Abstract
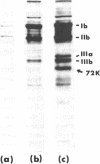
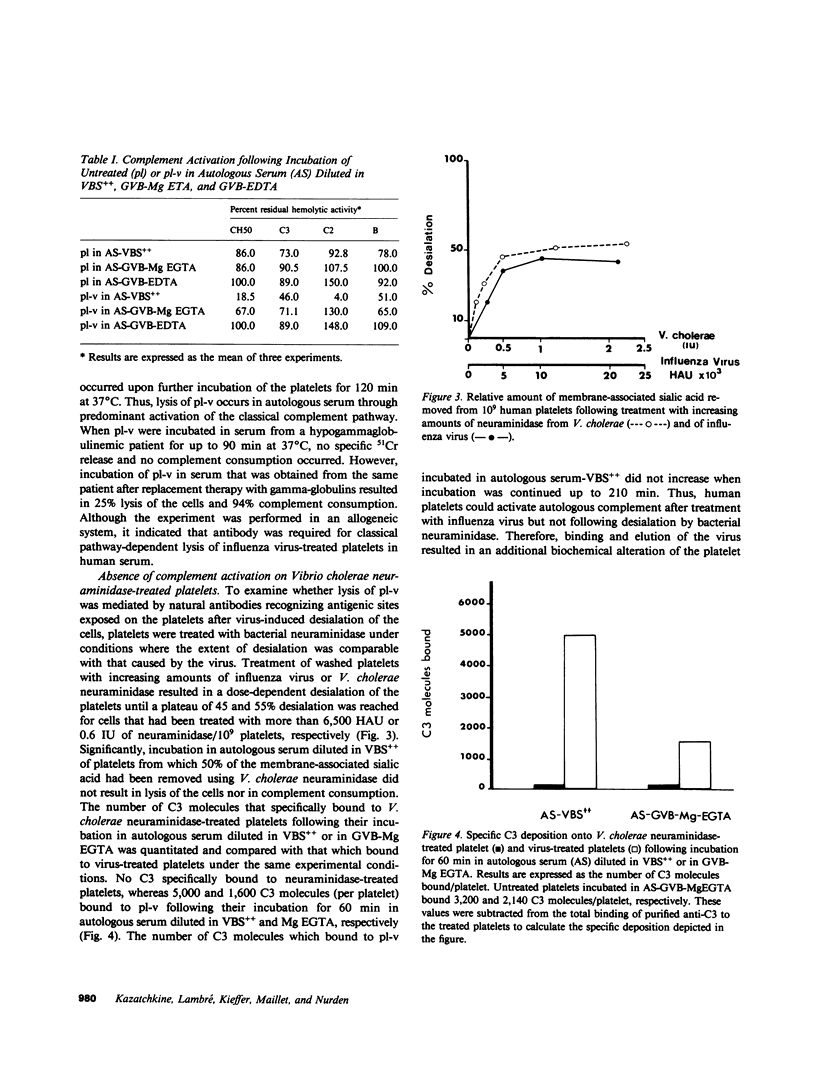
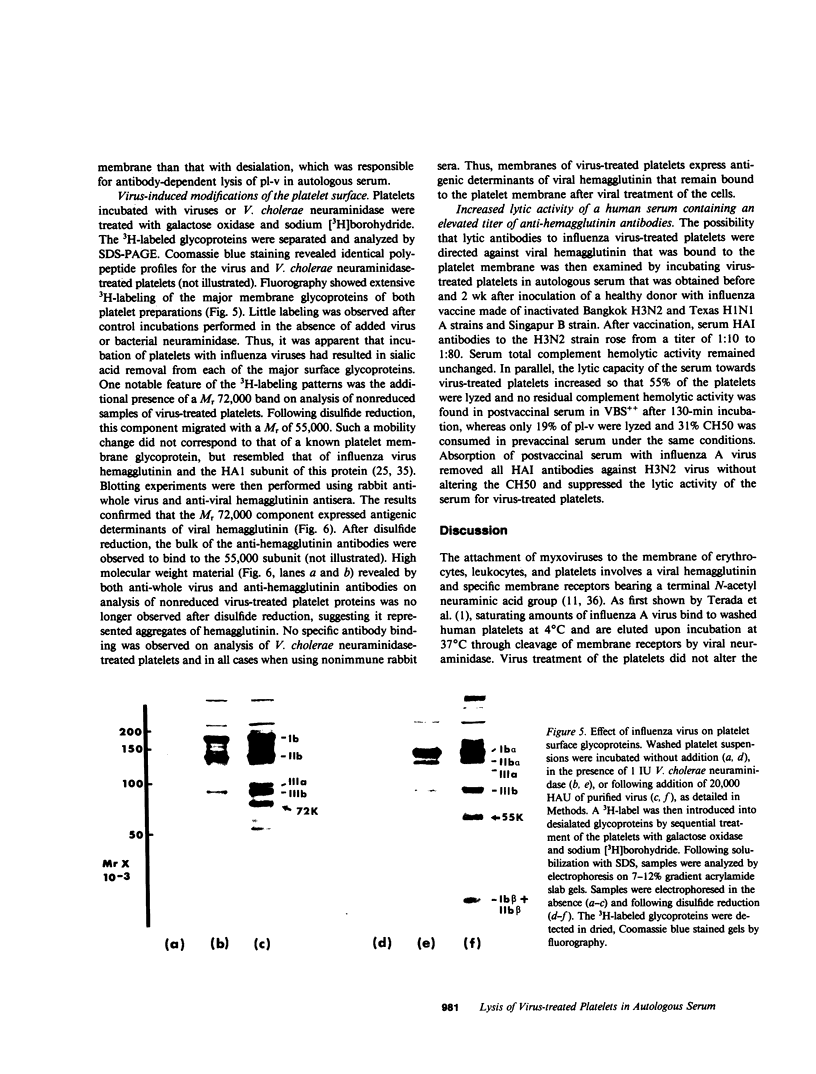
Influenza A virus-treated human platelets were lyzed in autologous serum. Lysis required the presence of antibody and occurred predominantly through activation of the classical complement pathway. Binding of the virus followed by its elution at 37 degrees C resulted in a dose-dependent desialation of the cells with a maximal release of 45% of total platelet sialic acid. In contrast, platelets that had been treated with Vibrio cholerae neuraminidase and from which 55% of total sialic acid had been removed were not lyzed in autologous serum and did not bind C3 as shown in binding assays using radiolabeled monoclonal anti-C3 antibody. Thus, the immune-mediated lysis of virus-treated platelets in autologous serum did not involve neoantigens expressed by desialated cells. To assess the effect of viruses on the platelet surface, treated platelets were incubated with galactose oxidase and sodium [3H]borohydride prior to separation and analysis of the labeled glycoproteins by SDS-PAGE. Viral treatment resulted in a desialation of each of the surface glycoproteins. At the same time, a labeled component of Mr 72,000 (nonreduced) and Mr 55,000 (reduced) was observed that was not present when V. cholerae-desialated platelets were examined in the same way. Immunoblotting experiments performed using antiwhole virus and anti-hemagglutinin antibodies demonstrated this component to be viral hemagglutinin. Involvement of membrane-bound hemagglutinin in antibody and in complement-mediated lysis of virus-treated platelets in autologous serum was supported by the increased lytic activity of a postvaccinal serum containing an elevated titer of complement fixing anti-hemagglutinin antibodies. Binding of a viral protein to the platelet surface provides a model for immune thrombocytopenias occurring during acute viral infections at the time of the specific immune response.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett J. S., Vilaire G., Cines D. B. Identification of the fibrinogen receptor on human platelets by photoaffinity labeling. J Biol Chem. 1982 Jul 25;257(14):8049–8054. [PubMed] [Google Scholar]
- Brand C. M., Skehel J. J. Crystalline antigen from the influenza virus envelope. Nat New Biol. 1972 Aug 2;238(83):145–147. doi: 10.1038/newbio238145a0. [DOI] [PubMed] [Google Scholar]
- Brown E. J., Joiner K. A., Frank M. M. Interaction of desialated guinea pig erythrocytes with the classical and alternative pathways of guinea pig complement in vivo and in vitro. J Clin Invest. 1983 Jun;71(6):1710–1719. doi: 10.1172/JCI110925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHARKES N. D. Purpuric chickenpox: report of a case, review of the literature, and classification by clinical features. Ann Intern Med. 1961 Apr;54:745–759. doi: 10.7326/0003-4819-54-4-745. [DOI] [PubMed] [Google Scholar]
- Cabezas J. A., Calvo P., Eid P., Martin J., Perez E., Reglero A., Hannoun C. Neuraminidase from influenza virus A (H3N2): specificity towards several substrates and procedure of activity determination. Biochim Biophys Acta. 1980 Dec 4;616(2):228–238. doi: 10.1016/0005-2744(80)90141-2. [DOI] [PubMed] [Google Scholar]
- Choi S. I., Simone J. V., Jorney L. J. Neuraminidase-induced thrombocytopenia in rats. Br J Haematol. 1972 Jan;22(1):93–101. doi: 10.1111/j.1365-2141.1972.tb08790.x. [DOI] [PubMed] [Google Scholar]
- Fearon D. T., Daha M. R., Strom T. B., Weiler J. M., Carpenter C. B., Austen K. F. Pathways of complement activation in membranoproliferative glomerulonephritis and allograft rejection. Transplant Proc. 1977 Mar;9(1):729–739. [PubMed] [Google Scholar]
- Fearon D. T. Regulation by membrane sialic acid of beta1H-dependent decay-dissociation of amplification C3 convertase of the alternative complement pathway. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1971–1975. doi: 10.1073/pnas.75.4.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUDSON J. B., WEINSTEIN L., CHANG T. W. Thrombocytopenic purpura in measles. J Pediatr. 1956 Jan;48(1):48–56. doi: 10.1016/s0022-3476(56)80116-9. [DOI] [PubMed] [Google Scholar]
- Kazatchkine M. D., Fearon D. T., Austen K. F. Human alternative complement pathway: membrane-associated sialic acid regulates the competition between B and beta1 H for cell-bound C3b. J Immunol. 1979 Jan;122(1):75–81. [PubMed] [Google Scholar]
- Kelton J. G., Keystone J., Moore J., Denomme G., Tozman E., Glynn M., Neame P. B., Gauldie J., Jensen J. Immune-mediated thrombocytopenia of malaria. J Clin Invest. 1983 Apr;71(4):832–836. doi: 10.1172/JCI110836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb H., Schudt C., Kolb-Bachofen V., Kolb H. A. Cellular recognition by rat liver cells of neuraminidase-treated erythrocytes. Demonstration and analysis of cell contacts. Exp Cell Res. 1978 May;113(2):319–325. doi: 10.1016/0014-4827(78)90372-5. [DOI] [PubMed] [Google Scholar]
- Lambre C. R., Thibon M., Le Maho S., Di Bella G. Auto-antibody dependent activation of the autologous classical complement pathway by guinea-pig red cells treated with influenza virus or neuraminidase: in vitro and in vivo study. Immunology. 1983 Jun;49(2):311–319. [PMC free article] [PubMed] [Google Scholar]
- Lambre C., Kasturi K. N. A microplate immunoenzyme assay for anti-influenza antibodies. J Immunol Methods. 1979;26(1):61–67. doi: 10.1016/0022-1759(79)90041-3. [DOI] [PubMed] [Google Scholar]
- Lambré C. R., Kazatchkine M. D., Maillet F., Thibon M. Guinea pig erythrocytes, after their contact with influenza virus, acquire the ability to activate the human alternative complement pathway through virus-induced desialation of the cells. J Immunol. 1982 Feb;128(2):629–634. [PubMed] [Google Scholar]
- Lambré C., Thibon M. Ability to activate the alternative complement pathway acquired by human and guinea-pig erythrocytes after contact with influenza virus. Ann Immunol (Paris) 1980 Mar-Apr;131C(2):213–221. [PubMed] [Google Scholar]
- Leroy C., Peltier A. P. Fixations quantitatives comparées du complément total et de ses quatre premiers composants (C1, C4, C2 et C3) au cours des réactions antigéne-anticorps in vitro. Ann Immunol (Paris) 1973 Feb;124(1):87–96. [PubMed] [Google Scholar]
- Morse E. E., Zinkham W. H., Jackson D. P. Thrombocytopenic purpura following rubella infection in children and adults. Arch Intern Med. 1966 Apr;117(4):573–579. [PubMed] [Google Scholar]
- NELSON E. R., BIERMAN H. R. DENGUE FEVER: A THROMBOCYTOPENIC DISEASE? JAMA. 1964 Oct 12;190:99–103. doi: 10.1001/jama.1964.03070150009002. [DOI] [PubMed] [Google Scholar]
- Nelson R. A., Jr, Jensen J., Gigli I., Tamura N. Methods for the separation, purification and measurement of nine components of hemolytic complement in guinea-pig serum. Immunochemistry. 1966 Mar;3(2):111–135. doi: 10.1016/0019-2791(66)90292-8. [DOI] [PubMed] [Google Scholar]
- Nurden A. T., Dupuis D., Kunicki T. J., Caen J. P. Analysis of the glycoprotein and protein composition of Bernard-Soulier platelets by single and two-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis. J Clin Invest. 1981 May;67(5):1431–1440. doi: 10.1172/JCI110172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurden A. T., Dupuis D., Pidard D., Kieffer N., Kunicki T. J., Cartron J. P. Surface modifications in the platelets of a patient with alpha-N-acetyl-D-galactosamine residues, the Tn-syndrome. J Clin Invest. 1982 Dec;70(6):1281–1291. doi: 10.1172/JCI110727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nydegger U. E., Fearon D. T., Austen K. F. The modulation of the alternative pathway of complement in C2-deficient human serum by changes in concentration of the component and control proteins. J Immunol. 1978 Apr;120(4):1404–1408. [PubMed] [Google Scholar]
- Nydegger U. E., Kazatchkine M. D. The role of complement in immune clearance of blood cells. Springer Semin Immunopathol. 1983;6(4):373–398. doi: 10.1007/BF02116281. [DOI] [PubMed] [Google Scholar]
- Osborn J. E., Shahidi N. T. Thrombocytopenia in murine cytomegalovirus infection. J Lab Clin Med. 1973 Jan;81(1):53–63. [PubMed] [Google Scholar]
- Paulson J. C., Sadler J. E., Hill R. L. Restoration of specific myxovirus receptors to asialoerythrocytes by incorporation of sialic acid with pure sialyltransferases. J Biol Chem. 1979 Mar 25;254(6):2120–2124. [PubMed] [Google Scholar]
- Ruddy S., Austen K. F. C3 inactivator of man. I. Hemolytic measurement by the inactivation of cell-bound C3. J Immunol. 1969 Mar;102(3):533–543. [PubMed] [Google Scholar]
- Scott S., Reimers H. J., Chernesky M. A., Greenberg J. P., Kinolugh-Rathbone R. L., Packham M. A., Mustard J. F. Effect of viruses on platelet aggregation and platelet survival in rabbits. Blood. 1978 Jul;52(1):47–55. [PubMed] [Google Scholar]
- Stanley P., Crook N. E., Streader L. G., Davidson B. E. The polypeptides of influenza virus. 8. Large-scale purification of the hemagglutinin. Virology. 1973 Dec;56(2):640–645. doi: 10.1016/0042-6822(73)90066-4. [DOI] [PubMed] [Google Scholar]
- Terada H., Baldini M., Ebbe S., Madoff M. A. Interaction of influenza virus with blood platelets. Blood. 1966 Aug;28(2):213–228. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALLACE S. J. Thrombocytopenic purpura after rubella. Lancet. 1963 Jan 19;1(7273):139–141. doi: 10.1016/s0140-6736(63)91023-7. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Wiley D. C., Wilson I. A., Skehel J. J. Structural identification of the antibody-binding sites of Hong Kong influenza haemagglutinin and their involvement in antigenic variation. Nature. 1981 Jan 29;289(5796):373–378. doi: 10.1038/289373a0. [DOI] [PubMed] [Google Scholar]
- Wilson A. B., Planterose D. N., Nagington J., Park J. R., Barry R. D., Coombs R. R. Influenza A antigens on human lymphocytes in vitro and probably in vivo. Nature. 1976 Feb 19;259(5544):582–584. doi: 10.1038/259582a0. [DOI] [PubMed] [Google Scholar]
- Wilson I. A., Skehel J. J., Wiley D. C. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 A resolution. Nature. 1981 Jan 29;289(5796):366–373. doi: 10.1038/289366a0. [DOI] [PubMed] [Google Scholar]
- Zimmerman T. S., Kolb W. P. Human platelet-initiated formation and uptake of the C5-9 complex of human complement. J Clin Invest. 1976 Jan;57(1):203–211. doi: 10.1172/JCI108261. [DOI] [PMC free article] [PubMed] [Google Scholar]