Abstract
Neuromodulation underlies the flexibility of neural circuit operation and behavior. Individual neuromodulators can have divergent actions in a neuron by targeting multiple physiological mechanisms. Conversely, multiple neuromodulators may have convergent actions through overlapping targets. Additionally, the divergent and convergent neuromodulator actions can be unambiguously synergistic or antagonistic. Neuromodulation often entails balanced adjustment of nonlinear membrane and synaptic properties by targeting ion channel and synaptic dynamics rather than just excitability or synaptic strength. In addition, neuromodulators can exert effects at multiple timescales, from short-term adjustments of neuron and synapse function to persistent long-term regulation. This short review summarizes some highlights of the diverse actions of neuromodulators on ion channel and synaptic properties.
Introduction
The current understanding of nervous system function holds a prominent place for the role of neuromodulators in shaping electrophysiological activity. All nervous system function, from simple reflexes to sleep, memory and higher cognitive tasks, ultimately result from the activity of neural circuits. A wide variety of substances, including small molecule transmitters, biogenic amines, neuropeptides and others can be released in modes other than classical fast synaptic transmission, and modify neural circuit output to produce extensive adaptability in behaviors [1]. They do so by changing the properties of a circuit’s constituent neurons, their synaptic connections or the inputs to the circuit. Such functional reconfiguration of hard-wired circuits is essential for the adaptability of the nervous system.
Neuromodulators are often thought to convey global control of brain states that underlie different behaviors, such as sleep and arousal. Implicit in this view is that one or a few modulators can dominate the operation of a large number of neurons and interconnected circuits, and that the global presence or absence of a neuromodulator is equivalent to a specific behavioral state. However, this view appears to contradict studies at the cellular level which show that multiple neuromodulators can act simultaneously on any single neuron, that intrinsic excitability and synaptic efficacy are always under neuromodulatory influence and, therefore, reconfiguration of neural circuits by neuromodulators is an intricately balanced process that involves multiple synergistic or antagonistic pathways. These conflicting views do not arise from contradictory experimental results, but rather from the challenge to bridge multiple levels of analysis from cellular to circuit to behavior. A comprehensive description of the variety of neuromodulator actions at these different levels is beyond the scope of a single review. Here we summarize findings that highlight the diversity of neuromodulatory effects on cellular and synaptic properties and discuss them in the context of local circuit activity.
Neuromodulation of synapses
Neuromodulators modify synaptic communication through a number of mechanisms which can be broadly divided into effects that target synapses directly and those that indirectly modify synaptic interactions by changing the excitability of neurons. Indirect effects include presynaptic modulation that can lead to changes in action potential shape [2–4••], and postsynaptic modulation that for example increases voltage-gated inward currents to enhance EPSPs [5–7•]. We will discuss these effects in detail in the next section.
Direct effects on synaptic interactions can also be divided into pre- and postsynaptic mechanisms. Presynaptically, neuromodulators often target the probability of vesicular release by modifying presynaptic Ca2+ influx, the size of the reserve pool, or proteins in the active zone [8–11]. On the postsynaptic side, the expression and properties of transmitter receptors can be modified to change postsynaptic responses independent of neurotransmitter release [12,13]. Modulation of neurotransmitter release can also occur through local feedback that alters the level of release through retrograde messengers [14] or autoreceptors [15–17]. Finally, neuromodulator release itself can be subject to modulation. For example, nitric oxide (NO) can modify modulatory actions of glutamate or serotonin (5-HT) [18,19], an example of a broader category of neuromodulatory actions referred to as meta-modulation [20–22].
Neuromodulation of synaptic strength
The simplest functional consequence of synapse modulation is the modification of synaptic strength. Multiple modulators can act on the same synapse to modify its strength, presumably depending on the behavioral need [24,25]. Such effects can be drastic: 5-HT can functionally silence synapses in the crustacean stomatogastric ganglion (STG), whereas dopamine can unmask synapses that are normally silent [26]. The combined action of multiple neuromodulators on synapses can be more than simply additive [27], and the same neuromodulator can have opposing actions on synaptic strength, depending on the network state [28–30].
An important lesson from small invertebrate networks is that neuromodulation of synaptic strength does not always have obvious consequences at the network level. In the STG, the inhibitory synaptic connection from one follower neuron to another is strengthened by dopamine without changing its influence on the postsynaptic activity [31]. In the same system, the inhibitory feedback connection from a follower neuron to pacemaker neurons is greatly strengthened by two neuropeptides [32,33•] with no apparent consequence for network frequency. However, the increase in synaptic efficacy acts to stabilize network oscillations [33•].
Neuromodulation of gap-junctions is less explored [34]. However, early work in the retina showed that electrical synapses are also subject to neuromodulation [35,36] and more recent work has shown that electrical synapses in other vertebrate and invertebrate systems are modulated by monoamines and NO [37–42], although the main influence of a modulator on the functional strength of the electrical synapse may be due to changes in neuronal input conductance [38,43].
Neuromodulation of synaptic dynamics
In many systems, neuromodulators also act on synaptic dynamics (short-term synaptic plasticity, STP) [30,44–46]. The effect of modulators can be drastic and in some cases can switch the sign of synaptic dynamics from depression to facilitation [33•,47–49]. If the presynaptic neuron is active repetitively, STP can act as a gain-control mechanism, modifying synaptic strength as a function of the frequency of presynaptic activity [50,51]. The modulation of STP can therefore be as important as the modulation of synaptic strength in determining the efficacy of the synapse during network activity (Fig. 1a).
Figure 1.
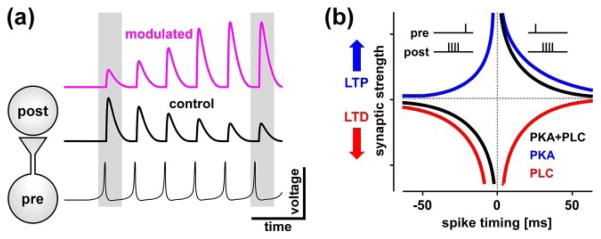
Neuromodulation of short- and long-term synaptic plasticity. (a) Neuromodulators can target synaptic strength, which modifies the response to a single presynaptic input (left gray box), as well as short-term synaptic plasticity, which modifies the synaptic efficacy in response to sustained input (right gray box). These two effects can be functionally opposite. (b) The LTP and LTD components of STDP are controlled by modulators. Normally, the relative timing of pre- and postsynaptic spikes determines the direction of the modification in synaptic strength. However, activation of the PKA pathway promotes LTP and activation of the PLC pathway promotes LTD, independent of the relative spike timing. Each suppresses the other, producing a push-pull rule for the control of STDP. Panel modified from [23].
The effects of neuromodulators on synaptic strength and dynamics are best understood in the Aplysia gill and siphon withdrawal reflex [52]. This reflex is mediated by a sensory to motor neuron connection which normally habituates but becomes sensitized by serotonergic modulation activated by pairing touch with a noxious tail shock [53]. 5-HT facilitates synaptic strength through activation of protein kinase A (PKA), resulting in a reduction of presynaptic potassium currents. This leads to an increase in the width of the presynaptic action potential and increased transmitter release [54]. A longer training paradigm, equivalent to prolonged exposure to 5-HT, results in an intermediate-term facilitation that involves presynaptic protein kinase C (PKC) and postsynaptic Ca2+-dependent pathways and leads to the insertion of AMPA-like receptors [55,56]. Long-term facilitation of the synapse, associated with long-term sensitization of the behavior, involves a PKA-dependent modification of transcription through CREB phosphorylation, which results in new protein expression and structural changes at the synapse [57].
The Aplysia example demonstrates that neuromodulators can act at different time scales and can also affect more persistent changes in synaptic function in the context of learning and memory. In insects, aminergic modulation is an important mechanism in the formation of associative memory [58]. A number of studies over the last two decades have also shown that neuromodulators play an important role in long-term potentiation (LTP) and depression (LTD) of mammalian central synapses [59–63]. Recently, a unifying narrative has emerged that describes how different neuromodulators change the balance of LTP and LTD. The exploration of neuromodulator effects on spike-timing-dependent plasticity (STDP) unmasked a simple rule: the activation of the PKA pathway, e.g. by β-adrenergic receptors, promotes and gates LTP, whereas the activation of the phospholipase C (PLC) pathway, e.g. by M1 muscarinic receptors, promotes LTD [23,64,65]. These modulatory actions require pairing with the induction protocols that enable NMD-Adependent synaptic plasticity and the simultaneous activation of both modulatory pathways enables the bidirectional long-term changes in STDP [65] (Fig. 1b). Interestingly, activation of each pathway also suppresses the other, suggesting a push-pull rule for the neuromodulation of long-term synaptic plasticity [66] that seems to be independent of the underlying mechanisms of LTP and LTD [67•].
Neuromodulation of neuronal excitability
Responses to synaptic input, as well as spontaneous activity, critically depend on input conductance and the complement of voltage-gated currents. Differences in these properties across and within cell types can be due to differences in the types and spatial distribution of ion channels [68], in their relative expression levels [69], or in the gating properties of similar channels [70]. Accordingly, neuromodulators can change activity and excitability by adding or subtracting ionic currents, changing the relative magnitudes across the complement of currents, or changing voltage- and time dependence of channel gating. It has long been known that neuromodulators affect the availability of voltage-gated ion channels and the gating properties underlying activation and inactivation [71] and, in some cases, also the magnitude of unitary conductances [72]. Over the last two decades, work on a large number of neuron types in different model systems has shown that ion channel modulation is ubiquitous and that each channel is likely to be under modulatory control at all times. Any given neuromodulator can affect multiple types of ion channels, and any given ion channel type can be affected by multiple neuromodulators [1].
The canonical mechanism of ion channel modulation involves binding of a neuromodulator to G protein coupled receptors, subsequent activation of second messengers, and activation of kinases and phosphatases that phosphorylate or dephosphorylate target channel proteins. However, ion channel modulation can occur through other mechanisms, such as direct gating by cyclic nucleotides [73,74] or G proteins [75], or direct and indirect effects of tyrosine kinases [76,77]. All these mechanisms share the common theme that different phosphorylation sites control different aspects of channel gating. Even voltage-gated sodium channels, long thought not to be modulated, are now known to respond to a wide variety of modulators [78] which target multiple phosphorylation sites at the α-subunits [79–81]. The sensitivity to multiple signaling pathways suggests a balanced modulation by many neuromodulators.
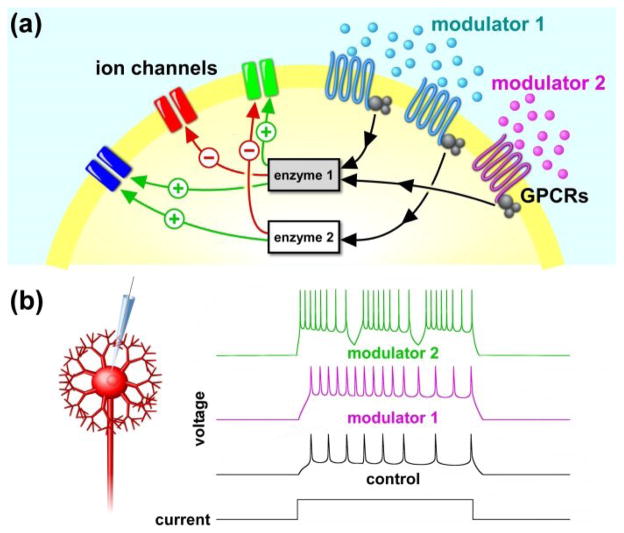
Even though neuromodulators are often released diffusely or reach target neurons through the circulatory system, they can have specific effects across different cell types and different compartments within one cell. Neuromodulators can target multiple ion channel types in the same cell, either through shared signaling pathways or different receptors. Similarly, the same signaling pathway can have different effects on different channels (Fig. 2a). Gq-coupled receptors, for example, can have opposite effects on different types of Na+-dependent K+ channels, both mediated by PKC [82]. The same modulator can also act through different signaling pathways. Dopamine, for instance, acts through different second messenger systems that synergistically modulate Ca2+ currents in teleost retinal horizontal cells [83], and the same is true for μ-opioid receptor mediated modulation of Na+ channels in the prefrontal cortex, which depends on both PKA and PKC [84].
Figure 2.
Neuromodulation of ion channels and membrane excitability. (a) Even in a single cell, neuromodulators can have convergent or divergent actions. Different modulators can converge on the same intracellular pathway to target the same or different ion channel types. Conversely, one modulator may activate different intracellular pathways depending on receptor type. (b) The modulatory effect on excitability may be straightforward (modulator 1) or change the membrane properties qualitatively in a nonlinear fashion (modulator 2).
Different neuromodulators often affect different subsets of ion channels in a neuron, but there can be convergence of modulatory effects onto the same channel. If these effects have the same sign, they can either be occluding, i.e. simply additive [85], or synergistic [86••].
The pattern of differential modulation of ion channels across different cell types is difficult to unravel in large circuits with ambiguous cell types, but has been relatively straightforward in invertebrate circuits with identified cells. In the STG, at least two principles have emerged. Biogenic amines, such as dopamine, affect multiple currents in each cell, but the subset of target currents is cell-type specific and so is the sign of the effect on a given current [87]. In contrast, different neuropeptides converge onto the same voltage-dependent channel type, but each peptide affects different subsets of cells in the circuit [85,88]. Therefore, in order to understand neuromodulator effects on neuron and circuit activity, one has to identify the pattern of convergence and divergence seen both at the single neuron level and across different neurons [1].
A major contributing factor to the specificity of neuromodulator actions is that intracellular signaling, despite global effects of second messenger pathways, is often restricted to local intracellular domains [89,90]. Consequently, neuromodulation can be restricted to subsets of ion channels in specific cell compartments, even if channels of the same type in different compartments, or channels of a different type in the same compartment could potentially respond to the same pathways [91•,92]. This specificity may arise from the organization of both ion channels and enzymes into local signaling complexes, associated with specific anchoring proteins [93–95] and may give rise to local differences in gating properties within the same cell [96].
While modulation of voltage-gated channels is particularly important because it directly affects the nonlinear dynamics underlying membrane excitability, neuromodulators can have equally significant effects on other types of ion channels. Neuronal input resistance and resting potential are important determinants of excitability and are changed when modulators activate ligand-gated channels or modify “leak” channels. Modulation of leak conductance is best understood for the family of two-pore domain K+ channels. These channels are responsible for a substantial portion of leak conductance in a variety of neurons, are modulated by a number of intracellular signaling pathways [97], and can be the target of convergent actions of neuromodulators [98]. Inhibition of these channels can be important in the control of excitability [99], whereas their activation, which leads to membrane hyperpolarization, is a molecular target for anesthetics [100].
Ionotropic receptors are best known for mediating postsynaptic responses during fast synaptic transmission. However, ionotropic receptors for small-molecule transmitters can also function in an unambiguously modulatory context. Acetylcholine, the classic fast transmitter at the vertebrate neuromuscular junction, predominantly acts as a neuromodulator in the vertebrate CNS, even when activating ionotropic receptors [101–103]. GABAA receptors, which mediate fast postsynaptic inhibitory responses and fast presynaptic inhibition [104], are often found in non-synaptic membrane, where they respond to low-concentration tonic GABA signals [104–106][107]. Even AMPA receptors, the ubiquitous mediators of fast glutamatergic transmission in the brain, have recently been shown to have presynaptic neuromodulatory actions [4••]. Apart from presynaptic effects, modulation of axonal excitability through ionotropic receptors can also have a substantial influence on action potential generation and propagation [108,109••].
Although the neuromodulation of ion channel properties has been best described in terms of acute effects, there is evidence that neuromodulators are also involved in long-term modifications of ion channels [110]. The removal of endogenous modulators from STG neurons is known to affect both the expression and the co-regulation of ion channels [111,112]. These modifications depend on both translation [113•] and transcription [114•]. Similarly, disruption of aminergic modulation in spinalized animals drastically increases the sensitivity of spinal interneurons to modulators [115] and enables motor neurons to recover persistent Ca2+ currents as amine receptors become constitutively active [6,7•].
From cellular and synaptic properties to circuit function
The modulation of neural circuits depends on the type, location and temporal dynamics of neuromodulator release [116]. The examples discussed above show that even a single neuromodulator can have complex effects on ion channels in each cell and on the strength and dynamics of synapses and, therefore, its effect on circuit output is not straightforward.
Changes in excitability are not always unequivocal. In the simplest case, a neuron’s firing response to presynaptic activity increases or decreases. Consequently, the divergent effects of a neuromodulator on multiple ion channels or synaptic parameters become intuitive if the changes are additive or synergistic; for example, enhanced excitability due to decreased outward currents and increased inward currents, as seen for 5-HT modulation of many motor neurons [117]. However, electrical activity in most neurons is nonlinear, giving rise to a repertoire of membrane behaviors, including post-inhibitory rebound, plateau potentials, bistability, resonance and endogenous bursting. For such neurons, translating changes in synaptic strength and excitability to a firing rate is too simplistic. In this context, neuromodulation fine tunes specific nonlinear response properties and activity patterns [118] (Fig. 2b). For example, some neurons in the rhythmically-active STG respond to dopamine with an increase in a subset of both inward and outward currents and a decrease in others, and effects on pre-and postsynaptic parameters can be functionally opposing. Additionally, dopamine changes the synaptic strength and dynamics in these circuits [87].
Such fine-tuning clearly implies that neuromodulatory effects can only really be understood in the context of how neurons are activated and how they function within a circuit, which is a nontrivial task. Although some computational studies have examined the role of neuromodulation on synaptic or neuronal properties [119,120], there are few modeling studies that explore the integrative role of neuromodulators on network output [121–123]. A full description of neuromodulatory actions requires a new generation of theoretical and computational models [124] that take into consideration the dynamic short- and long-term actions of neuromodulators on their multiple targets.
Summary and conclusions
Neuromodulators target ion channels and synaptic interactions to modify circuit dynamics, which allows for adaptability of circuit operation in different behavioral contexts. Synaptic modulation is not limited to changes in the strength of connections, but involves modifications of short- and long-term synaptic plasticity. Similarly, neuromodulation of intrinsic excitability is not limited to simple amplification or reduction of responsiveness to input, but can shape the nonlinear interactions between different currents to give rise to qualitatively different membrane behaviors. A single modulator can control multiple aspects of synaptic and intrinsic dynamics in a single neuron, and multiple modulators can affect these properties through converging and diverging intracellular pathways. The complexity of cell-type specific effects, their highly nonlinear dynamics, as well as the fact that multiple neuromodulators may act at the same time, presents a challenge in trying to understand consequences for circuit output. Even if much of the modulatory effects are described quantitatively in a given circuit, their functional synthesis will require new theoretical approaches and computational modeling.
Highlights.
Modulation of synaptic strength can be through changes in short-term plasticity.
Modulators can shape or induce long-term synaptic plasticity.
Modulators can have spatially specific and divergent targets in single neurons.
Modulators can change neuronal excitability qualitatively and nonlinearly.
Classical ionotropic receptors can exert unambiguously modulatory effects.
Acknowledgments
We thank Isabel Soffer, Diana Martinez and Jorge Golowasch for their helpful comments. This work was supported in part by NIH grants NS083319 and MH060605.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
- 1.Bucher D, Marder E. SnapShot: Neuromodulation. Cell. 2013;155:482–482. e481. doi: 10.1016/j.cell.2013.09.047. [DOI] [PubMed] [Google Scholar]
- 2.Rosen SC, Susswein AJ, Cropper EC, Weiss KR, Kupfermann I. Selective modulation of spike duration by serotonin and the neuropeptides, FMRFamide, SCPB, buccalin and myomodulin in different classes of mechanoafferent neurons in the cerebral ganglion of Aplysia. J Neurosci. 1989;9:390–402. doi: 10.1523/JNEUROSCI.09-02-00390.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sakurai A, Darghouth NR, Butera RJ, Katz PS. Serotonergic enhancement of a 4-AP-sensitive current mediates the synaptic depression phase of spike timing-dependent neuromodulation. J Neurosci. 2006;26:2010–2021. doi: 10.1523/JNEUROSCI.2599-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4••.Sasaki T, Matsuki N, Ikegaya Y. Action-potential modulation during axonal conduction. Science. 2011;331:599–601. doi: 10.1126/science.1197598. In CA3 pyramidal neurons, glial release of glutamate depolarizes long stretches of axonal membrane through activation of AMPA receptors. This depolarization inactivates Kv1 potassium channels, which broadens action potential waveform and increases transmitter release. [DOI] [PubMed] [Google Scholar]
- 5.Heckman CJ, Mottram C, Quinlan K, Theiss R, Schuster J. Motoneuron excitability: the importance of neuromodulatory inputs. Clin Neurophysiol. 2009;120:2040–2054. doi: 10.1016/j.clinph.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murray KC, Stephens MJ, Ballou EW, Heckman CJ, Bennett DJ. Motoneuron excitability and muscle spasms are regulated by 5-HT2B and 5-HT2C receptor activity. J Neurophysiol. 2010;105:731–748. doi: 10.1152/jn.00774.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7•.Rank MM, Murray KC, Stephens MJ, D’Amico J, Gorassini MA, Bennett DJ. Adrenergic receptors modulate motoneuron excitability, sensory synaptic transmission and muscle spasms after chronic spinal cord injury. J Neurophysiol. 2010;105:410–422. doi: 10.1152/jn.00775.2010. EPSPs in motor neurons are enhanced by persistent Ca2+ currents that are activated by norepinephrine through NEα1A receptors. Following spinal cord injury, the enhanced EPSPs in motorneurons are restored after several weeks due to the fact that the NEα1A receptors become constitutively active. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKay BE, Placzek AN, Dani JA. Regulation of synaptic transmission and plasticity by neuronal nicotinic acetylcholine receptors. Biochem Pharmacol. 2007;74:1120–1133. doi: 10.1016/j.bcp.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higley MJ, Sabatini BL. Competitive regulation of synaptic Ca2+ influx by D2 dopamine and A2A adenosine receptors. Nat Neurosci. 2010;13:958–966. doi: 10.1038/nn.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Regehr WG, Carey MR, Best AR. Activity-dependent regulation of synapses by retrograde messengers. Neuron. 2009;63:154–170. doi: 10.1016/j.neuron.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Logsdon S, Johnstone AF, Viele K, Cooper RL. Regulation of synaptic vesicles pools within motor nerve terminals during short-term facilitation and neuromodulation. J Appl Physiol (1985) 2006;100:662–671. doi: 10.1152/japplphysiol.00580.2005. [DOI] [PubMed] [Google Scholar]
- 12.Sun X, Zhao Y, Wolf ME. Dopamine receptor stimulation modulates AMPA receptor synaptic insertion in prefrontal cortex neurons. J Neurosci. 2005;25:7342–7351. doi: 10.1523/JNEUROSCI.4603-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng J, Cai X, Zhao J, Yan Z. Serotonin receptors modulate GABA(A) receptor channels through activation of anchored protein kinase C in prefrontal cortical neurons. J Neurosci. 2001;21:6502–6511. doi: 10.1523/JNEUROSCI.21-17-06502.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-Mediated Control of Synaptic Transmission. Physiological Reviews. 2009;89:309–380. doi: 10.1152/physrev.00019.2008. [DOI] [PubMed] [Google Scholar]
- 15.Fu WM, Liou HC, Chen YH. Nerve terminal currents induced by autoreception of acetylcholine release. J Neurosci. 1998;18:9954–9961. doi: 10.1523/JNEUROSCI.18-23-09954.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cui LN, Coderre E, Renaud LP. GABA(B) presynaptically modulates suprachiasmatic input to hypothalamic paraventricular magnocellular neurons. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1210–1216. doi: 10.1152/ajpregu.2000.278.5.R1210. [DOI] [PubMed] [Google Scholar]
- 17.Langer SZ. Presynaptic autoreceptors regulating transmitter release. Neurochem Int. 2008;52:26–30. doi: 10.1016/j.neuint.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 18.Pinard A, Robitaille R. Nitric oxide dependence of glutamate-mediated modulation at a vertebrate neuromuscular junction. Eur J Neurosci. 2008;28:577–587. doi: 10.1111/j.1460-9568.2008.06355.x. [DOI] [PubMed] [Google Scholar]
- 19.Straub VA, Grant J, O’Shea M, Benjamin PR. Modulation of serotonergic neurotransmission by nitric oxide. J Neurophysiol. 2007;97:1088–1099. doi: 10.1152/jn.01048.2006. [DOI] [PubMed] [Google Scholar]
- 20.Ribeiro JA, Sebastiao AM. Modulation and metamodulation of synapses by adenosine. Acta Physiol (Oxf) 2010;199:161–169. doi: 10.1111/j.1748-1716.2010.02115.x. [DOI] [PubMed] [Google Scholar]
- 21.Katz P, Edwards D. Metamodulation: the control and modulation of neuromodulation. In: Katz P, editor. Beyond Neurotransmission. 1999. pp. 349–381. [Google Scholar]
- 22.Mesce KA. Metamodulation of the biogenic amines: second-order modulation by steroid hormones and amine cocktails. Brain Behav Evol. 2002;60:339–349. doi: 10.1159/000067793. [DOI] [PubMed] [Google Scholar]
- 23.Pawlak V, Wickens JR, Kirkwood A, Kerr JN. Timing is not everything: neuromodulation opens the STDP gate. Front Synaptic Neurosci. 2010;2:146. doi: 10.3389/fnsyn.2010.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson BR, Brown JM, Kvarta MD, Lu JY, Schneider LR, Nadim F, Harris-Warrick RM. Differential modulation of synaptic strength and timing regulate synaptic efficacy in a motor network. J Neurophysiol. 2011;105:293–304. doi: 10.1152/jn.00809.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katz PS, Getting PA, Frost WN. Dynamic neuromodulation of synaptic strength intrinsic to a central pattern generator circuit. Nature. 1994;367:729–731. doi: 10.1038/367729a0. [DOI] [PubMed] [Google Scholar]
- 26.Johnson BR, Peck JH, Harris-Warrick RM. Differential modulation of chemical and electrical components of mixed synapses in the lobster stomatogastric ganglion. J Comp Physiol A. 1994;175:233–249. doi: 10.1007/BF00215119. [DOI] [PubMed] [Google Scholar]
- 27.Koh HY, Vilim FS, Jing J, Weiss KR. Two neuropeptides colocalized in a command-like neuron use distinct mechanisms to enhance its fast synaptic connection. J Neurophysiol. 2003;90:2074–2079. doi: 10.1152/jn.00358.2003. [DOI] [PubMed] [Google Scholar]
- 28.Gu Z, Yan Z. Bidirectional regulation of Ca2+/calmodulin-dependent protein kinase II activity by dopamine D4 receptors in prefrontal cortex. Mol Pharmacol. 2004;66:948–955. doi: 10.1124/mol.104.001404. [DOI] [PubMed] [Google Scholar]
- 29.Cai X, Flores-Hernandez J, Feng J, Yan Z. Activity-dependent bidirectional regulation of GABAA receptor channels by the 5-HT4 receptor-mediated signalling in rat prefrontal cortical pyramidal neurons. J Physiol. 2002;540:743–759. doi: 10.1113/jphysiol.2001.013391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sakurai A, Katz PS. State-, timing-, and pattern-dependent neuromodulation of synaptic strength by a serotonergic interneuron. J Neurosci. 2009;29:268–279. doi: 10.1523/JNEUROSCI.4456-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson BR, Schneider LR, Nadim F, Harris-Warrick RM. Dopamine modulation of phasing of activity in a rhythmic motor network: contribution of synaptic and intrinsic modulatory actions. J Neurophysiol. 2005;94:3101–3111. doi: 10.1152/jn.00440.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thirumalai V, Prinz AA, Johnson CD, Marder E. Red pigment concentrating hormone strongly enhances the strength of the feedback to the pyloric rhythm oscillator but has little effect on pyloric rhythm period. J Neurophysiol. 2006;95:1762–1770. doi: 10.1152/jn.00764.2005. [DOI] [PubMed] [Google Scholar]
- 33•.Zhao S, Sheibanie AF, Oh M, Rabbah P, Nadim F. Peptide neuromodulation of synaptic dynamics in an oscillatory network. J Neurosci. 2011;31:13991–14004. doi: 10.1523/JNEUROSCI.3624-11.2011. Neuropeptide modulation of the inhibitory synaptic feedback to stomatogastric pyloric pacemaker neurons increases the synaptic strength and, for certain stimuli, even flips the direction of short-term plasticity from depression into facilitation. Surprisingly, the change in synaptic strength has no effect on the network frequency but it does act to reduce its variability. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pereda AE, Curti S, Hoge G, Cachope R, Flores CE, Rash JE. Gap junction-mediated electrical transmission: regulatory mechanisms and plasticity. Biochim Biophys Acta. 2013;1828:134–146. doi: 10.1016/j.bbamem.2012.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lasater EM. Retinal horizontal cell gap junctional conductance is modulated by dopamine through a cyclic AMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1987;84:7319–7323. doi: 10.1073/pnas.84.20.7319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piccolino M, Neyton J, Gerschenfeld HM. Decrease of gap junction permeability induced by dopamine and cyclic adenosine 3′:5′-monophosphate in horizontal cells of turtle retina. J Neurosci. 1984;4:2477–2488. doi: 10.1523/JNEUROSCI.04-10-02477.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zsiros V, Maccaferri G. Noradrenergic modulation of electrical coupling in GABAergic networks of the hippocampus. J Neurosci. 2008;28:1804–1815. doi: 10.1523/JNEUROSCI.4616-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson BR, Peck JH, Harris-Warrick RM. Amine modulation of electrical coupling in the pyloric network of the lobster stomatogastric ganglion. J Comp Physiol A. 1993;172:715–732. doi: 10.1007/BF00195397. [DOI] [PubMed] [Google Scholar]
- 39.O’Donnell P, Grace AA. Dopaminergic modulation of dye coupling between neurons in the core and shell regions of the nucleus accumbens. J Neurosci. 1993;13:3456–3471. doi: 10.1523/JNEUROSCI.13-08-03456.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pereda A, Triller A, Korn H, Faber DS. Dopamine enhances both electrotonic coupling and chemical excitatory postsynaptic potentials at mixed synapses. Proc Natl Acad Sci U S A. 1992;89:12088–12092. doi: 10.1073/pnas.89.24.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rorig B, Sutor B. Nitric oxide-stimulated increase in intracellular cGMP modulates gap junction coupling in rat neocortex. Neuroreport. 1996;7:569–572. doi: 10.1097/00001756-199601310-00046. [DOI] [PubMed] [Google Scholar]
- 42.Hatton GI, Yang QZ. Synaptically released histamine increases dye coupling among vasopressinergic neurons of the supraoptic nucleus: mediation by H1 receptors and cyclic nucleotides. J Neurosci. 1996;16:123–129. doi: 10.1523/JNEUROSCI.16-01-00123.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Szabo TM, Caplan JS, Zoran MJ. Serotonin regulates electrical coupling via modulation of extrajunctional conductance: H-current. Brain Res. 2010;1349:21–31. doi: 10.1016/j.brainres.2010.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rush AM, Kilbride J, Rowan MJ, Anwyl R. Presynaptic group III mGluR modulation of short-term plasticity in the lateral perforant path of the dentate gyrus in vitro. Brain Res. 2002;952:38–43. doi: 10.1016/s0006-8993(02)03188-8. [DOI] [PubMed] [Google Scholar]
- 45.Carey MR, Myoga MH, McDaniels KR, Marsicano G, Lutz B, Mackie K, Regehr WG. Presynaptic CB1 receptors regulate synaptic plasticity at cerebellar parallel fiber synapses. J Neurophysiol. 2011;105:958–963. doi: 10.1152/jn.00980.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parker D. Spinal-cord plasticity. Molecular Neurobiology. 2000;22:055–080. doi: 10.1385/MN:22:1-3:055. [DOI] [PubMed] [Google Scholar]
- 47.Barriere G, Tartas M, Cazalets JR, Bertrand SS. Interplay between neuromodulator-induced switching of short-term plasticity at sensorimotor synapses in the neonatal rat spinal cord. J Physiol. 2008;586:1903–1920. doi: 10.1113/jphysiol.2008.150706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bevan S, Parker D. Metaplastic facilitation and ultrastructural changes in synaptic properties are associated with long-term modulation of the lamprey locomotor network. J Neurosci. 2004;24:9458–9468. doi: 10.1523/JNEUROSCI.3391-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baimoukhametova DV, Hewitt SA, Sank CA, Bains JS. Dopamine modulates use-dependent plasticity of inhibitory synapses. J Neurosci. 2004;24:5162–5171. doi: 10.1523/JNEUROSCI.4979-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rothman JS, Cathala L, Steuber V, Silver RA. Synaptic depression enables neuronal gain control. Nature. 2009;457:1015–1018. doi: 10.1038/nature07604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abbott LF, Varela JA, Sen K, Nelson SB. Synaptic depression and cortical gain control. Science. 1997;275:220–224. doi: 10.1126/science.275.5297.221. [DOI] [PubMed] [Google Scholar]
- 52.Hawkins RD, Kandel ER, Bailey CH. Molecular mechanisms of memory storage in Aplysia. Biol Bull. 2006;210:174–191. doi: 10.2307/4134556. [DOI] [PubMed] [Google Scholar]
- 53.Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- 54.Klein M, Camardo J, Kandel ER. Serotonin modulates a specific potassium current in the sensory neurons that show presynaptic facilitation in Aplysia. Proc Natl Acad Sci U S A. 1982;79:5713–5717. doi: 10.1073/pnas.79.18.5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jin I, Kandel ER, Hawkins RD. Whereas short-term facilitation is presynaptic, intermediate-term facilitation involves both presynaptic and postsynaptic protein kinases and protein synthesis. Learn Mem. 2011;18:96–102. doi: 10.1101/lm.1949711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jin I, Udo H, Rayman JB, Puthanveettil S, Kandel ER, Hawkins RD. Spontaneous transmitter release recruits postsynaptic mechanisms of long-term and intermediate-term facilitation in Aplysia. Proc Natl Acad Sci U S A. 2012;109:9137–9142. doi: 10.1073/pnas.1206846109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim JH, Udo H, Li HL, Youn TY, Chen M, Kandel ER, Bailey CH. Presynaptic activation of silent synapses and growth of new synapses contribute to intermediate and long-term facilitation in Aplysia. Neuron. 2003;40:151–165. doi: 10.1016/s0896-6273(03)00595-6. [DOI] [PubMed] [Google Scholar]
- 58.Giurfa M. Associative learning: the instructive function of biogenic amines. Curr Biol. 2006;16:R892–895. doi: 10.1016/j.cub.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 59.Otani S, Daniel H, Roisin MP, Crepel F. Dopaminergic modulation of long-term synaptic plasticity in rat prefrontal neurons. Cereb Cortex. 2003;13:1251–1256. doi: 10.1093/cercor/bhg092. [DOI] [PubMed] [Google Scholar]
- 60.Kulla A, Manahan-Vaughan D. Modulation by serotonin 5-HT4 receptors of long-term potentiation and depotentiation in the dentate gyrus of freely moving rats. Cerebral Cortex. 2002;12:150–162. doi: 10.1093/cercor/12.2.150. [DOI] [PubMed] [Google Scholar]
- 61.Sakai N, Tanaka C. Inhibitory modulation of long-term potentiation via the 5-HT1A receptor in slices of the rat hippocampal dentate gyrus. Brain Research. 1993;613:326–330. doi: 10.1016/0006-8993(93)90921-9. [DOI] [PubMed] [Google Scholar]
- 62.O’Dell TJ, Connor SA, Gelinas JN, Nguyen PV. Viagra for your synapses: Enhancement of hippocampal long-term potentiation by activation of betaadrenergic receptors. Cell Signal. 2010;22:728–736. doi: 10.1016/j.cellsig.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shinoe T, Matsui M, Taketo MM, Manabe T. Modulation of synaptic plasticity by physiological activation of M1 muscarinic acetylcholine receptors in the mouse hippocampus. J Neurosci. 2005;25:11194–11200. doi: 10.1523/JNEUROSCI.2338-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Choi SY, Chang J, Jiang B, Seol GH, Min SS, Han JS, Shin HS, Gallagher M, Kirkwood A. Multiple receptors coupled to phospholipase C gate long-term depression in visual cortex. J Neurosci. 2005;25:11433–11443. doi: 10.1523/JNEUROSCI.4084-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Seol GH, Ziburkus J, Huang S, Song L, Kim IT, Takamiya K, Huganir RL, Lee HK, Kirkwood A. Neuromodulators control the polarity of spike-timing-dependent synaptic plasticity. Neuron. 2007;55:919–929. doi: 10.1016/j.neuron.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang S, Trevino M, He K, Ardiles A, Pasquale R, Guo Y, Palacios A, Huganir R, Kirkwood A. Pull-push neuromodulation of LTP and LTD enables bidirectional experience-induced synaptic scaling in visual cortex. Neuron. 2012;73:497–510. doi: 10.1016/j.neuron.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67•.Huang S, Huganir RL, Kirkwood A. Adrenergic gating of hebbian spike-timing-dependent plasticity in cortical interneurons. J Neurosci. 2013;33:13171–13178. doi: 10.1523/JNEUROSCI.5741-12.2013. The modulatory rules for regulating long-term plasticity are similar for synapses targeting pyramidal neurons or parvalbumin-positive fast-spiking (FS) interneurons in which STDP relies on mGluR5, rather than NMDA, receptors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nusser Z. Variability in the subcellular distribution of ion channels increases neuronal diversity. Trends Neurosci. 2009;32:267–274. doi: 10.1016/j.tins.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 69.Prinz AA, Billimoria CP, Marder E. Alternative to hand-tuning conductance-based models: construction and analysis of databases of model neurons. J Neurophysiol. 2003;90:3998–4015. doi: 10.1152/jn.00641.2003. [DOI] [PubMed] [Google Scholar]
- 70.Baranauskas G. Ionic channel function in action potential generation: current perspective. Mol Neurobiol. 2007;35:129–150. doi: 10.1007/s12035-007-8001-0. [DOI] [PubMed] [Google Scholar]
- 71.Kaczmarek LK, Levitan IB, editors. Neuromodulation: The Biochemical Control of Neuronal Excitability. New York: Oxford University Press; 1986. [Google Scholar]
- 72.Pfeiffer-Linn CL, Lasater EM. Dopamine modulates unitary conductance of single PL-type calcium channels in Roccus chrysops retinal horizontal cells. J Physiol. 1996;496 (Pt 3):607–616. doi: 10.1113/jphysiol.1996.sp021712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Podda MV, Grassi C. New perspectives in cyclic nucleotide-mediated functions in the CNS: the emerging role of cyclic nucleotide-gated (CNG) channels. Pflugers Arch. 2013 doi: 10.1007/s00424-013-1373-2. [DOI] [PubMed] [Google Scholar]
- 74.He C, Chen F, Li B, Hu Z. Neurophysiology of HCN channels: from cellular functions to multiple regulations. Prog Neurobiol. 2014;112:1–23. doi: 10.1016/j.pneurobio.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 75.Dascal N. Ion-channel regulation by G proteins. Trends Endocrinol Metab. 2001;12:391–398. doi: 10.1016/s1043-2760(01)00475-1. [DOI] [PubMed] [Google Scholar]
- 76.Ahn M, Beacham D, Westenbroek RE, Scheuer T, Catterall WA. Regulation of Na(v)1.2 channels by brain-derived neurotrophic factor, TrkB, and associated Fyn kinase. J Neurosci. 2007;27:11533–11542. doi: 10.1523/JNEUROSCI.5005-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee JH, Choi SH, Lee BH, Hwang SH, Kim HJ, Rhee J, Chung C, Nah SY. Activation of lysophosphatidic acid receptor by gintonin inhibits Kv1.2 channel activity: involvement of tyrosine kinase and receptor protein tyrosine phosphatase alpha. Neurosci Lett. 2013;548:143–148. doi: 10.1016/j.neulet.2013.05.048. [DOI] [PubMed] [Google Scholar]
- 78.Cantrell AR, Catterall WA. Neuromodulation of Na+ channels: an unexpected form of cellular plasticity. Nat Rev Neurosci. 2001;2:397–407. doi: 10.1038/35077553. [DOI] [PubMed] [Google Scholar]
- 79.Berendt FJ, Park KS, Trimmer JS. Multisite phosphorylation of voltage-gated sodium channel alpha subunits from rat brain. J Proteome Res. 2010;9:1976–1984. doi: 10.1021/pr901171q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Catterall WA. Voltage-gated sodium channels at 60: structure, function and pathophysiology. J Physiol. 2012;590:2577–2589. doi: 10.1113/jphysiol.2011.224204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hourez R, Azdad K, Vanwalleghem G, Roussel C, Gall D, Schiffmann SN. Activation of protein kinase C and inositol 1,4,5-triphosphate receptors antagonistically modulate voltage-gated sodium channels in striatal neurons. Brain Res. 2005;1059:189–196. doi: 10.1016/j.brainres.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 82.Santi CM, Ferreira G, Yang B, Gazula VR, Butler A, Wei A, Kaczmarek LK, Salkoff L. Opposite regulation of Slick and Slack K+ channels by neuromodulators. J Neurosci. 2006;26:5059–5068. doi: 10.1523/JNEUROSCI.3372-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pfeiffer-Linn CL, Lasater EM. Multiple second-messenger system modulation of voltage-activated calcium currents in teleost retinal horizontal cells. J Neurophysiol. 1998;80:377–388. doi: 10.1152/jn.1998.80.1.377. [DOI] [PubMed] [Google Scholar]
- 84.Witkowski G, Szulczyk P. Opioid mu receptor activation inhibits sodium currents in prefrontal cortical neurons via a protein kinase A- and C-dependent mechanism. Brain Res. 2006;1094:92–106. doi: 10.1016/j.brainres.2006.03.119. [DOI] [PubMed] [Google Scholar]
- 85.Swensen AM, Marder E. Multiple peptides converge to activate the same voltage-dependent current in a central pattern-generating circuit. J Neurosci. 2000;20:6752–6759. doi: 10.1523/JNEUROSCI.20-18-06752.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86••.Park JY, Spruston N. Synergistic actions of metabotropic acetylcholine and glutamate receptors on the excitability of hippocampal CA1 pyramidal neurons. J Neurosci. 2012;32:6081–6091. doi: 10.1523/JNEUROSCI.6519-11.2012. In CA1 pyramidal neurons, both activation of group I metabotropic glutamate receptors and muscarinic acetylcholine receptors increases activation of Cav2.3 R-type Ca2+ channels and converts post-burst after-hyperpolarization into after-depolarization. When activated together, glutamate and acetylcholine act synergistically, as the magnitude of the after-depolarization is enhanced highly nonlinearly. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Harris-Warrick RM, Johnson BR. Checks and balances in neuromodulation. Front Behav Neurosci. 2010;4 doi: 10.3389/fnbeh.2010.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Swensen AM, Marder E. Modulators with convergent cellular actions elicit distinct circuit outputs. J Neurosci. 2001;21:4050–4058. doi: 10.1523/JNEUROSCI.21-11-04050.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nikolaev VO, Bunemann M, Schmitteckert E, Lohse MJ, Engelhardt S. Cyclic AMP imaging in adult cardiac myocytes reveals far-reaching beta1-adrenergic but locally confined beta2-adrenergic receptor-mediated signaling. Circ Res. 2006;99:1084–1091. doi: 10.1161/01.RES.0000250046.69918.d5. [DOI] [PubMed] [Google Scholar]
- 90.Rich TC, Fagan KA, Tse TE, Schaack J, Cooper DM, Karpen JW. A uniform extracellular stimulus triggers distinct cAMP signals in different compartments of a simple cell. Proc Natl Acad Sci U S A. 2001;98:13049–13054. doi: 10.1073/pnas.221381398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91•.Bender KJ, Ford CP, Trussell LO. Dopaminergic modulation of axon initial segment calcium channels regulates action potential initiation. Neuron. 2010;68:500–511. doi: 10.1016/j.neuron.2010.09.026. In brainstem interneurons, dopamine acts through PKC to inhibit T-type Ca2+ channels that are directly involved in regulating action potential initiation at the axon initial segment (AIS). This effect is selective both for the channel type and the cell compartment, as neither Na+ channels at the AIS nor T-type Ca2+ channels in somatodendritic compartments are affected. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Carr DB, Cooper DC, Ulrich SL, Spruston N, Surmeier DJ. Serotonin receptor activation inhibits sodium current and dendritic excitability in prefrontal cortex via a protein kinase C-dependent mechanism. J Neurosci. 2002;22:6846–6855. doi: 10.1523/JNEUROSCI.22-16-06846.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Catterall WA, Hulme JT, Jiang X, Few WP. Regulation of sodium and calcium channels by signaling complexes. J Recept Signal Transduct Res. 2006;26:577–598. doi: 10.1080/10799890600915100. [DOI] [PubMed] [Google Scholar]
- 94.Chen Y, Cantrell AR, Messing RO, Scheuer T, Catterall WA. Specific modulation of Na+ channels in hippocampal neurons by protein kinase C epsilon. J Neurosci. 2005;25:507–513. doi: 10.1523/JNEUROSCI.4089-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Few WP, Scheuer T, Catterall WA. Dopamine modulation of neuronal Na(+) channels requires binding of A kinase-anchoring protein 15 and PKA by a modified leucine zipper motif. Proc Natl Acad Sci U S A. 2007;104:5187–5192. doi: 10.1073/pnas.0611619104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gasparini S, Magee JC. Phosphorylation-dependent differences in the activation properties of distal and proximal dendritic Na+ channels in rat CA1 hippocampal neurons. J Physiol. 2002;541:665–672. doi: 10.1113/jphysiol.2002.020503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Enyedi P, Czirjak G. Molecular background of leak K+ currents: two-pore domain potassium channels. Physiol Rev. 2010;90:559–605. doi: 10.1152/physrev.00029.2009. [DOI] [PubMed] [Google Scholar]
- 98.Talley EM, Lei Q, Sirois JE, Bayliss DA. TASK-1, a two-pore domain K+ channel, is modulated by multiple neurotransmitters in motoneurons. Neuron. 2000;25:399–410. doi: 10.1016/s0896-6273(00)80903-4. [DOI] [PubMed] [Google Scholar]
- 99.Perrier JF, Alaburda A, Hounsgaard J. 5-HT1A receptors increase excitability of spinal motoneurons by inhibiting a TASK-1-like K+ current in the adult turtle. J Physiol. 2003;548:485–492. doi: 10.1113/jphysiol.2002.037952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sirois JE, Lei Q, Talley EM, Lynch C, 3rd, Bayliss DA. The TASK-1 two-pore domain K+ channel is a molecular substrate for neuronal effects of inhalation anesthetics. J Neurosci. 2000;20:6347–6354. doi: 10.1523/JNEUROSCI.20-17-06347.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Picciotto MR, Higley MJ, Mineur YS. Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron. 2012;76:116–129. doi: 10.1016/j.neuron.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lendvai B, Vizi ES. Nonsynaptic chemical transmission through nicotinic acetylcholine receptors. Physiol Rev. 2008;88:333–349. doi: 10.1152/physrev.00040.2006. [DOI] [PubMed] [Google Scholar]
- 103.Mansvelder HD, Mertz M, Role LW. Nicotinic modulation of synaptic transmission and plasticity in corticolimbic circuits. Semin Cell Dev Biol. 2009;20:432–440. doi: 10.1016/j.semcdb.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- 105.Trigo FF, Marty A, Stell BM. Axonal GABAA receptors. Eur J Neurosci. 2008;28:841–848. doi: 10.1111/j.1460-9568.2008.06404.x. [DOI] [PubMed] [Google Scholar]
- 106.Belelli D, Harrison NL, Maguire J, Macdonald RL, Walker MC, Cope DW. Extrasynaptic GABAA receptors: form, pharmacology, and function. J Neurosci. 2009;29:12757–12763. doi: 10.1523/JNEUROSCI.3340-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kullmann DM, Ruiz A, Rusakov DM, Scott R, Semyanov A, Walker MC. Presynaptic, extrasynaptic and axonal GABAA receptors in the CNS: where and why? Prog Biophys Mol Biol. 2005;87:33–46. doi: 10.1016/j.pbiomolbio.2004.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bucher D, Goaillard JM. Beyond faithful conduction: short-term dynamics, neuromodulation, and long-term regulation of spike propagation in the axon. Prog Neurobiol. 2011;94:307–346. doi: 10.1016/j.pneurobio.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109••.Bahner F, Weiss EK, Birke G, Maier N, Schmitz D, Rudolph U, Frotscher M, Traub RD, Both M, Draguhn A. Cellular correlate of assembly formation in oscillating hippocampal networks in vitro. Proc Natl Acad Sci U S A. 2011;108:E607–616. doi: 10.1073/pnas.1103546108. Complex network interactions can critically depend on axonal neuromodulation. High-frequency network oscillations occur in defined subsets of CA1 pyramidal neurons. In these neurons, GABAA receptors play a dual role. During oscillations, firing resulting from somatodendritic synaptic integration is inhibited by GABAergic inputs. At the same time, spikes are generated ectopically in remote axonal compartments resulting from depolarizing responses to tonic GABAA receptor activation. Synchronization then occurs through electrical coupling between axons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chan CS, Glajch KE, Gertler TS, Guzman JN, Mercer JN, Lewis AS, Goldberg AB, Tkatch T, Shigemoto R, Fleming SM, et al. HCN channelopathy in external globus pallidus neurons in models of Parkinson’s disease. Nat Neurosci. 2011;14:85–92. doi: 10.1038/nn.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mizrahi A, Dickinson PS, Kloppenburg P, Fenelon V, Baro DJ, Harris-Warrick RM, Meyrand P, Simmers J. Long-term maintenance of channel distribution in a central pattern generator neuron by neuromodulatory inputs revealed by decentralization in organ culture. J Neurosci. 2001;21:7331–7339. doi: 10.1523/JNEUROSCI.21-18-07331.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Khorkova O, Golowasch J. Neuromodulators, not activity, control coordinated expression of ionic currents. J Neurosci. 2007;27:8709–8718. doi: 10.1523/JNEUROSCI.1274-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113•.Rodgers EW, Krenz WD, Baro DJ. Tonic dopamine induces persistent changes in the transient potassium current through translational regulation. J Neurosci. 2011;31:13046–13056. doi: 10.1523/JNEUROSCI.2194-11.2011. Low concentrations of dopamine, referred to dopamine tone, can result in a long term increase of IA in a different STG neuron through a translation-dependent mechanism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114•.Temporal S, Desai M, Khorkova O, Varghese G, Dai A, Schulz DJ, Golowasch J. Neuromodulation independently determines correlated channel expression and conductance levels in motor neurons of the stomatogastric ganglion. J Neurophysiol. 2012;107:718–727. doi: 10.1152/jn.00622.2011. Long-term regulation of different ion channel types depends on the presence of endogenously released neuromodulators. Removal of modulatory inputs disrupts or modifies the coordinated expression of different voltage-gated ion channels and the associated mRNA levels in a cell-type-dependent manner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Husch A, Van Patten GN, Hong DN, Scaperotti MM, Cramer N, Harris-Warrick RM. Spinal cord injury induces serotonin supersensitivity without increasing intrinsic excitability of mouse V2a interneurons. J Neurosci. 2012;32:13145–13154. doi: 10.1523/JNEUROSCI.2995-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Marder E. Neuromodulation of neuronal circuits: back to the future. Neuron. 2012;76:1–11. doi: 10.1016/j.neuron.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Perrier JF, Rasmussen HB, Christensen RK, Petersen AV. Modulation of the intrinsic properties of motoneurons by serotonin. Curr Pharm Des. 2013;19:4371–4384. doi: 10.2174/13816128113199990341. [DOI] [PubMed] [Google Scholar]
- 118.Pape HC, McCormick DA. Noradrenaline and serotonin selectively modulate thalamic burst firing by enhancing a hyperpolarization-activated cation current. Nature. 1989;340:715–718. doi: 10.1038/340715a0. [DOI] [PubMed] [Google Scholar]
- 119.Oh M, Zhao S, Matveev V, Nadim F. Neuromodulatory changes in short-term synaptic dynamics may be mediated by two distinct mechanisms of presynaptic calcium entry. J Comput Neurosci. 2012;33:573–585. doi: 10.1007/s10827-012-0402-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dreyer JK, Hounsgaard J. Mathematical model of dopamine autoreceptors and uptake inhibitors and their influence on tonic and phasic dopamine signaling. J Neurophysiol. 2013;109:171–182. doi: 10.1152/jn.00502.2012. [DOI] [PubMed] [Google Scholar]
- 121.Li G, Cleland TA. A two-layer biophysical model of cholinergic neuromodulation in olfactory bulb. J Neurosci. 2013;33:3037–3058. doi: 10.1523/JNEUROSCI.2831-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hasselmo ME, Sarter M. Modes and models of forebrain cholinergic neuromodulation of cognition. Neuropsychopharmacology. 2011;36:52–73. doi: 10.1038/npp.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cohen MX, Frank MJ. Neurocomputational models of basal ganglia function in learning, memory and choice. Behav Brain Res. 2009;199:141–156. doi: 10.1016/j.bbr.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Doya K, Dayan P, Hasselmo ME. Introduction for 2002 Special Issue:Computational Models of Neuromodulation. Neural Networks. 2002;15:475–477. [Google Scholar]