Abstract
BACKGROUND/OBJECTIVES
Adipogenesis is part of the cell differentiation process in which undifferentiated fibroblasts (pre-adipocytes) become mature adipocytes with the accumulation of lipid droplets and subsequent cell morphological changes. Several transcription factors and food components have been suggested to be involved in adipogenesis. The aim of this study was to determine whether mulberry leaf ethanol extract (MLEE) affects adipogenesis in 3T3-L1 adipocytes.
MATERIALS/METHODS
The 3T3-L1 adipocytes were treated with different doses of MLEE for 8 days starting 2 days post-confluence. Cell viability, fat accumulation, and adipogenesis-related factors including CCAAT-enhancer-binding protein alpha (C/EBPα), peroxisome proliferator-activated receptor gamma (PPARγ), PPARγ coactivator 1 alpha (PGC-1α), fatty acid synthase (FAS), and adiponectin were analyzed.
RESULTS
Results showed that MLEE treatments at 10, 25, 50, and 100 µg/ml had no effect on cell morphology and viability. Without evident toxicity, all MLEE treated cells had lower fat accumulation compared with control as shown by lower absorbances of Oil Red O stain. MLEE at 50 and 100 µg/ml significantly reduced protein levels of PPARγ, PGC-1α, FAS, and adiponectin in differentiated adipocytes. Furthermore, protein level of C/EBPα was significantly decreased by the treatment of 100 µg/ml MLEE.
CONCLUSION
These results demonstrate that MLEE treatment has an anti-adipogenic effect in differentiated adipocytes without toxicity, suggesting its potential as an anti-obesity therapeutic.
Keywords: Adipocytes, adipogenesis, anti-obesity, mulberry leaf extract
INTRODUCTION
Obesity is a global epidemic caused by abnormal energy metabolism and is associated with increased metabolic diseases worldwide. Obesity represents an imbalance between fat synthesis and fat degradation. Differentiation of undifferentiated fibroblasts (pre-adipocytes) to mature adipocytes, which is termed adipogenesis, is a central area of obesity research [1]. The main characteristics of cellular adipogenesis are continuous fat mobilization and subsequent cell morphological changes in size and shape. During adipogenesis, fat droplets accumulate in adipocytes and cells become more insulin-responsive [2]. Also, gene expressions of fat-related factors are changed; for example, expressions of adiponectin and fatty acid synthase (FAS) increase as adipogenesis progresses [3,4]. Adipogenesis is tightly regulated by the network of transcription factors and other effector proteins [2]. CCAAT-enhancer-binding proteins (C/EBPs), peroxisome proliferator-activated receptor gamma (PPARγ), and PPARγ coactivator 1 alpha (PGC-1α) stimulate adipogenesis [2,5,6]. However, Wnt and beta-catenin impair adipocyte differentiation by inhibiting adipocyte development from mesenchymal precursors and by repressing C/EBPβ and PPARγ [7,8,9]. The balance between adipogenic factors and anti-adipogenic factors decides the fate of adipocyte development and the extent of adiposity.
Mulberry has been used as a part of traditional oriental medicine. It contains phenolic compounds including 1-deoxynojirimycin, rutin, quercetin, isoquercitrin, and resveratrol [10,11]. Raw material and extracts of mulberry leaves and fruits are commonly consumed in the diet as a type of herbal tea and dietary supplement. Water and ethanol extracts of mulberry leaves and fruits exert anti-oxidative and anti-diabetic effects in cell culture and animal models [12,13,14,15]. Moreover, vascular protective effects of mulberry water extract were observed in rats fed an atherogenic diet, as shown as reduced levels of blood pressure and acetylcholine-induced relaxation of aortic rings [16]. These protective effects were accompanied by improvements in plasma lipid profiles and cell adhesion molecules expression associated with vascular dysfunction in the aorta [16].
Previous reports demonstrated the anti-obesity effects of mulberry in rodents [11,17,18]. In diet-induced obese mice, ethanol extract of mulberry leaves inhibited weight gain acting as a melanin-concentrating hormone-1 antagonist [17]. And combined treatment of mulberry leaf and fruit ethanol extract decreased body weight gain and obesity-related inflammation [11]. Mulberry water extract treatment for 12 weeks decreased weight gain and adiposity, and serum and liver lipids; especially, hepatic fat was reduced with the alterations of lipogenesis- and lipolysis-related gene expression [18].
Compared to the anti-obesity effects of mulberry, evidence regarding mulberry and adipogenesis is limited. We hypothesized that mulberry leaf ethanol extract (MLEE) alters adipogenesis and related markers in 3T3-L1 adipocytes. To test the hypothesis, 3T3-L1 adipocytes were treated with different doses of MLEE for 8 days starting at 2 days after post-confluence, and cell viability, fat accumulation, and markers of adipogenesis were analyzed.
MATERIALS AND METHODS
Plant extraction
Mulberry leaves were collected from Yang Pyeong Agricultural Development & Technology Center (Yang Pyeong-gun, South Korea). The dried leaves (1.0 kg) were extracted with 70% ethanol. The mixture was filtered, evaporated in rotary evaporator and lyophilized. Using this procedure, the yield was 20% of the starting dry weight of mulberry leaves. The obtained ethanol extract of mulberry leaves was kept at -20℃ until used.
Cell culture and differentiation
The 3T3-L1 fibroblasts (American Type Culture Collection, Manassas, VA, USA) were cultured to confluence in Dulbecco's modified Eagle medium (DMEM; GIBCO, New York, NY, USA) supplemented with 10% (v/v) bovine calf serum (BCS; GIBCO) and 1% penicillin-streptomycin (GIBCO) in a CO2 incubator at 37℃. On day 2 post-confluence (designated as day 0), cells were induced to differentiate with DMEM containing 10% fetal bovine serum (FBS; GIBCO), 5 µg/ml insulin (Sigma, St. Louis, MO, USA), 1 µmol/L dexamethasone (Sigma), and 0.05 mmol/L 3-isobutyl-1-methylxanthine (IBMX; Sigma). After 2 days, the medium was replaced with DMEM supplemented with 10% FBS and 5 µg/ml insulin. The cells were subsequently re-fed every 48 h with DMEM containing 10% FBS. To examine the anti-adipogenic effect of mulberry leaf, MLEE was added to medium at different concentrations (10, 25, 50, and 100 µg/ml) during medium changes. Cells without the MLEE treatment were considered as a control.
MTT assay
The 3T3-L1 fibroblasts were seeded at a density of 1 × 104 cells/well in 96-well plates. The cells were treated with different concentrations of dimethyl sulfoxide (DMSO) or MLEE for 48 h. After completion of the treatment, the cells were incubated with 0.5 mg/ml MTT (3-4,5-dimethylthiazol-2-yl-2, 3-diphenyl tetrazolium bromide) for 4 h at 37℃. The supernatants were carefully aspirated and 100 µL of DMSO was added to dissolve the formazan crystal product. Absorbances were measured at 560 nm using a microplate reader.
Oil Red O staining
Adipocyte cell monolayers were gently rinsed twice with phosphate-buffered saline (PBS), fixed in a with 4% paraformaldehyde-PBS solution for 1 h at room temperature, stained with the 0.5% Oil Red O-isopropyl alcohol for 1 h, and then washed with distilled water. The cells were checked by a bright-field optical microscope (HS-100, OPTICAL, China). The cells were eluted with isopropyl alcohol and absorbances were measured at 520 nm with a microplate reader.
Preparation of nuclear fractions
Treated 3T3-L1 adipocytes were homogenized with buffer A [10 mM HEPES-KOH (pH 7.9), 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol (DTT), and 0.2 mM phenylmethanesulfonyl fluoride (PMSF)] and centrifuged. Pellets were resuspended in buffer C [20 mM HEPES-KOH (pH 7.9), 25% glycerol, 1.5 mM MgCl2, 420 mM NaCl, 0.2 mM EDTA, 0.5 mM DTT, and 0.2 mM PMSF] followed by incubation on ice for 20 min. After vortex mixing, the resulting suspension was centrifuged, and the supernatant was stored at -80℃ after determination of protein concentrations.
Western blot analysis
Cells were harvested by scraping in 120 µL lysis buffer [50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1% NP40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), and protease inhibitor cocktail]. They were then incubated on ice for 20 min and centrifuged at 13,000 rpm for 15 min. The supernatant was then transferred to a fresh tube. Protein concentrations were determined using a NanoPhotometer (Implen, Germany). Equal concentrations of protein (30 µg per lane) were loaded in the wells of 6-12% polyacrylamide gels. After the electrophoretic run, proteins on gels were transferred to a polyvinylidene difluoride membrane (Millipore, Marlborough, MA, USA) and incubated in 5% non-fat milk at room temperature. The membrane was incubated with polyclonal antibody against FAS (Abcam, Cambridge, UK, 1:500), adiponectin (Cell Signaling Technology, Danvers, MA, USA, 1:1000), PGC-1α (Santa Cruz Biotechnology, CA, USA, 1:2000), PPARγ (Abcam, 1:500), C/EBPα (Cell Signaling Technology, 1:1000), and β-actin (Santa Cruz Biotechnology, 1:200) overnight at 4℃. The blots were incubated with horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology) for 1 h. The blots were developed by enhanced chemiluminescence (Santa Cruz Biotechnology). The chemiluminescence signal was recorded and quantified with the Syngene G box (Syngene, Cambridge, UK).
Statistical analyses
Results are expressed as mean ± SD. Statistical significance was determined by one-way analysis of variance followed by a Duncan's test for multiple comparisons using SPSS 20. P < 0.05 was considered statistically significant.
RESULTS
Effect of MLEE on cell viability
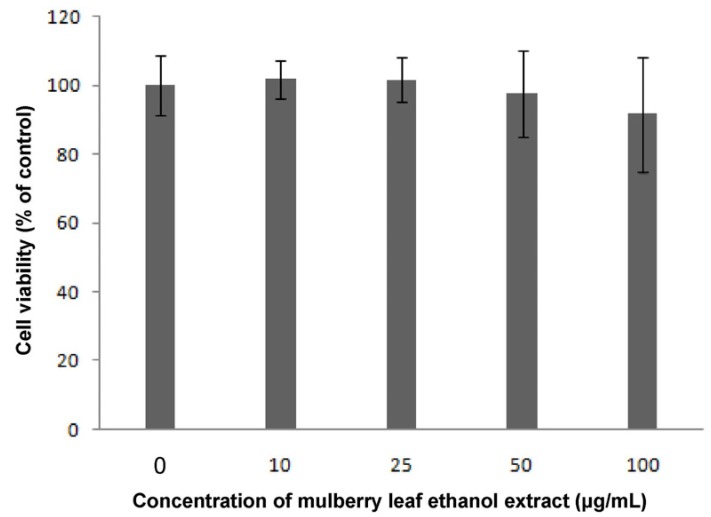
On day 2 of post-confluence, 3T3-L1 cells were induced to differentiate and treated with various concentrations of MLEE every 2 days for 8 days. Cell viability was measured by MTT assay. As shown in Fig. 1, MLEE at 10, 25, 50, and 100 µg/ml showed no significant effects on cell viability after 48 h treatment. During differentiation process, cell morphology changed from small and spindle shape to round shape with lipid droplets accumulation. However, the treatments did not alter cell morphology, showing no toxicity of MLEE treatment.
Fig. 1.
Effect of mulberry leaf ethanol extract (MLEE) on the cell viability in 3T3-L1 adipocytes. Cells were incubated with MLEE at the indicated concentrations (0-100 µg/ml) for 48 h; growth rate was assessed by MTT (3-4,5-dimethylthiazol-2-yl-2, 3-diphenyl tetrazolium bromide) assay. All values are mean ± SD.
MLEE inhibits fat accumulation
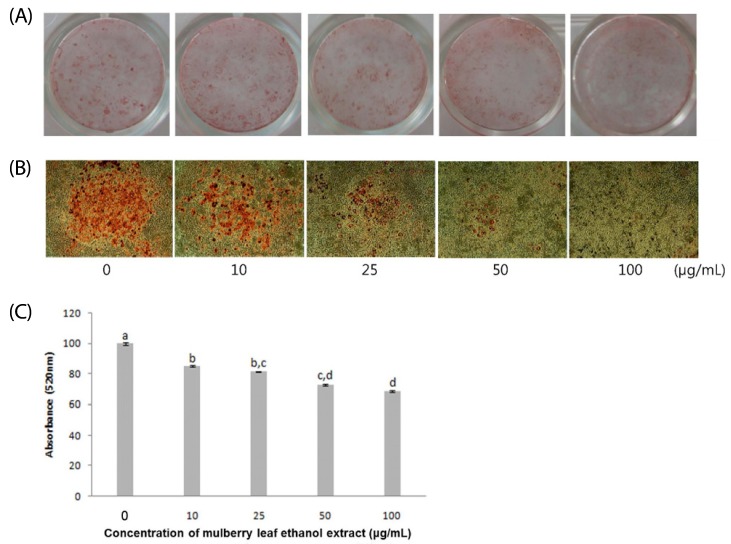
The effects of MLEE on fat accumulation were examined by Oil Red O staining of 3T3-L1 adipocytes. All cells treated with MLEE reduced fat accumulation, as indicated by decreased Oil Red O staining in 3T3-L1 adipocytes (Fig. 2). The result of the absorbance measurements of extracted Oil Red O showed that the effect of MLEE on Oil Red O staining was dose-dependent.
Fig. 2.
Mulberry leaf ethanol extract (MLEE) treatment inhibits lipid accumulation in 3T3-L1 adipocytes. At 2 days after confluence, differentiation was induced in 3T3-L1 adipocytes, and different concentrations of MLEE (0-100 µg/ml) were treated for 8 days. Eight days after differentiation and MLEE treatment, fat contents were analyzed by oil red O staining. (A-B) Representative images of Oil Red O staining. (C) Quantification of lipid accumulation based on the optical density values at 520 nm of destained Oil Red O extracted from the adipocytes. All values are mean ± SD. Mean values with different letters are significantly different at P < 0.05.
MLEE alters the protein expression levels of adipogenesis-related factors
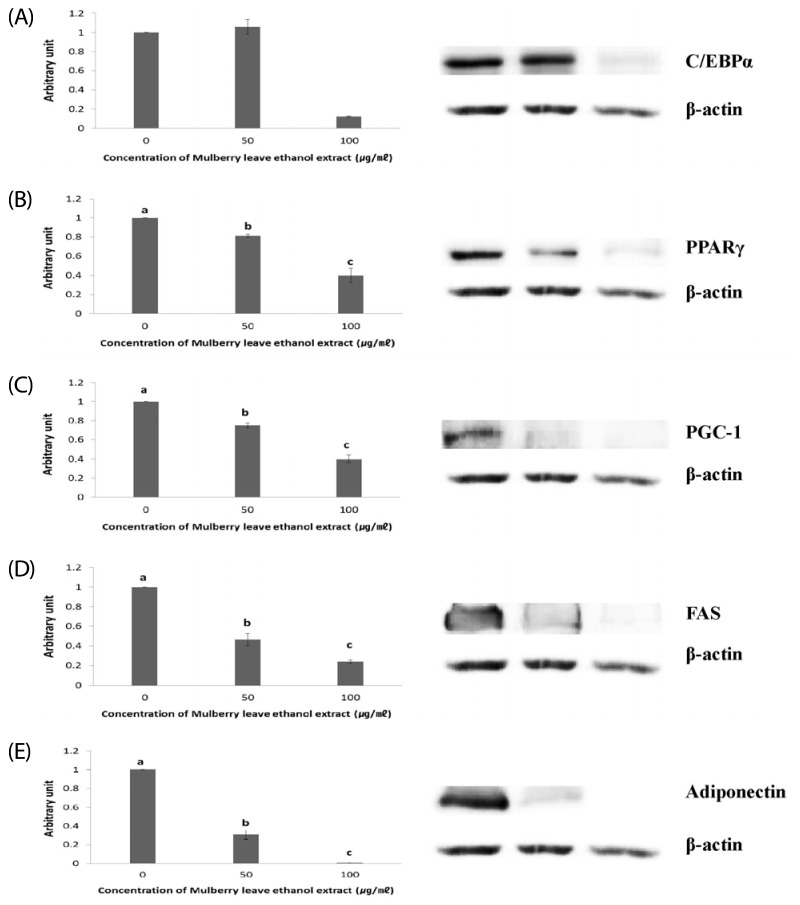
Western blot analysis was performed to determine whether MLEE alters protein expression levels of adipogenesis-related factors. C/EBPα and PPARγ are master regulators of initial stages of adipogenesis [19]. The protein expression of C/EBPα was significantly decreased by the treatment of differentiated adipocytes with 100 µg/ml MLEE (Fig. 3A). MLEE at 50 and 100 µg/ml significantly reduced protein expression of PPARγ (Fig. 3B). PGC-1α, which stimulates mitochondrial biogenesis and adaptive thermogenesis, increases its expression as adipogenesis progresses [20]. MLEE treatments for 8 days decreased PGC-1α expression in a dose-dependent way (Fig. 3C). The expressions of FAS and adiponectin were inhibited by the MLEE treatments in differentiated adipocytes (Fig. 3D and E).
Fig. 3.
The effects of mulberry leaf ethanol extract (MLEE) on the protein expression of adipogenesis-related factors in 3T3-L1 adipocytes. Representative western blots and densitometric analysis for (A) CCAAT-enhancer-binding protein alpha (C/EBPα), (B) peroxisome proliferator-activated receptor gamma (PPARγ), (C) PPARγ coactivator 1 alpha (PGC-1α), (D) fatty acid synthase (FAS), and (E) adiponectin were shown and the expression of beta-actin was analyzed to confirm an equal protein loading control. All values are mean ± SD. Mean values with different letters are significantly different at P < 0.05.
DISCUSSION
Mulberry as a functional food with various phenolic compounds has been suggested to exert anti-oxidative and anti-diabetic effects [12,13,14]. In addition, recent studies reported the anti-obesity and anti-inflammatory effects in rodents [11,17,18]. In the present study, MLEE inhibited adipogenesis and the expression of adipogenesis-related factors in 3T3-L1 adipocytes. Adipogenesis is a part of cell differentiation process in which undifferentiated fibroblasts (pre-adipocytes) become mature adipocytes with the accumulation of lipid droplets and the coordinated changes in cell morphology, gene expression and hormone sensitivity [1,2]. The 3T3-L1 cell line has been considered as an ideal cell line to study adipogenesis and to identify key regulatory potential of functional foods components.
Anti-obesity effect of mulberry has been suggested in various rodent models [11,17,18]. A previous study reported that MLEE decreases weight gain acting as a melanin-concentrating hormone-1 antagonist in diet-induced obese mice [17]. Another finding utilizing three herbs (Melissa officinalis L., Morus alba L., and Artemisia capillaris Thunb) showed their inhibitory effect on body weight gain and adipose tissue mass (both visceral and subcutaneous adipose tissues), which was accompanied by anti-angiogenic and matrix metalloproteinase-inhibitory activities in obese mice [21]. Mulberry water extract treatment for 12 weeks decreased weight gain and adiposity, and serum and liver lipids; especially, hepatic fat was reduced with the alterations of lipogenesis- and lipolysis-related gene expression [18]. Previous reports from our research group demonstrated that combined treatment of mulberry leaf and fruit ethanol extract decreased body weight gain and obesity-related inflammation and oxidative stress in diet-induced obese mice [11,12].
Compared to the anti-obesity effects of mulberry, evidence regarding mulberry and adipogenesis is limited. Based on the in vivo results that mulberry leaf and fruit extract exerts anti-obesity effects shown as reduced body weight gain and adipose tissue mass, whether MLEE alters adipogenesis and related markers was tested in 3T3-L1 adipocytes.
According to the previously reported HPLC data, the total contents of Deoxynojirimycin (DNJ) and resveratrol were approximately 3.75% and 0.015%, respectively in the MLE [11,12]. The natural compounds were shown to exert anti-obesity effects by inhibiting differentiation of preadipocytes [22] and stimulating the β-oxidation system [23].
Without affecting cell viability, MLEE inhibited 3T3-L1 adipogenesis and fat accumulation. Likely, the anti-adipogenic effect of MLEE is an expected result considering the previously reported anti-obesity effects of mulberry. However, there is a contrasting report hypothesizing that the anti-diabetic effects of mulberry may be mediated via the alteration of adiponectin and adipogenesis is available [24].
Adipogenesis is divided into two phases (determination phase and terminal differentiation phase). In the determination phase, C/EBP-β and C/EBP-δ begin to accumulate and directly induce the expression of C/EBPα and PPARγ [25,26,27]. Especially, the second phase of the adipogenesis involves a series of transcriptional processes. C/EBPα and PPARγ initiate to induce their own expression and also activate downstream target genes including PGC-1α and FAS [19,28,29]. PGC-1α, which stimulates mitochondrial biogenesis and adaptive thermogenesis, increases its expression as adipogenesis progresses [20]. FAS is a multienzyme protein that catalyzes fatty acid synthesis from acetyl-CoA and malonyl-CoA into long-chain fatty acids, and also is increased during adipogenesis process. Adiponectin, a highly expressed adipokine, is induced during adipogenesis and is involved with insulin signaling and inflammation [30,31,32,33]. Adiponectin is considered as a marker of mature and functional adipocytes [34]. In addition to the regulation of adipogenic factors, adipocyte differentiation is modulated by the action of anti-adipogenic factors. Wnt and beta-catenin impairs adipocyte differentiation by inhibiting adipocyte development from mesenchymal precursors and by repressing C/EBPβ and PPARγ and [7,8,9]. The balance between adipogenic factors and antiadipogenic factors decides the fate of adipocyte development and the extent of adiposity.
In the present study, MLEE treatment significantly reduced protein expression of the transcription factors of initial adipogenesis (C/EBPα and PPARγ), their downstream targets (PGC-1α and FAS), and marker of mature and functional adipocytes (adiponectin) in differentiated adipocytes. These results demonstrate that MLEE treatment exerts anti-adipogenic effects in differentiated adipocytes without toxicity, suggesting the possibility of its potential as anti-obesity therapeutics. These results are still controversial [24]. A previous report showed the up-regulated mRNA expression of C/EBPα, PPARγ, and adiponectin [24]. As previously described, there are possible differences in extraction condition and concentrations of mulberry leaf extracts. Moreover, protein levels in the present study are more functional than mRNA expression because mRNA expression can be altered by post-transcriptional- and translational regulation.
Collectively, our study clearly demonstrates the anti-adipogenic effects of MLEE shown as Oil Red O staining and Western blot analysis of adipogenic targets. Nevertheless, the current study warrants future study to investigate whether mulberry affects anti-adipogenic profiles (Wnt and beta-catenin) and the functional mechanisms of anti-adipogenic effects of MLEE. Although more mechanistic insights are necessary, these findings may provide additional evidence to understand the functional role of mulberry and to suggest it as an effective nutritional intervention for obesity and obesity-related complications.
Footnotes
This work was supported by a grant from Kyung Hee University (KHU-20100631).
References
- 1.Ali AT, Hochfeld WE, Myburgh R, Pepper MS. Adipocyte and adipogenesis. Eur J Cell Biol. 2013;92:229–236. doi: 10.1016/j.ejcb.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Lefterova MI, Lazar MA. New developments in adipogenesis. Trends Endocrinol Metab. 2009;20:107–114. doi: 10.1016/j.tem.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Skurk T, Alberti-Huber C, Herder C, Hauner H. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab. 2007;92:1023–1033. doi: 10.1210/jc.2006-1055. [DOI] [PubMed] [Google Scholar]
- 4.Fu Y, Luo N, Klein RL, Garvey WT. Adiponectin promotes adipocyte differentiation, insulin sensitivity, and lipid accumulation. J Lipid Res. 2005;46:1369–1379. doi: 10.1194/jlr.M400373-JLR200. [DOI] [PubMed] [Google Scholar]
- 5.Lehrke M, Lazar MA. The many faces of PPARgamma. Cell. 2005;123:993–999. doi: 10.1016/j.cell.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 6.Lefterova MI, Zhang Y, Steger DJ, Schupp M, Schug J, Cristancho A, Feng D, Zhuo D, Stoeckert CJ, Jr, Liu XS, Lazar MA. PPARgamma and C/EBP factors orchestrate adipocyte biology via adjacent binding on a genome-wide scale. Genes Dev. 2008;22:2941–2952. doi: 10.1101/gad.1709008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moldes M, Zuo Y, Morrison RF, Silva D, Park BH, Liu J, Farmer SR. Peroxisome-proliferator-activated receptor gamma suppresses Wnt/beta-catenin signalling during adipogenesis. Biochem J. 2003;376:607–613. doi: 10.1042/BJ20030426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ross SE, Hemati N, Longo KA, Bennett CN, Lucas PC, Erickson RL, MacDougald OA. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289:950–953. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- 9.Liu J, Wang H, Zuo Y, Farmer SR. Functional interaction between peroxisome proliferator-activated receptor gamma and beta-catenin. Mol Cell Biol. 2006;26:5827–5837. doi: 10.1128/MCB.00441-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi EM, Hwang JK. Effects of Morus alba leaf extract on the production of nitric oxide, prostaglandin E2 and cytokines in RAW264.7 macrophages. Fitoterapia. 2005;76:608–613. doi: 10.1016/j.fitote.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Lim HH, Lee SO, Kim SY, Yang SJ, Lim Y. Anti-inflammatory and antiobesity effects of mulberry leaf and fruit extract on high fat diet-induced obesity. Exp Biol Med (Maywood) 2013;238:1160–1169. doi: 10.1177/1535370213498982. [DOI] [PubMed] [Google Scholar]
- 12.Lim HH, Yang SJ, Kim Y, Lee M, Lim Y. Combined treatment of mulberry leaf and fruit extract ameliorates obesity-related inflammation and oxidative stress in high fat diet-induced obese mice. J Med Food. 2013;16:673–680. doi: 10.1089/jmf.2012.2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naowaboot J, Pannangpetch P, Kukongviriyapan V, Prawan A, Kukongviriyapan U, Itharat A. Mulberry leaf extract stimulates glucose uptake and GLUT4 translocation in rat adipocytes. Am J Chin Med. 2012;40:163–175. doi: 10.1142/S0192415X12500139. [DOI] [PubMed] [Google Scholar]
- 14.Tanabe K, Nakamura S, Omagari K, Oku T. Repeated ingestion of the leaf extract from Morus alba reduces insulin resistance in KK-Ay mice. Nutr Res. 2011;31:848–854. doi: 10.1016/j.nutres.2011.09.023. [DOI] [PubMed] [Google Scholar]
- 15.Sugimoto M, Arai H, Tamura Y, Murayama T, Khaengkhan P, Nishio T, Ono K, Ariyasu H, Akamizu T, Ueda Y, Kita T, Harada S, Kamei K, Yokode M. Mulberry leaf ameliorates the expression profile of adipocytokines by inhibiting oxidative stress in white adipose tissue in db/db mice. Atherosclerosis. 2009;204:388–394. doi: 10.1016/j.atherosclerosis.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 16.Lee YJ, Choi DH, Kim EJ, Kim HY, Kwon TO, Kang DG, Lee HS. Hypotensive, hypolipidemic, and vascular protective effects of Morus alba L. in rats fed an atherogenic diet. Am J Chin Med. 2011;39:39–52. doi: 10.1142/S0192415X11008634. [DOI] [PubMed] [Google Scholar]
- 17.Oh KS, Ryu SY, Lee S, Seo HW, Oh BK, Kim YS, Lee BH. Melanin-concentrating hormone-1 receptor antagonism and anti-obesity effects of ethanolic extract from Morus alba leaves in diet-induced obese mice. J Ethnopharmacol. 2009;122:216–220. doi: 10.1016/j.jep.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 18.Peng CH, Liu LK, Chuang CM, Chyau CC, Huang CN, Wang CJ. Mulberry water extracts possess an anti-obesity effect and ability to inhibit hepatic lipogenesis and promote lipolysis. J Agric Food Chem. 2011;59:2663–2671. doi: 10.1021/jf1043508. [DOI] [PubMed] [Google Scholar]
- 19.Cowherd RM, Lyle RE, McGehee RE., Jr Molecular regulation of adipocyte differentiation. Semin Cell Dev Biol. 1999;10:3–10. doi: 10.1006/scdb.1998.0276. [DOI] [PubMed] [Google Scholar]
- 20.Puigserver P. Tissue-specific regulation of metabolic pathways through the transcriptional coactivator PGC1-alpha. Int J Obes (Lond) 2005;29(Suppl 1):S5–S9. doi: 10.1038/sj.ijo.0802905. [DOI] [PubMed] [Google Scholar]
- 21.Kim MY, Park BY, Lee HS, Park EK, Hahm JC, Lee J, Hong Y, Choi S, Park D, Lee H, Yoon M. The anti-angiogenic herbal composition Ob-X inhibits adipose tissue growth in obese mice. Int J Obes (Lond) 2010;34:820–830. doi: 10.1038/ijo.2010.13. [DOI] [PubMed] [Google Scholar]
- 22.Rayalam S, Yang JY, Ambati S, Della-Fera MA, Baile CA. Resveratrol induces apoptosis and inhibits adipogenesis in 3T3-L1 adipocytes. Phytother Res. 2008;22:1367–1371. doi: 10.1002/ptr.2503. [DOI] [PubMed] [Google Scholar]
- 23.Tsuduki T, Nakamura Y, Honma T, Nakagawa K, Kimura T, Ikeda I, Miyazawa T. Intake of 1-deoxynojirimycin suppresses lipid accumulation through activation of the beta-oxidation system in rat liver. J Agric Food Chem. 2009;57:11024–11029. doi: 10.1021/jf903132r. [DOI] [PubMed] [Google Scholar]
- 24.Naowaboot J, Chung CH, Pannangpetch P, Choi R, Kim BH, Lee MY, Kukongviriyapan U. Mulberry leaf extract increases adiponectin in murine 3T3-L1 adipocytes. Nutr Res. 2012;32:39–44. doi: 10.1016/j.nutres.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Tang QQ, Grønborg M, Huang H, Kim JW, Otto TC, Pandey A, Lane MD. Sequential phosphorylation of CCAAT enhancer-binding protein beta by MAPK and glycogen synthase kinase 3beta is required for adipogenesis. Proc Natl Acad Sci U S A. 2005;102:9766–9771. doi: 10.1073/pnas.0503891102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamm JK, Park BH, Farmer SR. A role for C/EBPbeta in regulating peroxisome proliferator-activated receptor gamma activity during adipogenesis in 3T3-L1 preadipocytes. J Biol Chem. 2001;276:18464–18471. doi: 10.1074/jbc.M100797200. [DOI] [PubMed] [Google Scholar]
- 27.Darlington GJ, Ross SE, MacDougald OA. The role of C/EBP genes in adipocyte differentiation. J Biol Chem. 1998;273:30057–30060. doi: 10.1074/jbc.273.46.30057. [DOI] [PubMed] [Google Scholar]
- 28.Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, Spiegelman BM, Mortensen RM. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell. 1999;4:611–617. doi: 10.1016/s1097-2765(00)80211-7. [DOI] [PubMed] [Google Scholar]
- 29.Wu Z, Rosen ED, Brun R, Hauser S, Adelmant G, Troy AE, McKeon C, Darlington GJ, Spiegelman BM. Cross-regulation of C/EBP alpha and PPAR gamma controls the transcriptional pathway of adipogenesis and insulin sensitivity. Mol Cell. 1999;3:151–158. doi: 10.1016/s1097-2765(00)80306-8. [DOI] [PubMed] [Google Scholar]
- 30.Trujillo ME, Scherer PE. Adiponectin--journey from an adipocyte secretory protein to biomarker of the metabolic syndrome. J Intern Med. 2005;257:167–175. doi: 10.1111/j.1365-2796.2004.01426.x. [DOI] [PubMed] [Google Scholar]
- 31.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26:439–451. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 32.Pajvani UB, Scherer PE. Adiponectin: systemic contributor to insulin sensitivity. Curr Diab Rep. 2003;3:207–213. doi: 10.1007/s11892-003-0065-2. [DOI] [PubMed] [Google Scholar]
- 33.Ota T. Chemokine systems link obesity to insulin resistance. Diabetes Metab J. 2013;37:165–172. doi: 10.4093/dmj.2013.37.3.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Körner A, Wabitsch M, Seidel B, Fischer-Posovszky P, Berthold A, Stumvoll M, Blüher M, Kratzsch J, Kiess W. Adiponectin expression in humans is dependent on differentiation of adipocytes and down-regulated by humoral serum components of high molecular weight. Biochem Biophys Res Commun. 2005;337:540–550. doi: 10.1016/j.bbrc.2005.09.064. [DOI] [PubMed] [Google Scholar]