8+ T-cell responses. Cellular microbiology. 2004;6:1057–1070. doi: 10.1111/j.1462-5822.2004.00418.x. [DOI] [PubMed] [Google Scholar]
- 75.Hansen MR, et al. Xanthosine utilization in Salmonella enterica serovar Typhimurium is recovered by a single aspartate-to-glycine substitution in xanthosine phosphorylase. Journal of bacteriology. 2006;188:4153–4157. doi: 10.1128/JB.01926-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chopra AK, et al. Nucleotide sequence analysis of purH and purD genes from Salmonella typhimurium. Biochimica et biophysica acta. 1991;1090:351–354. doi: 10.1016/0167-4781(91)90202-w. [DOI] [PubMed] [Google Scholar]
- 77.Phan MD, et al. Variation in Salmonella enterica serovar Typhi IncHI1 plasmids during the global spread of resistant typhoid fever. Antimicrobial agents and chemotherapy. 2009;53:716–727. doi: 10.1128/AAC.00645-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sharma A, Qadri A. Vi polysaccharide of Salmonella Typhi targets the prohibitin family of molecules in intestinal epithelial cells and suppresses early inflammatory responses. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:17492–17497. doi: 10.1073/pnas.0407536101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Seth-Smith HM. SPI-7: Salmonella 's Vi-encoding pathogenicity island. Journal of infection in developing countries. 2008;2:267–271. doi: 10.3855/jidc.220. [DOI] [PubMed] [Google Scholar]
- 80.Winter SE, et al. The Salmonella enterica serotype Typhi regulator TviA reduces interleukin-8 production in intestinal epithelial cells by repressing flagellin secretion. Cellular microbiology. 2008;10:247–261. doi: 10.1111/j.1462-5822.2007.01037.x. [DOI] [PubMed] [Google Scholar]
- 81.Pickard D, et al. Composition, acquisition, and distribution of the Vi exopolysaccharide-encoding Salmonella enterica pathogenicity island SPI-7. Journal of bacteriology. 2003;185:5055–5065. doi: 10.1128/JB.185.17.5055-5065.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lindow JC, et al. Antibodies in action: role of human opsonins in killing Salmonella enterica serovar Typhi. Infection and immunity. 2011;79:3188–3194. doi: 10.1128/IAI.05081-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Szu SC, et al. Phase I clinical trial of O-acetylated pectin conjugate, a plant polysaccharide based typhoid vaccine. Vaccine. 2014;32:2618–2622. doi: 10.1016/j.vaccine.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bhutta ZA, et al. Immunogenicity and safety of the Vi-CRM197 conjugate vaccine against typhoid fever in adults, children, and infants in south and southeast Asia: results from two randomised, observer-blind, age de-escalation, phase 2 trials. The Lancet infectious diseases. 2014;14:119–129. doi: 10.1016/S1473-3099(13)70241-X. [DOI] [PubMed] [Google Scholar]
- 85.Scobie HM, et al. Impact of a targeted typhoid vaccination campaign following Cyclone Tomas, Republic of Fiji, 2010. The American journal of tropical medicine and hygiene. 2014;90:1031–1038. doi: 10.4269/ajtmh.13-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cui C, et al. Physical and chemical characterization and immunologic properties of Salmonella enterica serovar Typhi capsular polysaccharide-diphtheria toxoid conjugates. Clinical and vaccine immunology : CVI. 2010;17:73–79. doi: 10.1128/CVI.00266-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Micoli F, et al. Production of a conjugate vaccine for Salmonella enterica serovar Typhi from Citrobacter Vi. Vaccine. 2012;30:853–861. doi: 10.1016/j.vaccine.2011.11.108. [DOI] [PubMed] [Google Scholar]
- 88.Levine MM, Robins-Browne RM. Factors that explain excretion of enteric pathogens by persons without diarrhea. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012;55(Suppl 4):S303–S311. doi: 10.1093/cid/cis789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cruickshank JG, Humphrey TJ. The carrier food-handler and non-typhoid salmonellosis. Epidemiology and infection. 1987;98:223–230. doi: 10.1017/s0950268800061975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sirinavin S, et al. Duration of nontyphoidal Salmonella carriage in asymptomatic adults. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2004;38:1644–1645. doi: 10.1086/421027. [DOI] [PubMed] [Google Scholar]
- 91.Punpanich W, et al. Invasive salmonellosis in urban Thai children: a ten-year review. The Pediatric infectious disease journal. 2012;31:e105–e110. doi: 10.1097/INF.0b013e31825894b0. [DOI] [PubMed] [Google Scholar]
- 92.Buchwald DS, Blaser MJ. A review of human salmonellosis: II. Duration of excretion following infection with nontyphi Salmonella. Reviews of infectious diseases. 1984;6:345–356. doi: 10.1093/clinids/6.3.345. [DOI] [PubMed] [Google Scholar]
- 93.Murase T, et al. Fecal excretion of Salmonella enterica serovar Typhimurium following a food-borne outbreak. Journal of clinical microbiology. 2000;38:3495–3497. doi: 10.1128/jcm.38.9.3495-3497.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Musher DM, Rubenstein AD. Permanent carriers of nontyphosa salmonellae. Archives of internal medicine. 1973;132:869–872. [PubMed] [Google Scholar]
- 95.Kingsley RA, et al. Epidemic multiple drug resistant Salmonella Typhimurium causing invasive disease in sub-Saharan Africa have a distinct genotype. Genome research. 2009;19:2279–2287. doi: 10.1101/gr.091017.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Morpeth SC, et al. Invasive non-Typhi Salmonella disease in Africa. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2009;49:606–611. doi: 10.1086/603553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.McCarron B. A 3-year retrospective review of 132 patients with Salmonella enterocolitis admitted to a regional infectious diseases unit. The Journal of infection. 1998;37:136–139. doi: 10.1016/s0163-4453(98)80167-9. [DOI] [PubMed] [Google Scholar]
- 98.Ruiz-Rebollo ML, et al. Acalculous cholecystitis due to Salmonella enteritidis. World journal of gastroenterology : WJG. 2008;14:6408–6409. doi: 10.3748/wjg.14.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]