Abstract
BACKGROUND/OBJECTIVES
Kimchi is a traditional Korean fermented vegetable containing several ingredients. We investigated the protective activity of methanol extract of kimchi under different fermentation stages against oxidative damage.
MATERIALS/METHODS
Fresh kimchi (Fresh), optimally ripened kimchi (OptR), and over ripened kimchi (OvR) were fermented until the pH reached pH 5.6, pH 4.3, and pH 3.8, respectively. The radical scavenging activity and protective activity from oxidative stress of kimchi during fermentation were investigated under in vitro and cellular systems using LLC-PK1 cells.
RESULTS
Kimchi exhibited strong radical scavenging activities against 1,1-diphenyl-2-picrylhydrazyl, nitric oxide, superoxide anion, and hydroxyl radical. In addition, the free radical generators led to loss of cell viability and elevated lipid peroxidation, while treatment with kimchi resulted in significantly increased cell viability and decreased lipid peroxidation. Furthermore, the protective effect against oxidative stress was related to regulation of cyclooxygenase-2, inducible nitric oxide synthase, nuclear factor-κB p65, and IκB expression. In particular, OvR showed the strongest protective effect from cellular oxidative stress among other kimchi.
CONCLUSION
The current study indicated that kimchi, particularly OptR and OvR, played a protective role against free radical-induced oxidative stress. These findings suggest that kimchi is a promising functional food with an antioxidative effect and fermentation of kimchi led to elevation of antioxidative activity.
Keywords: Kimchi, fermentation, oxidative stress, nitrosative stress, free radical
INTRODUCTION
Oxidative stress and nitrosative stress are known as precursors for chronic and degenerative diseases, such as cardiovascular disease, atherosclerosis, diabetes, Alzheimer's, and Parkinson's diseases. They are defined as an imbalance between excessive production of reactive oxygen species (ROS) and reactive nitrogen species (RNS), and depletion of the biological antioxidative defense system to detoxify and eliminate the reactive intermediates [1]. These stressors are also important mediators of cellular damage, including DNA damage and protein modification [1,2]. Antioxidants that prevent oxidative and nitrosative stress-induced cellular damage have been considered as therapeutic agents for treatment of degenerative diseases. However, synthetic antioxidants with preventive effects against oxidative stress related diseases showed toxicity and side effects [3]. Therefore, safe and effective natural antioxidants from dietary agents are of great interest [4].
Kimchi is a traditional Korean fermented vegetable in which salt, spices, and other condiments have undergone lactic acid fermentation. It contains high levels of vitamins, minerals, dietary fibers, and phytochemicals from the ingredients. Optimally ripened kimchi (OptR) (pH 4.2-4.3, total acidity 0.6-0.7%) contains higher levels of vitamin B complex, phytochemicals, and lactic acid bacteria [5]. The functional activities of kimchi include antimutagenic/anticancer [6], antiobesity [7], antiatherosclerotic, and immunomodulatory effects [8]. Several beneficial effects of kimchi have been reported and fermentation is known to affect the functional activity of kimchi. However, there is a lack of scientific evidence to address the relationship between fermentation state of kimchi and functionality. Therefore, the current study was conducted for comparison of the protective role of kimchi under different fermentation stages against nitric oxide (NO), superoxide anion (O2-), and peroxynitrite (ONOO-)-induced oxidative damage under an in vitro and cellular system.
MATERIALS AND METHODS
Preparation of kimchi and kimchi extract
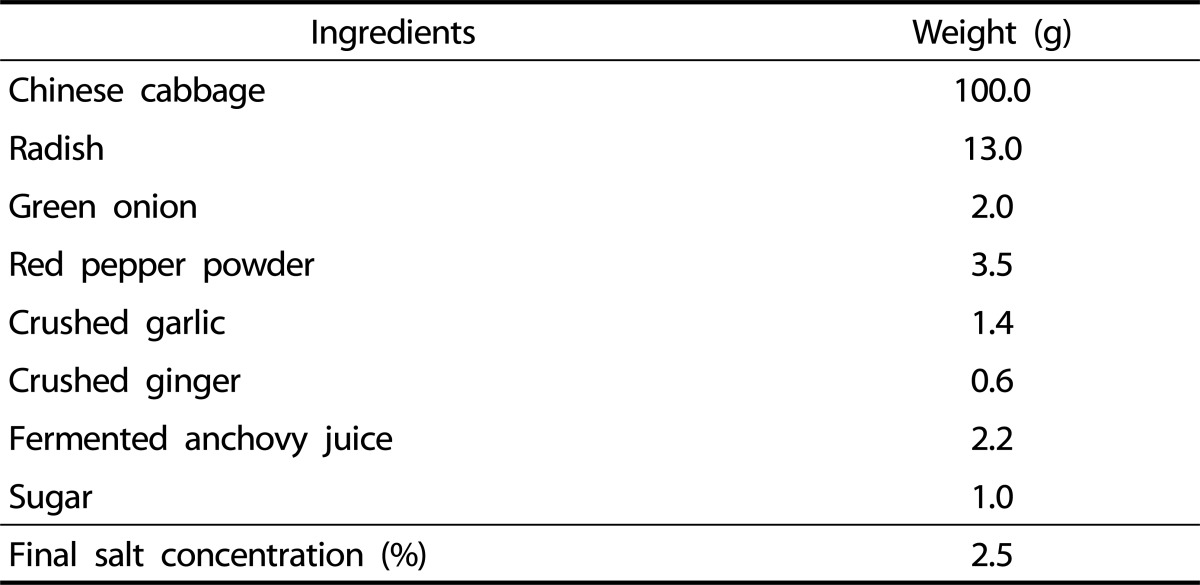
Preparation of kimchi was based on the standardized kimchi recipe of the Kimchi Research Institute at Pusan National University (Table 1) [9]. Fresh kimchi (Fresh), OptR, and over ripened kimchi (OvR) were fermented at 5℃ until reaching pH 5.6, pH 4.3, and pH 3.8, respectively. All kimchi samples were freeze-dried, powdered, and extracted with 20 volumes of methanol (MeOH) for 24 h at room temperature. The MeOH extraction was repeated three times. The extract of kimchi was concentrated using a rotary evaporator and dissolved in dimethyl sulfoxide.
Table 1.
Ingredients ratio of standardized kimchi
1,1-Diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity
Different concentrations (10-1000 µg/mL) of kimchi extracts were prepared in 96-well plates and added to a 60 mM DPPH solution. Following incubation for 30 min at room temperature, the DPPH radicals were determined at 540 nm using a microplate reader (model SPECTRAmax 340PC, Molecular Devices, Sunnyvale, CA, USA). Scavenging activity was calculated using the following formula: Scavenging activity (%) = [1 - (Asample - Asample blank)/Acontrol] × 100. Where Acontrol is the absorbance of the DPPH solution without sample extracts, Asample is the absorbance of DPPH solution + sample extracts, and the Asample blank is the absorbance of the sample extracts without DPPH solution. Scavenging activity was plotted against concentration, and the equation for the line was used to calculate the IC50 value [10].
Nitric oxide scavenging activity
Nitric oxide was generated from sodium nitroprusside (SNP) and measured using the Griess reaction according to the method of Sreejayan and Rao [11]. Sodium nitroprusside (5 mM) in phosphate buffered saline (PBS) was mixed with different concentrations of kimchi and incubated at 25℃ for 150 min. The amount of NO produced by SNP was assayed by measuring the accumulation of nitrite, using a microplate assay method based on the Griess reaction.
Superoxide anion scavenging activity
Superoxide anion levels were measured following a previously described method [12]. All kimchi samples were added to microplate wells containing 200 µL of freshly prepared 0.125 mM ethylenediaminetetraacetic acid (EDTA), 62 mM nitro blue tetrazolium (NBT), and 98 mM reduced nicotinamide adenine dinucleotide phosphate in 50 mM phosphate buffer (pH 7.4). The reaction was initiated with the addition of 25 mL of freshly prepared 33 mM 5-methylphenazium methyl sulfate in 50 mM PBS (pH 7.4). The absorbance was continuously monitored at 540 nm over 5 min as an index of NBT reduction using a microplate reader.
Hydroxyl radical (·OH) scavenging activity
The reaction mixture (2 mL) contained 0.2 M PBS (pH 7.0), 10 mM 2-deoxyribose, 10 mM FeSO4-EDTA, 10 mM hydrogen peroxide, distilled water, and 75 µL of kimchi extract solutions. After incubation of the reaction mixture at 37℃ for 4 h, and 1 mL of 2.8% trichloroacetic acid (TCA) and 1 mL of 1% of 2-tribarbituric acid (TBA) solution were added. The solution was boiled for 10 min, and the absorbance of the solution was measured at 520 nm [13].
Cell culture
LLC-PK1 renal tubular epithelial cells were obtained from the American Type Culture Collection (Manassas, VA, USA). This cell line, which is susceptible to oxidative stress, is widely used for the study of materials with antioxidative activity [14]. Cells were cultured in a culture flask with Dulbecco's modified eagle medium (pH 7.2) supplemented with 5% fetal bovine serum and maintained at 37℃ and 5% CO2. When cells were 80% confluent, they were sub-cultured with 0.05% trypsin-EDTA.
Treatment of radical generators and measurement of cell viability
The cells were plated into 96-well plates at 104cells/mL and allowed to adhere for 2 h. Next, 1.2 mM of SNP, 1.2 mM of pyrogallol, and 1 mM of 3-morpholinosydnonimine (SIN-1) were applied for generation of NO, O2-, and ONOO-, respectively. Kimchi extract was applied in the test wells at various concentrations for 24 h. Cell viability was assessed using the MTT colorimetric assay [15].
Determination of thiobarbituric acid reactive substances (TBARS)
The level of lipid peroxidant released from cultured cells was estimated as TBARS according to a previously described method [12]. One aliquot of medium was mixed with 1.5 mL of 0.67% TBA aqueous solution and 1.5 mL of 20% TCA, and boiled at 95-100℃ for 45 min. The mixture was cooled with water and shaken vigorously with 3 mL of n-butanol. After the mixture was centrifuged at 4000 × g for 10 min, the n-butanol layer was removed, and the absorbance was measured at 520 nm on a fluorescence spectrophotometer (Model FR-550, Shimadzu, Kyoto, Japan) [16].
Assay of NO levels
The amount of NO production in cells was assayed by measuring the accumulation of nitrite, using a microplate assay method based on the Griess reaction [11]. Briefly, 100 µL of culture supernatant was allowed to react with 100 µL of Griess reagent, followed by incubation at room temperature for 5 min. Optical density of the samples was measured at 540 nm using a microplate reader.
Western blot analysis
Western blot analysis was performed as previously described [12]. Antibodies against cyclooxygenase-2 (COX-2), inducible nitric oxide synthase (iNOS), nuclear factor-κB (NF-κB) p65, and IκB were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). β-actin (Sigma, St. Louis, MO, USA) was used as a loading control. For determination of NF-κB translocation to the nucleus, nuclear protein was extracted using a nuclear extraction kit (Sigma, St. Louis, MO, USA) by following the manufacturer's method. The membranes were subjected to immunoblot analysis with desired antibodies and the proteins were visualized using the enhanced chemiluminescence method.
Reverse transcription (RT)-polymerase chain reaction (PCR)
RT-PCR analysis for gene expression was performed as previously described [12] using the Mastercycler (Eppendorf, Hamburg, Germany) and the primers used in this research were as follows: COX-2: 5'-TTC-AAA-TGA-GAT-TGT-GGG-AAA-AT-3' (sense) and 5'-AGA-TCA-TCT-CTG-CCT-GAG-TAT-CTT-3' (antisense); iNOS: 5'-AGA-GAG-ATC-CGG-TTC-ACA-3' (sense) and 5'-CAC-AGA-GCT-GAG-GGT-ACA-3' (antisense); GAPDH: 5'-CGG-AGT-CAA-CGG-ATT-TGG-TCG-TAT-3' (sense) and 5'-AGC-CTT-CTC-CAT-GGT-GGT-GAA-GAC-3' (antisense). The amplified PCR products were run on 1.5% agarose gels and visualized by ethidium bromide (Sigma, St. Louis, MO, USA).
Statistical analysis
Significance was verified by performing Duncan's multiple range tests using SAS software (version 6.0, SAS Institute, Cary, NC, USA).
RESULTS
Radical scavenging activity
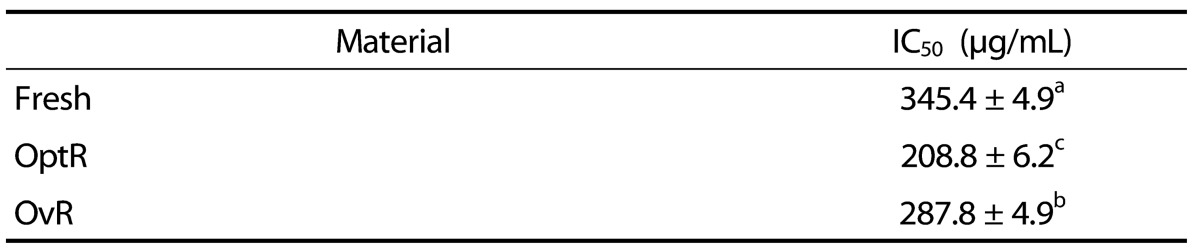
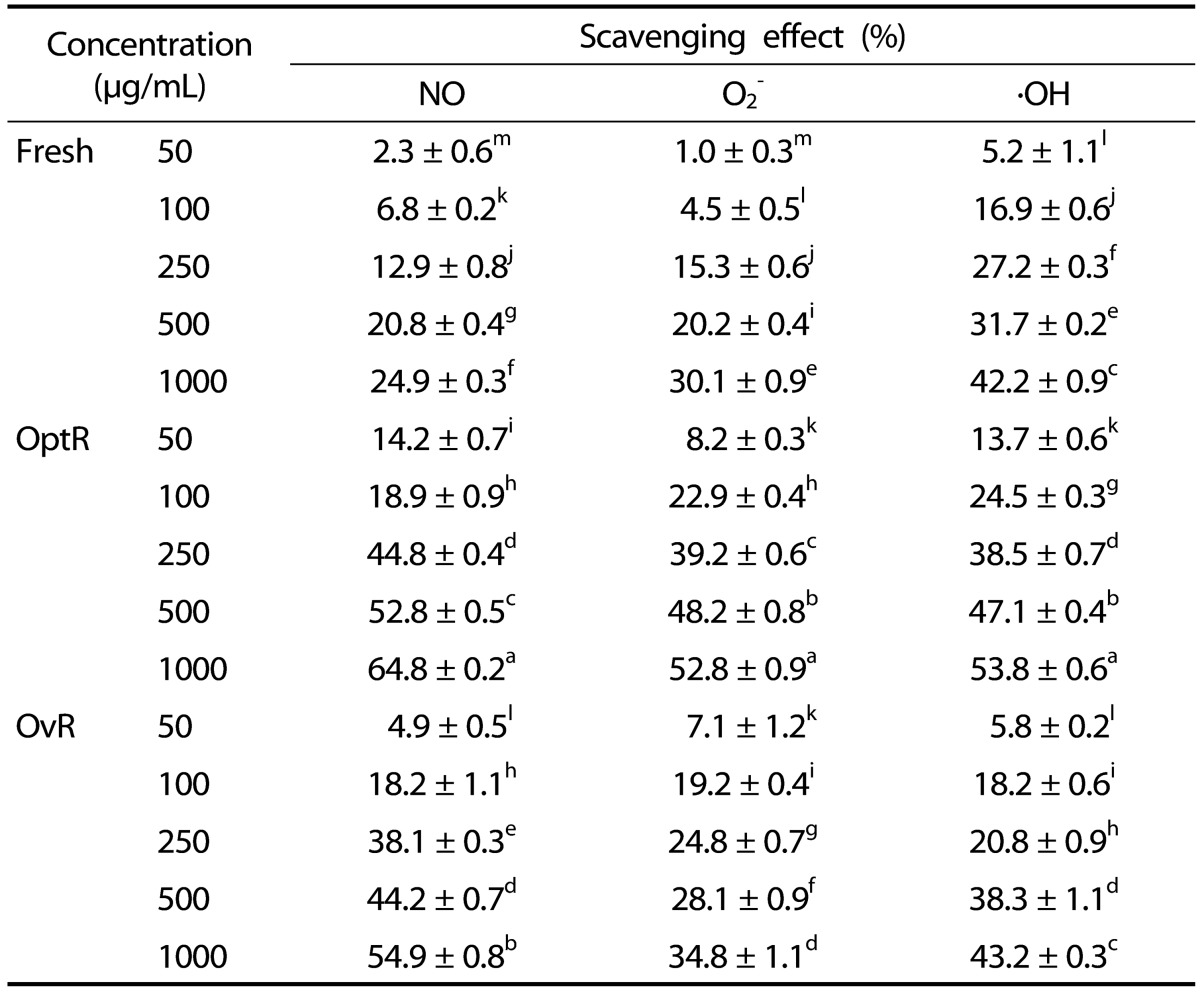
The DPPH radical scavenging activity of kimchi under different fermentation stages is shown in Table 2. Treatment with Fresh, OptR, and OvR inhibited DPPH formation by 50% at a concentration (IC50) of 345.4 µg/mL, 208.8 µg/mL, and 287.8 µg/mL, respectively. The scavenging effects of kimchi on NO, O2-, and ·OH radicals were also measured under in vitro conditions (Table 3). Treatment with kimchi under different fermentation stages showed strong dose-dependent scavenging activity against radicals. At a concentration of 1000 µg/mL of Fresh, OptR, and OvR, NO was scavenged by 24.9%, 64.8%, and 54.9%, respectively. In addition, O2- was also scavenged by 30.1%, 52.8%, and 34.8%, respectively. Fresh, OptR, and OvR also exerted ·OH radical scavenging effects of 42.2%, 53.8%, and 43.2%, respectively.
Table 2.
DPPH radical scavenging effect of kimchi under different fermentation stage
Values are expressed as mean ± SD (n = 3).
a-cMeans with different letters are significantly different (P < 0.05) by Duncan's multiple range.
Table 3.
NO, O2-, and ·OH radical scavenging effects of kimchi under different fermentation stage
Values are expressed as mean ± SD (n = 3).
a-mMeans with different letters are significantly different (P < 0.05) by Duncan's multiple range.
Protective effects of kimchi against NO-induced oxidative stress
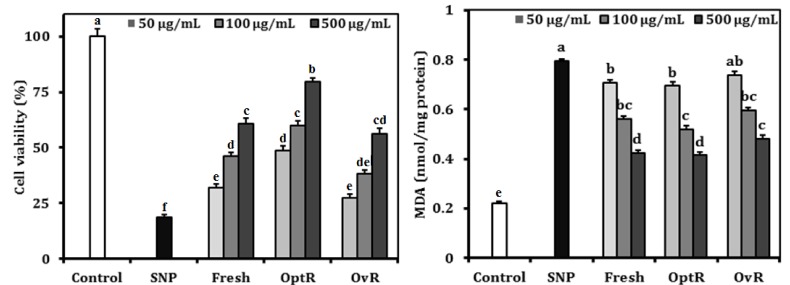
The protective effects of kimchi against NO-induced oxidative stress by SNP are shown in Fig. 1. NO generation by SNP resulted in markedly reduced cell viability compared to control of non-treated cells. However, kimchi increased cell viability in a dose-dependent manner. At the concentration of 500 µg/mL, treatment with Fresh, OptR, and OvR elevated cell viability from 18.7% to 60.8%, 79.7%, and 56.1%, respectively. The cellular lipid peroxidation levels were increased in SNP-treated cells from 0.220 nmol malondialdehyde (MDA)/mg protein to 0.796 nmol MDA/mg protein. At the concentration of 500 µg/mL, Fresh, OptR, and OvR decreased lipid peroxidation to 0.424 nmol MDA/mg protein, 0.415 nmol MDA/mg protein, and 0.483 nmol MDA/mg protein, respectively.
Fig. 1.
Protective effect of kimchi under different fermentation stage on cell viability and TBARS generation in SNP-treated LLC-PK1 cells. Values are expressed as mean ± SD (n = 3). a-fMeans with different letters are significantly different (P < 0.05) by Duncan's multiple range.
Protective effects of kimchi against O2--induced oxidative stress
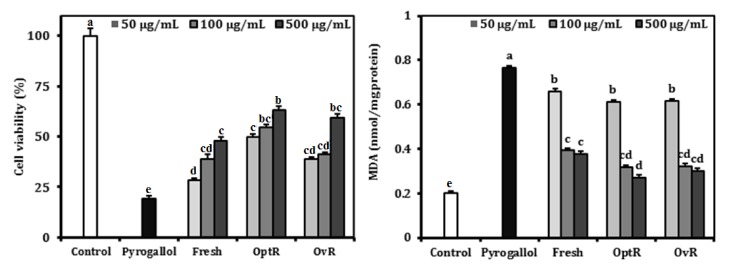
The protective activity of kimchi under different stages of fermentation from oxidative stress by pyrogallol is shown in Fig. 2. Treatment with kimchi resulted in amelioration of pyrogallol-induced cytotoxicity in LLC-PK1 cells. Cell viability declined to 19.3% by O2- generated by pyrogallol. However, treatment with Fresh, OptR, and OvR at a concentration of 500 µg/mL resulted in recovery of cell viability to 48.1%, 63.2%, and 59.4%, respectively. O2- elevated MDA formation from 0.203 nmol MDA/mg protein to 0.764 nmol MDA/mg protein. However, treatment with Fresh, OptR, and OvR resulted in inhibition of MDA level to 0.376 nmol MDA/mg protein, 0.271 nmol MDA/mg protein, and 0.301 nmol MDA/mg protein, respectively.
Fig. 2.
Protective effect of kimchi under different fermentation stage on cell viability and TBARS generation in pyrogallol-treated LLC-PK1 cells. Values are expressed as mean ± SD (n = 3). a-eMeans with different letters are significantly different (P < 0.05) by Duncan's multiple range.
Protective effects of kimchi against ONOO--induced oxidative stress
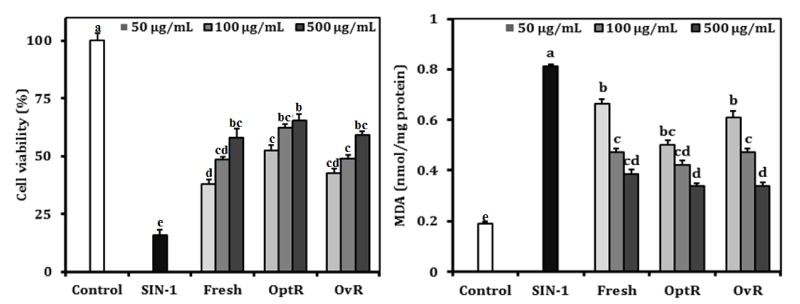
Generation of ONOO- by SIN-1 decreased cell viability to 15.8% of non-treated control cells. Treatment with kimchi resulted in significant, dose-dependent inhibition of reduction of cell viability from ONOO--induced oxidative stress. Cells exposed to Fresh, OptR, and OvR at 500 µg/mL recovered the viability to 58.1%, 65.4%, and 59.1%, respectively. The cellular lipid peroxidation levels in SIN-1-treated LLC-PK1 cells were increased from 0.189 nmol MDA/mg protein to 0.813 nmol MDA/mg protein, while treatment with kimchi resulted in a decrease in MDA levels. At the concentration of 500 µg/mL, Fresh, OptR, and OvR decreased lipid peroxidation from 0.813 nmol MDA/mg protein to 0.387 nmol MDA mg/protein, 0.337 nmol MDA mg/protein, and 0.338 nmol MDA mg/protein, respectively (Fig. 3). The protective effects of kimchi against SIN-1 induced production of NO in LLC-PK1 cells are shown in Table 4. NO production was increased by SIN-1 treatment compared to non-treated cells. However, treatment with kimchi resulted in significantly decreased production of NO. Fresh, OptR, and OvR caused a reduction of NO level from 57.3% to 50.6%, 40.8%, and 50.2%, respectively.
Fig. 3.
Protective effect of kimchi under different fermentation stage on cell viability and TBARS generation in SIN-1-treated LLC-PK1 cells. Values are expressed as mean ± SD (n = 3). a-eMeans with different letters are significantly different (P < 0.05) by Duncan's multiple range.
Table 4.
Protective effect of kimchi under different fermentation stage against SIN-1-induced NO production in LLC-PK1 cells
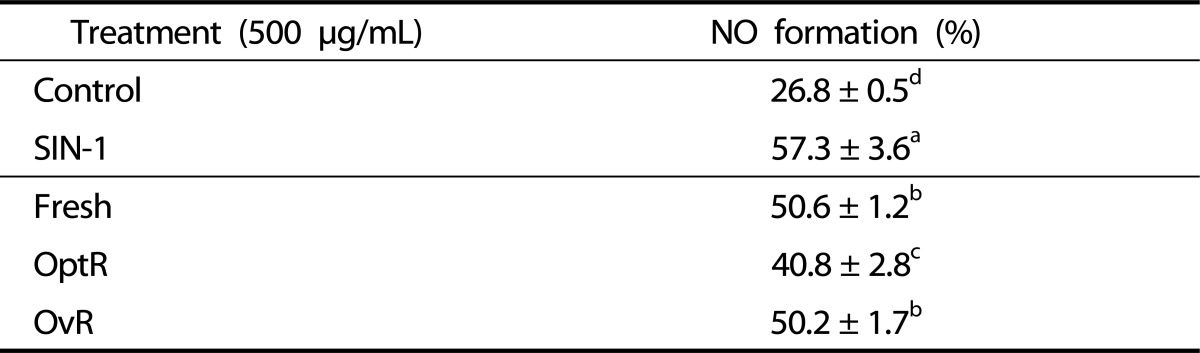
Values are expressed as mean ± SD (n = 3).
a-dMeans with different letters are significantly different (P < 0.05) by Duncan's multiple range.
Effects of kimchi on expression of oxidative stress-related genes
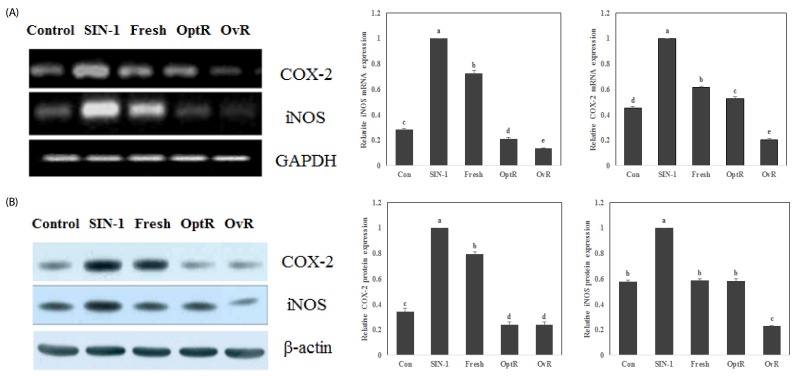
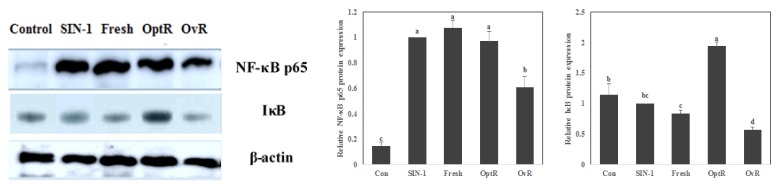
We observed the protective effect of kimchi on mRNA and protein expression of COX-2, iNOS, NF-κB p65, and IκB against SIN-1 (Fig. 4 and Fig. 5). The mRNA and protein expressions of COX-2 and iNOS were elevated in SIN-1-treated LLC-PK1 cells (Fig. 4). However, treatment with 500 µg/mL of kimchi resulted in markedly decreased mRNA and protein expression of COX-2 and iNOS. In particular, OvR caused more significant downregulation of these expressions than other kimchi extract-treated groups. SIN-1 also increased the protein expression of NF-κB p65, while OvR caused a marked decrease of this expression (Fig. 5). In parallel, IκB expression was decreased against the SIN-1-treated group, however, treatment with kimchi resulted in up-regulated expression of IκB.
Fig. 4.
Effect of kimchi under different fermentation stage on mRNA and protein expression of COX-2 and iNOS in SIN-1 treated LLC-PK1 cells. COX-2 and iNOS mRNA expressions and protein levels were measured by RT-PCR (A), and western blot analysis (B). Values are expressed as mean ± SD (n = 3). a-dMeans with different letters are significantly different (P < 0.05) by Duncan's multiple range.
Fig. 5.
Effect of kimchi under different fermentation stage on NF-κB p65 and IκB protein levels in SIN-1 treated LLC-PK1 cells. NF-κB p65 and IκB protein levels were measured by western blot analysis. Values are expressed as mean ± SD (n = 3). a-dMeans with different letters are significantly different (P < 0.05) by Duncan's multiple range.
DISCUSSION
Kimchi is the traditional Korean fermented food; Chinese cabbage is the main ingredient and other ingredients include radish, green onion, red pepper, fermented anchovy sauce, ginger, and garlic. These ingredients contain a large amount of vitamin C, β-carotene, dietary fiber, chlorophylls, and polyphenols [17,18]. Several active components of ingredients with known health beneficial effects include β-sitosterol from Chinese cabbage, capsaicin from red pepper, sulfur compounds, S-methylcysteinsulfoxide and S-allylcysteinsulfoxide from garlic, etc. In addition, beneficial lactic acid bacteria are increased by fermentation. The fermentation process affects the elevation of the health beneficial effect as well as the taste of kimchi [19]. Therefore, in the current study, we prepared the different fermentation stages of kimchi and compared their protective role against oxidative stress.
In pathophysiological conditions, electron-transport mechanisms in the mitochondrial respiratory chain are impaired, resulting in mitochondria damage with over production of ROS and RNS. These are also causes of oxidative stress and nitrosative stress that are well-known critical risk factors of degenerative disease [20]. LLC-PK1 renal epithelial cells are vulnerable to free radical-induced oxidative stress and are widely used as a cellular model for study of oxidative stress-associated molecular changes [21]. Therefore, cellular oxidative stress in LLC-PK1 cells induced by free radical generators is considered useful for examining the protective effects of kimchi against free radicals. The current results indicate that kimchi, particularly OvR, attenuated oxidative damage induced by NO through inhibition of lipid peroxidation and elevation of cell viability. Nitric oxide and O2- react rapidly to form ONOO-, a powerful oxidation, resulting in occurrence of oxidation and nitration of proteins [22]. Our results showed that NO, O2-, and ONOO--induced cellular damage by SNP, pyrogallol, and SIN-1 in LLC-PK1 cells through a decrease in viability and increases of lipid peroxidation. However, treatment with kimchi resulted in amelioration of free radical induced oxidative stress by recovery of cell viability and decrease of lipid peroxidation.
NF-κB, an oxidative-stress responsive transcription factor, plays a critical role in cellular signaling under oxidative stress [23,24]. It is known to mediate the expression of inducible genes such as iNOS, COX-2, VCAM-1, and ICAM-1 in immune and inflammatory responses. In an unstimulated state, NF-κB is present in the cytosol as a NF-κB/IκB complex, through the p65 unit. Extracellular signals trigger phosphorylation of IκB and are ubiquinated and degraded by ubiquitine-proteasome. Removal of IκB allows translocation of NF-κB from the cytosol to the nucleus for induction of its downstream genes such as COX-2 and iNOS [25,26,27]. NF-κB is activated by ROS and oxidative stress by the IκB kinase (IKK)-dependent pathway. IKK complex subunit is regulated by an essential NF-κB protein modulator and it can also regulate IKK complex activation [28,29]. In endothelial cells, ONOO- is known to increase iNOS through the NF-κB pathway [30]. Therefore, the expression of SIN-1-induced NF-κB and its downstream genes, COX-2 and iNOS, was investigated under cellular oxidative stress in LLC-PK1 cells. NF-κ B protein level was markedly increased by ONOO- generated by SIN-1, whereas treatment with kimchi decreased this expression. In particular, OvR caused significant down-regulation of this protein expression, indicating that kimchi, particularly OptR and OvR, would regulate expression of COX-2 and iNOS through the NF-κB pathway. In addition, the fermentation state would also be related to the NF-κB pathway. Several studies have also demonstrated that OvR exerted a stronger antioxidative effect than Fresh [31]. Total phenolic content, DPPH and nitrite scavenging activity of long-term fermented kimchi was higher than those of short-term fermented kimchi. Although further study on the relationship between fermentation and antioxidative effect has to be supported, the bioactive compounds would be produced more during the fermentation period and would be responsible for the stronger biological activity.
In the current study, treatment with kimchi, particularly OptR and OvR, showed strong radical scavenging activity against DPPH, NO, O2-, and ·OH under in vitro conditions. In addition, treatment with kimchi resulted in attenuation of cellular oxidative stress through increases in cell viability and inhibition of lipid peroxidation under the cellular system. In addition, this study indicated that protective effects of kimchi against oxidative stress were related to regulation of COX-2, iNOS, NF-κ B p65, and IκB expression. In addition, findings of this study implied that fermentation of kimchi would affect the protective role from oxidative stress, suggesting that kimchi is a promising functional food with a protective effect against oxidative stress, although the in vivo and human study has to be supported. Further research on the antioxidative mechanisms of kimchi in relation to fermentation stage in detail should be conducted.
Footnotes
This research (912001-2) was supported by grants from the Globalization of Korean Foods R&D program, funded by the Ministry of Food, Agriculture, Forestry and Fisheries, Republic of Korea.
References
- 1.Nakamura T, Cho DH, Lipton SA. Redox regulation of protein misfolding, mitochondrial dysfunction, synaptic damage, and cell death in neurodegenerative diseases. Exp Neurol. 2012;238:12–21. doi: 10.1016/j.expneurol.2012.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Y, Tocchetti CG, Krieg T, Moens AL. Oxidative and nitrosative stress in the maintenance of myocardial function. Free Radic Biol Med. 2012;53:1531–1540. doi: 10.1016/j.freeradbiomed.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 3.Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn Rev. 2010;4:118–126. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh B, Bhat TK, Singh B. Potential therapeutic applications of some antinutritional plant secondary metabolites. J Agric Food Chem. 2003;51:5579–5597. doi: 10.1021/jf021150r. [DOI] [PubMed] [Google Scholar]
- 5.Park KY, Rhee SH. Functional foods from fermented vegetable products: Kimchi (Korean fermented vegetables) and functionality. In: Shi J, Ho CT, Shahidi F, editors. Asian Functional Foods. Boca Raton (FL): CRC Press; 2006. pp. 341–380. [Google Scholar]
- 6.Kong CS, Bahn YE, Kim BK, Lee KY, Park KY. Antiproliferative effect of chitosan-added Kimchi in HT-29 human colon carcinoma cells. J Med Food. 2010;13:6–12. doi: 10.1089/jmf.2009.1213. [DOI] [PubMed] [Google Scholar]
- 7.Kim EK, An SY, Lee MS, Kim TH, Lee HK, Hwang WS, Choe SJ, Kim TY, Han SJ, Kim HJ, Kim DJ, Lee KW. Fermented Kimchi reduces body weight and improves metabolic parameters in overweight and obese patients. Nutr Res. 2011;31:436–443. doi: 10.1016/j.nutres.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 8.Noh JS, Choi YH, Song YO. Beneficial effects of the active principle component of Korean cabbage Kimchi via increasing nitric oxide production and suppressing inflammation in the aorta of apoE knockout mice. Br J Nutr. 2013;109:17–24. doi: 10.1017/S0007114512000633. [DOI] [PubMed] [Google Scholar]
- 9.Cho EJ, Lee SM, Rhee SH, Park KY. Studies on standardization of Chinese cabbage Kimchi. Korean J Food Sci Technol. 1998;30:324–332. [Google Scholar]
- 10.Hatano T, Edamatsu R, Hiramatsu M, Mori A, Fujita Y, Yasuhara T, Yoshida T, Okuda T. Effects of the interaction of tannins with co-existing substances. VI.: effects of tannins and related polyphenols on superoxide anion radical, and on 1, 1-Diphenyl-2-picrylhydrazyl radical. Chem Pharm Bull (Tokyo) 1989;37:2016–2021. [Google Scholar]
- 11.Sreejayan, Rao MN. Nitric oxide scavenging by curcuminoids. J Pharm Pharmacol. 1997;49:105–107. doi: 10.1111/j.2042-7158.1997.tb06761.x. [DOI] [PubMed] [Google Scholar]
- 12.Kim BK, Choi MJ, Park KY, Cho EJ. Protective effects of Korean mistletoe lectin on radical-induced oxidative stress. Biol Pharm Bull. 2010;33:1152–1158. doi: 10.1248/bpb.33.1152. [DOI] [PubMed] [Google Scholar]
- 13.Kim JW, Minamikawa T. Hydroxyl radical-scavenging effects of spices and scavengers from brown mustard (Brassica nigra) Biosci Biotechnol Biochem. 1997;61:118–123. [Google Scholar]
- 14.Yokozawa T, Satoh A, Cho EJ, Kashiwada Y, Ikeshiro Y. Protective role of Coptidis Rhizoma alkaloids against peroxynitrite-induced damage to renal tubular epithelial cells. J Pharm Pharmacol. 2005;57:367–374. doi: 10.1211/0022357055470. [DOI] [PubMed] [Google Scholar]
- 15.Carmichael J, DeGraff WG, Gazdar AF, Minna JD, Mitchell JB. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 1987;47:936–942. [PubMed] [Google Scholar]
- 16.Yokode M, Kita T, Kikawa Y, Ogorochi T, Narumiya S, Kawai C. Stimulated arachidonate metabolism during foam cell transformation of mouse peritoneal macrophages with oxidized low density lipoprotein. J Clin Invest. 1988;81:720–729. doi: 10.1172/JCI113377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee HA, Song YO, Jang MS, Han JS. Alleviating effects of Baechu Kimchi added Ecklonia cava on postprandial hyperglycemia in diabetic mice. Prev Nutr Food Sci. 2013;18:163–168. doi: 10.3746/pnf.2013.18.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park KY, Cho EJ, Rhee SH, Jung KO, Yi SJ, Jhun BH. Kimchi and an active component, beta-sitosterol, reduce oncogenic H-Ras(v12)-induced DNA synthesis. J Med Food. 2003;6:151–156. doi: 10.1089/10966200360716544. [DOI] [PubMed] [Google Scholar]
- 19.Choi IH, Noh JS, Han JS, Kim HJ, Han ES, Song YO. Kimchi, a fermented vegetable, improves serum lipid profiles in healthy young adults: randomized clinical trial. J Med Food. 2013;16:223–229. doi: 10.1089/jmf.2012.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalyanaraman B. Teaching the basics of redox biology to medical and graduate students: oxidants, antioxidants and disease mechanisms. Redox Biol. 2013;1:244–257. doi: 10.1016/j.redox.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yokozawa T, Kim YA, Kim HY, Lee YA, Nonaka G. Protective effect of persimmon peel polyphenol against high glucose-induced oxidative stress in LLC-PK(1) cells. Food Chem Toxicol. 2007;45:1979–1987. doi: 10.1016/j.fct.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 22.Yokozawa T, Cho EJ, Rhyu DY, Shibahara N, Aoyagi K. Glycyrrhizae Radix attenuates peroxynitrite-induced renal oxidative damage through inhibition of protein nitration. Free Radic Res. 2005;39:203–211. doi: 10.1080/10715760400027888. [DOI] [PubMed] [Google Scholar]
- 23.Schreck R, Albermann K, Baeuerle PA. Nuclear factor κB: an oxidative stress-responsive transcription factor of eukaryotic cells (a review) Free Radic Res Commun. 1992;17:221–237. doi: 10.3109/10715769209079515. [DOI] [PubMed] [Google Scholar]
- 24.Oh YC, Cho WK, Im GY, Jeong YH, Hwang YH, Liang C, Ma JY. Anti-inflammatory effect of Lycium Fruit water extract in lipopolysaccharide-stimulated RAW 264.7 macrophage cells. Int Immunopharmacol. 2012;13:181–189. doi: 10.1016/j.intimp.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 25.Aktan F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004;75:639–653. doi: 10.1016/j.lfs.2003.10.042. [DOI] [PubMed] [Google Scholar]
- 26.Biswas SK, Lopes de Faria JB. Does peroxynitrite sustain nuclear factor-κB? Cardiovasc Res. 2005;67:745–746. doi: 10.1016/j.cardiores.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 27.Baeuerle PA, Baltimore D. NF-κB: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 28.Salminen A, Kaarniranta K. Genetics vs. entropy: longevity factors suppress the NF-κB-driven entropic aging process. Ageing Res Rev. 2010;9:298–314. doi: 10.1016/j.arr.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 29.Salminen A, Huuskonen J, Ojala J, Kauppinen A, Kaarniranta K, Suuronen T. Activation of innate immunity system during aging: NF-κB signaling is the molecular culprit of inflamm-aging. Ageing Res Rev. 2008;7:83–105. doi: 10.1016/j.arr.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 30.Yokozawa T, Cho EJ, Rhyu DY, Nakagawa T. Novel approaches to oxidative stress-induced renal failure: therapeutic potentials of Sanguisorbae Radix, Wen-Pi-Tang and green tea. J Trad Med. 2003;20:83–101. [Google Scholar]
- 31.Park JM, Shin JH, Gu JG, Yoon SJ, Song JC, Jeon WM, Suh HJ, Chang UJ, Yang CY, Kim JM. Effect of antioxidant activity in Kimchi during a short-term and over-ripening fermentation period. J Biosci Bioeng. 2011;112:356–359. doi: 10.1016/j.jbiosc.2011.06.003. [DOI] [PubMed] [Google Scholar]