Summary
People find it easier to learn about topics that interest them, but little is known about the mechanisms by which intrinsic motivational states affect learning. We used functional magnetic resonance imaging to investigate how curiosity (intrinsic motivation to learn) influences memory. In both immediate and one-day delayed memory tests, participants showed improved memory for information that they were curious about, and also for incidental material learned during states of high curiosity. FMRI results revealed that activity in the midbrain and the nucleus accumbens was enhanced during states of high curiosity. Importantly, individual variability in curiosity-driven memory benefits for incidental material was supported by anticipatory activity in the midbrain and hippocampus and by functional connectivity between these regions. These findings suggest a link between the mechanisms supporting extrinsic reward motivation and intrinsic curiosity and highlight the importance of stimulating curiosity in order to create more effective learning experiences.
Keywords: intrinsic, motivation, curiosity, dopamine, midbrain, nucleus, accumbens, ventral, striatum, states, anticipation, synaptic tagging hypothesis, pre-stimulus, learning, encoding, memory, episodic, consolidation, reward
Introduction
In a typical day, most of the events that a person experiences will be forgotten. What differentiates those occasions that are successfully remembered? It is clear that learning is influenced by the characteristics of particular stimuli and how they are processed. Much less is known about whether, in addition to such stimulus-related processing, particular motivational states can also influence the likelihood of memory formation and later consolidation processes. Consistent with this possibility, recent evidence suggests that neural activity in the midbrain (i.e. the substantia nigra/ventral tegmental area complex [SN/VTA]) along with hippocampal activity during anticipation of a reward can influence memory formation for a preceding (Wittmann et al., 2005) or subsequent stimulus (Adcock et al., 2006; Wolosin et al., 2012). In addition, it has been shown that increased functional connectivity between the SN/VTA and hippocampus supports reward-related memory benefits (Adcock et al., 2006; Wolosin et al., 2012).
In real-life situations, learning is often self-motivated, driven by intrinsic curiosity in a particular topic, rather than by external rewards (Berlyne, 1966; Reeve and Reeve, 1996; Ryan and Deci, 2000). Little is known about how intrinsic motivation affects brain activity and learning, but an initial study by Kang et al. (2009) provided important clues. In this study, the authors found that curiosity to learn the answers to trivia questions was associated with enhanced activation in the caudate nucleus. In a separate behavioral study, Kang and colleagues found that, after an 11–16 day retention period, participants were better able to recall answers to questions that they were highly curious about.
The results of Kang et al. raise important questions about the mechanisms by which intrinsic motivation can modulate brain activity and memory performance, and also the degree to which intrinsic motivation influences memory in a manner that is similar to extrinsic motivation. Here, we used functional magnetic resonance imaging (fMRI) to address three critical questions: (i) Is curiosity associated with activity in key brain regions that are responsive to extrinsic motivation? (ii) What are the neural mechanisms that promote the influence of curiosity on learning? (iii) Most importantly, does a curious state enhance learning of incidental material, and if so, what are the brain areas that support curiosity-related memory benefits?
First, each participant rated his/her curiosity to learn the answer to a series of trivia questions (see Figure 1A for an example). Next, they were scanned during encoding of the answers to these questions, along with a set of neutral, unrelated face stimuli (Figure 1B). Each trial commenced with presentation of a selected trivia question, and the participant anticipated the associated answer during a 14s delay. During this anticipation period, the participant incidentally encoded a face. After the scan session, participants performed a surprise recognition memory test for the faces that were presented during the anticipation period, followed by a memory test for the answers to the trivia questions. The critical analyses focused on activity that preceded the presentation of the face or the trivia answer, which we interpret to reflect anticipatory states of high or low curiosity.
Figure 1.
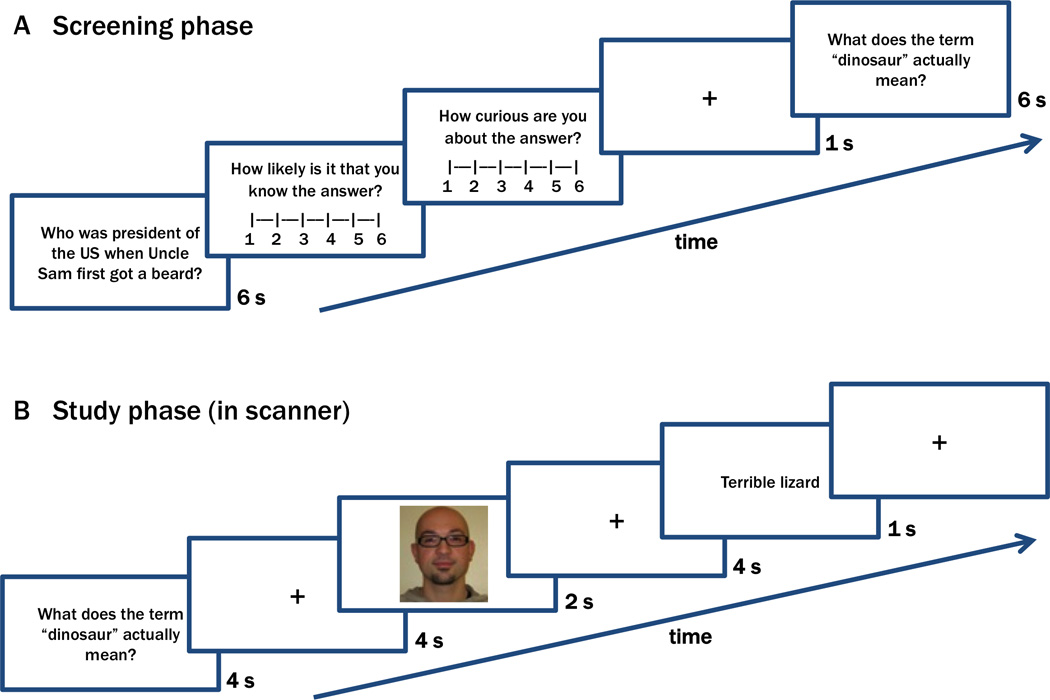
Example trials from screening and study phases. (A) Screening phase: On each trial, participants rated how likely they knew the answer to a trivia question and how curious they were to learn the answer. Questions associated with high and low curiosity, for which participants did not know the answer, were used for the next phase. Answers were not presented in this phase. (B) Study phase (performed in the MRI scanner): On each trial, a selected trivia question was presented and the participant anticipated presentation of the answer. During this anticipation period, participants were required to make an incidental judgment to a face. Following the study phase, participants completed memory tests (not shown) on both the trivia answers and the faces that were studied in the scanner.
For the fMRI analyses, we focused our hypotheses on three major regions of interest (ROIs)—the SN/VTA, the nucleus accumbens, and the hippocampus. These three regions show high intrinsic functional connectivity (Kahn and Shohamy, 2013) and have been hypothesized to comprise a functional loop in the service of learning (Düzel et al., 2010; Lisman and Grace, 2005; Lisman et al., 2011). According to these views, the SN/VTA (particularly, the VTA) modulates learning of salient information in the hippocampus via enhanced dopamine release, whereas the nucleus accumbens incorporates additional information related to novelty and goal-relevance into this functional circuit. Although the hippocampal-VTA loop theory has primarily been used to explain effects of stimulus-related salience on learning, we predicted that the same circuit might also mediate effects of intrinsic motivational states. Accordingly, we used the NeuroSynth tool (Yarkoni et al., 2011) to conduct a meta-analysis based on 329 studies of reward processing in order to identify voxels within the three regions that are reliably recruited during extrinsic motivational states (see Methods and Figure S1). If curiosity indeed relies on similar brain mechanisms as extrinsic reward motivation, we would expect that activity in these ROIs to be modulated by curiosity. In turn, curiosity-driven memory benefits should be supported by increased activity in our ROIs. Finally, based on work suggesting that extrinsic salience promotes stable memories by enhancing the late stage of long-term potentiation (LTP), we hypothesized that curiosity-driven memory benefits should persist even after a 1-day retention delay.
Results
What are the brain areas that support curiosity?
Our first analyses identified brain areas that are recruited during states of high curiosity. Based on studies of extrinsic reward anticipation (Adcock et al., 2006; Knutson et al., 2001), we hypothesized that activity in the SN/VTA and nucleus accumbens ROIs should be enhanced during states of high curiosity. We were less certain about effects of curiosity in the hippocampal ROI, as it is unclear from prior studies whether hippocampal activity is generally reward-sensitive or if it more specifically reflects motivational influences on learning (Shohamy and Adcock, 2010). To quantify the positive relationship between curiosity and brain activity (cf. Kang et al., 2009), we ran an analysis testing for parametric modulation of activation during each trial as a function of curiosity ratings (see Methods for details). Because of directed hypotheses, we performed one-tailed t-tests (note that this is the approach routinely used in voxel-based fMRI analyses). As we did not have strong predictions about whether effects would be seen in the left or right hemispheres, we corrected for multiple comparisons across hemispheres by using a Bonferroni-adjusted alpha level of 0.025 per analysis.
During presentation of trivia questions (when curiosity was elicited), activity in the bilateral nucleus accumbens (left: t(18)=3.16, p=0.003; right: t(18)=2.60, p=0.009) and the left SN/VTA (left: t(18)=2.23, p=0.020; right: t(18)=1.52, p=0.073) increased linearly with curiosity ratings (Figure 2A–B). In contrast, no significant modulation was seen in these regions during presentation of trivia answers, when curiosity was satisfied (left and right nucleus accumbens: t(18)=0.54, and t(18)=0.73, respectively; left and right SN/VTA: t(18)=0.23 and t(18)=−0.06, respectively; p’s>0.05). Activity in the hippocampal ROIs was not significantly modulated by curiosity during presentation of trivia questions (left: t(18)=0.31; right: t(18)=−0.28; p’s>0.05) or answers (left: t(18)=−0.61; right: t(18)=−0.43; p’s>0.05).
Figure 2.
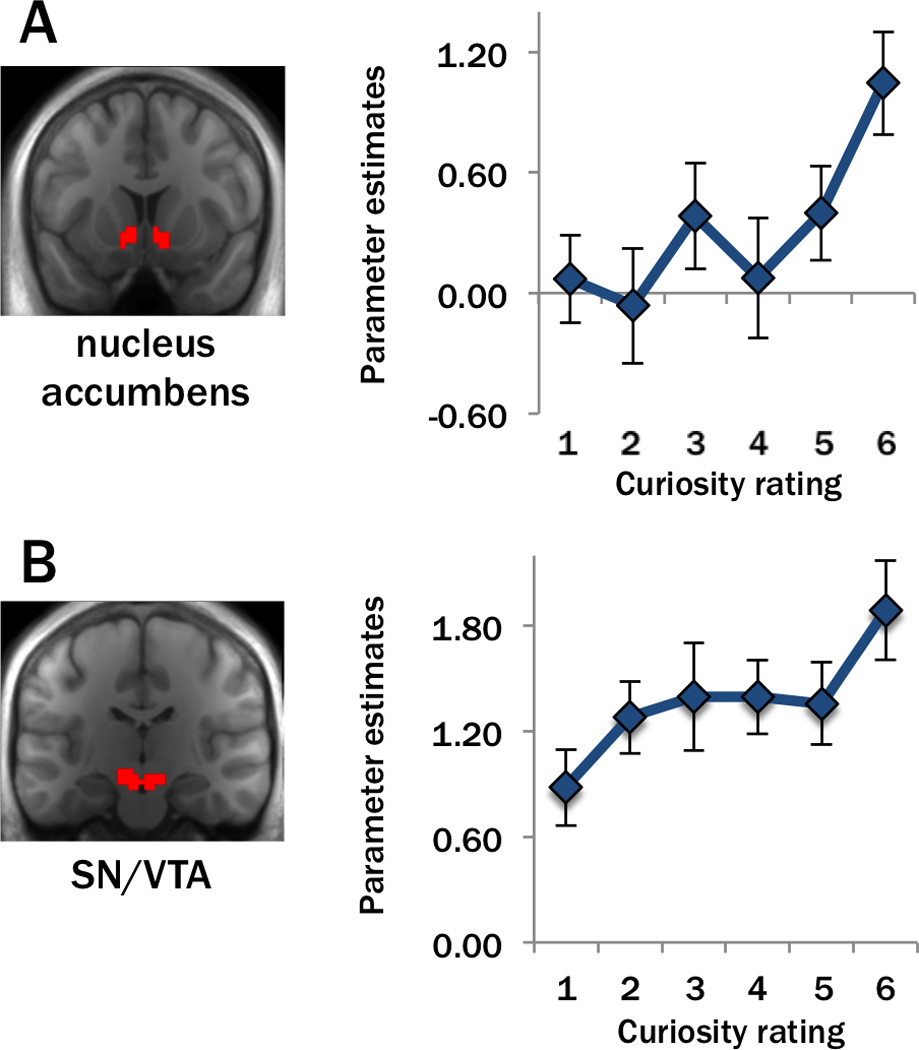
Curiosity modulated activity in the dopaminergic circuit. Curiosity ratings were associated with activity increases in the bilateral nucleus accumbens ROI (A) and left SN/VTA ROI (B). On the left, ROIs are shown in red on the average, normalized anatomical image in our group of participants. On the right, to visualize the effects modeled by the parametric modulation analysis, mean BOLD parameter estimates related to the onset of the trivia questions are plotted on the y-axis against the curiosity rating given during the screening phase on the x-axis. Note: In this and all other figures, error bars indicate ±1 SEM.
In addition to the parametric modulation analysis, we also performed a simpler analysis in which we directly contrasted activation following the presentation of questions associated with high (4–6) and low (1–3) curiosity ratings. Consistent with findings from the parametric modulation analyses, we found significantly increased question-related activation for high compared to low curiosity conditions in the left SN/VTA (left: t(18)=2.53, p=0.010; right: t(18)=0.95, p=0.177), and similar trends in the bilateral nucleus accumbens that did not exceed the Bonferroni-corrected threshold (left: t(18)=2.05, p=0.027; right: t(18)=1.70, p=0.053). Again, no significant effects were seen in the hippocampal ROIs (left: t(18)=−0.91; right: t(18)=−0.68; p’s>0.05). As in the parametric modulation analysis, no significant effects of curiosity were seen during presentation of the trivia answers (left nucleus accumbens: t(18)=1.68, p=0.055; all other ROIs: t’s≤1.22, p’s>0.05).
To characterize activation outside of the a priori ROIs and enable comparison to prior fMRI studies of motivation (e.g., Kang et al., 2009; Knutson et al., 2001), we performed whole-brain, voxel-based analyses testing for parametric modulation of activity following presentation of trivia questions as a function of curiosity. This analysis revealed suprathreshold clusters of the bilateral striatum (i.e. dorsal and ventral), left inferior frontal gyrus, left superior gyrus and the cerebellum (for details, see Table S1 and Figure S2). These results are highly consistent with the work of Kang et al. (2009), who also showed curiosity-related activation in the dorsal striatum, inferior frontal gyrus, and superior frontal gyrus.
In summary, the results described above suggest that curiosity modulates activity in the nucleus accumbens and SN/VTA, along with a possible set of SN/VTA afferents across the striatum and prefrontal cortex.
How does curiosity benefit learning of interesting material?
We next investigated the effect of curiosity on learning. We first compared recall rates for answers to trivia questions associated with high and low curiosity. Participants recalled significantly more answers to high curiosity questions, compared to low curiosity questions (70.6% SE=±2.60 vs. 54.1% SE=±3.04; paired-sample t(17)=5.64, p<0.001; Figure 3A) replicating earlier findings (Kang et al., 2009).
Figure 3.
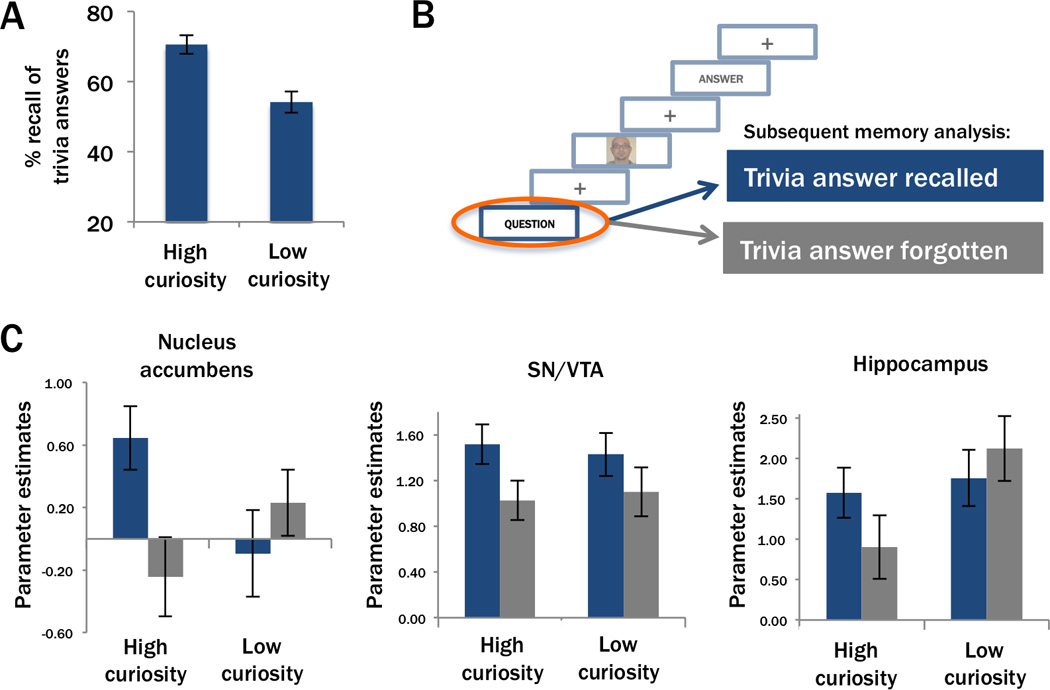
Curiosity benefits learning of trivia answers via the nucleus accumbens and hippocampus. (A) Recall was higher for answers to high curiosity trivia questions than for answers to low curiosity trivia questions. (B) Brain activity elicited by the onset of each trivia question was analyzed according to whether the associated answer was recalled in the post-scan memory test. These analyses therefore tested the relationship between activation prior to the processing of trivia answers and successful encoding of those answers. (C) Anticipatory brain activity (across-participant mean BOLD parameter estimates) in our three ROIs sorted by curiosity ratings and memory for the trivia answer. In the bilateral nucleus accumbens (left), activation evoked by the trivia question was increased for high-curiosity questions whose answers were later recalled compared to all remaining conditions. In the bilateral SN/VTA (middle), question-elicited activation was enhanced for later recalled compared to later forgotten answers independent of curiosity. In the right hippocampus (right), question-evoked activation predicted later memory performance only for trivia answers associated with high curiosity.
Based on studies of extrinsic motivation on memory (Adcock et al., 2006; Wittmann et al., 2006; Wolosin et al., 2012), we hypothesized that activation in the nucleus accumbens, SN/VTA, and hippocampus evoked by presentation of high-curiosity trivia questions (i.e., the point at which participants began to anticipate the answer) would be predictive of successful recall of the answers to those questions in the post-scan memory test. Within each ROI, we analyzed activity evoked by high and low curiosity trivia questions depending on whether the associated trivia answers were later recalled or forgotten (see Figure 3B). In the nucleus accumbens, significant Curiosity x Memory interactions were observed (left: F(1,17)=6.75, p=0.019; right: F(1,17)=8.56, p=0.009; Figure 3C left), revealing that activity during anticipation of trivia answers predicted later memory for high curiosity (left: t(17)=2.23, p=0.020; right: t(17)=2.79, p=0.006), but not low curiosity trivia answers (left: t(17)=−0.80; right: t(17)=−1.15, p’s>0.05). Activity in the SN/VTA was predictive of successful memory formation in both curiosity conditions (main effect memory: left: F(1,17)=7.17, p=0.016; right: F(1,17)=6.46, p=0.021; Figure 3C middle), and no significant interactions were observed (left: F(1,17)=0.06, p=0.810; right: F(1,17)=0.48, p=0.496). Findings for the hippocampus paralleled those for the nucleus accumbens, exhibiting a trend for a Curiosity x Memory interaction in the right hippocampus (F(1,17)=5.25, p=0.035; Figure 3C right), but not in the left (F(1,17)=2.01, p=0.175). Further analyses showed that activity during anticipation of trivia answers predicted later memory on high curiosity trials in the right hippocampus (t(17)=2.12, p=0.0247), with a similar effect in the left that did not exceed the Bonferroni-corrected threshold (t(17)=2.04, p=0.029). No subsequent memory effect was evident on low curiosity trials (left: t(17)=0.00; right: t(17)=−1.37; p>0.05).
Analyses of activity directly evoked by the trivia answers revealed subsequent memory effects that did not differentiate between high and low curiosity conditions (see Supplemental Results and Figure S3), consistent with results from fMRI studies of stimulus-related encoding activity (Paller and Wagner, 2002). Thus, the results indicate that curiosity-driven memory benefits were driven by anticipatory activity, rather than activity elicited during processing of interesting trivia answers (cf. Adcock et al., 2006).
How does a curious state modulate learning of incidental items?
Our next analyses focused on incidental learning of faces presented during states of high or low curiosity. We predicted that neural processes that are elicited by the presentation of a high curiosity question would enhance incidental learning of faces that were presented during this period. Consistent with this prediction, recognition performance was higher for faces that were encoded during states of high curiosity (Pr=42.4% (Hits – False Alarms), SE=±2.68) than for faces encoded during low curiosity trials (Pr=38.2%, SE=±2.37; t(18)=1.97, p=0.032; Figure 4A). This small, but significant effect is in line with the idea that a curious state can benefit learning of incidental information.
Figure 4.
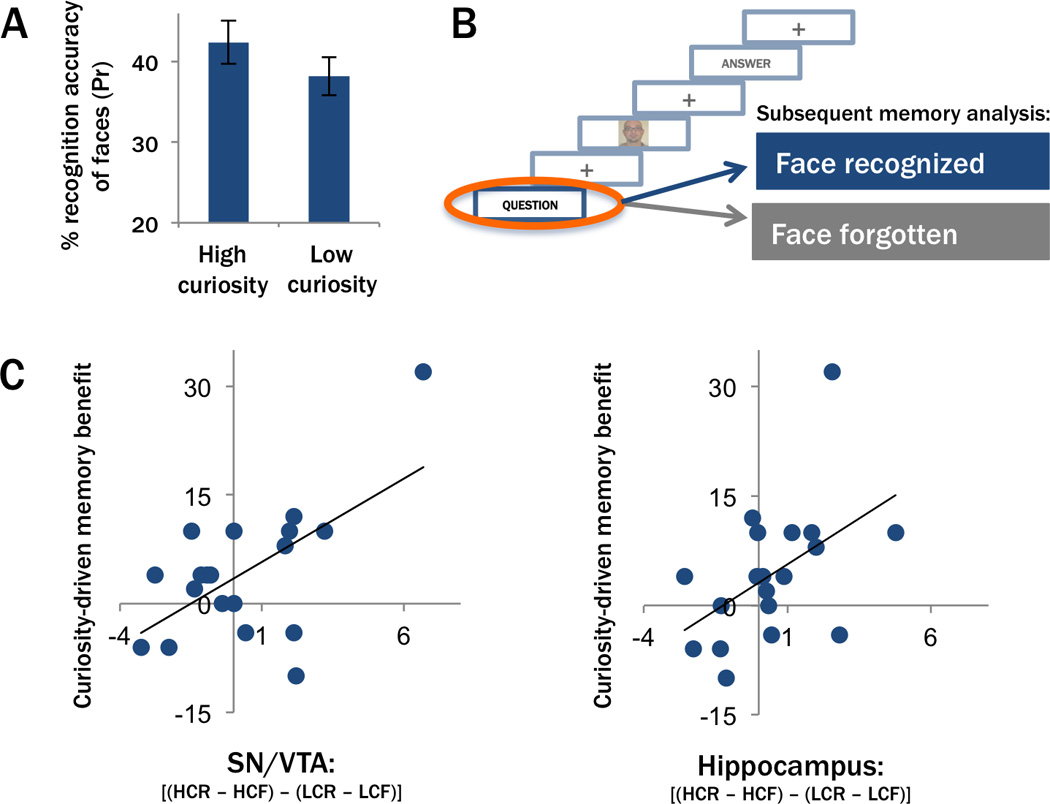
Enhanced incidental learning of faces encoded during states of high curiosity. (A) Recognition discriminability (Pr values) was higher for faces presented during states of high curiosity compared to low curiosity trials. (B) Brain activity elicited by onset of each trivia question was analyzed based on whether the face that was subsequently presented on the same trial was recognized or forgotten on the post-scan face recognition test. (C) The neural interaction between anticipatory curiosity and memory was highly correlated with the curiosity-driven memory benefit for neutral faces. The scatter plots show significant, positive correlations between the inter-subject variability in the curiosity-driven memory benefit (plotted on the y-axis) and in activity for the bilateral SN/VTA (left) and the right hippocampus (right). Each data point represents one participant. HCR/ HCF = High curiosity recognized / forgotten, LCR/ LCF = low curiosity recognized / forgotten.
We then tested whether activity in our ROIs during states of high curiosity (i.e. question-evoked activity) supports the memory benefits for faces that were incidentally encoded during high compared to low curiosity states. We did not, however, find significant interactions between curiosity and memory or main effects of memory in the ROIs (all F’s≤2.01, p’s≥0.173), possibly due to high inter-subject variability in behavioral effects of curiosity on face encoding. Stimulus-related activity was predictive of successful memory formation, but this effect was independent of whether a face was presented during high or low curiosity states (see Supplemental Results).
Given the large inter-subject variability in curiosity-driven memory benefits for incidentally presented faces, we investigated whether this variability might be driven by inter-individual variations in activation during states of high curiosity. That is, if a curious state promotes learning of incidental information via activity in our ROIs, we might expect that those participants who exhibited the largest activation increase during states of high curiosity to show the largest memory benefits for neutral faces. As with the earlier analyses, this analysis was again performed on fMRI data during anticipation of answers to trivia questions (and therefore prior to face encoding; see Figure 4B).
To test this prediction, we computed the Pearson product moment correlation between the curiosity-driven memory benefit for faces (i.e., the difference in recognition memory performance between faces presented on high vs. low curiosity trials) and the neural interaction between curiosity and subsequent memory (i.e. [subsequently recognized – forgotten faces on high curiosity trials] – [subsequently recognized – forgotten faces on low curiosity trials]). Results revealed strong relationships between the behavioral effect of curious states on subsequent memory for faces and curiosity-driven activation increases during anticipation of trivia answers in the bilateral SN/VTA (left: r=0.618, p=0.002; right: r=0.537, p=0.009) and right hippocampus (right: r=0.493, p=0.016; see Figure 4C). Correlations were not significant for the left hippocampus (r=0.176, p=0.236) or nucleus accumbens (left: r=− 0.189; right: r=−0.108; p’s>0.05). To confirm that correlations were not driven by extreme values, we repeated these analyses using Spearman’s rank order correlation coefficient, which is robust to outliers. These analyses revealed a similar pattern of results, such that the neural interaction between curiosity and memory showed a trend for a positive correlation with curiosity-driven memory benefits for faces in the bilateral SN/VTA (left: r=0.384, p=0.052; right: r=0.393, p=0.048) and right hippocampus ROI (left: r=0.149, p=0.272; right: r=0.447, p=0.028), but not in the bilateral nucleus accumbens (left: r=−0.154; right: r=0.084; p’s>0.05). A further mediation analysis revealed that the relationship between hippocampal memory-predicting activity and curiosity-driven memory benefits was mediated by midbrain memory-predicting activity (see Supplemental Information and Figure S4). Importantly, the correlations described above were solely driven by neural memory-predicting effects on high curiosity trials (left SN/VTA: Pearson’s r=0.760, p=0.001; Spearman’s r=0.588, p=0.004; right SN/VTA: Pearson’s r=0.710, p<0.001; Spearman’s r=0.638, p=0.002; right hippocampus: Pearson’s r=0.504, p=0.014; Spearman’s r=0.371, p=0.059; see Figure S5). No significant relationships were observed between the behavioral curiosity-driven memory benefit and memory-predicting activity in the low curiosity condition (all Pearson’s and Spearman’s r’s≤−0.107, p’s>0.05).
Given that between-individual variations in activity in the SN/VTA and hippocampus predicted memory benefits for unrelated, neutral faces, it is reasonable to speculate that these relationships were driven by functional connectivity between the two regions (Adcock et al., 2006; Wolosin et al., 2012). We therefore performed psychophysiological interaction analyses (PPI) to investigate whether the SN/VTA ROIs (i.e. seed region) show increased functional correlations with the hippocampus ROIs during successful, as compared with unsuccessful incidental encoding of faces. We performed separate PPI analyses for the high and low curiosity conditions targeting the critical time period following onset of the trivia question (for details, see Experimental Procedures). Results revealed a positive correlation between curiosity-driven memory benefits for faces and the magnitude of memory-predicting enhanced functional connectivity between the left SN/VTA and left hippocampus. Although the Pearson’s r-value (r=0.432, p=0.032) did not reach the Bonferroni-corrected significance threshold, the correlation was significant when calculated with Spearman’s rank order correlation coefficient (r=0.4768, p=0.020). In contrast, we did not find significant correlations with memory benefits for faces in the low curiosity condition (Pearson’s r=0.134, Spearman’s r=−0.005, both p’s>0.05). Additional analyses on functional connectivity between the right SN/VTA and right hippocampus did not reveal any significant findings.
In summary, the findings suggest that individual differences in activity in the SN/VTA and hippocampus, and functional connectivity between the two regions accounted for between-individual variability in incidental face encoding during states of high curiosity.
Do curiosity-driven memory benefits for incidental events persist after a long delay?
Behavioral results from the fMRI study revealed that curiosity influenced memory for trivia answers and also for incidentally encoded faces. On average, the interval between initial encoding of an item and presentation of that item at test was around 53 minutes (range: 20–85 minutes) for faces and 70 minutes (range: 40–100 minutes) for trivia answers, though the interval varied across items and also across subjects. This time frame is consistent with the possibility that LTP was enhanced for stimuli presented on high-curiosity trials. If LTP was enhanced for stimuli on high-curiosity trials, then we would expect that curiosity-related memory benefits should extend across longer retention intervals. Accordingly, in a second, behavioral experiment, we tested whether curiosity-driven memory benefits would be evident after a one-day delay between study and test (for details, see Experimental Procedures). Results of this experiment replicated the behavioral findings of the fMRI experiment. Recall of trivia answers to high curiosity questions was higher than recall of answers to low curiosity questions (45.9% SE=±3.35 vs. 28.1% SE=±2.84; paired-sample t(26)=11.11, p<0.001; Figure 5A), consistent with the findings of Kang et al. (2009) and the findings of our fMRI study. Results also replicated the small, but reliable recognition advantage for faces that were presented during high curiosity states, although this finding was specific to confidently recognized faces. The rate of confidently recognized faces was significantly higher for faces encoded during high curiosity states than for faces encoded during low curiosity states (Pr=35.2% SE=±2.39 vs. Pr=31.2% SE=±2.38; t(27)=2.44, p=0.011; Figure 5B), whereas the difference was not significant for overall hit rates (Pr=39.6% SE=±2.76 vs. Pr=38.0% SE=±2.68; t(27)=0.96, p=0.173; see also Table S3 for the full pattern of memory responses). These findings are consistent with the idea that curiosity can influence memory consolidation of interesting material and also incidental material encoded during high curiosity states.
Figure 5.
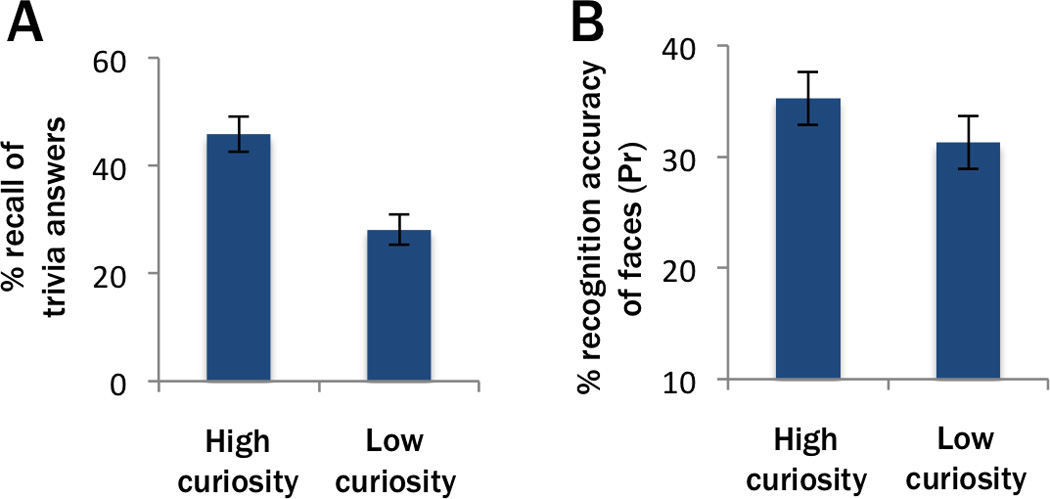
Follow-up behavioral experiment replicates curiosity-driven memory benefits for interesting and incidental material over a one-day retention interval. (A) Participants recalled more answers to high curiosity trivia questions than answers to low curiosity questions. (B) Participants showed higher rates of confident recognition for faces that were encoded during states of high curiosity than for faces encoded on low curiosity trials.
Discussion
The goal of the present study was to examine how intrinsic motivation benefits learning of interesting and incidental information. Behavioral results from two studies revealed that states of high curiosity enhance not only learning of interesting information, but also learning of incidental material. Imaging results demonstrated that these learning benefits are related to anticipatory brain activity in the mesolimbic dopaminergic circuit including the hippocampus. In particular, curiosity-driven memory benefits for incidental material were supported by activity in the SN/VTA and the hippocampus and by increased midbrain-hippocampus functional connectivity. Importantly, the effects of curiosity on memory for incidental material were correlated with activity in the SN/VTA prior to the encoding event, accounting for more than half of the behavioral variance in incidental encoding during high curiosity states. These findings are consistent with the idea that curiosity enhances learning, at least in part, through increased dopaminergic modulation of hippocampal activity.
Parallels between intrinsic and extrinsic motivation
The current findings complement results from a study on the neurocognitive mechanisms of curiosity by Kang et al. (2009). Both our whole-brain analyses and their results demonstrated that curiosity to learn answers to trivia questions was associated with increased activation in focal clusters in the ventrolateral prefrontal cortex and dorsal striatum. Using regions identified from a synthesis of published fMRI studies of reward, we additionally demonstrated that the specific ventral striatum and midbrain regions that were consistently recruited during reward anticipation also show increased activity during anticipation of interesting information (c.f. Knutson et al., 2001; O’Doherty et al., 2002). Results from the behavioral study conducted by Kang et al. (2009), like our study, also demonstrated that curiosity influences memory for trivia answers even across long retention intervals.
One major difference between Kang et al.’s (2009) study and the present study is that Kang et al. investigated how curiosity interacts with prior knowledge, whereas our study investigated how curiosity influences new learning. In their study, participants guessed answers to the questions and activity during the answer was contrasted between trials associated with correct and incorrect guesses. Activation in the midbrain, putamen, and the medial temporal lobe was enhanced for incorrectly guessed answers, if the participant was curious about the answer, leading the authors to conclude that the effect was driven by a reward prediction error. In contrast, the present study revealed that activation in the midbrain and nucleus accumbens was enhanced during anticipation of answers, but not during the presentation of the answer itself. Thus, our findings speak more to the influences of a curious state on memory, rather than to the phasic reinforcing influence of satisfying one’s curiosity.
Given that activity in the midbrain and nucleus accumbens has been reliably linked to presentation of rewards, the fact that we did not see curiosity-related modulation of responses in these areas to trivia answers might seem surprising. However, we note that responses to external rewards in the dopaminergic circuit scale with reward prediction errors and value (Dayan and Balleine, 2002; Schultz, 2013). In the present study, we could not assess the extent to which answers to trivia questions satisfied participants’ curiosity, and it is likely that this variance contributed to variability in SN/VTA activity during presentation of the answers. Consistent with this explanation, Kang et al. (2009) found responses to trivia answers that resembled reward prediction errors.
Dopaminergic mechanisms of motivated memory
Although we cannot make strong conclusions about whether fMRI signals in the midbrain and nucleus accumbens in our study reflect increased release of dopamine, there is reason to believe that dopamine might have played an important role. First, recent evidence indicates that BOLD fMRI signals in the dopaminergic midbrain and nucleus accumbens are positively correlated with dopamine release in the striatum (Knutson and Gibbs, 2007; Schott et al., 2008). Second, our whole-brain analyses confirmed that curious states were associated with relatively restricted activation in regions that are thought to be targets of midbrain dopaminergic nuclei (Haber and Fudge, 1997). Third, as we describe below, the findings linking activity in the SN/VTA and hippocampus to memory formation during curious states strongly parallel theoretical accounts and findings in rodents showing that dopaminergic activity can modulate hippocampus-dependent learning (for reviews, see Düzel et al., 2010; Lisman and Grace, 2005; Lisman et al., 2011; Shohamy and Adcock, 2010).
It has been shown that dopamine stimulates local protein synthesis in the dendrites of hippocampal neurons, which in turn is necessary for the late phase of LTP (e.g., Smith et al., 2005). Blockade of D1 receptors, in turn, can inhibit hippocampal synaptic plasticity (e.g., Frey et al., 1990; O’Carroll and Morris, 2004). Accordingly, several models (Frey and Morris, 1998; Lisman and Grace, 2005; Lisman et al., 2011; Redondo and Morris, 2011; Shohamy and Adcock, 2010) propose that stabilization of learning-induced hippocampal plasticity depends on dopaminergic neuromodulation, in addition to synaptic activity. Critically, research has indicated that weak learning events can elicit LTP if they are preceded by events that upregulate dopaminergic activity (Wang et al., 2010). Thus, dopaminergic activity might influence encoding “not only of specific salient events, but also the contexts in which they occur” (Shohamy and Adcock, 2010, p. 470).
The present results are consistent with this proposal, in that anticipatory activity in the hippocampal-VTA circuit was related to subsequent memory for trivia answers and also for temporally contiguous faces. These increases in BOLD signal could have been driven by increased dopaminergic input to the hippocampus during anticipation of the answer (Shohamy and Adcock, 2010). If so, then dopaminergic activity during states of high curiosity might have “rescued” memories for incidentally encoded faces that would otherwise been forgotten (Lisman et al., 2011; Redondo and Morris, 2011). This result is in line with recent studies showing similar memory enhancements on temporally contiguous information with extrinsic rewards (Murty and Adcock, 2013; Mather and Schoeke, 2011; Murayama and Kitagami, 2014). In addition, activity that predicted curiosity-driven memory benefits for interesting and incidental material was the activity during the anticipatory state, which is also consistent with findings from reward-motivated learning (Adcock et al., 2006; Gruber and Otten, 2010; Gruber et al., 2013; Murty and Adcock, 2013). Collectively, these findings suggest that both intrinsic and extrinsic motivational states can modulate memory formation.
If the effects of curiosity on learning were driven, at least in part, by dopaminergic modulation of hippocampal activity, it would imply a specific effect of curiosity on the late phase of LTP. The lower bound of the timescale for late LTP is not clear, but the retention intervals tested here are potentially consistent with such a mechanism. In the first experiment, memory was tested almost an hour after its initial encoding (on average, 53 min for each face, 70 min for each trivia answer). Importantly, we replicated the curiosity-driven memory benefits with a 1-day retention interval, which is definitely consistent with the timescale of late LTP. The findings are therefore in line with the idea that curiosity influenced memory for trivia answers and incidental memory for faces via dopaminergic facilitation of hippocampal LTP.
Different roles of the dopaminergic circuit for intentional and incidental learning
Although curiosity enhanced encoding of both trivia answers and incidentally presented faces, our results revealed some differences between effects of curiosity on intentional and incidental learning. Anticipatory activation in the nucleus accumbens predicted later memory performance only for high curiosity trivia answers, whereas activation in the SN/VTA was related to memory for high and low curiosity trivia answers and to faces incidentally encoded during states of high curiosity. We did not predict this difference between the nucleus accumbens and SN/VTA, but we speculate that this may reflect different roles for the accumbens and SN/VTA in intentional and incidental encoding. Anticipatory activity in the nucleus accumbens may set the stage for encoding of upcoming information that is goal-relevant. In contrast, anticipatory activity in the midbrain may promote memory for goal-relevant information, temporally contiguous goal-irrelevant information (e.g., faces shown during high curiosity trials), and other information that is somehow salient but irrelevant to current goals (e.g., subsequently remembered answers to low curiosity trivia questions). This account is admittedly speculative, but it aligns with models (Goto and Grace, 2008; Lisman and Grace, 2005; Scimeca and Badre, 2012) proposing that the VTA signals salience, whereas the nucleus accumbens integrates information about salience from the VTA with information about goal-relevance conveyed by the prefrontal cortex.
Future Directions
Further research is needed to explore the relationship between extrinsic and intrinsic motivation. Although there is reason to think that they share common mechanisms, they might also interact in counter-intuitive ways. For instance, behavioral studies have shown that extrinsic rewards can undermine intrinsic motivation (for a review, see Deci et al., 1999), an effect that has been linked to decreased activation in the striatum and prefrontal cortex (Murayama et al., 2010). Furthermore, Murayama and Kuhbandner (2011) demonstrated that the effects of extrinsic rewards and curiosity on memory encoding are not additive. In their study, extrinsic rewards were associated with enhanced memory for uninteresting trivia answers, but rewards did not improve memory for answers of questions that participants were highly curious about. These findings suggest that it would be useful to directly assess interactions between intrinsic and extrinsic motivational processes in relation to dopaminergic activity and learning.
Another important question concerns the cognitive processes that are influenced by intrinsic motivation. One possibility is that curiosity was associated with increased arousal or attentional processes. Although this is certainly possible, we do not believe that the relationship between curiosity and memory can be solely attributed to increased attentional processing. Behavioral studies have revealed direct influences of reward motivation on memory that cannot be explained by attentional processes per se (Wittmann et al., 2011). Consistent with this idea, the effects of curiosity identified in the whole-brain analyses bore little resemblance to the frontoparietal networks seen in whole-brain analyses of anticipatory attention (cf. Corbetta and Schulman, 2002). Furthermore, an attentional account would predict that curiosity should enhance intentional encoding of trivia answers but impair incidental encoding of faces, as these faces were irrelevant to the questions that stimulated participants’ curiosity. It is conceivable that the encoding task, which required participants to rate the faces for potential knowledge of the trivia answers, made the faces seem relevant. However, participants knew that the faces did not correspond to people who would actually provide an answer, and, irrespective of the rating, the answer was always presented only a few seconds after the face. Thus, the faces were more likely to be seen as distracters that were not relevant to satisfying their curiosity. Accordingly, the fact that we found enhanced memory for faces on high curiosity trials is not obviously consistent with a purely attentional account. Nonetheless, further research is needed to more extensively characterize how states of curiosity affect attentional and mnemonic processing.
Perhaps the most interesting finding to emerge from these experiments is that states of curiosity enhance encoding of temporally contiguous, but otherwise incidental information. These effects were relatively subtle, but reliable across experiments. Additionally, the high inter-subject variability in this effect was related to variability in hippocampal and midbrain activity and to functional connectivity between the two regions. Findings of high inter-subject variability are common in studies that investigate the influence of the dopaminergic circuit on learning in both animals (Flagel et al., 2011) and humans (Krebs et al., 2009; Wimmer and Shohamy, 2012; Zald et al., 2008). Further research is needed to better understand the sources of this inter-subject variability, which might reflect different genotypes, personality traits, or other influences on motivation and/or dopaminergic function.
Implications
The present findings have potential implications for understanding memory deficits in the elderly and in patients with psychiatric and neurological disorders that affect dopaminergic transmission (Chowdhury et al., 2013; Düzel et al., 2010; Goto and Grace, 2008; Lisman et al., 2011). We found that curiosity had large and long-lasting effects on memory for interesting information. Although effects on memory for incidental information were more subtle, it should be noted that our trivia question paradigm might only weakly approximate the effects of an individual’s idiosyncratic interests and motivation to learn. If anything, it is likely that our results may be underestimating the effects of curiosity on learning in daily life.
Given that healthy aging, and several neurological and psychiatric disorders are associated with changes in dopaminergic function, it is possible that these conditions affect memory, in part, through changes in intrinsic motivation to learn. In addition, the results are pertinent to learning in educational and occupational settings. For example, our findings suggest that, in addition to optimizing instructional methods, stimulating curiosity ahead of knowledge acquisition could enhance learning success (Lisman et al., 2011). Furthermore, teaching of detailed material that may not be of broad interest might be best done in the context of instruction on topics that students are highly motivated to learn.
Experimental Procedures
The details about the participants, stimulus material and the presentation are presented in the Supplemental Experimental Procedures.
Task Procedures
In both experiments, participants underwent a 4–stage paradigm with (1) a screening phase, (2) a study phase, (3) a surprise recognition test phase for incidental items (i.e. faces), and (4) a surprise recall test for trivia answers presented during the study phase. The delay between the study phase and the first memory test was on average 20 minutes in the fMRI experiment and one-day (22.5 hours) in the follow-up behavioral experiment. There were no other differences in respect to task procedures between both experiments.
(1) Screening phase
Because the level of curiosity elicited by different trivia questions is likely to vary between participants, we used participants’ ratings to sort trivia questions into participant-specific high and low curiosity categories (56 questions each). Trivia questions were randomly selected from a pool of 375 trivia questions and were consecutively presented. After the presentation of a trivia question, participants had to give two self-paced ratings on six-point scales (see Figure 1A). First, they had to rate how confident they were that they knew the answer to a trivia question (extreme points: 1 = “I am confident that I do not know the answer” and 6 = “I am confident that I know the answer”). Second, participants rated their level of curiosity about the answer to a trivia question (extreme points: 1 = “I am not interested at all in the answer” and 6 = “I am very much interested in the answer”). If participants did not indicate that they knew the answer to a trivia question (i.e. they did not give a 6 response on the answer confidence rating), trivia questions with responses 1–3 of the curiosity rating were allocated to the low curiosity condition and responses 4–6 to the high curiosity condition. The screening phase lasted until 56 trivia questions were allocated for each curiosity condition. On average (min-max), participants gave a high curiosity rating on 85 (56–173) and a low curiosity rating on 58 (56–68) trivia questions.
(2) Study phase
In the subsequent study phase that took place in an MRI scanner for the fMRI experiment, the selected 112 trivia questions were presented along with the associated answers (see Figure 1B). A trial started with the presentation of a trivia question, followed by an anticipation period that preceded the presentation of the associated trivia answer. Six of the 56 trials (~10%) in each condition were catch trials in order to ensure participants’ attention throughout the scanning session. In these trials, the letter string ‘xxxxx’ was presented instead of the trivia answer. During the anticipation period, a cross hair was presented that was replaced by an image of an emotionally neutral face (incidental item) during the middle of the anticipation period. During the presentation of the face, participants had to give a yes/ no response as to whether this particular person would be knowledgeable about the trivia topic and could help them figure out the answer. ‘Yes’ responses were given with the right index finger and ‘no’ responses with the right middle finger on an MRI compatible response box in the fMRI experiment and on a computer keyboard for the behavioral experiment. This encoding judgment was used to ensure that faces were likely to be encoded with a similar level of attention across both curiosity conditions. The study phase was divided into four scanning runs (9 min each).
(3) Recognition memory test for incidental items
Approximately 20 min (fMRI experiment) or 22.5 h (behavioral experiment) after the end of the study phase, a surprise recognition memory test for the faces was administered. All 112 faces from the study phase and 56 new faces were randomly presented. Participants made confidence judgments on whether they thought the face was presented during the study phase or was not presented earlier (i.e. ‘confident new’, ‘unconfident new’, ‘unconfident old’, and ‘confident old’). Participants were encouraged to try to give a response as accurately and quickly as possible.
(4) Recall test for trivia answers
After the recognition memory test for faces, participants were given a list with all trivia questions from the study phase in random order. Participants were encouraged to take approximately 20 min to write down the correct answers without guessing any answers.
Behavioral analyses
To assess whether memory improved for the high compared to the low curiosity condition and whether memory was above chance, we performed one-tailed paired-sample t-tests. Catch trials were not included in any analyses.
FMRI methods
FMRI acquisition
We used a 3T Siemens Skyra scanner with a 32-channel phased array head coil to acquire anatomical and functional MRI images. A multiband Echo-Planar Imaging sequence was used to acquire whole brain T2*-weighted images (TR=1.22 s, TE=24ms; 38 slices per volume; multiband factor=2; voxel size=3 mm isotropic) with 441 volumes for each of the four scanning runs. In addition, a T1-weighted MP-RAGE with whole brain coverage was acquired. Inside the head coil, the participant’s head was padded to restrict excessive motion. Stimuli were displayed on a mirror attached to the head coil above the participant’s eyes. During the scanning, the participant’s eyes were monitored by the experimenter via an eye tracker to ensure that the participant attended to all stimuli.
FMRI preprocessing
The functional and anatomical images were preprocessed and analyzed using the SPM8 software (The Wellcome Trust Centre for Neuroimaging, London, UK). The functional images were first realigned and then coregistered to the anatomical images. Anatomical images were segmented into grey and white matter images and imported into DARTEL to create a template anatomical image that was specific to the participants in this study. We then used DARTEL to normalize functional and anatomical images into MNI space. Functional images were spatially smoothed with a 6 mm full-width half-maximum Gaussian kernel. The ART repair toolbox (http://cibsr.stanford.edu/tools/human-brain-project/artrepair-software.html) was used to identify individual scans that showed abrupt movements (spikes).
FMRI analyses
General linear models (GLMs) were estimated by modeling blood oxygen level-dependent (BOLD) signal changes using a stick function (0 s duration) to model the onset of the particular events. We convolved these stick functions with a canonical hemodynamic response function and included motion covariates to account for motion-related noise in the data (i.e. three rigid-body translation and three rigid-body rotation parameters and additional spike regressors for scans that were identified by the ART repair toolbox). Catch trials were modeled separately for all event onsets and were not included in any analyses.
Regions-of-interest approach
We focused our analyses on three regions of interest (ROIs): the SN/VTA, the nucleus accumbens, and the hippocampus. First, the SN/VTA ROI was derived from a probabilistic mask based on magnetization transfer images (Guitart-Masip et al., 2011) containing the whole SN/VTA complex. Second, the nucleus accumbens ROI was traced on the mean anatomical images according to accepted guidelines (Haber and Knutson, 2010) (Center for Morphometric Analyses, Massachusetts General Hospital, Charlestown, MA, USA; http://www.cma.mgh.harvard.edu/). Third, the hippocampus ROI was derived from the hippocampal mask from the SPM Anatomy Toolbox (Amunts et al., 2005). In order to have a sensitive measure within these anatomical ROIs we conducted a meta-analysis using the NeuroSynth tool (neurosynth.org) (Yarkoni et al., 2011). We performed a term-based search on “reward” that included 329 studies (retrieved: July 2nd 2013) and generated a reverse inference mask (i.e. probability of the term “reward” given the observed activation). The reverse inference mask was chosen because of its increased selectivity in brain activation related to the search term (Yarkoni et al., 2011). We then inclusively masked this functional “reward” mask with our three anatomical masks. Figure S1 shows the resulting ROIs that indicate the overlap between the functional “reward” mask and the anatomical masks. Using this approach, the SN/VTA ROI potentially captured the whole VTA and parts of the SN, the nucleus accumbens ROI was used as a whole, and the hippocampus ROI was restricted to clusters in the hippocampal head and body. Importantly, our reward-sensitive hippocampus ROI overlapped with a hippocampal region that shows high functional connectivity with the VTA and the nucleus accumbens (Kahn and Shohamy, 2013). Analyses are based on activity in the left and right hemisphere separately.
Curiosity-related activation
The first GLM tested whether curiosity ratings parametrically modulated activity in our ROIs. Separate regressors were used for onsets of the trivia questions, faces, and trivia answers. The analyses of interest were the onsets of the trivia questions and answers (i.e. when curiosity was elicited and satisfied). That is, for each participant, activation in response to the question was modeled with one regressor modeling mean activation across all trials, and a mean-centered parametric modulation regressor whose magnitude scaled linearly with curiosity ratings given for the question during the screening phase. Because we hypothesized that activity in our ROIs might linearly increase with curiosity ratings, fMRI beta estimates were entered into one-tailed one-sample t-tests and tested against the value 0 (i.e. per ROI and events of interest). Because we did not have strong predictions about laterality, tests for left and right hemisphere ROIs were evaluated using a Bonferroni-corrected threshold of 0.025 per analysis. For additional whole brain analyses, we used 3DClustSim (Cox, 1996; http://afni.nimh.nih.gov/pub/dist/doc/program_help/3dClustSim.html) to determine a cluster correction of p<0.05 for the whole brain (p<0.005 and k=65 voxels using a gray matter mask based on the subjects’ mean anatomical image).
Activation predicting later recall of trivia answers
The second GLM modeled activation depending on later memory performance for trivia answers. Event onsets for trivia questions, faces, and trivia answers were modeled according to trials in which trivia answers were later correctly recalled or forgotten. All regressors were further separated into the low (curiosity ratings 1–3 during the screening phase) and high curiosity condition (curiosity ratings 4–6) (for trial numbers, see Supplemental Information). Our main analyses of interest targeted the onset of the trivia questions (when a curious state was elicited). We hypothesized that our ROIs would support learning of interesting – but not uninteresting – material via increased activity for later recalled compared to later forgotten trivia answers. Importantly, we hypothesized such memory-predicting activity at the time interval when curiosity was elicited (i.e. at the time of trivia questions) (see Figure 3B). We therefore performed a 2 × 2 repeated measures ANOVA with the factors curiosity (high/ low) and memory (recalled/ forgotten). If an interaction was present, paired-sample one-tailed t-tests were performed on memory-predicting activity in both curiosity conditions separately. In addition to activity elicited by trivia questions, we hypothesized that stimulus-related activity (i.e. during the actual learning of trivia answers) should also predict later recall of trivia answers (see Figure S3A). ANOVAs on stimulus-related activity in our ROIs were performed in the same way as the analyses for activity elicited by trivia questions.
Activation predicting later recognition of faces
The third GLM modeled activity depending on later memory performance of faces that were presented during the anticipation phase. As in the previous GLMs, all event onsets for trivia questions, faces, and trivia answers were modeled separately, but in this GLM separate regressors were used according to whether a face in a given trial was later correctly recognized (i.e. a ‘confident old’ or ‘unconfident old’ response) or forgotten (i.e. a ‘confident new’ or ‘unconfident new’ response) (for trial numbers, see Supplemental Information). Regressors were further separated into both curiosity conditions. Our main analyses of interest again targeted the time when curiosity was elicited (i.e. at the time of the trivia questions). This way, we could ask how memory benefits for incidental stimuli (i.e. faces) that were presented during states of high compared to low curiosity would be supported by activity in our ROIs (see Figure 4B). To test whether memory for neutral faces was supported by question-evoked activity, ANOVAs were performed using an identical approach as for the analyses concerning memory for trivia answers. In addition, we performed Pearson’s and Spearman’s correlations to investigate relationships between participants’ behavioral curiosity-driven memory benefit for faces (i.e. recognition accuracy for faces: high – low curiosity condition) and the neural interaction between curiosity and subsequent memory (i.e. [high curiosity condition: faces recognized – forgotten] – [low curiosity condition: faces recognized – forgotten]).
Functional connectivity analyses
Psychophysiological interaction (PPI) analyses were performed to investigate how functional connectivity between the SN/VTA and hippocampus ROIs predicted memory benefits for incidental, unrelated faces. PPI general linear models included the raw time course of a seed region (i.e. the physiological term; here: the left or right SN/VTA ROI), the onsets of either high or low curiosity questions convolved with an HRF (i.e., the psychological term; contrasting trials with later recognized (1) and later forgotten faces (−1)), the critical interaction term (i.e. physiological term multiplied by the unconvolved psychological term) and movement-related regressors. For each participant, we then extracted the PPI beta weights from the hippocampal ROI on the same hemisphere as the SN/TVA seed region. In the first analysis, we performed one-tailed paired-sample t-tests examining whether PPI beta weights differed significantly from chance in order to test whether functional connectivity between the seed region and ROIs predicts later memory for faces. Furthermore, we performed correlations between individual PPI beta weights and individual memory benefits for faces in order to investigate how individual variability in the strength of connectivity between seed region and ROIs predicted memory benefits for faces.
Supplementary Material
Highlights.
People are better at learning information that they are curious about
Memory for incidental material presented during curious states also enhanced
Curiosity associated with anticipatory activity in nucleus accumbens and midbrain
Memory benefits for incidental material depend on midbrain-hippocampus involvement
Acknowledgements
We thank each of the reviewers for their helpful suggestions and comments. We also thank Mason Oliver for help with the data collection; Manoj Doss and Maria Montchal for help with creating the stimuli; and Brian Wiltgen, Mariam Aly, Eva Bauch, Laura Libby, and Maureen Ritchey for comments on earlier versions of the manuscript. This work was supported by NIH grant 1R01MH083734, a Guggenheim Fellowship, a Parke-Davis Exchange Fellowship from the University of Cambridge, and a Visiting Professorship from the Leverhulme Trust awarded to C.R. and a postdoctoral fellowship from the German Academic Exchange Service (Deutscher Akademischer Austausch Dienst) to M.J.G. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: M.J.G and C.R. designed the experiment, B.D.G and M.J.G. collected the data, M.J.G. analyzed the data, and M.J.G and C.R. wrote the manuscript.
References
- Adcock RA, Thangavel A, Whitfield-Gabrieli S, Knutson B, Gabrieli JDE. Reward-motivated learning: mesolimbic activation precedes memory formation. Neuron. 2006;50:507–517. doi: 10.1016/j.neuron.2006.03.036. [DOI] [PubMed] [Google Scholar]
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, Habel U, Schneider F, Zilles K. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat Embryol. 2005;210:343–352. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- Berlyne DE. Curiosity and Exploration. Science. 1966;153:25–33. doi: 10.1126/science.153.3731.25. [DOI] [PubMed] [Google Scholar]
- Chowdhury R, Guitart-Masip M, Lambert C, Dayan P, Huys Q, Düzel E, Dolan RJ. Dopamine restores reward prediction errors in old age. Nat Neurosci. 2013;16:648–653. doi: 10.1038/nn.3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dayan P, Balleine BW. Reward, Motivation, and Reinforcement Learning. Neuron. 2002;36:285–298. doi: 10.1016/s0896-6273(02)00963-7. [DOI] [PubMed] [Google Scholar]
- Deci EL, Koestner R, Ryan RM. A meta-analytic review of experiments examining the effects of extrinsic rewards on intrinsic motivation. Psychological Bulletin. 1999;125:627–668. doi: 10.1037/0033-2909.125.6.627. [DOI] [PubMed] [Google Scholar]
- Düzel E, Bunzeck N, Guitart-Masip M, Düzel S. NOvelty-related motivation of anticipation and exploration by dopamine (NOMAD): implications for healthy aging. Neuroscience & Biobehavioral Reviews. 2010;34:660–669. doi: 10.1016/j.neubiorev.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Fiorillo CD, Tobler PN, Schultz W. Discrete coding of reward probability and uncertainty by dopamine neurons. Science. 2003;299:1898–1902. doi: 10.1126/science.1077349. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, Akers CA, Clinton SM, Phillips PEM, Akil H. A selective role for dopamine in stimulus-reward learning. Nature. 2011;469:53–57. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey U, Morris RG. Synaptic tagging: implications for late maintenance of hippocampal long-term potentiation. Trends Neurosci. 1998;21:181–188. doi: 10.1016/s0166-2236(97)01189-2. [DOI] [PubMed] [Google Scholar]
- Frey U, Schroeder H, Matthies HR. Dopaminergic antagonists prevent long-term maintenance of posttetanic LTP in the CA1 region of rat hippocampal slices. Brain Research. 1990;522:69–75. doi: 10.1016/0006-8993(90)91578-5. [DOI] [PubMed] [Google Scholar]
- Gruber MJ, Otten LJ. Voluntary Control over Prestimulus Activity Related to Encoding. J. Neurosci. 2010;30:9793–9800. doi: 10.1523/JNEUROSCI.0915-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber MJ, Watrous AJ, Ekstrom AD, Ranganath C, Otten LJ. Expected reward modulates encoding-related theta activity before an event. Neuroimage. 2013;64:68–74. doi: 10.1016/j.neuroimage.2012.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Grace AA. Limbic and cortical information processing in the nucleus accumbens. Trends Neurosci. 2008;31:552–558. doi: 10.1016/j.tins.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guitart-Masip M, Fuentemilla L, Bach DR, Huys QJM, Dayan P, Dolan RJ, Düzel E. Action dominates valence in anticipatory representations in the human striatum and dopaminergic midbrain. J. Neurosci. 2011;31:7867–7875. doi: 10.1523/JNEUROSCI.6376-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Fudge JL. The primate substantia nigra and VTA: integrative circuitry and function. Crit Rev Neurobiol. 1997;11:323–342. doi: 10.1615/critrevneurobiol.v11.i4.40. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn I, Shohamy D. Intrinsic connectivity between the hippocampus, nucleus accumbens, and ventral tegmental area in humans. Hippocampus. 2013;23:187–192. doi: 10.1002/hipo.22077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang MJ, Hsu M, Krajbich IM, Loewenstein G, McClure SM, Wang JT-Y, Camerer CF. The wick in the candle of learning: epistemic curiosity activates reward circuitry and enhances memory. Psychol Sci. 2009;20:963–973. doi: 10.1111/j.1467-9280.2009.02402.x. [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J. Neurosci. 2001;21:RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Gibbs SEB. Linking nucleus accumbens dopamine and blood oxygenation. Psychopharmacology (Berl.) 2007;191:813–822. doi: 10.1007/s00213-006-0686-7. [DOI] [PubMed] [Google Scholar]
- Krebs RM, Schott BH, Düzel E. Personality traits are differentially associated with patterns of reward and novelty processing in the human substantia nigra/ventral tegmental area. Biol. Psychiatry. 2009;65:103–110. doi: 10.1016/j.biopsych.2008.08.019. [DOI] [PubMed] [Google Scholar]
- Li S, Cullen WK, Anwyl R, Rowan MJ. Dopamine-dependent facilitation of LTP induction in hippocampal CA1 by exposure to spatial novelty. Nat Neurosci. 2003;6:526–531. doi: 10.1038/nn1049. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Grace AA. The Hippocampal-VTA Loop: Controlling the Entry of Information into Long-Term Memory. Neuron. 2005;46:703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Lisman J, Grace AA, Düzel E. A neoHebbian framework for episodic memory; role of dopamine-dependent late LTP. Trends Neurosci. 2011;34:536–547. doi: 10.1016/j.tins.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Schoeke A. Positive outcomes enhance incidental learning for both younger and older adults. Front Neurosci. 2011;5:129. doi: 10.3389/fnins.2011.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murayama K, Kitagami S. Consolidation power of extrinsic rewards: Reward cues enhance long-term memory for irrelevant past events. J Exp Psychol Gen. 2014;143:15–20. doi: 10.1037/a0031992. [DOI] [PubMed] [Google Scholar]
- Murayama K, Kuhbandner C. Money enhances memory consolidation - But only for boring material. Cognition. 2011;119:120–124. doi: 10.1016/j.cognition.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Murayama K, Matsumoto M, Izuma K, Matsumoto K. Neural basis of the undermining effect of monetary reward on intrinsic motivation. Proc. Natl. Acad. Sci. U.S.A. 2010;107:20911–20916. doi: 10.1073/pnas.1013305107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murty VP, Adcock RA. Enriched Encoding: Reward Motivation Organizes Cortical Networks for Hippocampal Detection of Unexpected Events. Cereb. Cortex. 2013 doi: 10.1093/cercor/bht063. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Carroll CM, Morris RGM. Heterosynaptic co-activation of glutamatergic and dopaminergic afferents is required to induce persistent long-term potentiation. Neuropharmacology. 2004;47:324–332. doi: 10.1016/j.neuropharm.2004.04.005. [DOI] [PubMed] [Google Scholar]
- O'Doherty JP, Deichmann R, Critchley HD, Dolan RJ. Neural responses during anticipation of a primary taste reward. Neuron. 2002;33:815–826. doi: 10.1016/s0896-6273(02)00603-7. [DOI] [PubMed] [Google Scholar]
- Paller KA, Wagner AD. Observing the transformation of experience into memory. In: Regul, editor. Trends Cogn. Sci. Vol. 6. 2002. pp. 93–102. [DOI] [PubMed] [Google Scholar]
- Redondo RL, Morris RGM. Making memories last: the synaptic tagging and capture hypothesis. Nat Rev Neurosci. 2011;12:17–30. doi: 10.1038/nrn2963. [DOI] [PubMed] [Google Scholar]
- Reeve J, Reeve JM. Motivating others: Nurturing inner motivational resources. Boston, MA: Allyn and Bacon; 1996. [Google Scholar]
- Ryan R, Deci E. Intrinsic and Extrinsic Motivations: Classic Definitions and New Directions. Contemp Educ Psychol. 2000;25:54–67. doi: 10.1006/ceps.1999.1020. [DOI] [PubMed] [Google Scholar]
- Schott BH, Minuzzi L, Krebs RM, Elmenhorst D, Lang M, Winz OH, Seidenbecher CI, Coenen HH, Heinze H-J, Zilles K, et al. Mesolimbic functional magnetic resonance imaging activations during reward anticipation correlate with reward-related ventral striatal dopamine release. J. Neurosci. 2008;28:14311–14319. doi: 10.1523/JNEUROSCI.2058-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Updating dopamine reward signals. Curr. Opin. Neurobiol. 2013;23:229–238. doi: 10.1016/j.conb.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scimeca JM, Badre D. Striatal contributions to declarative memory retrieval. Neuron. 2012;75:380–392. doi: 10.1016/j.neuron.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohamy D, Adcock RA. Dopamine and adaptive memory. In: Regul, editor. Trends Cogn. Sci. Vol. 14. 2010. pp. 464–472. [DOI] [PubMed] [Google Scholar]
- Smith WB, Starck SR, Roberts RW, Schuman EM. Dopaminergic Stimulation of Local Protein Synthesis Enhances Surface Expression of GluR1 and Synaptic Transmission in Hippocampal Neurons. Neuron. 2005;45:765–779. doi: 10.1016/j.neuron.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Wang S-H, Redondo RL, Morris RGM. Relevance of synaptic tagging and capture to the persistence of long-term potentiation and everyday spatial memory. Proc. Natl. Acad. Sci. U.S.A. 2010;107:19537–19542. doi: 10.1073/pnas.1008638107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer GE, Shohamy D. Preference by association: how memory mechanisms in the hippocampus bias decisions. Science. 2012;338:270–273. doi: 10.1126/science.1223252. [DOI] [PubMed] [Google Scholar]
- Wittmann BC, Schott BH, Guderian S, Frey JU, Heinze H-J, Düzel E. Reward-related FMRI activation of dopaminergic midbrain is associated with enhanced hippocampus-dependent long-term memory formation. Neuron. 2005;45:459–467. doi: 10.1016/j.neuron.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Wittmann BC, Dolan RJ, Düzel E. Behavioral specifications of reward-associated long-term memory enhancement in humans. Learn Mem. 2011;18:296–300. doi: 10.1101/lm.1996811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolosin SM, Zeithamova D, Preston AR. Reward modulation of hippocampal subfield activation during successful associative encoding and retrieval. J Cogn Neurosci. 2012;24:1532–1547. doi: 10.1162/jocn_a_00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nat. Methods. 2011;8:665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald DH, Cowan RL, Riccardi P, Baldwin RM, Ansari MS, Li R, Shelby ES, Smith CE, McHugo M, Kessler RM. Midbrain dopamine receptor availability is inversely associated with novelty-seeking traits in humans. J. Neurosci. 2008;28:14372–14378. doi: 10.1523/JNEUROSCI.2423-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


