Abstract
BACKGROUND/OBJECTIVES
The type of sweet snack incorporated into an energy-restricted diet (ERD) may produce differential effects on metabolic improvements associated with body weight (BW) loss. This study compared effects of incorporating either twice daily energy-controlled dark chocolate snacks plus once daily sugar-free cocoa beverage (DC) to non-chocolate snacks plus sugar-free non-cocoa beverage (NC) into an ERD on BW loss and metabolic outcomes.
MATERIALS/METHODS
In an 18-week randomized comparative trial, 60 overweight/obese premenopausal women were assigned to DC (n = 30) or NC group (n = 30). Dietary intake was measured at baseline and week 18, and BW, anthropometrics, blood pressure (BP) and serum glucose, insulin and lipid concentrations were measured at baseline, and weeks 6, 12 and 18. Data were analyzed using repeated measures ANOVA.
RESULTS
Using intention-to-treat analysis, women in DC and NC groups reduced energy intake (both P < 0.001) and lost 4.4 ± 0.6 kg and 5.0 ± 0.9 kg (both P < 0.001), respectively. Both groups lowered systolic and diastolic BP [DC = 2.7 (P < 0.05), 2.7 (P < 0.01); NC = 3.4 (P < 0.01), 4.2 (P < 0.01) mmHg, respectively]. Glucose and insulin concentrations decreased by 0.72 mmol/L (P < 0.001) and 13.20 pmol/L (P < 0.01) in DC group and by 0.83 mmol/L (P < 0.001) and 13.20 pmol/L (P < 0.01), respectively, in NC group. Total cholesterol increased in NC group (P < 0.05), with no significant lipid changes in DC group. There were no significant differences in biomarker outcomes between groups.
CONCLUSIONS
Overweight/obese premenopausal women following an 18-week ERD that included either DC or NC sweet snack and sugar-free beverage lost equivalent amounts of BW and improved BP measurements and glucose and insulin concentrations.
Keywords: Cocoa, dark chocolate, dietary intervention, obesity, randomized comparative trial
INTRODUCTION
Overweight and obesity have become major public health concerns as 67 to 75% of adults in the United States (U.S.) are now affected [1]. Individuals with excess body weight (BW) spend 30% more on healthcare than those of normal BW, due to the increased incidence of co-morbidities such as hypertension, diabetes and heart disease [2]. To improve current health status and prevent future complications, the primary treatment for these individuals is reduction of excess adiposity through moderate BW loss [3]. The key dietary objective for inducing BW loss is a reduction in total daily energy intake below energy needs [4].
Many weight-loss diets and diet programs restrict all high-fat and/or high-sugar snack foods [5,6,7,8]. Allowing individuals to consume snacks that are normally enjoyed in energy- and portion-controlled amounts as part of an energy-restricted diet (ERD) may make adherence easier and potentially increase diet satisfaction, because habitual eating patterns are emphasized, and there is less dramatic alteration in food choices [9]. Chocolate is one of the most commonly liked and widely consumed sweet snacks among women in the U.S. and around the world [10,11], with approximately one-half of women reporting weekly consumption [12]. In addition, U.S. women are more likely than men to consume sweet foods such as ice cream, pastries and non-chocolate candy on a regular basis [11].
Chocolate as a sweet snack food is of particular interest, due to its volume of consumption [13], likeability [10] and pleasing sensory properties [12]. Epidemiologic studies suggest that chocolate intake is inversely related to body mass index (BMI) [14] and reduced risk of low high-density lipoprotein-cholesterol (HDL-C) and other metabolic syndrome indicators [13,15]. Further, previous studies suggest that consumption of chocolate and cocoa, specifically dark chocolate, may have beneficial effects on blood pressure (BP) [16,17,18,19,20,21], fasting blood glucose [20,22], insulin sensitivity [16,23,24] and blood lipids [17,19,25,26]. Cocoa is rich in minerals and phytonutrients, namely flavanols, including catechin, epicatechin and proanthocyanidins (PACs), and due to the higher cocoa content, dark chocolate may confer the greatest metabolic benefits when compared to milk or white chocolate [27,28].
Dark chocolate is commonly regarded as an energy-dense food [29] and excess consumption of any energy-dense food may have adverse metabolic effects, including weight gain. Therefore, women attempting BW loss often withhold chocolate and other sweet snacks from their diet. While several short-term (i.e., 2 to 8 weeks) studies have examined changes in BW following consumption of dark chocolate [30], only one study has compared the effects of dark chocolate against non-chocolate intake specifically on changes in BW and body composition during energy restriction in overweight/obese women [9]. In this feasibility study, inclusion of a dark chocolate or non-chocolate sweet snack as part of an ERD resulted in losses in BW, fat mass (FM) and body fat percentage (BF%), with no significant differences between the two snack groups. The sample size of this pilot study was small, and outcomes were limited to body composition without further exploration of biomarkers of metabolic health [9].
The aim of the current 18-week randomized intervention was to compare effects of incorporating twice daily dark chocolate snacks plus once daily sugar-free cocoa beverage (DC group) to twice daily non-chocolate snacks plus once daily sugar-free non-cocoa beverage (NC group) into an ERD with a typical macronutrient distribution [31] on BW loss and metabolic outcomes in free-living premenopausal women with overweight/obesity. Outcome measures included estimated energy intake, BW, anthropometric and BP measurements as well as serum glucose, insulin and lipid concentrations. Based on emerging evidence that dark chocolate and cocoa may modulate obesity, it was hypothesized that inclusion of dark chocolate and cocoa into an ERD would result in a significantly greater decrease in BW and more favorable improvements in metabolic indicators of health, compared to inclusion of non-chocolate and non-cocoa products.
SUBJECTS AND METHODS
Participants
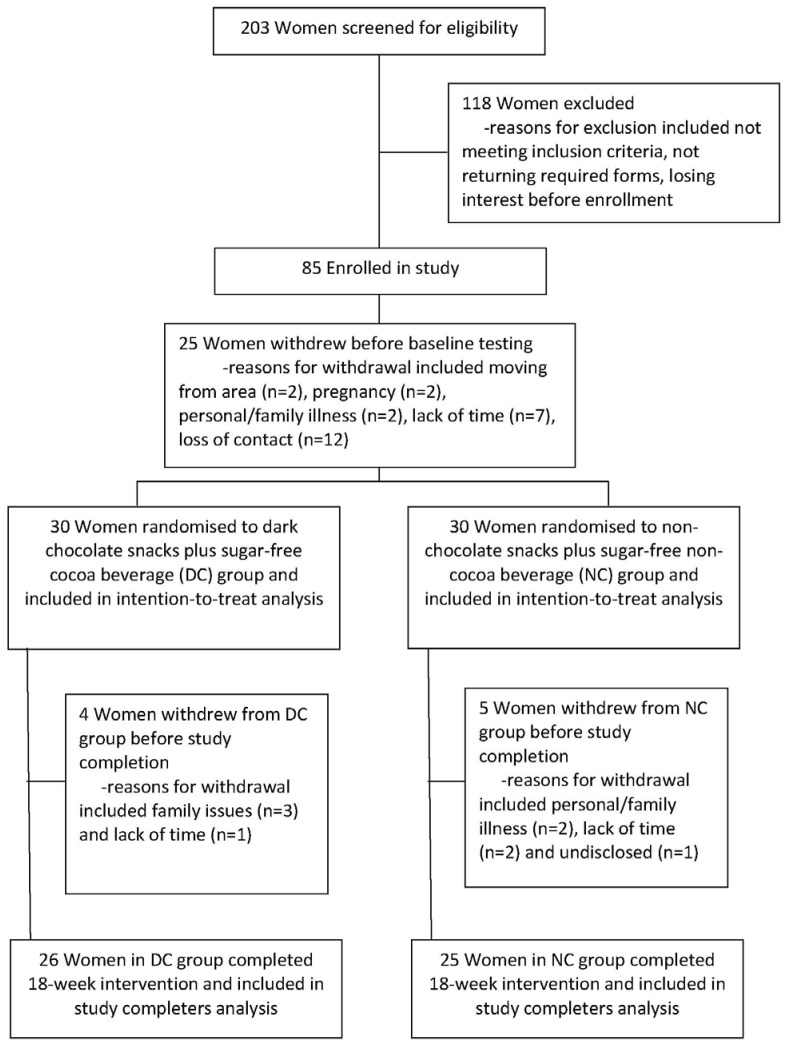
Participants were recruited from central Pennsylvania, U.S., by word-of-mouth, newsletter and newspaper advertisements, electronic-mail notices and flyers posted in the local community. Two-hundred three women provided verbal consent for an initial telephone screening and were assessed for study eligibility; 85 were enrolled in the study. Due to a relatively lengthy recruitment interval, 25 women withdrew before baseline testing was completed for a final sample size of 60 women (Fig. 1).
Fig. 1.
Flow diagram of participant enrollment in a study of premenopausal women with overweight/obesity, designed to evaluate changes in body weight, blood pressure and selected metabolic biomarkers with an energy-restricted diet including twice daily dark chocolate snacks plus once daily sugar-free cocoa beverage or twice daily non-chocolate snacks plus once daily sugar-free non-cocoa beverage.
The current dietary intervention included women ages 25 to 45 years with a BMI of ≥ 25.0 and < 43.0 kg/m2. Women were moderately physically active (≤ 5 hours of planned exercise/week), eumenorrheic (≥ 8 menstrual cycles/year) and of self-reported stable BW (< 5% change in BW for at least six months before study participation). Further inclusion criteria included a score of < 50 on the Zung Self-Rating Depression Scale/Status Inventory and no intolerance, aversion or allergy to chocolate. Exclusion criteria included women who currently smoked, were pregnant or attempting to become pregnant, had a hysterectomy and/or ovariectomy without hormone replacement therapy and those who used oral contraceptives for < 2 years in duration (if used). Women who used medications, including steroid or thyroid hormones, bisphosphonates, anticonvulsants and glucocorticoids, or consumed ≥ 40 grams of chocolate per day (i.e., equivalent of one standard chocolate bar or more/day) were also excluded. All study participants underwent medical examinations by their personal healthcare providers to obtain measured and reliable values for height, BW and BMI to ascertain inclusion criteria was met.
Written informed consent was provided by all participants before entry into the study. The Institutional Review Board (IRB) for Research Involving Human Subjects at The Pennsylvania State University (PSU; University Park, PA, U.S.) conducted a full review of the study procedures and approved the study protocol (PSU-IRB#29543).
Study design
This was an 18-week randomized, parallel-arm, comparative dietary intervention in which participants were enrolled in two cohorts (July-December 2009 and March-July 2010). After enrollment of each cohort, women were stratified by baseline age, BMI and physical activity and then randomly assigned to either the DC group (n = 30) or the NC group (n = 30).
Dietary intervention
Participants in both groups followed an ERD with a macronutrient composition of 50% carbohydrate, 30% fat and 20% protein designed to induce approximately 0.91 kg of BW loss per week by consuming 2,092 fewer kJ per day than required for energy balance. Baseline energy levels were set between 5,439 and 7,531 kJ per day as determined using the Harris-Benedict equation [32].
Women in both groups were administered portion-controlled and energy-matched snacks and beverages. Women in the DC group consumed one, 236 mL sugar-free natural cocoa beverage (The Hershey Company, Hershey, PA, U.S.) each day (272 kJ/day) and one 1.45 oz dark chocolate tasting square (Hershey's® Extra Dark® dark chocolate, The Hershey Company) at two intervals each day (377 kJ/day). Women in the NC group drank one, 236 mL sugar-free cocoa-free vanilla beverage (The Hershey Company; 272 kJ/day) each day and consumed two non-chocolate sweet snacks (fruit-flavored licorice stick; The Hershey Company) each day (377 kJ/day) at the same daily intervals as the DC group. Participants in both groups were instructed to not consume additional cocoa or chocolate products throughout the 18-week intervention beyond the snack and beverage assignments. Women in the DC group consumed 270 mg of flavanols (PACs 1-10) per day from dark chocolate snacks and the sugar-free cocoa beverage [16,17], and women in the NC group consumed 0 mg of flavanols per day from non-chocolate snacks and the sugar-free non-cocoa beverage.
A registered dietitian educated participants on how to follow the ERD, which was based on a food exchange system. Women were assigned a certain number of servings from each of six exchange groups to promote a flexible dietary approach that approximated usual intake except for energy restriction and inclusion of assigned beverages and twice daily snacks. Three main principles were emphasized, including portion control [33,34], planning ahead for meals [35] and consuming vegetables to satisfy an acute hunger sensation [36]. Handouts that contained food options, dietary patterns and individualized meal plans specific to energy levels were provided.
All women attended weekly nutrition education classes that covered topics such as general nutrition information, dining in restaurants, food selection, food preparation and recipe modification. Problem-solving and motivational concerns also were discussed and addressed. Education sessions were specific to DC or NC group; however, topics were the same for both groups, and one registered dietitian led all of the education sessions to maintain consistency between groups. Snacks and beverage mixes were dispensed at education sessions, and compliance with snack and beverage intervention was assessed by participant self-report and concurrent investigator-conducted snack counts; compliance was defined as intake of ≥ 85% of weekly snacks and beverages based on group assignment. Upon completion of the study, participants received monetary compensation of $80 (U.S.).
Outcome measures
Dietary intake was evaluated at baseline and week 18. Anthropometric, physical activity and BP measurements were completed and whole blood samples were collected at baseline, week 6, week 12 and week 18.
Dietary intake assessment
Dietary intake was estimated using 4-day food records. Women recorded all foods and beverages consumed on three non-consecutive weekdays and one weekend day in the week before measurement sessions at baseline and week 18. Handouts containing pictures of standard serving sizes of different foods and beverages were provided to aid in recording intake. Food records were evaluated using Food Processor® dietary analysis software (version 10.6.0, 2010, esha Research, Salem, OR, U.S.) for estimated average daily intake of total energy (kJ); carbohydrate, fat, protein and alcohol (% of total kJ); total sugar (g), fiber (g), saturated fat (g) and cholesterol (g); and sodium (mg).
Anthropometric measurements
Standing height (cm) was measured using a stadiometer (Seca 700, Hamburg, Germany), and BW (kg) and BF% were measured using an electronic scale (410GS, Tanita Corporation, Arlington Heights, IL, U.S.); height and BW measurements were used to calculate BMI for each participant. Using a spring-calibrated measuring tape (Gulik II, Country Technology, Gay Mills, WI, U.S.), two measurements each of the waist at the narrowest point above the belly button and hips at the widest part of the buttocks were taken to the nearest 0.1 cm and averaged. For all measurements, women were dressed in lightweight clothing without shoes.
Physical activity
Physical activity was estimated using the Stanford 7-day Physical Activity Recall Scale [37]. For seven consecutive days before a measurement session, participants recorded number of hours slept, spent in front of a television or computer screen and engaged in moderate, hard and very hard activity. Total hours of moderate, hard and very hard activity were summed from the recall scale and divided by seven to estimate hours of physical activity per day.
Blood pressure
Seated systolic and diastolic BP (mmHg) was measured by a registered nurse using a standard sphygmomanometer (Baumanometer® Desk Model, Copiague, NY, U.S.). Two BP measurements were recorded with a 2- to 3-minute rest period between readings; values were averaged.
Sample collection
Venous blood samples were obtained by a registered nurse between the hours 0700-0930 after a 12-hour fast. Samples were centrifuged at 810 × P for 12 minutes, after clotting. Serum was pipetted into cryovials and stored at -80℃ until completion of bioassays.
Metabolic profile including serum glucose, insulin and lipids
Serum glucose (Kit #1070, Stanbio Labs, Boerne, TX, U.S.) was measured (mmol/L) using ultra-violet (UV) spectrophotometry (version 3.0, Simple Reads Software, Varian, Santa Clara, CA, U.S.), and serum insulin (Catalog #IS130D, CalBiotech, Spring Valley, CA, U.S.) was measured (pmol/L) using enzyme-linked immunosorbent assay (GEN5 version 1.10, Epoch, BioTek, Winooski, VT, U.S.). Insulin resistance was estimated by homeostasis model of assessment-insulin resistance (HOMA-IR) using the formula: fasting insulin concentration (µIU/mL) × fasting glucose concentration (mg/dL) × 0.0555/22.5 [38].
Serum total cholesterol (mmol/L), HDL-C (mmol/L) and triacylglycerides (mmol/L) concentrations (Kits #1010, #0599, and #2100, respectively, Stanbio Labs) were measured using UV spectrophotometry (Varian). Low-density lipoprotein-cholesterol (LDL-C) was calculated using the equation: LDL-C = total cholesterol-HDL-C-(triacylglycerides/5) [39].
All serum samples were analyzed in duplicate. Intra-assay coefficients of variation (CVs) for glucose and insulin were 7.4 and 6.0%, respectively. Intra-assay CVs for serum total cholesterol, HDL-C and triacylglycerides were 6.0, 5.9 and 7.9%, respectively.
Statistical analyses
Using BW change from baseline to week 18 as the primary outcome, 21 participants per group were required to detect a treatment difference with 80% power when using t-tests and a 2-sided type I error of 5%. Using data from the 60 women who completed baseline measurements, data were first analyzed using intention-to-treat model. The nine women who did not complete the study (i.e., non-completers) were included in intention-to-treat analyses by replacing missing data with the last available measurement value. A secondary efficacy analysis was conducted by including only the 51 women who completed the 18-week intervention.
Data are presented as means ± SEM unless otherwise indicated. Differences between the two cohorts were analyzed using independent t-tests. Differences in characteristics at baseline between DC and NC groups and between study-completers and those who withdrew also were analyzed using independent t-tests.
Using intention-to-treat data, a 2 × 4 ANOVA with repeated measures on the time factor was performed to assess differences in anthropometrics and BP measurements and metabolic indicators between DC and NC groups over four intervals. The interaction of group (Treatment) by interval (Time) also was assessed. Data were analyzed using the Statistical Package for the Social Sciences (version 17.0, 2008, SPSS Inc., Chicago, IL, U.S.). All tests were two-sided with significance set at P < 0.05.
RESULTS
Statistically significant differences were not observed between the two cohorts of women (July-December, March-July) in baseline characteristics or estimated dietary intakes, with the exception of self-reported physical activity. Therefore, data from both cohorts were combined and used in analyses. Sixty women (one Native American, two African American and 57 Caucasian), with a mean ± SEM age of 35.9 ± 0.8 years and BMI of 31.0 ± 0.6 kg/m2 began the intervention. Fifty-one of the women (85%) completed the intervention with no difference between DC and NC groups in discontinuation rate. No statistically significant differences in race, age, height, BW, BMI, waist and hip circumferences and physical activity between the DC (n = 30) and NC (n = 30) groups were found at baseline. There were no differences in these same characteristics at baseline for women randomly assigned to DC or NC group who completed (n = 51) the study compared to those who withdrew (n = 9).
Snack compliance and class attendance
Snack and beverage compliance was 90 and 90%, respectively, for the DC group and 92 and 94%, respectively, for the NC group. Attendance at nutrition education classes was 74 and 75% for the DC group and NC group, respectively. Neither snack and beverage compliance nor class attendance differed between groups.
Intention-to-treat analysis
Dietary intake assessment
Fifty-nine women completed 4-day food records at baseline. Table 1 displays estimated dietary intake of these participants at baseline and week 18 and changes over time. Within the DC and NC groups, women decreased estimated total energy, total sugar, saturated fat and cholesterol intakes, supporting that participants were successful in complying with energy restriction. Women in the DC group also reduced estimated sodium intake, and women in the NC group increased estimated dietary protein intake. Significant differences in estimated dietary intake variables were not found between groups at baseline and week 18 or in changes in nutrient intakes over time.
Table 1.
Estimated dietary intake of premenopausal women with overweight/obesity at baseline, week 18 and change over time in a study evaluating changes in body weight, blood pressure and selected metabolic biomarkers with an energy-restricted diet including twice daily dark chocolate snacks plus once daily sugar-free cocoa beverage or twice daily non-chocolate snacks plus once daily sugar-free non-cocoa beverage
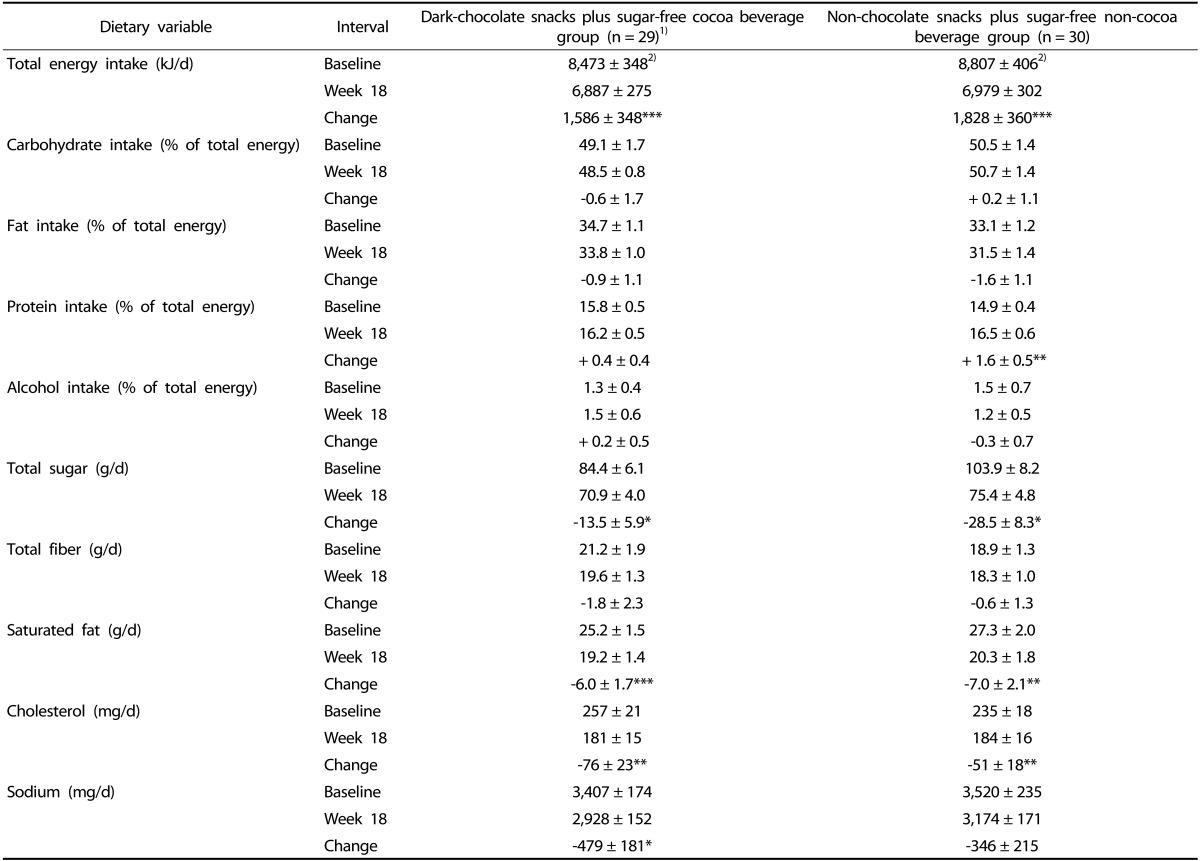
1)Missing data for n = 1 participant.
2)Values are means ± SEM. P-values from intention-to-treat analysis, using analysis of covariance with repeated measures on the time factor.
*P < 0.05, **P < 0.01, ***P < 0.001 within group change from baseline. There were no significant differences in estimated dietary intake variables between snack/beverage groups at any interval or for the change over time between snack/beverage groups.
Anthropometric measurements
Women in the DC group lost 5.3% (P < 0.001) of BW, while women in the NC group lost 5.9% (P < 0.001) of BW from baseline to week 18 (Table 2). The rate of BW change over time did not differ between groups. Women within both groups significantly reduced BMI, waist and hip circumferences and BF% over time (Table 2), again suggesting compliance with energy restriction. Changes in these anthropometric measurements did not differ between groups at any interval or over time.
Table 2.
Anthropometric measurements of premenopausal women with overweight/obesity at baseline and change from baseline at weeks 6, 12 and 18 in a study evaluating changes in body weight, blood pressure and selected metabolic biomarkers with an energy-restricted diet including twice daily dark chocolate snacks plus once daily sugar-free cocoa beverage or twice daily non-chocolate snacks plus once daily sugar-free non-cocoa beverage
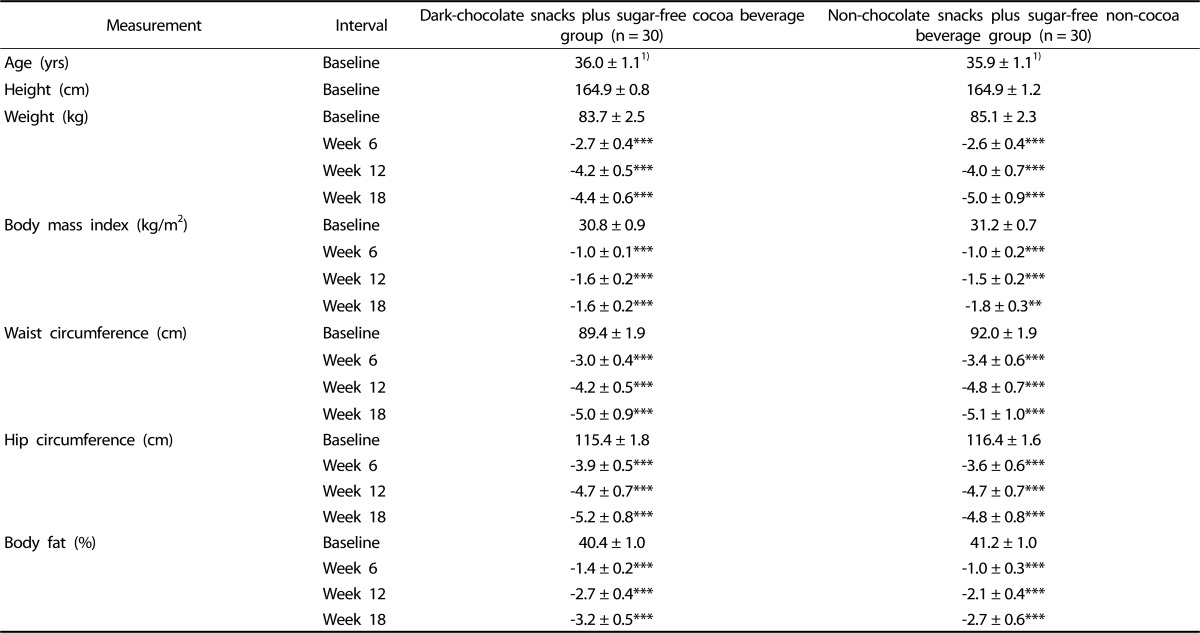
1)Values are means ± SEM for baseline values and mean ± SEM for change from baseline values. P-values from intention-to-treat analysis, using analysis of covariance with repeated measures on the time factor.
*P < 0.05, **P < 0.01, ***P < 0.001 within group change from baseline. There were no significant differences in measurements between snack/beverage groups at any interval or for the change over time between snack/beverage groups.
Physical activity
Self-reported physical activity (hr/day) was greater in the first cohort of women compared to second cohort (P < 0.01) at baseline. Therefore, a physical activity change variable was created for each treatment group- (DC and NC) by-cohort (1 and 2). The change variable was then compared among these four groups using ANOVA. No significant difference in the change in physical activity from baseline to week 18 among treatment group-by-cohort was observed. The effect of time on physical activity was assessed within each group using repeated measures ANOVA. Self-reported physical activity did not differ within the four treatment group-by-cohort categories or between treatment groups over time.
Blood pressure
From baseline to week 18, women in both DC and NC groups experienced significant reductions in systolic and diastolic BP (Table 3). For women in the DC group, the significant change in diastolic BP occurred by week 12. For women in the NC group, significant changes in systolic and diastolic BP occurred by week 12. Differences in BP measurements between groups were not found, and the changes within DC and NC groups over time were not different between groups.
Table 3.
Blood pressure measurements and selected metabolic biomarkers of premenopausal women with overweight/obesity at baseline and change from baseline at weeks 6, 12 and 18 in a study evaluating changes in body weight, blood pressure and selected metabolic biomarkers with an energy-restricted diet including twice daily dark chocolate snacks plus once daily sugar-free cocoa beverage or twice daily non-chocolate snacks plus once daily sugar-free non-cocoa beverage
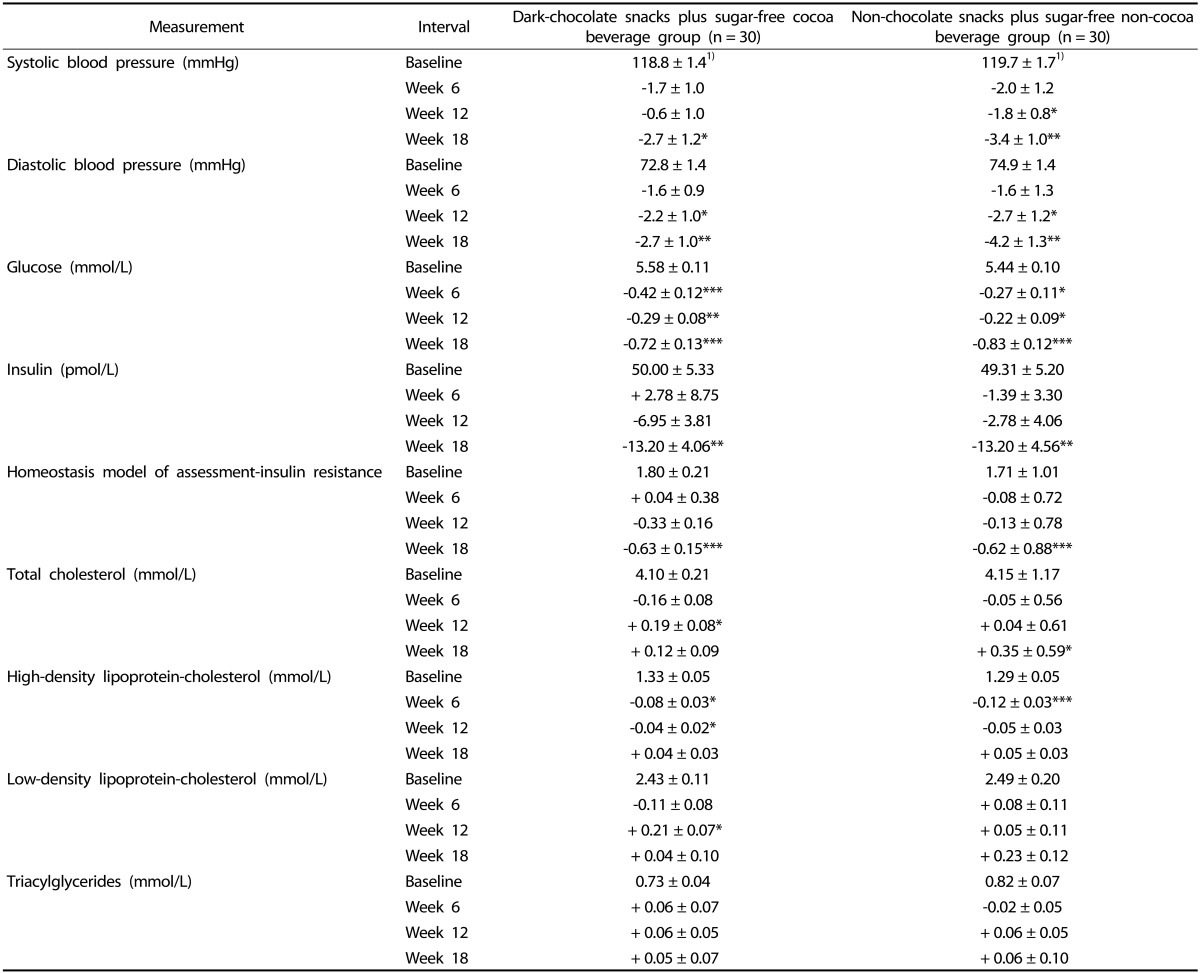
1)Values are means ± SEM for baseline values and mean ± SEM for change from baseline values. P-values from intention-to-treat analysis, using analysis of covariance with repeated measures on the time factor.
*P < 0.05, **P < 0.01, ***P < 0.001 within group change from baseline. There were no significant differences in measurements between snack/beverage groups at any interval or for the change over time between snack/beverage groups. Homeostasis model of assessment-insulin resistance calculated by fasting insulin concentration (µIU/mL) × fasting glucose concentration (mg/dL) × 0.0555/22.5.
Metabolic profile
Women in the DC group had decreases in serum glucose concentration by week 6 (7.5% ↓, P < 0.001), and at week 12 (5.3% ↓, P < 0.01) and week 18 (12.9% ↓, P < 0.001) compared to baseline (Table 3). Women in the NC group also experienced decreases in serum glucose by week 6 (4.9% ↓, P < 0.05), at week 12 (4.0% ↓, P < 0.05) and week 18 (15.2% ↓, P < 0.001) compared to baseline. Serum glucose concentrations did not differ between groups at any interval, and the change over time within each group did not differ between groups.
Serum insulin concentration decreased in the DC group (26.4% ↓, P < 0.01) and in the NC group (26.8% ↓, P < 0.01) from baseline to week 18 (Table 3). Differences between groups at any interval or for the change over time between groups in serum insulin were not statistically significant. HOMA-IR followed a pattern similar to serum insulin for both DC and NC groups.
Serum lipid concentrations did not differ between groups at any interval (Table 3). In the DC group, serum total and LDL-C increased by week 12 but returned to baseline levels at week 18. HDL-C decreased by week 6 but returned to baseline level at week 18 for women in the DC group. Women in the NC group had an increase in serum total cholesterol from baseline that persisted to week 18 (8.5% ↑, P < 0.05). Women in the NC group also had a decrease in HDL-C from baseline to week 6 (9.5% ↓, P < 0.001) that returned to baseline level at week 18. Changes over time in serum lipids were not different between DC and NC groups.
Efficacy analysis
Dietary intake assessment
For those participants who completed the study (n = 51), women in the DC (n = 26) and NC (n = 25) groups reduced estimated energy intake by 1,900 kJ/day (P < 0.001) and 2,213 kJ/day (P < 0.001), respectively. Macronutrient intake did not change in the DC group, while percent of energy from protein increased for women in the NC group (P < 0.01). Changes in total sugar, fiber, saturated fat, cholesterol and sodium intakes within and between groups over time were similar to results previously reported using the intention-to-treat analysis.
Anthropometric and blood pressure measurements and metabolic profile
Due to the low number of dropouts from each group, changes in anthropometric and BP measurements and glucose, insulin, HOMA-IR and lipid concentrations in the efficacy analysis were greater but had similar statistical significance to changes observed in intention-to-treat analysis. From baseline to week 18, BW decreased by 5.1 ± 1.4 kg (P < 0.001) and 5.9 ± 0.9 kg (P < 0.001) in the DC group and NC group, respectively. In the DC group, BMI, waist circumference, hip circumference and BF% decreased by 1.9 ± 0.2 kg/m2 (P < 0.001), 5.8 ± 0.9 cm (P < 0.001), 6.0 ± 0.7 cm (P < 0.001) and 3.7 ± 0.4% (P < 0.001), respectively, from baseline to week 18. In the NC group, BMI, waist circumference, hip circumference and BF% decreased by 2.2 ± 0.3 kg/m2 (P < 0.001), 6.1 ± 1.0 cm (P < 0.001), 5.8 ± 0.7 cm (P < 0.001) and 3.3 ± 0.6% (P < 0.001), respectively, from baseline to week 18. These changes over time were not significantly different between DC and NC groups.
Systolic and diastolic BP, respectively, decreased by 3.6 ± 1.4 mmHg (P < 0.05) and 3.5 ± 1.1 mmHg (P < 0.05) and by 4.3 ± 1.1 mmHg (P < 0.01) and 5.3 ± 1.4 mmHg (P < 0.01) in the DC group and NC group, respectively, from baseline to week 18. Serum glucose and insulin concentrations and HOMA-IR, respectively, decreased by 14.7% (P < 0.001), 30.6% (P < 0.01) and 40.2% (P < 0.001) and by 17.9% (P < 0.001), 28.2% (P < 0.05) and 39.6% (P < 0.001) in the DC group and NC group, respectively, from baseline to week 18. Values for serum lipid concentrations for participants who completed the study by DC or NC group were within 15% of values for intention-to-treat analysis. Changes over time in BP measurements and metabolic biomarkers did not differ between groups.
DISCUSSION
Premenopausal women with overweight/obesity who followed an 18-week ERD that included twice daily dark chocolate or non-chocolate sweet snacks plus once daily sugar-free beverage were able to achieve an energy deficit, reduce BW and significantly improve BP and glucose and insulin concentrations. Participants in both groups were compliant with snack and beverage intake, as well as reducing overall energy intake as evidenced by improvements in BW and other anthropometric measurements. Although the hypothesis was not supported, and differential effects between the DC group and NC group were not found in this randomized comparative study, the two snack and beverage assignments were equally effective in promoting significant BW loss and improvements in metabolic parameters while following a dietary approach that did not appreciably alter macronutrient composition of the habitual diet or completely eliminate sweet snacks. Because the current study did not include a non-snack and non-beverage control group, further evaluation is needed.
Golomb et al. [14] found an inverse relationship between frequency of chocolate consumption and BMI among nearly 1,000 adults, ages 20 to 85 years, even after adjusting for age, gender, physical activity, dietary components and energy intake. Using National Health and Nutrition Examination Survey, 1999-2004, data, O'Neil et al. [13] reported that chocolate consumers had significantly lower BW and waist circumference compared to non-consumers. Conversely, a prospective analysis by Greenberg and Buijsse [40] found more frequent consumption of chocolate to be significantly associated with greater weight gain over the long term. These epidemiological studies cannot draw causal inferences regarding effects of chocolate intake on BW or BMI but rather provide direction for future research to confirm these results. Further, these studies rely on self-reported data and not all distinguished the type of chocolate consumed. Randomized clinical trials exploring the impact of dark chocolate and cocoa on anthropometric measurements as the primary outcome measure are few; however, Desch et al. [41] observed a slight weight gain after three months of consuming 25 g of dark chocolate per day. Conversely, Taubert et al. [18] did not detect a change in BW after 18 weeks of daily consumption of 6.3 g of dark chocolate.
In an experimental study, Matsui et al. [42] demonstrated that cocoa intake for three weeks led to lower BW and white adipose tissue weight in male Wistar rats fed a high-fat diet compared to rats fed mimetic cocoa and high-fat diet. Cocoa consumption in these animals suppressed fatty acid synthase and other liver enzymes required for fatty acid synthesis. In addition, fatty acid binding protein and fatty acid synthase were lowered in white adipose tissue of cocoa-fed rats, suggesting altered lipid metabolism with cocoa intake in the presence of high dietary fat (-50% of total energy) [42]. Min et al. [43] cultured 3T3-L1 preadipocytes with cocoa polyphenol extract and found that signaling systems for cell proliferation were blunted. In a complementary 5-week whole animal study, Min et al. [43] further showed that C57BL/6N mice fed a high-fat diet and cocoa polyphenol extract had lesser BW gain and adiposity compared to mice fed only the high-fat diet. These experimental studies suggest biologically plausible mechanisms by which cocoa may moderate BW and FM. Results of the current comparative trial are somewhat inconsistent with human epidemiologic and clinical trials [13,14,18,40,41], experimental animal [42,43] and cell culture [43] studies, in that women in the NC group also experienced benefits to BW and BF%. Discordant results are likely due to the design of the current study conducted in free-living women, where the total diet was not controlled and the lack of a control group. Nonetheless significant BW loss and improved BF% was achieved by incorporating preferred sweet snacks within an ERD.
Effects on BP, glucose, insulin, HOMA-IR, total cholesterol, HDL-C, triacylglycerides and LDL-C did not differ between the DC group and NC group. Several meta-analyses have indicated that consumption of 500 to 1,000 mg of cocoa flavanols per day results in acute (i.e., 2 to 12 weeks, with one 18-week trial) health benefits, including lowering of SBP [21,22,28] and DBP [21,24,28] as well as moderating insulin [24], HOMA-IR [22,24], total cholesterol [44] and LDL-C [22,24,44] and improving HDL-C [22,24], although one meta-analysis did not find any consistent effects on HDL-C [44]. One systematic review has reported no effect of cocoa intake on total cholesterol [22], and two reviews have indicated a lack of effect of cocoa on triacylglycerides [22,44]. Changes in the DC group were consistent with previous findings for SBP (-2.77 mmHg) [21] and DBP (-2.20 mmHg) [21], although mean differences for SBP and DBP were slightly better in the NC group. The change in HOMA-IR for the DC group was similar to the finding (-0.67) reported in the meta-analysis by Hooper et al. [24]. Although these benefits also were apparent for women in the NC group, the flavanol dosage for the DC group (270 mg) was less than the 500 to 1,000 mg used in previous studies. Blood lipids did not change or changed in expected directions when compared to other studies [16,20,45]. BW reduction has been shown to produce favorable changes in metabolic risk factors for disease [46,47]. Therefore, the weight loss achieved by women in the DC group may have overshadowed any differential effect or potentially additive effect of dark chocolate and cocoa intake on metabolic markers, given that comparable BW loss occurred in the NC group with similar metabolic outcomes.
This study is limited to premenopausal women of specific age and BMI ranges and cannot be generalized to others. The duration of 18 weeks was shorter than the ideal length of ≥ 24 weeks for a short-term weight-loss study; however, 18 weeks was of sufficient duration to detect glucose, insulin and blood lipid changes measured in the current study and this duration was consistent or longer than previous trials examining dark chocolate and cocoa. Nutrition education classes included in the current intervention make it difficult to distinguish between positive outcomes attributed to weekly visits with a registered dietitian from benefits of dietary components. The current study was not a metabolic feeding trial and consequently relied on self-reported dietary intakes that have previously been shown to be underreported in overweight/obese individuals [48]. The current intervention was conducted with free-living women to mimic a real world scenario, and positive changes in BW, FM and metabolic markers demonstrated compliance with the dietary intervention, including energy restriction. Long-term intervention studies are needed, and future studies should compare inclusion of cocoa-based sweet snacks and sugar-free cocoa beverages into an ERD against a control ERD that does not include snacks or beverages to make appropriate recommendations for weight management programs. While the lack of a control group limits the generalizability of results, the design of the current study was to compare a sweet, widely consumed snack to one that is not as widely consumed. However, future interventions should compare an ERD that includes sweet snacks and beverages as well as sugar-free snacks and beverages to an ERD that does not.
While emerging evidence from animal, human and in vitro studies suggests dark chocolate and cocoa may have beneficial effects on body weight and other anthropometric measures [30], the current study found that an 18-week ERD that included twice daily dark chocolate snacks plus once daily sugar-free cocoa beverage resulted in a magnitude of BW loss and changes in BP and metabolic markers that were comparable to an ERD that included a sweet snack without dark chocolate or cocoa and sugar-free cocoa-free beverage. Premenopausal women with overweight/obesity and without established hypertension or hyperglycemia may experience clinically significant improvements in BP and glucose and insulin concentrations with a moderate 6.5% decrease in BW facilitated by an ERD that includes two energy- and portion-controlled sweet snacks and one sugar-free beverage daily. Portion- and energy-controlled sweet snacks, including dark chocolate or non-chocolate snacks, may be included in diets intended for BW modification.
ACKNOWLEDGMENTS
Authors thank the participants for their engagement in this study and undergraduate student research assistants who contributed to participant recruitment and retention. This research was supported by a grant from The Hershey Company, including provision of dark chocolate and non-chocolate snacks and cocoa and non-cocoa beverage mixes in addition to Graduate Research Assistant support. Graduate Research Fellowships were provided through the American Dietetic Association, The American Association of Family and Consumer Sciences, and The Pennsylvania State University.
References
- 1.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 2.Withrow D, Alter DA. The economic burden of obesity worldwide: a systematic review of the direct costs of obesity. Obes Rev. 2011;12:131–141. doi: 10.1111/j.1467-789X.2009.00712.x. [DOI] [PubMed] [Google Scholar]
- 3.Hankey CR. Session 3 (joint with the British Dietetic Association): management of obesity: weight-loss interventions in the treatment of obesity. Proc Nutr Soc. 2010;69:34–38. doi: 10.1017/S0029665109991844. [DOI] [PubMed] [Google Scholar]
- 4.Wu T, Gao X, Chen M, van Dam RM. Long-term effectiveness of diet-plus-exercise interventions vs. diet-only interventions for weight loss: a meta-analysis. Obes Rev. 2009;10:313–323. doi: 10.1111/j.1467-789X.2008.00547.x. [DOI] [PubMed] [Google Scholar]
- 5.Sears B, Lawren B. Enter the Zone: A Dietary Road Map. New York (NY): Harper Collins; 1995. [Google Scholar]
- 6.Ornish D. Eat More, Weigh Less: Dr. Dean Ornish's Program for Losing Weight Safely While Eating Abundantly. New York (NY): Harper Collins; 2001. [Google Scholar]
- 7.Atkins RC. Dr. Atkins' New Diet Revolution. New York (NY): Harper Collins; 2002. [Google Scholar]
- 8.Agatston A. The South Beach Diet : The Delicious, Doctor-Designed, Foolproof Plan for Fast and Healthy Weight Loss. Emmaus (PA): Rodale; 2003. [Google Scholar]
- 9.Piehowski KE, Preston AG, Miller DL, Nickols-Richardson SM. A reduced-calorie dietary pattern including a daily sweet snack promotes body weight reduction and body composition improvements in premenopausal women who are overweight and obese: a pilot study. J Am Diet Assoc. 2011;111:1198–1203. doi: 10.1016/j.jada.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rozin P, Levine E, Stoess C. Chocolate craving and liking. Appetite. 1991;17:199–212. doi: 10.1016/0195-6663(91)90022-k. [DOI] [PubMed] [Google Scholar]
- 11.Zellner DA, Garriga-Trillo A, Rohm E, Centeno S, Parker S. Food liking and craving: a cross-cultural approach. Appetite. 1999;33:61–70. doi: 10.1006/appe.1999.0234. [DOI] [PubMed] [Google Scholar]
- 12.Lewis JR, Prince RL, Zhu K, Devine A, Thompson PL, Hodgson JM. Habitual chocolate intake and vascular disease: a prospective study of clinical outcomes in older women. Arch Intern Med. 2010;170:1857–1858. doi: 10.1001/archinternmed.2010.396. [DOI] [PubMed] [Google Scholar]
- 13.O'Neil CE, Fulgoni VL, 3rd, Nicklas TA. Candy consumption was not associated with body weight measures, risk factors for cardiovascular disease, or metabolic syndrome in US adults: NHANES 1999-2004. Nutr Res. 2011;31:122–130. doi: 10.1016/j.nutres.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Golomb BA, Koperski S, White HL. Association between more frequent chocolate consumption and lower body mass index. Arch Intern Med. 2012;172:519–521. doi: 10.1001/archinternmed.2011.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khawaja O, Gaziano JM, Djoussé L. Chocolate and coronary heart disease: a systematic review. Curr Atheroscler Rep. 2011;13:447–452. doi: 10.1007/s11883-011-0203-2. [DOI] [PubMed] [Google Scholar]
- 16.Grassi D, Lippi C, Necozione S, Desideri G, Ferri C. Short-term administration of dark chocolate is followed by a significant increase in insulin sensitivity and a decrease in blood pressure in healthy persons. Am J Clin Nutr. 2005;81:611–614. doi: 10.1093/ajcn/81.3.611. [DOI] [PubMed] [Google Scholar]
- 17.Grassi D, Desideri G, Necozione S, Lippi C, Casale R, Properzi G, Blumberg JB, Ferri C. Blood pressure is reduced and insulin sensitivity increased in glucose-intolerant, hypertensive subjects after 15 days of consuming high-polyphenol dark chocolate. J Nutr. 2008;138:1671–1676. doi: 10.1093/jn/138.9.1671. [DOI] [PubMed] [Google Scholar]
- 18.Taubert D, Roesen R, Lehmann C, Jung N, Schomig E. Effects of low habitual cocoa intake on blood pressure and bioactive nitric oxide: a randomized controlled trial. JAMA. 2007;298:49–60. doi: 10.1001/jama.298.1.49. [DOI] [PubMed] [Google Scholar]
- 19.Allen RR, Carson L, Kwik-Uribe C, Evans EM, Erdman JW., Jr Daily consumption of a dark chocolate containing flavanols and added sterol esters affects cardiovascular risk factors in a normotensive population with elevated cholesterol. J Nutr. 2008;138:725–731. doi: 10.1093/jn/138.4.725. [DOI] [PubMed] [Google Scholar]
- 20.Almoosawi S, Fyfe L, Ho C, Al-Dujaili E. The effect of polyphenol-rich dark chocolate on fasting capillary whole blood glucose, total cholesterol, blood pressure and glucocorticoids in healthy overweight and obese subjects. Br J Nutr. 2010;103:842–850. doi: 10.1017/S0007114509992431. [DOI] [PubMed] [Google Scholar]
- 21.Ried K, Sullivan TR, Fakler P, Frank OR, Stocks NP. Effect of cocoa on blood pressure. Cochrane Database Syst Rev. 2012;8:CD008893. doi: 10.1002/14651858.CD008893.pub2. [DOI] [PubMed] [Google Scholar]
- 22.Shrime MG, Bauer SR, McDonald AC, Chowdhury NH, Coltart CE, Ding EL. Flavonoid-rich cocoa consumption affects multiple cardiovascular risk factors in a meta-analysis of short-term studies. J Nutr. 2011;141:1982–1988. doi: 10.3945/jn.111.145482. [DOI] [PubMed] [Google Scholar]
- 23.Davison K, Coates AM, Buckley JD, Howe PR. Effect of cocoa flavanols and exercise on cardiometabolic risk factors in overweight and obese subjects. Int J Obes (Lond) 2008;32:1289–1296. doi: 10.1038/ijo.2008.66. [DOI] [PubMed] [Google Scholar]
- 24.Hooper L, Kay C, Abdelhamid A, Kroon PA, Cohn JS, Rimm EB, Cassidy A. Effects of chocolate, cocoa, and flavan-3-ols on cardiovascular health: a systematic review and meta-analysis of randomized trials. Am J Clin Nutr. 2012;95:740–751. doi: 10.3945/ajcn.111.023457. [DOI] [PubMed] [Google Scholar]
- 25.Mursu J, Voutilainen S, Nurmi T, Rissanen TH, Virtanen JK, Kaikkonen J, Nyyssönen K, Salonen JT. Dark chocolate consumption increases HDL cholesterol concentration and chocolate fatty acids may inhibit lipid peroxidation in healthy humans. Free Radic Biol Med. 2004;37:1351–1359. doi: 10.1016/j.freeradbiomed.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Jia L, Liu X, Bai YY, Li SH, Sun K, He C, Hui R. Short-term effect of cocoa product consumption on lipid profile: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2010;92:218–225. doi: 10.3945/ajcn.2009.28202. [DOI] [PubMed] [Google Scholar]
- 27.Corti R, Flammer AJ, Hollenberg NK, Lüscher TF. Cocoa and cardiovascular health. Circulation. 2009;119:1433–1441. doi: 10.1161/CIRCULATIONAHA.108.827022. [DOI] [PubMed] [Google Scholar]
- 28.Fernández-Murga L, Tarín JJ, García-Perez MA, Cano A. The impact of chocolate on cardiovascular health. Maturitas. 2011;69:312–321. doi: 10.1016/j.maturitas.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 29.Tey SL, Brown RC, Gray AR, Chisholm AW, Delahunty CM. Long-term consumption of high energy-dense snack foods on sensory-specific satiety and intake. Am J Clin Nutr. 2012;95:1038–1047. doi: 10.3945/ajcn.111.030882. [DOI] [PubMed] [Google Scholar]
- 30.Farhat G, Drummond S, Fyfe L, Al-Dujaili EA. Dark chocolate: an obesity paradox or a culprit for weight gain? Phytother Res. 2014;28:791–797. doi: 10.1002/ptr.5062. [DOI] [PubMed] [Google Scholar]
- 31.Panel on Macronutrients; Panel on the Definition of Dietary Fiber; Subcommittee on Upper Reference Levels of Nutrients; Subcommittee on Interpretation and Uses of Dietary Reference Intakes; Standing Committee on the Scientific Evaluation of Dietary Reference Intakes; Food and Nutrition Board; Institute of Medicine (US) Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids. Washington, D.C.: National Academies Press; 2005. [Google Scholar]
- 32.Harris JA, Benedict FG. A Biometric Study of Basal Metabolism in Man. Washington, D.C.: Carnegie Institute of Washington; 1919. [Google Scholar]
- 33.Hill JO, Peters JC. Environmental contributions to the obesity epidemic. Science. 1998;280:1371–1374. doi: 10.1126/science.280.5368.1371. [DOI] [PubMed] [Google Scholar]
- 34.Stroebele N, Ogden LG, Hill JO. Do calorie-controlled portion sizes of snacks reduce energy intake? Appetite. 2009;52:793–796. doi: 10.1016/j.appet.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sciamanna CN, Kiernan M, Rolls BJ, Boan J, Stuckey H, Kephart D, Miller CK, Jensen G, Hartmann TJ, Loken E, Hwang KO, Williams RJ, Clark MA, Schubart JR, Nezu AM, Lehman E, Dellasega C. Practices associated with weight loss versus weight-loss maintenance results of a national survey. Am J Prev Med. 2011;41:159–166. doi: 10.1016/j.amepre.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 36.Rolls BJ. Plenary lecture 1: dietary strategies for the prevention and treatment of obesity. Proc Nutr Soc. 2010;69:70–79. doi: 10.1017/S0029665109991674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sallis JF, Haskell WL, Wood PD, Fortmann SP, Rogers T, Blair SN, Paffenbarger RS., Jr Physical activity assessment methodology in the Five-City Project. Am J Epidemiol. 1985;121:91–106. doi: 10.1093/oxfordjournals.aje.a113987. [DOI] [PubMed] [Google Scholar]
- 38.Tsai MC, Chang CM, Huang TL. Changes in high-density lipoprotein and homeostasis model assessment of insulin resistance in medicated schizophrenic patients and healthy controls. Chang Gung Med J. 2010;33:613–618. [PubMed] [Google Scholar]
- 39.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 40.Greenberg JA, Buijsse B. Habitual chocolate consumption may increase body weight in a dose-response manner. PLoS One. 2013;8:e70271. doi: 10.1371/journal.pone.0070271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Desch S, Kobler D, Schmidt J, Sonnabend M, Adams V, Sareban M, Eitel I, Bluher M, Schuler G, Thiele H. Low vs. higher-dose dark chocolate and blood pressure in cardiovascular high-risk patients. Am J Hypertens. 2010;23:694–700. doi: 10.1038/ajh.2010.29. [DOI] [PubMed] [Google Scholar]
- 42.Matsui N, Ito R, Nishimura E, Yoshikawa M, Kato M, Kamei M, Shibata H, Matsumoto I, Abe K, Hashizume S. Ingested cocoa can prevent high-fat diet-induced obesity by regulating the expression of genes for fatty acid metabolism. Nutrition. 2005;21:594–601. doi: 10.1016/j.nut.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 43.Min SY, Yang H, Seo SG, Shin SH, Chung MY, Kim J, Lee SJ, Lee HJ, Lee KW. Cocoa polyphenols suppress adipogenesis in vitro and obesity in vivo by targeting insulin receptor. Int J Obes (Lond) 2013;37:584–592. doi: 10.1038/ijo.2012.85. [DOI] [PubMed] [Google Scholar]
- 44.Tokede OA, Gaziano JM, Djoussé L. Effects of cocoa products/dark chocolate on serum lipids: a meta-analysis. Eur J Clin Nutr. 2011;65:879–886. doi: 10.1038/ejcn.2011.64. [DOI] [PubMed] [Google Scholar]
- 45.Engler MB, Engler MM, Chen CY, Malloy MJ, Browne A, Chiu EY, Kwak HK, Milbury P, Paul SM, Blumberg J, Mietus-Snyder ML. Flavonoid-rich dark chocolate improves endothelial function and increases plasma epicatechin concentrations in healthy adults. J Am Coll Nutr. 2004;23:197–204. doi: 10.1080/07315724.2004.10719361. [DOI] [PubMed] [Google Scholar]
- 46.Nordmann AJ, Nordmann A, Briel M, Keller U, Yancy WS, Jr, Brehm BJ, Bucher HC. Effects of low-carbohydrate vs low-fat diets on weight loss and cardiovascular risk factors: a meta-analysis of randomized controlled trials. Arch Intern Med. 2006;166:285–293. doi: 10.1001/archinte.166.3.285. [DOI] [PubMed] [Google Scholar]
- 47.Makris A, Foster GD. Dietary approaches to the treatment of obesity. Psychiatr Clin North Am. 2011;34:813–827. doi: 10.1016/j.psc.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schoeller DA. How accurate is self-reported dietary energy intake? Nutr Rev. 1990;48:373–379. doi: 10.1111/j.1753-4887.1990.tb02882.x. [DOI] [PubMed] [Google Scholar]