Abstract
We examined functional activation across the adult lifespan in 316 healthy adults aged 20–89 years on a judgment task that, across conditions, drew upon both semantic knowledge and ability to modulate neural function in response to cognitive challenge. Activation in core regions of the canonical semantic network (e.g., left IFG) were largely age-invariant, consistent with cognitive aging studies that show verbal knowledge is preserved across the lifespan. However, we observed a steady linear increase in activation with age in regions outside the core network, possibly as compensation to maintain function. Under conditions of increased task demands, we observed a stepwise reduction across the lifespan of modulation of activation to increasing task demands in cognitive control regions (frontal, parietal, anterior cingulate), paralleling the neural equivalent of “processing resources” described by cognitive aging theories. Middle-age was characterized by decreased modulation to task-demand in subcortical regions (caudate, nucleus accumbens, thalamus), and very old individuals showed reduced modulation to task difficulty in midbrain/brainstem regions (ventral tegmental, substantia nigra). These novel findings suggest aging of activation to demand follows a gradient along the dopaminergic/nigrostriatal system, with earliest manifestation in fronto-parietal regions, followed by deficits in subcortical nuclei in middle-age and then to midbrain/brainstem dopaminergic regions in the very old.
Keywords: aging, cognitive control, fMRI, lifespan, nigrostriatal system, semantic judgment
Introduction
Aging of the brain’s structure over the course of the adult lifespan is characterized by precipitous declines in gray matter volume in association cortices, especially in the prefrontal cortex, and relative sparing of the primary sensory cortices and other regions associated with early perceptual processing (for review Rodrigue & Kennedy, 2011; Raz & Kennedy, 2009). Similarly, a broad array of cognitive functions decline with age, including speed of processing, working memory, episodic memory, and reasoning, but semantic knowledge and vocabulary tend to be preserved, or even increase with age (Ackerman & Rolfhus, 1999; Park et al., 2002; Puglisi et al., 1988). In contrast to our understanding of brain structure and cognition function, considerably less is known about the lifespan trajectory of brain function.
Typical cross-sectional comparisons pit young (usually 20–30 year olds) against old adults (usually 60–70 year olds). Such extreme group contrasts tend to find evidence for task-related BOLD “underactivation” in older adults relative to younger adults in some regions (e.g., hippocampus) and “overactivation” in many other regions, particularly frontal-parietal regions, even when task performance is roughly equivalent for old and young (for review: Cabeza, 2002; Grady, 2000; Park & Reuter-Lorenz, 2009; Reuter-Lorenz & Lustig, 2005). Thus far, we know little about brain function during critical life periods such as middle age and very old age. Hence, the focus of the present study is to characterize neural activity cross-sectionally across the adult lifespan (utilizing age as a continuous variable as well as a discrete variable with several levels: young, middle, old and very old ages) to investigate when in the lifespan and where in the brain shifts in activation occur as we age. We investigate these questions on two tasks of theoretical importance: an easy (canonical) and a difficult (ambiguous) semantic judgment task.
The canonical (easy) task involves a semantic judgment (“living or nonliving?”) about a highly familiar concrete target such as “walrus”, truck”, or “asphalt.” In young adults, this task broadly engages the left inferior frontal gyrus and a supporting semantic network that includes posterior inferior parietal lobe, middle temporal gyrus, fusiform and parahippocampal gyri, dorsomedial prefrontal cortex, ventromedial prefrontal cortex, and the middle cingulate gyrus (Binder et al., 2009; Spreng, et al 2010; Thompson-Schill, 2003; Wagner et al., 2001). In the aging literature, semantic judgment tasks (e.g., living/non-living judgments) have been used to study memory encoding and/or retrieval (i.e., Andrews-Hanna et al., 2007; Cabeza et al., 2004; Lustig et al., 2003; Morcom et al., 2007; Bucur et al., 2008; Grady et al., 2006; Stebbins et al., 2002) and repetition priming (e.g., Bergerbest et al., 2009; Daselaar et al., 2005; Lustig et al., 2004). The majority of these studies used extreme age groups and have generally reported greater right prefrontal cortex activation in older adults compared to their younger counterparts suggesting that older adults require more neural activity to make even simple, knowledge-based judgments (Cabeza 2002). It has been proposed that this additional activation acts as a type of scaffolding to preserve behavioral function (Park & Reuter-Lorenz, 2009). How and when in the lifespan functional activation is altered is largely unknown (although see Park et al., 2013) and thus the first focus of the present study was to examine the developmental course of brain activation across the adult lifespan in a canonical semantic judgment task condition where individuals made living/nonliving judgments about straightforward (easy) concrete nouns.
The other major focus of the present study is on the construct of mental effort or processing resources—a concept that plays a critical role in prominent theories of cognitive aging. A central theme of these theories is that aging is characterized by reduced cognitive resources available to allocate to complex mental tasks (e.g., Craik & Byrd, 1982), resulting in steeper age-related behavioral decline on difficult compared to easy tasks (McDowd & Craik, 1988). Despite a large literature on this topic in the cognitive domain (e.g., Glisky 2007), the impact of increased processing demand on older adults has received considerably less attention in neuroimaging studies of aging, even with extreme age group designs (see Reuter-Lorenz & Cappell 2008 for a discussion of this issue). Hence, a second goal of the present study is to investigate, across the adult life course, what regions modulate activation to increased task processing demands. To assess this, we increased task processing demands and examined individuals’ ability to increase neural response when faced with a challenge. Specifically, we created a second task condition where individuals were presented with ambiguous nouns that required more deliberative processing for a response. In this condition, items were typically poor exemplars of living and nonliving categories or had multiple meanings that required more processing, e.g., “ghost”, “virus”, “coral”, “speaker”, “orange”.
There are many reports on the neural substrates associated with increased processing demands in young adults, with evidence that cognitive control regions show increased activation, including a frontal-parietal network as well as the anterior cingulate cortex (Braver, et al 2009; Braver, 2012; Brown & Braver, 2005). The broader prefrontal-striatal executive function network is dopaminergic in nature and includes regions of heteromodal association cortices involved in loops with the striatum (Alexander, DeLong, & Strick, 1986). The circuitry of this dopaminergic pathway originates in the substantia nigra and ventral tegmental area, innervates the neostriatum (caudate, putamen and nucleus accumbens), thalamus (anterior and dorsomedial nuclei), and prefrontal cortex in partially reciprocal loops (Groenewegen et al., 1990). Importantly, these are all areas known to undergo the most structural change during the course of normal aging (Raz & Kennedy, 2009; Rodrigue & Kennedy, 2011), with a precipitous decline in volume (Raz et al., 2003; Raz et al., 2010) and in neurochemical receptor structure and function (Volkow et al., 1998). Thus, we tentatively hypothesized that regions of this dopaminergic nigrostriatal system would be most likely to display age-related alterations in brain activity across the lifespan, as these regions are most sensitive to task demands and also most vulnerable to the effects of aging.
There are relatively few studies of age-related differences in neural activation to increasing task demands (see e.g., Reuter-Lorenz & Cappell, 2008 for a review). Functional imaging studies comparing younger and older adults generally find that in more demanding task conditions, younger adults can better modulate neural activity to task demands, often in prefrontal and parietal regions (Cappell et al., 2010; Davis et al., 2008; Grady et al., 1998; Konishi et al. 1998; Persson et al., 2004; Rypma & D’Esposito, 2000; Schneider-Garces et al., 2010; however see Nagel et al., 2009), but there is not yet information about when these shifts in modulation to processing demand occur during the lifespan, and whether this shift is incremental across the lifespan (i.e., linear) or punctuated by larger, discrete changes at certain ages (i.e., stepwise). In particular, we would expect altered modulation of activation to processing demand to be most evident in the malleable prefrontal and parietal heteromodal association cortices involved in cognitive control in middle-age as this is an important period in which structural changes to these brain structures become evident and exert cognitive consequences (Raz et al., 2005; Rodrigue & Kennedy, 2011).
With these considerations in mind, the current study investigated the following research questions: 1) How does functional brain activation to a canonical semantic judgment task differ across the adult lifespan? What regions are age-sensitive and is the relationship linear or nonlinear? Given the relative lifespan stability of semantic knowledge, we hypothesize that activation in the core brain regions of the semantic network will not differ with age, but rather activity outside this network will increase or decrease with age. 2) When processing demands are increased, how does age affect modulation of brain activity (comparing low-demand to high-demand conditions)? We hypothesize that the ability to modulate neural activation to task demands will decline with age and, given the vulnerability of the brain regions comprising the dopaminergic executive system, these age-related declines in modulation of activation to task demand will be seen primarily in heteromodal frontal-parietal association regions. 3) When in the lifespan do these alterations to demand-modulation emerge and do different brain regions show different age-trajectories? To fully explore functional activation modulation differences in the periods of middle age and very old age, about which little is currently known, we will separately examine modulation to task demand in the following discrete age ranges: young adulthood (age 20–39), middle age (40–59), older adulthood (60–79), and very old (80–89). We hypothesize that different regions of the cognitive control system will show different age trajectories. 4) Similarly, we examine whether task-related demand modulation is altered linearly with age or whether are there greater stepwise shifts between specific age periods (e.g. between middle and old or between old vs. very old individuals)? Although there is almost no literature on functional activation in very old adults (i.e., 80+ years), we suspect that the age trajectory of altered demand modulation may be nonlinear with particularly steep drops in the ability to modulate activation to task demand in this very old population.
Methods
Participants
Participants were 316 individuals aged 20–89 (mean 54.07 ± 20.21 years; uniform age distribution with 44–48 subjects per decade; 201 women, 155 men) from the Dallas Lifespan Brain Study (DLBS). These participants were recruited through media advertisements and flyers and underwent health history screening via a health questionnaire as well as telephone and personal interviews. All participants were screened against cardiovascular, neurological and psychiatric disorders, head injury with loss of consciousness > 10 min, and drug/alcohol abuse. Participants were native English speakers and strongly right-handed (on the Edinburgh Handedness Questionnaire (Oldfield, 1971)). The participants were well-educated (mean 16.55 ± 2.68 years) and scored highly (28.35 ± 1.27) on the Mini Mental State Examination (MMSE; Folstein et al., 1975). MMSE score (r = −.38) and years of formal education (r = −.14) decreased with increasing age. See Table 1 for a categorical age breakdown of sample demographics. All participants provided written informed consent and were debriefed in accord with university human investigations committee guidelines.
Table 1.
Demographic information for the sample by decade
| Age Group |
Decade | Mean Age |
N | % Women |
yrs Education |
MMSE |
|---|---|---|---|---|---|---|
| Young | 20–29 | 24.2 | 48 | 65% | 16.2 | 29.0 |
| 30–39 | 34.0 | 44 | 60% | 17.8 | 28.6 | |
| Middle | 40–49 | 45.2 | 44 | 66% | 16.1 | 28.6 |
| 50–59 | 54.5 | 46 | 65% | 17.5 | 28.8 | |
| Old | 60–69 | 64.8 | 43 | 58% | 17.0 | 28.1 |
| 70–79 | 73.4 | 46 | 65% | 15.9 | 27.9 | |
| Very Old | 80–89 | 83.9 | 45 | 67% | 15.5 | 27.4 |
| Total | 54.1 | 316 | 64% | 16.6 | 28.3 | |
MRI protocol
MRI Acquisition
All participants were scanned on a single 3T Philips Achieva scanner equipped with an 8-channel head coil. High-resolution anatomical images were collected with a T1-weighted MP-RAGE sequence with 160 sagittal slices, 1×1×1mm3 voxel; 204×256×160 matrix, TR=8.1ms, TE=3.7ms, flip-angle=12°. Blood Oxygen Level Dependent (BOLD) fMRI data were acquired using a T2*-weighted echo-planar imaging sequence (with SENSE encoding) with 43 interleaved axial slices per volume providing full brain coverage and acquired parallel to the AC-PC line, 64×64×43 matrix (3.4×3.4×3.5mm3), FOV=220mm2, TE=25ms, TR=2sec, FA=80°. Five dummy volumes were discarded at scan time to allow for T1 stabilization.
fMRI Task
In the blocked-design fMRI experiment, participants viewed blocks of “easy” (unambiguous) and “hard” (ambiguous) words with regards to their living/nonliving categorization. Upon seeing each word the participant made a living or nonliving judgment with a button press via their right index (“yes”, was living) or middle finger (“no”, was not living). The hard words were intended to impose greater cognitive and neural processing demand. Pretesting indicated the hard words had a longer response time and were judged to be greater in difficulty than the easy words. A total of 128 words were presented, with eight words of one condition within each block for a total of 16 blocks (eight easy and eight hard blocks). Additionally, there were three 24-second fixation blocks, which provided baseline data for the scan session. Blocks were presented in pseudo-random order. Each word was displayed for 2500 ms followed by a 500 ms fixation (crosshair). Total scan time was 7.7 min, including a 6s fixation interval before the first stimulus block. Words were randomly distributed within the easy and hard condition blocks, and responses were recorded using a fiber-optic button box held in the right hand. Accuracy was assessed for the easy (“canonical”) condition. We note that the difficult or ambiguous living/nonliving judgments often had no “correct” answer, so we did not assess accuracy for this condition. Visual stimuli were presented using E-prime (Psychology Software Tools, Pittsburgh, PA, USA) software projected through the back of the scanner and viewed though a mirror attached to the head coil.
fMRI Data Processing
SPM8 (Wellcome Department of Cognitive Neurology, London, UK) was used for data preprocessing and statistical analyses were performed using in-house scripts and SPM. For preprocessing, functional images were corrected for slice acquisition time followed by motion correction. Using the T1-weighted anatomical image for each subject as a coregistered intermediary, functional images were normalized to standard MNI template space and resampled into 3 mm3 voxels. Normalized images were smoothed with an isotropic 8mm FWHM Gaussian kernel. At the individual subject level, neural activity for each task condition was modeled as a block convolved with a canonical hemodynamic response function. In addition, six estimates of motion were included as covariates of no interest. An AR(1) model was used to handle time-series autocorrelations. Two planned group level contrasts were developed for subsequent analysis: easy words vs. fixation (to examine the age trajectory of activation to the canonical semantic judgment) and hard words vs. easy words (to examine the age trajectory of activation under increased task demand).
fMRI Data Analysis. At the group level, data analysis proceeded in four steps:
In the first analysis, we examined patterns of activation associated with the two major contrasts of interest collapsed across age: (a) the Easy vs. Fixation contrast allowed us to document that we observed the well-established patterns of activation evoked in the canonical semantic judgment task and (b) the Hard vs. Easy contrast allowed us to observe that the demand manipulation activated appropriate cognitive control regions. Because these were coarse-grained analyses that collapsed across age and utilized the entire sample size (N = 316), we used a stringent FWE corrected p < .01, k > 30 threshold. These analyses provide overview brain maps of the broad network of regions activated and deactivated by the canonical semantic judgment task and the demand manipulation. The maps are reported as Supplemental Data Figures S1 and S2.
The next analyses addressed how these two primary contrasts were modified by age. Thus, we ran two second-level multiple regression analyses. For the Easy vs. fixation contrast, age was treated as a continuous variable, allowing us to assess the main effect of age on the canonical semantic judgment task. For the Easy vs. Hard contrast, age was again treated as a continuous variable and Demand (Easy vs. Hard) was a two-level categorical variable, allowing us to assess an Age x Demand interaction to examine whether there were age differences in modulation of activation in response to increasing task processing demand. These two analyses result in statistical parametric maps of linear age-related increases and decreases in activation across the lifespan. These omnibus maps were thresholded at p < .05, FWE corrected, k > 30 based on bootstrapping simulation estimates (via 3DClustSim in AFNI).
The third step of the analysis plan explored planned follow-up post-hoc comparisons for decomposing the omnibus Age x Demand interaction found in step two. To accomplish this, we reconfigured age from a continuous variable to a categorical factor with four age levels: young (ages 20–39), middle-aged (40–59), old (60–79), very old (80–89). Conducting three age group comparisons (young vs. middle; middle vs. old; old vs. very old) allowed us to decompose when in the lifespan age differences become apparent and in which brain regions. As these were planned “simple main effects” and because examining categorical age group levels reduces power relative to the full sample of 316 these follow-up comparisons are thresholded at p < .001, k > 30.
Finally, we examined whether there was evidence for nonlinear (e.g., accelerating or decelerating) age effects on the two contrasts (Easy vs. fixation and Easy vs. Hard). We tested a quadratic age polynomial (age-squared) for significance while treating age as a continuous variable in the Easy vs. fixation contrast (thresholded at p < .001, k > 30 to maximize detection of this additional polynomial component). We conducted an a priori weighted orthogonal contrast [1 1 −1 −1] across the four categorical age groups in a factorial model on the Hard vs. Easy contrast to examine a hypothesized stepwise age reduction in modulation after middle age (i.e., between middle and old age and/or between old and very old ages, thresholded at p < .05, FWE corrected).
Results
Behavioral Results from the fMRI Task
To examine behavioral performance on the judgment task we conducted a mixed-model GLM on response times (RT) with age as a continuous predictor variable and Task Demand (Easy, Hard) as a repeated measures dependent vector. As expected, RTs for the living-nonliving judgment increased with age (main effect of age: F[1,314]=23, p < .001) and were greater for hard words (mean RT = 1276±154 ms) than for easy words (mean RT = 1021±146 ms; main effect of task: F[1, 314]=289, p < .001) confirming the task demand manipulation was effective. Older adults were also disproportionately slower for hard judgments than easy judgments compared to younger adults (age x task interaction: F[1,314]=4.72, p =.03). Because this interaction reached significance, we used RT as a covariate in subsequent imaging analyses. We also assessed living/nonliving judgment accuracy in the easy condition. Mean accuracy for the easy living/nonliving judgments was 86% (.05 SD) and did not differ with age (r = .01, p = .85). Because the hard judgments were designed to be ambiguous there were no “correct” responses and therefore they were not scored for accuracy.
Neuroimaging Results
Brain Activity Associated with the Canonical Semantic Judgment Task
The semantic judgment task network activated in the whole sample (Easy vs. fixation collapsed across age) is illustrated in Supplementary Figure S1A and showed the expected effects. These included widespread activation in regions typically associated with verbal semantic processing, including the left inferior frontal cortex involved in semantics, ventral temporal regions involved in lexical processing, and primary visual and motor cortex involved with stimulus viewing and response preparation, respectively. There was also robust activation in a subcortical network including caudate, putamen, thalamus, midbrain, and anterior cerebellum. Additionally, substantial deactivations occurred in regions generally associated with the “default mode network” (brain regions that are typically active in young adults during baseline but are actively suppressed under conditions of mental challenge (Gusnard & Raichle, 2001; Greicius et al., 2004) and include medial frontal/anterior cingulate and medial parietal/posterior cingulate regions along with regions in the lateral parietal and lateral temporal cortex.
Age Effects on the Canonical Semantic Judgment Task: Which Regions Differ in Activation Across the Lifespan?
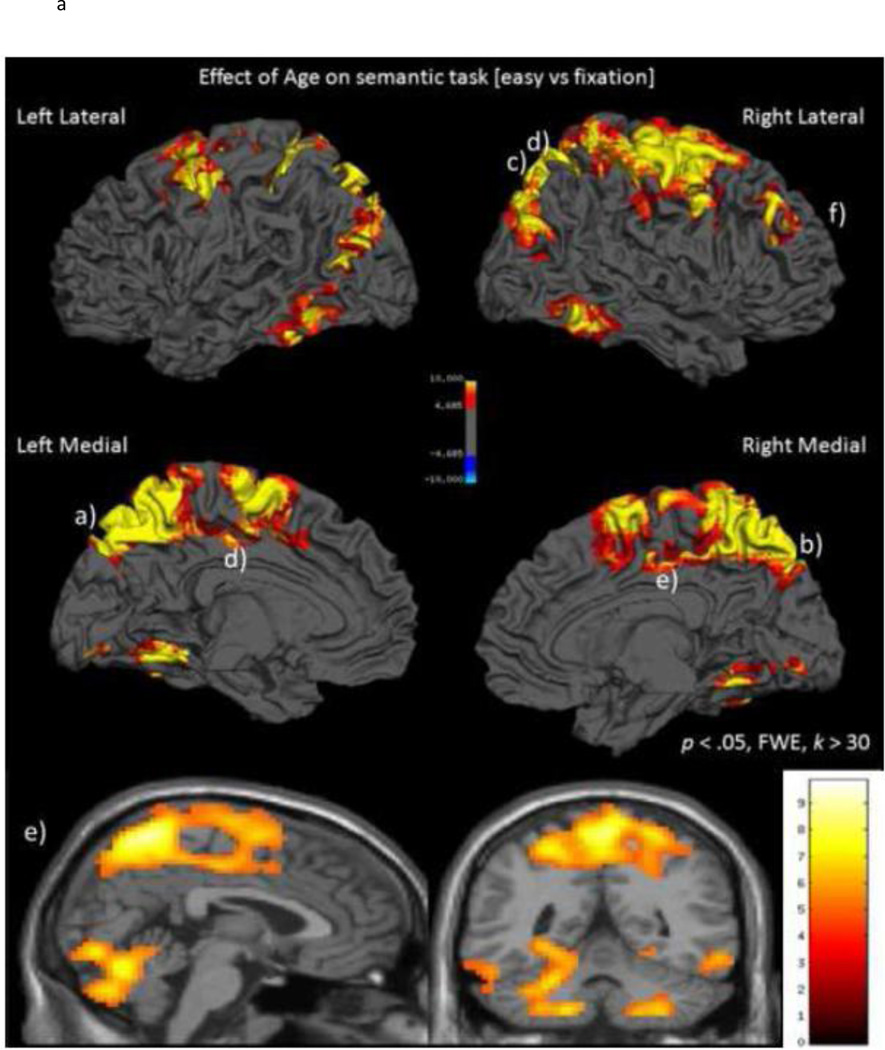
To examine the effects of age on activation during the canonical semantic judgment task (Easy vs. fixation) we computed a GLM with age as a continuous variable to determine in which brain regions activation differed as a function of increasing age. The main effect of age was significant and was due solely to increases in activation with age; there were no regions of the brain where activation decreased linearly with age. Figure 1A displays the statistical parametric map of the linear age-related increase in activation to semantic judgments of words1. Age-related increases in activation were evident in three clusters: a large cluster of 7661 voxels centered on the bilateral parietal cortices, a large 3003 voxel cluster centered in the bilateral cerebellum and extending into temporal cortices, and a 170 voxel cluster centered on the right middle frontal gyrus. To specify further the large parietal and cerebellar clusters, we sampled local maxima subpeaks greater than 4 mm apart throughout these two clusters. Peak coordinate information from the three significant clusters and representative local maxima subpeaks is displayed in Table 2.
Figure 1.
A) Activation to canonical semantic judgments (Easy vs. fixation) increases with age. B). Scatterplots of BOLD parameter estimates as a function of age. Increased activation with age stems both from activation in younger adults increasing to older adulthood as well as a decrease of task-induced deactivation with age. Lowercase letter annotation on the brain map and on the scatterplots indicates their correspondence.
Table 2.
Coordinate table for effect of age on canonical semantic task (Easy vs. fixation) and representative subpeaks
| Region | k | peak (mm) x y z |
t | r.age |
|---|---|---|---|---|
| L/R parietal cluster: | 7661 | |||
| R precuneus (BA 7) | 9 −60 63 | 9.84 | .49 | |
| R precuneus | 12 −69 60 | 9.64 | .48 | |
| R precentral | 36 −18 60 | 9.42 | .47 | |
| Additional representative subpeaks in cluster*: | ||||
| L precuneus | −6 −57 63 | 8.96 | .46 | |
| L superior frontal (BA 6) | −21 −3 63 | 8.85 | .45 | |
| R middle frontal | 39 0 54 | 8.67 | .45 | |
| R superior parietal (BA 7) | 24 −57 60 | 8.65 | .44 | |
| R superior frontal (BA 6) | 33 −6 63 | 8.48 | .44 | |
| R middle occipital (BA 19) | 33 −81 36 | 8.05 | .42 | |
| L superior parietal | −18 −54 51 | 8.03 | .42 | |
| L precentral | −30 −15 54 | 8.01 | .41 | |
| R postcentral (BA 2) | 39 −30 63 | 8.00 | .41 | |
| R supplemental motor area (BA 6) | 9 0 63 | 7.92 | .41 | |
| L postcentral | −45 −9 48 | 7.85 | .41 | |
| L supplemental motor area | −6 −6 63 | 7.84 | .40 | |
| L middle occipital | −27 −81 30 | 7.12 | .37 | |
| R middle cingulum (BA 24) | 3 −18 45 | 6.50 | .34 | |
| R inferior frontal (opercularis) | 42 6 27 | 6.21 | .34 | |
| L middle cingulum (BA 31) | −12 −24 39 | 5.78 | .31 | |
| R inferior parietal | 27 −45 45 | 4.98 | .31 | |
| R supramarginal gyrus | 57 −24 36 | 5.66 | .31 | |
| L/R cerebellar cluster: | 3003 | |||
| L Cerebellum (Lobule VI) | −15 −72 −24 | 8.09 | .41 | |
| R Cerebellum (Lobule VI) | 9 −69 −21 | 7.90 | .41 | |
| L Cerebellum (Lobule VI) | −18 −57 −15 | 7.72 | .40 | |
| Additional representative subpeaks in cluster*: | ||||
| L Cerebellum (Lobule IX) | −15 −54 −18 | 7.71 | .40 | |
| L Cerebellum (Lobule VII b) | −15 −81 −51 | 7.46 | .40 | |
| L Cerebellum (Lobule IV/V) | −9 −60 −12 | 7.20 | .38 | |
| L Cerebellum (Crus 2) | −3 −78 −36 | 7.18 | .38 | |
| R Occipital (BA 18) | 3 −81 −12 | 7.12 | .37 | |
| L Cerebellum (Lobule VIII) | −24 −42 −45 | 7.11 | .37 | |
| R inferior temporal (BA 37) | 57 −57 −12 | 7.08 | .37 | |
| R Cerebellum (Lobule IX) | 15 −54 −51 | 6.98 | .36 | |
| L lingual gyrus | −27 −51 −6 | 6.77 | .36 | |
| R fusiform gyrus (BA 19) | 24 −54 −12 | 6.63 | .35 | |
| R Cerebellum (Lobule VIII) | 30 −45 −48 | 6.61 | .35 | |
| R Cerebellum (Crus 1) | 48 −66 −24 | 6.60 | .35 | |
| L Cerebellum (Crus 1) | −48 −78 −27 | 6.58 | .34 | |
| L inferior temporal | −60 −57 −9 | 6.30 | .34 | |
| R middle frontal | 170 | 30 39 42 | 7.60 | .39 |
| R middle frontal (BA 10) | 30 45 30 | 6.51 | .35 |
Note. p < .05 FWE corrected, k threshold for cluster voxels > 30; r – Pearson correlation between age and BOLD parameter estimate;
subpeaks from 7661 voxel parietal cluster and 3003 voxel cerebellar cluster are local maxima greater than 4 mm apart
Table 2 reveals the age-related increases in activation to the canonical semantic judgment task occurred in several regions in the parietal cluster including bilateral precuneus/BA 7, precentral and postcentral gyri, superior parietal regions/BA 7, and right inferior parietal/supramarginal gyrus and into left middle occipital, middle cingulum (BA 24 and 31), as well as bilateral frontal regions including supplemental motor areas/BA 6 and superior frontal gyrus/BA 6, and in the right inferior frontal (opercularis). Interestingly, in prefrontal cortex, increased recruitment with age was exclusively evident in the right middle frontal gyrus (BA 9 and 10). See Figure 1A and for a categorical visualization across age groups see Supplementary Figure S1B–D. We also observed extensive age-related increases in activation in the bilateral cerebellar hemispheres and into bilateral lingual, inferior temporal and fusiform gyri. With the possible exception of middle cingulate and fusiform gyri, the effects of age on the core semantic network (Binder et al., 2009) are relatively modest. Instead, the age effects occur outside the core semantic regions in sensory, motor, response preparation and default mode network areas. This suggests that it was the processing/response components of the task that were affected by age rather than the knowledge-based, semantic component.
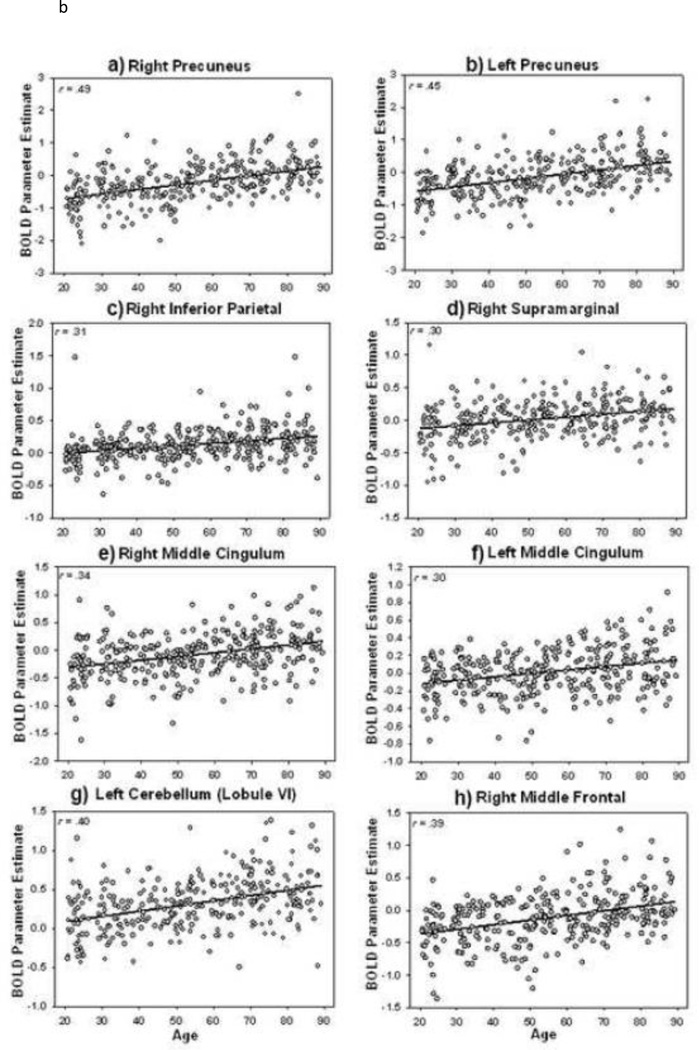
Based on this analysis, we examined these significant regions of age-related increase by extracting mean BOLD parameter estimates (beta weights) from 4 mm spheres around the peak voxels in three clusters from the large parietal cluster described in Table 2 including bilateral precuneus, bilateral inferior/supramarginal parietal and bilateral middle cingulate. Additionally, we selected the maxima from the cerebellar cluster (Lobule VI) and the right middle frontal gyrus cluster maxima. We then plotted BOLD parameter estimates as a function of age in Figure 1B, and Pearson correlations for the relation of age and BOLD estimate for all clusters/subclusters are provided in Table 2. Figures 1A and 1B illustrate that age-related increased activation appears generally greater in the right hemisphere than the left for most regions and for many of these regions an Age x Hemisphere interaction was significant (e.g., superior frontal Age x hemisphere F(1,314) = 7.69, P < .0001; precentral Age x Hemisphere F(1,314) = 23.86, p < .0001; trend for postcentral Age x Hemisphere F(1,314) = 3.51, p = .06; precuneus Age x Hemisphere F(1,314) = 11.05, p < .0001; cerebellum VI F(1,3140 = 5.91, p < .02; superior parietal Age x Hemisphere F(1,314) = 16.12, p < .001; supramarginal Age X Hemisphere F(1,314) = 10.19, p < .002; middle frontal Age x hemisphere F(1,314) = 13.58, p < .001). Given that the semantic task relies more heavily on the language-dominant left hemisphere, these age-related increases in the contralateral right hemisphere regions are in accord with HAROLD predictions (Cabeza 2002). Notably, it can also be seen from Figure 1B that some regional age-related increases in activation are driven by a shift from deactivation in young adults to less deactivation and even activation in older adults, especially in the precuneus and cingulate regions. Thus, we find that activation in both “default” and “task” regions increase with age; in other words there is a general increase in activity, stemming from either increased activation or reduced deactivation with age, and no regions of decreased activation with age.
Last, we investigated nonlinear age trajectories in increased activation by adding a quadratic polynomial term to the analysis of Easy vs. fixation with age-squared as a continuous variable. We found no evidence of nonlinear lifespan effects on activation in this contrast (even thresholded at p < .001, k > 30).
Age x Demand Condition (Hard vs. Easy) Interaction: Which Brain Regions Show Age-Related Differences in Modulation to Task Demand?
First, the broad network of regions comprising the main effect of demand condition (Hard vs. Easy collapsed across age) can be seen in Supplementary Figure S2 showing regions where brain activity was up- or down-regulated as a function of item processing demands. As expected, frontal and parietal association regions and the anterior cingulate, all regions of the cognitive control network, increased in response to increased processing demand. In contrast, the precuneus, lateral parietal and lateral temporal cortices showed deactivation in response to increased task demand.
Next, we focused on the primary question of interest: how age affected modulation of functional response to increased task demands. Modulation of activation to demand was indexed by the difference in activation from the Easy to the Hard condition; that is, by measuring increasing (or decreasing) activity from the canonical semantic judgment to the ambiguous judgment. We treated Age as a continuous variable and tested the Age x Demand (Hard vs. Easy) interaction. This interaction was significant and Figure 2A and Table 3 illustrate regions where modulation of activation to increased task demands differed with age. We found that the ability to modulate neural response to task-demand decreased with age (note in Figure 2a the cool scale for all regions of activity), particularly in the frontal and parietal cortex but also in the caudate nucleus and the cerebellum. Put another way, this age-related decrease reflects a diminishing response of the brain to increasing task demands as people age. To provide more detail about this effect, we plotted BOLD parameter estimates from 4 mm spheres around the peak voxel in representative significant clusters shown in Figure 2A and Table 3. Figure 2B illustrates these plots and shows consistent linear age-related decreases in modulation of activation to task demands in medial superior frontal, inferior frontal, superior parietal and angular gyrus, caudate nucleus and cerebellum.
Figure 2.
A). Regions with age-related decrease in modulation of activation to task demand: Age x Demand condition interaction. Modulation of activation to greater task demand decreases with age (i.e., the difference in neural response between Hard and Easy becomes smaller with age). B). Scatterplots depicting the decreased modulation to processing demand across the lifespan, predominately in frontal and parietal regions. Lowercase letter annotation on the brain map and on the scatterplots indicates their correspondence.
Table 3.
Age x Demand interaction (increased age and decreased modulation to task demand)
| Cluster | k | x y z peak (mm) | t |
|---|---|---|---|
| L/R medial superior frontal/anterior cingulate | 930 | 0 33 39 | 8.23 |
| R inferior frontal (orbital)/insula | 102 | 33 27 −6 | 6.35 |
| L inferior frontal (triangularis)/middle frontal/precentral | 310 | −48 27 18 | 6.20 |
| L inferior frontal (orbital) | 41 | −48 18 −6 | 5.91 |
| R inferior frontal (opercularis)/middle frontal | 259 | 54 21 36 | 7.17 |
| L inferior/superior/angular parietal | 162 | −33 −66 48 | 6.67 |
| R angular gyrus/inferior/superior parietal | 121 | 39 −57 45 | 5.82 |
| L/R caudate/L thalamus | 497 | 12 15 9 | 8.52 |
| R/L cerebellum (Crus2/Crus1) | 104 | 6 −84 −30 | 6.10 |
Note. p < .05 FWE corrected, k threshold for cluster voxels > 30
Decomposing the Age x Demand Interaction: When in the Lifespan are Neural Alterations to Increased Processing Demands Evident and Where in the Brain do they Occur?
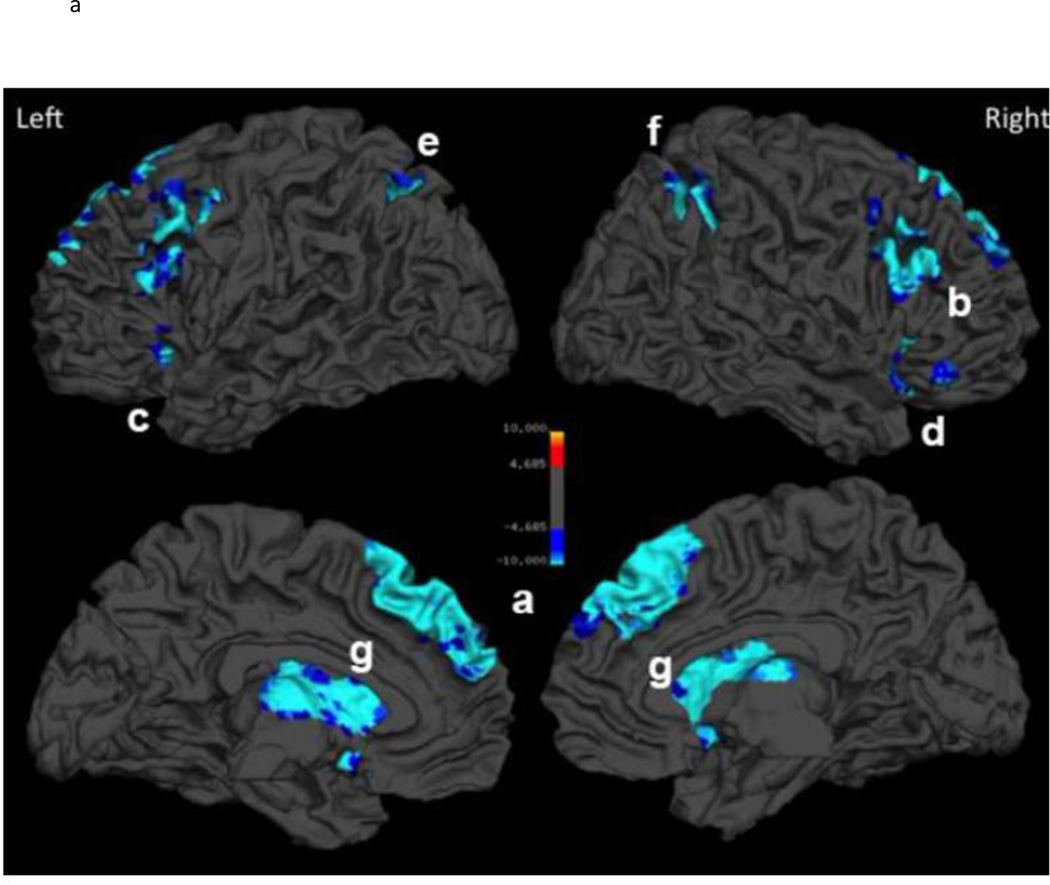
To decompose the significant Age x Demand interaction, we divided the sample into four age subgroups and examined how these age groups differed in their neural response to increased task demands. We conducted three separate voxel-wise contrasts of the age subgroups (“simple main effects”) to determine in which brain regions: (a) young adults differed from middle-age (20–39 year olds vs. 40–59 year olds), (b) middle-aged differed from old (60–79), and (c) old differed from very old (80–89). The results of these planned follow-up group comparisons are illustrated in Figure 3 and the coordinate information is provided in Table 4. As shown in Figure 3, we found minimal age differences between young and middle-aged adults in their ability to modulate activation to task demands, finding only an area in the superior frontal gyrus along the midline (see Figure 3a). However, the comparison of middle-to old-age revealed a substantial decrease in modulation of neural response to task demand that was largely localized to the frontal lobe. Effects were located in medial superior frontal cortex into the anterior cingulate gyrus, as well as bilateral middle/inferior frontal, and right middle/inferior temporal gyri and the caudate and putamen, illustrated in Figure 3b. We also noted an increase in modulation in occipital areas in this age comparison. Finally, comparison of old-age (60–79) to the very old-age (80+ year olds) subgroup revealed additional regions of decreased demand-related modulation that were largely left-lateralized and that included the left orbitofrontal cortex, the left supplemental, precentral, and postcentral gyri, and in the left subcortical nuclei that are broadly associated with the nigrostriatal dopaminergic system: caudate, putamen and dorsomedial nucleus of the thalamus, and in the midbrain (substantia nigra and ventral tegmentum) illustrated in Figure 3c.
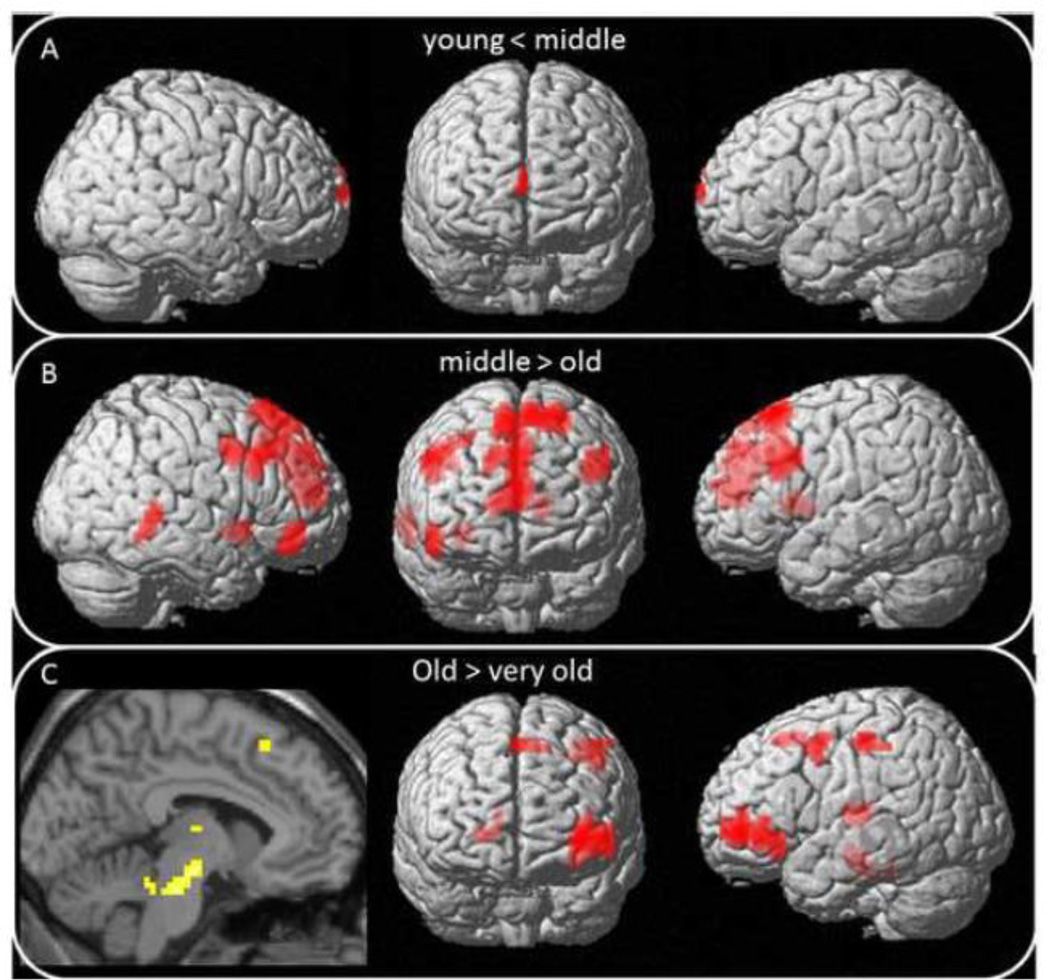
Figure 3.
Breakdown of Age x Demand interaction: Pairwise follow-up age-group comparisons showing when in the lifespan decreased modulation of activation to task demand occurs. Panel A) comparing modulation in young adults to middle-aged adults, B) comparing modulation in middle-aged to older adults, C) comparing modulation in older adults to the oldest adults.
Table 4.
Breakdown of Age x Demand interaction: Age group contrasts for Hard vs. Easy
| Cluster | k | x y z peak (mm) |
t |
|---|---|---|---|
| Young modulation > middle-aged | |||
| None | |||
| Middle-aged modulation > young | |||
| L/R/midline superior frontal | 36 | 0 72 27 | 4.57 |
| Middle-aged modulation > older | |||
| L/R/ medial superior frontal/anterior cingulate | 727 | −15 24 60 | 5.55 |
| R middle frontal/inferior frontal | 158 | 45 6 39 | 4.43 |
| L inferior frontal | 108 | −42 24 30 | 4.17 |
| R orbital frontal/inferior | 49 | 51 36 −18 | 3.98 |
| R middle/inferior temporal | 37 | 63 −39 0 | 3.63 |
| L caudate | 41 | −12 15 9 | 4.76 |
| R putamen/insula | 30 | 33 9 −6 | 3.87 |
| Older modulation > middle-aged | |||
| L cuneus/calcarine | 47 | 0 −93 24 | 4.09 |
| Older modulation > very old | |||
| L orbital frontal/middle/inferior | 124 | −36 51 −3 | 4.75 |
| L orbital frontal/inferior | 43 | −36 30 −6 | 3.79 |
| L supplemental motor | 32 | 0 15 54 | 3.66 |
| L precentral | 32 | −42 6 45 | 3.55 |
| L postcentral | 45 | −48 −24 51 | 3.74 |
| R caudate | 30 | 21 27 3 | 3.94 |
| L thalamus/putamen | 63 | −18 −18 9 | 3.73 |
| L midbrain/cerebellum | 75 | −6 −39 −21 | 4.11 |
| Very old modulate > older | |||
| None | |||
Note. p < .001, minimum k threshold for cluster voxels = 30; L = left; R = right; young = 20–39 years old; middle-aged = 40–59 years old; older = 60–79 years old; very old = 80–89 years old
Nonlinear Age Trajectories of Decreased Demand-Dependent Activity Modulation: Which Brain Regions Evidence Nonlinear Aging?
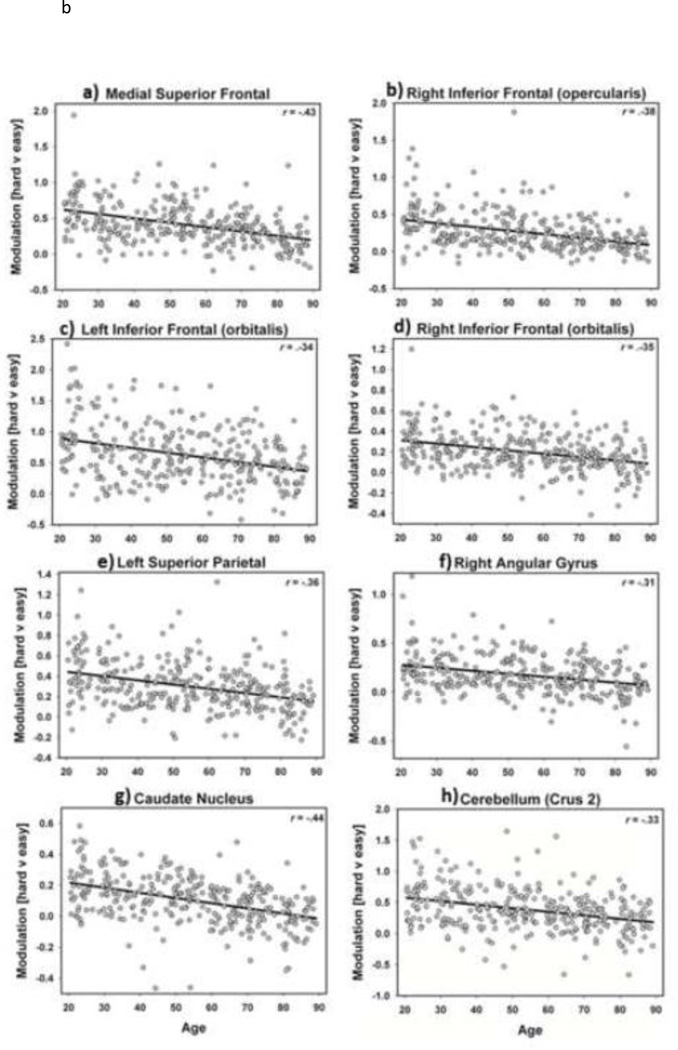
To address whether there was evidence for nonlinear age trajectories in the decreased task demand modulation we first added a quadratic polynomial term to the analyses of Hard vs. Easy with age-squared as a continuous variable. This analysis revealed an accelerating decline in demand modulation as age increased in an 85 voxel cluster in bilateral anterior cingulate gyrus (x=6, y=48, z=9) extending into bilateral medial superior frontal (x=-12, y=51, z=9), indicating that the reduced capacity to modulate activity to increased demands accelerates later in the lifespan in a key region of the cognitive control network.
Decreased Demand-Dependent Activity Modulation: Which Brain Regions Evidence Stepwise Shifts during Aging?
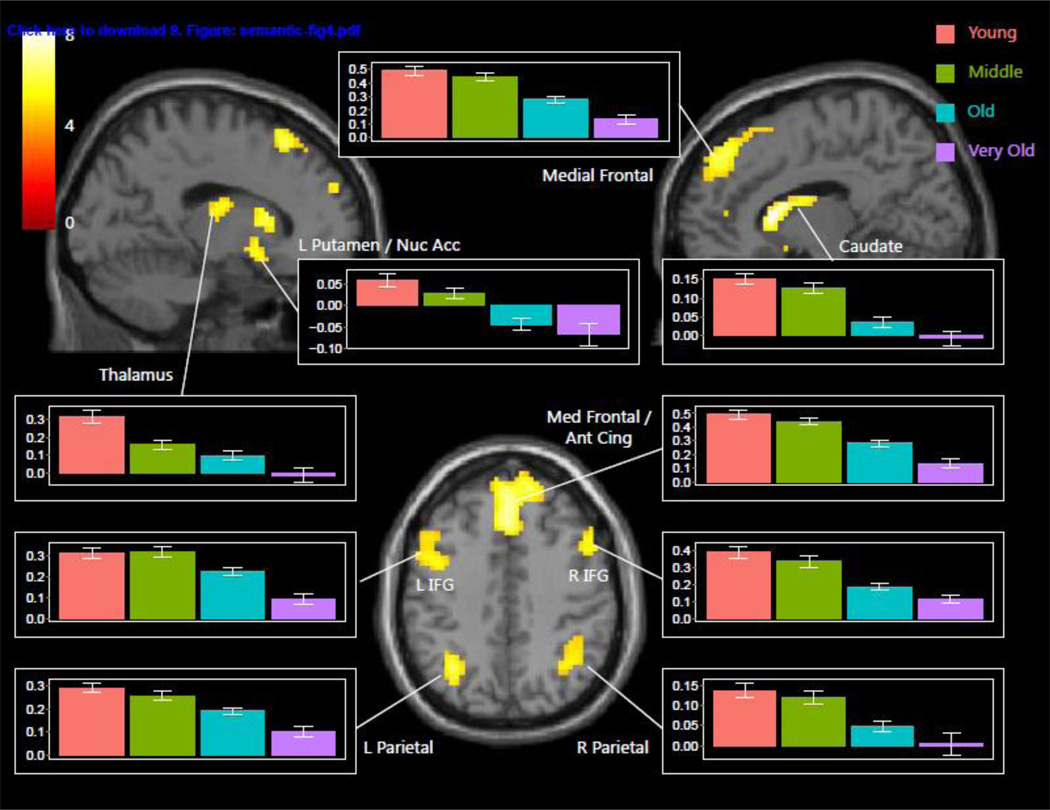
We also modeled an orthogonal stepwise function contrast in a factorial ANOVA of age on Hard vs. Easy using the four categorical age groups as levels of the age variable to further address whether there was evidence for stepwise age shifts in activation modulation drops (i.e., after middle-age) in the decreased task demand modulation. Interestingly, we found several significant clusters in the brain that were characterized by a stepwise shift (i.e., uneven magnitude of decline among age groups) in age-related decreases in activation modulation, illustrated in Figure 4. These regions of significant stepwise differences were observed in the caudate, putamen, nucleus accumbens, substantia nigra, thalamus (dorsomedial and ventrolateral nuclei), anterior cingulate, medial frontal, and lateral parietal and inferior frontal cortex, bilaterally. Figure 4 illustrates the stepwise decline across several regions stems from both a precipitous decline from old to very old age, as well as steep drops after middle-age. A permutation analysis confirmed that middle vs. old differed more than young vs. middle or old vs. very old for the majority of ROIs2. Interestingly, many of these regions displaying the age-related shift in modulation of activity to task demands are subcortical regions that are lower-order areas of the dopaminergic pathways that serve the cognitive control system, suggesting that at certain periods of aging, particularly very old age, these areas of the system show substantial losses in the ability to modulate to task demands. Finally, there is relative stability from young to old age in modulation to task demand in the left inferior frontal gyrus and substantia nigra, but then there is a sharp decline from old to very old age groups.
Figure 4.
Regions of age-related stepwise shifts in task demand-dependent activity modulation. Graph bars represent BOLD parameter estimate (± SEM) for Hard vs. Easy by age group (young in orange, middle aged in green, old in blue, very old in purple). Regions evidence sizable drops in modulation to task demand both after middle age and after old age.
Discussion
In this study we investigated age-related alterations in brain activation across the adult lifespan during a canonical semantic judgment task, and a more ambiguous judgment task that increased task processing demands. This task design allowed us not only to assess lifespan differences in activation to semantic processing under conditions of low demand, but also to understand age effects on how brain activation is modulated by tasks demand. Importantly, unlike prior studies that compared extreme age groups of younger and older adults, we tested a lifespan sample of adults from age 20 to 89, allowing us to identify when lifespan age differences arise and where in the brain such age-related alterations occur. The main findings are summarized and discussed below.
The Canonical Semantic Judgment Task is Characterized Solely by Age-related Increases in Activation
Across the adult lifespan, activation to simple living/non-living judgments increased with age. No decreases in activation were observed. Regions of increased activation with age were widespread including large regions of parietal cortices, along with more selective areas in the prefrontal cortex (BA 9 and 10). Age effects spanned both sensory-motor areas (supplemental motor, precentral and postcentral gyri) and association cortices (precuneus, supramarginal gyrus, inferior parietal), as well as bilateral middle cingulate, inferior temporal gyri and cerebellum.
There were three noteworthy aspects of the activation patterns. First, age-related increases in activation were more widespread and stronger in the right hemisphere, the side contralateral to that primarily used in this verbal task. This pattern is consistent with previous findings from extreme age group designs showing greater right hemispheric activity in older adults on tasks for which younger adults are left-lateralized (e.g., Cabeza, 2002; Grady, 2000; Grossman et al., 2002; Reuter-Lorenz et al., 2000). The activity increased continuously from young to very old age with no evidence of nonlinearity, suggesting that middle age samples would show intermediate levels of “over-activation”, between levels observed in younger and older groups in prior extreme group studies, adding now to this literature that “over-activation” is rather a linear phenomenon. Second, and perhaps most importantly, the increases in activation occurred outside of the left inferior frontal gyrus, the fundamental region typically activated in semantic judgments tasks. Quite strikingly, activation within the core regions of the canonical semantic network is largely age invariant. This result corresponds with evidence that semantic knowledge is generally preserved with age (Ackerman & Rolfhus, 1999; Puglisi et al., 1988). Third, the results showed that increased activation with advancing age is predominantly evident in multimodal association regions of the brain (cingulate, inferior temporal, cerebellum, and supramarginal, superior and precuneus portions of parietal lobe). We also find that prefrontal recruitment with age is selective to the right middle frontal gyrus (BA 9 and 10). Thus, we also here extend the contralateral (over)recruitment effect seen in age group studies to linear lifespan aging studies. This pattern of findings suggests that the increased contralateral recruitment is in highly malleable heteromodal association cortices, which is suggestive of the compensatory role of increased contralateral (usually prefrontal) cortex proposed by several aging theories (e.g., Cabeza, 2002; Park & Reuter-Lorenz, 2009; Reuter-Lorenz & Cappell, 2008) and in accord with studies that find that increased neural recruitment, that may be compensatory in nature, occurs in regions outside the frontal lobes (e.g., Berlingeri et al., 2012; Bergerbest et al., 2009; Huang et al., 2012).
Another interesting general finding is that overall pattern of age-related increases in activation included both the additional activation of regions already activated above baseline in younger ages, and increasing activation of regions that were deactivated in younger ages, for example in the precuneus (Figure 2B). In particular, aging may attenuate the ability to suppress or deactivate activity in some regions compared to younger adults, as shown in prior extreme groups studies of semantic processing (Persson et al., 2007) and episodic memory (Grady et al., 2006; H. Park et al., 2012), where older adults had difficulty suppressing activity in the “default mode network”—areas of the brain where young adults show more activation at rest. It has been suggested that activity in the default network regions are associated with off-task processing and that these regions are disengaged or suppressed for optimal performance on cognitive tasks (Gusnard & Raichle, 2001; Greicius et al., 2004; Lustig et al., 2003). Taken together it seems that, at least when examined cross-sectionally, aging is associated with a heightened level of brain activity, arising from increased activation, decreased deactivation or both, and the increases are linear across the adult lifespan. Consistent with the notion from behavioral studies that there is preservation of verbal knowledge across the lifespan, we found activation aging effects largely occur outside of canonical semantic processing regions.
Aging is Associated with Decreased Activity Modulation in Response to Increased Task Processing Demands
A prominent idea in theories of cognitive aging (e.g., Craik & Byrd, 1982) that has been relatively unexplored in the neuroimaging literature on aging (see Reuter-Lorenz & Cappell, 2008 and Reuter-Lorenz et al., 1999) is that older adults have an ever-shrinking pool of cognitive resources that increasingly limits their processing capacity (measured by speed, working memory, and other executive function tasks). Thus a second and particularly important focus of this study was to determine how aging affects the ability to modulate neural activity when a task becomes more demanding. The Age x Demand interaction we observed suggests that the capacity to modulate neural activity in response to increased task demands diminished with age. The diminished availability of neural resources for higher task demands was evident in the frontal and parietal cortex and in the anterior cingulate, as well as in subcortical structures including the caudate, thalamus and cerebellum. Critically, the network of brain regions that showed increased activity as a function of age (Figure 1A) evidenced minimal overlap with the regions that show decreased modulation to processing demand (Figure 2A), suggesting that two separate brain systems appear to be expressing distinct aging processes. Importantly, this also demonstrates that the same neural regions are not merely hitting a resource ceiling and then unable to activate to increased demand. The first set of regions, a semantic processing network manifested on the easy task, is characterized by additional recruitment of novel regions, and the second set of regions manifested in the demand contrast, is a cognitive control network that is characterized by decreasing modulatory ability as we age. Along these lines, we also found that after controlling for age, individual differences in activation to the easy canonical task were not negatively associated with the ability to modulate to the more demanding condition, providing further suggestion that individuals were not hitting a resource ceiling based on a higher starting level of activation at the easy task.
Our finding of age-related decrease in modulation to increased processing demand in the frontal, parietal and cingulate regions converges with previous evidence from extreme age group studies of young vs. older groups (Nagel et al., 2009; Persson et al., 2004; Stern et al., 2012) These brain regions are part of a control network engaged to meet increased task demands (Braver et al., 2009). The observed age-related decreases in these regions reflect a reduction of the aging brain’s ability to dynamically respond to increasing task demands and may represent the neural equivalent of “processing resources” described by Craik and colleagues (Craik & Byrd, 1982).
Interestingly, we note that in addition to the diminished ability to mobilize frontal-parietal regions with increased age and task demand, we also report diminished response to processing demand in several subcortical regions involved in the dopaminergic pathway, including the caudate, putamen, and thalamus. Quite a few studies have implicated the caudate nucleus in higher-order cognitive function, ostensibly via the frontal-striatal loops (Alexander, DeLong & Strick, 1986) and interestingly, the caudate shows dramatic reductions in both volume (Raz et al., 2003) and in dopamine receptor density with aging (Bäckman et al., 2000; Wong et al., 1984). Paralleling diminished structure and function in the caudate, we found age-related decline in modulation to task demands also in the thalamus—primarily the dorsomedial nucleus, which is the primary region of the thalamus involved in frontal cortex circuitry. Taken together, these results suggest that aging is associated with a reduction in function in the subcortical-prefrontal cortex circuitry necessary for higher-order cognition in response to increased task demands. We also found decreased modulation in the bilateral cerebellum, specifically in Crus 2 and into Crus 1.The cerebellum is increasingly recognized to be crucial for many higher-order cognitive functions (Bernard & Seidler, 2014; Cabeza & Nyberg, 2000; Desmond, 2001), putatively via cerebellar-association cortex loops projecting from lobules VI and VII. Indeed, the cerebellum evidences some of the most shrinkage of the entire brain with age (Raz et al., 2010). Our results from this semantic verbal task fit well with a recent cerebellar functional topography study showing language activations localized to Cerebellar Crus I and II (Stoodley et al., 2012), regions that project to frontal and parietal association cortices.
Life Stages and the Brain’s Functional Response to Increased Processing Demands
Note that we treated age as a categorical variable in decomposing the Age x Demand interaction, by dividing individuals into age groups and determining at what stages of the adult lifespan declines in modulation of activation occurred. Importantly, we found that the transition from middle- to old-age corresponded to the greatest differences, with fewer differences from young to middle and from old to very old age. Specifically, when we compared young (20 to 39) to middle age (40–59), the differences were few, with only one small region in the midline superior frontal cortex that showed a difference in modulation to demand. Surprisingly, the difference occurred because there was increased modulation in the middle-aged adults compared to the young adults, perhaps as a successful compensatory attempt that begins in this age period.
The greatest age differences in ability of the brain to respond to increased processing demand emerged in the comparison of middle-age (40–59) to old (60–79) adulthood, where modulation of activation to task demand decreased in anterior regions and a region in the right middle/inferior temporal gyrus. These anterior regions of decreased modulation include midline/bilateral superior frontal and anterior cingulate cortices (BA 32), left caudate nucleus, bilateral middle frontal into inferior frontal gyri, and right putamen into the insula. In this middle to old-age transition period we see modulation decreases emerge particularly in the frontal-striatal network, more so in the right compared to the left hemisphere.
In our final comparison of old (60–79) vs. very old (80–89) adults, we found further decreases in modulation to processing demands in this rarely-studied group of very old adults. These decreases were found predominantly in the left hemisphere in the oldest adults: middle/inferior/orbital frontal, precentral, postcentral and supplemental motor cortices, right caudate nucleus, left thalamus (predominately the dorsomedial nucleus), and left midbrain gray matter including substantia nigra and the ventral tegmentum. This circuitry (through substantia nigra, nucleus accumbens, caudate, putamen and thalamus on to prefrontal cortex) comprises the major portion of the dopaminergic pathway in the brain, underlies the executive function system, and undergoes significant changes with aging (Arnsten, 1993; Bäckman et al., 2006). Thus, in the oldest individuals, the decreased modulation effects to the dopaminergic/nigrostriatal system are manifested farther downstream when compared to earlier frontal and striatal effects observed in the younger and middle-aged groups (Figure 3). While the resolution of fMRI is not suited to fine-grained examination of this circuitry, the evidence for decline of different components of the dopaminergic system at different ages is intriguing in light of the importance of dopamine in cognition and in aging (Li, Lindenberger & Bäckman, 2010 for review) and warrants future exploration.
Interestingly, the progression of differences with increasing age appears to proceed from specific, demand-relevant regions in younger adults (midline prefrontal and anterior cingulate, middle and inferior frontal gyrus and posterior parietal cortices) based on the literature (e.g., Braver et al 2009, and based on our analyses in Supplemental Figure 2), to more widespread engagement of association cortices (superior frontal, middle/inferior frontal, anterior cingulate, middle/inferior temporal) in middle-age, and finally to less demand-specific but age-vulnerable regions in older adulthood (orbital frontal cortex, additional regions of parietal, and subcortical regions such as the thalamus, caudate, and putamen). This progression of activation differences suggests that as we age, reliance on particular brain networks shift over time, perhaps because the prior regions have been involved to maximum capacity, ostensibly as a compensatory mechanism to support performance (Reuter-Lorenz, 2002) or alternately because of dedifferentiation of brain regions with age (Goh, 2011). This is further supported by the present evidence of stepwise decreases in demand modulation with increasing age in the subcortical structures (substantia nigra, caudate, thalamus) and by a study that found an association between reduced prefrontal activation and reduced caudate dopamine receptor binding in older adults during a working memory task (Bäckman et al., 2011). Further supporting this pattern of functional activation decline, we found differences in the magnitude of demand-modulation decreases among the age groups. Some regions evidenced progressive decreases (multiple “drops”) in activation across examined age groups (e.g., from young to middle, middle to old, and old to very old), particularly subcortical regions (thalamus, putamen, caudate, nucleus accumbens). Other regions, primarily association cortices, showed stability from young to middle age with larger drops in activation observed from middle to old age and from old age to very old age: anterior cingulate, medial frontal, right inferior frontal, bilateral lateral parietal. Finally, the primary region involved in this semantic task, the left inferior frontal gyrus remained stable into old age and then showed a precipitous drop in activation in the very old individuals, suggesting a mechanism for the relative sparing of these verbal abilities, at least until very old age.
We note that these results are based on cross-sectional estimates of aging rather than within-person change over time. We also note that we chose one a priori stepwise function to examine, in a cross-sectional manner, whether there are age periods where age-related differences in activation are greater than others. Many other stepwise functions can also be fit to further investigate the shapes of trajectories these differences could assume. Aging studies that have utilized a longitudinal design found that an individual’s activation decreased over time, at least in a region of the right prefrontal cortex (Nyberg et al., 2010). However, around 8% of individuals in that sample did increase in frontal activation over time and showed several regions of increase outside of the frontal lobes (including regions in the parahippocampal gyrus, superior temporal, occipital, and motor cortices). Thus, further longitudinal research is certainly needed to explore these interesting within-person activation changes. Longitudinal follow-up is underway in the current sample.
In sum, we provide novel evidence of age-dependent alterations in brain activity observed over individuals across the adult lifespan from the 20s through the 80s. Specifically we show a continuously increasing level of activation with age during a canonical semantic judgment. Regions that were activated and those that were deactivated in young adults became more active with increasing age, suggesting a general pattern of increased activation with age, regardless of the starting point. For more demanding judgments (i.e., ambiguous living/non-living category membership) the increment in activation observed in the young adults (i.e. demand-dependent activity modulation) decreased with age. These demand-dependent modulation decreases emerge primarily in the transition from middle-aged to old adulthood and further decreases in neural modulation are evident into very old age (80–90 years old) particularly in regions of the dopaminergic midbrain-subcortical-prefrontal system. Thus, we demonstrate both incremental age-associated increases in activation (“over-recruitment”) and age-related decreases in neural modulation (“under-recruitment”) in different brain regions across the adult lifespan. We also demonstrated a pattern of regional differences in age-related demand-dependent modulation decline where middle-age decreases occurred in more task-specific brain regions and older age decreases progressed to more downstream regions such as midbrain and striatal portions of the dopaminergic pathway. Intriguingly, these functional age effects closely mirror structural effects: the so-called “last in-first out hypothesis” where, generally, the latest developing brain regions, both ontogenetically and phylogenetically, are the earliest to change with age (Raz, 2000; Raz & Kennedy, 2009). Our lifespan approach revealed this regional progression with age, which would have been unobservable in an extreme age group approach. Future studies will examine if these cross-sectional estimates of age-related alterations in neural activation are replicated in within-person changes in activation over time in our longitudinal follow-up currently underway.
Supplementary Material
Acknowledgments
This work was supported in part by National Institutes of Health National Institute on Aging (grants 4 R00 AG-036818-04 to KMK, 5R37AG-006265-27 to DCP, and 4 R00 AG-036848-04 to KMR). We thank several research assistants for help with data collection: Erin Wooden, Prasanna Tamil, Bela Bhatia, and Patrick Evans.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Note that controlling for task RT did not significantly alter the results.
Mean sampled differences for Middle-Old were higher than mean differences for Old-VeryOld and for Young-Middle in right angular gyrus, left inferior frontal orbitalis and triangularis, right inferior frontal orbitalis and opercularis, left superior medial frontal, left and right caudate, left putamen/accumbens, cerebellum, left precentral ROIs. Left thalamus ROI showed a larger step between Young-Middle. Left superior parietal, left middle frontal gyrus, and left inferior frontal triangularis (a second cluster) ROIs showed larger step differences between Old-VeryOld.
References
- Ackerman PL, Rolfhus EL. The locus of adult intelligence: Knowledge, abilities, and nonability traits. Psychology of Aging. 1999;14(2):314–330. doi: 10.1037//0882-7974.14.2.314. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Delong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neuroscience. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56(5):924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF. Catecholamine mechanisms in age-related cognitive decline. Neurobiol Aging. 1993;14(6):639–641. doi: 10.1016/0197-4580(93)90054-f. [DOI] [PubMed] [Google Scholar]
- Bäckman, et al. Age-related cognitive deficits mediated by changes in the striatal dopamine system. Am. J. Psychiat. 2000;157:635–637. doi: 10.1176/ajp.157.4.635. [DOI] [PubMed] [Google Scholar]
- Bäckman L, Karlsson S, Fischer H, Karlsson P, Brehmer Y, Rieckmann A, MacDonald SW, Farde L, Nyberg L. Dopamine D(1) receptors and age differences in brain activation during working memory. Neurobiol. Aging. 2011;32:1849–1856. doi: 10.1016/j.neurobiolaging.2009.10.018. [DOI] [PubMed] [Google Scholar]
- Bäckman L, Nyberg L, Lindenberger U, Li SC, Farde L. The correlative triad among aging, dopamine, and cognition: current status and future prospects. Neurosci Biobehav Rev. 2006;30(6):791–807. doi: 10.1016/j.neubiorev.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Berlingeri M, Danelli L, Bottini G, Sberna M, Paulesu E. Reassessing the HAROLD model: is the hemispheric asymmetry reduction in older adults a special case of compensatory-related utilisation of neural circuits? Exp Brain Res. 2013;224(3):393–410. doi: 10.1007/s00221-012-3319-x. [DOI] [PubMed] [Google Scholar]
- Bergerbest D, Gabrieli JDE, Whitfield-Gabrieli S, Kim H, Stebbins GT, Bennett DA, Fleischman DA. Age-associated reduction of asymmetry in prefrontal function and preservation of conceptual repetition priming. NeuroImage. 2009;45:237–246. doi: 10.1016/j.neuroimage.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JA, Seidler RD. Moving forward: Age effects on the cerebellum underlie cognitive and motor declines. Neurosci Biobehav Rev. 2014;42C:193–207. doi: 10.1016/j.neubiorev.2014.02.011. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex. 2009;19(12):2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Paxton JL, Locke HS, Barch DM. Flexible neural mechanisms of cognitive control within human prefrontal cortex. Proc Natl Acad Sci U S A. 2009;106(18):7351–7356. doi: 10.1073/pnas.0808187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS. The variable nature of cognitive control: a dual mechanisms framework. Trends Cogn Sci. 2012;16(2):106–113. doi: 10.1016/j.tics.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JW, Braver TS. Learned predictions of error likelihood in the anterior cingulate cortex. Science. 2005;307(5712):1118–1121. doi: 10.1126/science.1105783. [DOI] [PubMed] [Google Scholar]
- Bucur B, Madden DJ, Spaniol J, Provenzale JM, Cabeza R, White LE, Huettel SA. Age-related slowing of memory retrieval: contributions of perceptual speed and cerebral white matter integrity. Neurobiol Aging. 2008;29(7):1070–1079. doi: 10.1016/j.neurobiolaging.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12(1):1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging. 2002;17(1):85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Daselaar SM, Dolcos F, Prince SE, Budde M, Nyberg L. Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cereb Cortex. 2004;14(4):364–375. doi: 10.1093/cercor/bhg133. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Byrd M. Aging and cognitive deficits: The role of attentional resources. In: Craik FIM, Trehub SE, editors. Aging and cognitive processes. New York: Plenum; 1982. pp. 191–211. [Google Scholar]
- Cappell KA, Gmeindl L, Reuter-Lorenz PA. Age differences in prefontal recruitment during verbal working memory maintenance depend on memory load. Cortex. 2010;46:462–473. doi: 10.1016/j.cortex.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar SM, Veltman DJ, Rombouts SA, Raaijmakers JG, Jonker C. Aging affects both perceptual and lexical/semantic components of word stem priming: An event-related fMRI study. Neurobiol Learn Mem. 2005;83(3):251–262. doi: 10.1016/j.nlm.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Que PASA? The posterior-anterior shift in aging. Cereb Cortex. 2008;18:1201–1209. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmond J. Cerebellar involvement in cognitive function: evidence from neuroimaging. International Review of Psychiatry. 2001;13(4):283–294. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR, et al. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. Folstein et al 1975. [DOI] [PubMed] [Google Scholar]
- Glisky EL. Changes in cognitive function in human aging. In: Riddle DR, editor. Brain Aging: Models, Methods, and Mechanisms. Taylor & Francis; 2007. pp. 3–20. [PubMed] [Google Scholar]
- Goh JOS. Functional Dedifferentiation and Altered Connectivity in Older Adults: Neural Accounts of Cognitive Aging. Aging Dis. 2011;2:30–48. [PMC free article] [PubMed] [Google Scholar]
- Grady CL. Brain imaging and age-related changes in cognition. Exp Gerontol. 1998;33(7–8):661–673. doi: 10.1016/s0531-5565(98)00022-9. [DOI] [PubMed] [Google Scholar]
- Grady CL. Functional brain imaging and age-related changes in cognition. Biol Psychol. 2000;54(1–3):259–281. doi: 10.1016/s0301-0511(00)00059-4. [DOI] [PubMed] [Google Scholar]
- Grady CL, Springer MV, Hongwanishkul D, McIntosh AR, Winocur GJ. Age-related changes in brain activity across the adult lifespan. Cogn Neurosci. 2006 Feb;18(2):227–241. doi: 10.1162/089892906775783705. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode activity during a passive sensory task: Uncoupled from deactivation but impacting activation; Proceedings of the National Academy of Sciences of the United States of America; 2004. pp. 4637–4642. [Google Scholar]
- Groenewegen HJ, Berendse HW, Wolters J, Lohman AHM. The anatomical relationship of the prefrontal cortex with the striatopallidal system, the thalamus and the amygdala: evidence for a parallel organization, Ch 5. In: Uylings HBM, Van Eden CG, DeBruin JPC, Corner MA, Feenstra MGP, editors. Progress in Brain Research Vol 85: The Prefrontal Cortex: Its Structure, Function and Pathology. Elsevier; 1990. [DOI] [PubMed] [Google Scholar]
- Grossman M, Cooke A, DeVita C, Alsop D, Detre J, Chen W, Gee J. Age-Related Changes in Working Memory during Sentence Comprehension: An fMRI Study. NeuroImage. 2002;15:302–317. doi: 10.1006/nimg.2001.0971. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: Functional imaging and the resting human brain. Nature Review Neuroscience. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Huang CM, Polk TA, Goh JO, Park DC. Both left and right posterior parietal activations contribute to compensatory processes in normal aging. Neuropsychologia. 2012;50:55–66. doi: 10.1016/j.neuropsychologia.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi S, Nakajima K, Uchida I, Kameyama M, Nakahara K, Sekihara K, Miyashita Y. Transient activation of inferior prefrontal cortex during cognitive set shifting. Nat Neurosci. 1998;1(1):80–84. doi: 10.1038/283. [DOI] [PubMed] [Google Scholar]
- Li SC, Lindenberger U, Bäckman L. Dopaminergic modulation of cognition across the life span. Neurosci Biobehav Rev. 2010;34(5):625–630. doi: 10.1016/j.neubiorev.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Liu P, Hebrank AC, Rodrigue KM, Kennedy KM, Park DC, Lu H. A comparison of physiologic modulators of fMRI signals. Human Brain Mapping. 2013;34:2078–2088. doi: 10.1002/hbm.22053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig C, Buckner RL. Preserved neural correlates of priming in old age and dementia. Neuron. 2004;42(5):865–875. doi: 10.1016/j.neuron.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Lustig C, Snyder AZ, Bhakta M, O'Brien KC, McAvoy M, Raichle ME, Buckner RL. Functional deactivations: change with age and dementia of the Alzheimer type. Proc Natl Acad Sci U S A. 2003;100(24):14504–14509. doi: 10.1073/pnas.2235925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowd JM, Craik FI. Effects of aging and task difficulty on divided attention performance. J Exp Psychol Hum Percept Perform. 1988;14:267–280. doi: 10.1037/0096-1523.14.2.267. [DOI] [PubMed] [Google Scholar]
- Morcom AM, Li J, Rugg MD. Age effects on the neural correlates of episodic retrieval: increased cortical recruitment with matched performance. Cereb Cortex. 2007;17(11):2491–2506. doi: 10.1093/cercor/bhl155. [DOI] [PubMed] [Google Scholar]
- Nagel IE, Preuschhof C, Li SC, Nyberg L, Bäckman L, Lindenberger U, Heekeren HR. Performance level modulates adult age differences in brain activation during spatial working memory. Proc Natl Acad Sci U S A. 2009;106(52):22552–22557. doi: 10.1073/pnas.0908238106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg L, Salami A, Andersson M, Eriksson J, Kalpouzos G, Kauppi K, Nilsson LG. Longitudinal evidence for diminished frontal cortex function in aging. Proc Natl Acad Sci U S A. 2010;107(52):22682–22686. doi: 10.1073/pnas.1012651108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK. Models of visuospatial and verbal memory across the adult life span. Psychol. Aging. 2002;17:299–320. [PubMed] [Google Scholar]
- Park DC, Reuter-Lorenz P. The adaptive brain: aging and neurocognitive scaffolding. Annu Rev Psychol. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Kennedy KM, Rodrigue KM, Hebrank A, Park DC. An fMRI study of episodic encoding across the lifespan: Changes in subsequent memory effects are evident by middle-age. Neuropsychologia. 2013;51:448–456. doi: 10.1016/j.neuropsychologia.2012.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J, Sylvester CY, Nelson JK, Welsh KM, Jonides J, Reuter-Lorenz PA. Selection requirements during verb generation: differential recruitment in older and younger adults. Neuroimage. 2004;23(4):1382–1390. doi: 10.1016/j.neuroimage.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Puglisi JT, Park DC, Smith AD, Dudley WN. Age differences in encoding specificity. J Gerontol. 1988;43(6):P145–P150. doi: 10.1093/geronj/43.6.p145. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Kennedy KM, Head D, Gunning-Dixon F, Acker JD. Differential aging of the human striatum: longitudinal evidence. AJNR Am J Neuroradiol. 2003;24(9):1849–1856. [PMC free article] [PubMed] [Google Scholar]
- Raz N, Kennedy KM. A systems approach to age-related change: neuroanatomical changes, their modifiers, and cognitive correlates. In: Jagust W, D’Esposito M, editors. Imaging the Aging Brain. New York, NY: Oxford University Press; 2009. pp. 151–268. [Google Scholar]
- Raz N, Ghisletta P, Rodrigue KM, Kennedy KM, Lindenberger U. Trajectories of brain aging in middle-aged and older adults: regional and individual differences. Neuroimage. 2010;51:501–511. doi: 10.1016/j.neuroimage.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter-Lorenz PA. New visions of the aging mind and brain. TRENDS in Cognitive Science. 2002;6:394–400. doi: 10.1016/s1364-6613(02)01957-5. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Stanczak L, Miller AC. Neural Recruitment and Cognitive Aging: Two Hemispheres Are Better Than One, Especially as You Age. Psych Science. 1999;10:494–500. [Google Scholar]
- Reuter-Lorenz PA, Jonides J, Smith EE, Hartley A, Miller A, Marshuetz C, Koeppe RA. Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. J Cogn Neurosci. 2000;12(1):174–187. doi: 10.1162/089892900561814. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz P, Cappell KA. Neurocognitive aging and the compensation hypothesis. Curr Direct Psychol Sci. 2008;17(3):177–182. [Google Scholar]
- Rodrigue KM, Kennedy KM. The cognitive consequences of structural changes to the aging brain. In: Schaie KW, Willis SL, editors. Handbook of the Psychology of Aging. 7th ed. New York: Elsevier; 2011. pp. 73–92. Ch 5. [Google Scholar]
- Rypma B, D'Esposito M. Isolating the neural mechanisms of age-related changes in human working memory. Nat Neurosci. 2000;3(5):509–515. doi: 10.1038/74889. [DOI] [PubMed] [Google Scholar]
- Schneider-Garces NJ, Gordon BA, Brumback-Peltz CR, Shin E, Lee Y, Sutton BP, Maclin EL, Gratton G, Fabiani M. Span, CRUNCH, and beyond: working memory capacity and the aging brain. J Cogn Neuroscience. 2010;22:655–669. doi: 10.1162/jocn.2009.21230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Wojtowicz M, Grady CL. Reliable differences in brain activity between young and old adults: A quantitative meta-analysis across multiple cognitive domains. Neuroscience and Biobehavioral Reviews. 2010;34:1178–1194. doi: 10.1016/j.neubiorev.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Stebbins GT, Carrillo MC, Dorfman J, Dirksen C, Desmond JE, Turner DA, Gabrieli JD. Aging effects on memory encoding in the frontal lobes. Psychol Aging. 2002;17(1):44–55. doi: 10.1037//0882-7974.17.1.44. [DOI] [PubMed] [Google Scholar]
- Stern Y, Rakitin BC, Habeck C, Gazes Y, Steffener J, Kumar A, Reuben A. Task difficulty modulates young-old differences in network expression. Brain Research. 2012;1435:130–145. doi: 10.1016/j.brainres.2011.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ, Valera EM, Schmahmann JD. Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. NeuroImage. 2012;59:1560–1570. doi: 10.1016/j.neuroimage.2011.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill SL. Neuroimaging studies of semantic memory: inferring "how" from "where". Neuropsychologia. 2003;41(3):280–292. doi: 10.1016/s0028-3932(02)00161-6. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Gur RC, Wang GJ, Fowler JS, Moberg PJ, Ding YS, Hitzemann R, Smith G, Logan J. Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am. J. Psychiatry. 1998;155:344–349. doi: 10.1176/ajp.155.3.344. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Paré-Blagoev EJ, Clark J, Poldrack RA. Recovering meaning: left prefrontal cortex guides controlled semantic retrieval. Neuron. 2001;31:329–338. doi: 10.1016/s0896-6273(01)00359-2. [DOI] [PubMed] [Google Scholar]
- Wierenga CE, Benjamin M, Gopinath K, Perlstein WM, Leonard CM, Rothi LJ, Conway T, Cato MA, Briggs R, Crosson B. Age-related changes in word retrieval: role of bilateral frontal and subcortical networks. Neurobiol Aging. 2008;29(3):436–451. doi: 10.1016/j.neurobiolaging.2006.10.024. [DOI] [PubMed] [Google Scholar]
- Wong DF, et al. Effects of age on dopamine and serotonin receptors measured by positron tomography in the living human brain. Science. 1984;226:1393–1396. doi: 10.1126/science.6334363. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.