Abstract
Cripto-1 (CR-1)/Teratocarcinoma-derived growth factor1 (TDGF-1) is a cell surface glycosylphosphatidylinositol (GPI)-linked glycoprotein that can function either in cis (autocrine) or in trans (paracrine). The cell membrane cis form is found in lipid rafts and endosomes while the trans acting form lacking the GPI anchor is soluble. As a member of the epidermal growth factor (EGF)/Cripto-1-FRL-1-Cryptic (CFC) family, CR-1 functions as an obligatory co-receptor for the transforming growth factor-β (TGF-β) family members, Nodal and growth and differentiation factors 1 and 3 (GDF1/3) by activating Alk4/Alk7 signaling pathways that involve Smads 2, 3 and 4. In addition, CR-1 can activate non-Smad-dependent signaling elements such as PI3K, Akt and MAPK. Both of these pathways depend upon the 78 kDa glucose regulated protein (GRP78). Finally, CR-1 can facilitate signaling through the canonical Wnt/β-catenin and Notch/Cbf-1 pathways by functioning as a chaperone protein for LRP5/6 and Notch, respectively. CR-1 is essential for early embryonic development and maintains embryonic stem cell pluripotentiality. CR-1 performs an essential role in the etiology and progression of several types of human tumors where it is expressed in a population of cancer stem cells (CSCs) and facilitates epithelial-mesenchymal transition (EMT). In this context, CR-1 can significantly enhance tumor cell migration, invasion and angiogenesis. Collectively, these facts suggest that CR-1 may be an attractive target in the diagnosis, prognosis and therapy of several types of human cancer.
Keywords: Cancer, Stem cells, Epithelial-mesenchymal transition, GRP78, Cripto-1
1. Introduction
Tumors may be considered as caricatures of the process of normal embryonic development whereby oncogeny recapitulates ontogeny in an inappropriate spatiotemporal context [1, 2]. Specifically, the subversion and corruption of embryonic signaling pathways such as Wnt β-catenin, Notch/Cbf-1, Hedgehog/Gli and Nodal/CR-1 may be instrumental as drivers in the initiation and/or progression of multiple types of cancer especially if these pathways are operative in CSCs (the cells thought to propagate the tumor) or transit amplifying (TA) progenitor populations [2, 3]. The loss of appropriate genetic or epigenetic regulatory mechanisms that might occur in normal adult somatic tissue stem cells (SCs), TA cells or the surrounding niche cell populations is a likely contributor to the alteration in expression and/or aberrant activation of these embryonic signaling pathways observed in tumors. The consequences of these alterations may then lead to a disruption in the cell-cell communication between different tissue compartments (epithelial and stromal) and a loss in normal tissue architecture as mediated by the processes of EMT and mesenchymal-epithelial transition (MET).
The normal tissue microenvironment also has a significant influence on the suppression, initiation, and progression of tumor cells. For example, the embryonic microenvironment or the adult stem cell niche can reprogram tumor cells to acquire a more normal cellular lineage restriction and to differentiate [4, 5]. Reciprocally, the tumor microenvironment that consists of myeloid suppressor cells, mesenchymal stem cells which are derived from the bone marrow or surrounding cancer-associated fibroblasts (CAFs) can directly or indirectly through secreted factors reprogram SCs and induced-pluripotent stem cells (iPSCs) to acquire properties of CSCs or tumor initiating cells (TICs) [6, 7]. Identification of these factors that are expressed in cancer cells or by the surrounding niche compartment may provide unique drug targets for cancer therapy.
In this review, we discuss the novel biological properties of the embryonic gene CR-1 and the molecular signaling pathways that are regulated by CR-1 which may contribute to its pro-tumorigenic role in various types of cancer. The expression of CR-1 in potential CSCs or TICs suggests that CR-1 coupled with its capacity to facilitate EMT could prove to be an efficacious therapeutic target for the clinical management of malignant disease.
2. Structure and mechanisms regulating expression of Cripto-1
Cripto-1/TDGF-1 is the original member of the epidermal growth factor (EGF)-Cripto-1-FRL-1-Cryptic (CFC) family of vertebrate signaling molecules. It was initially isolated from human (CR-1) NTERA-2 and mouse (Cr-1) F9 undifferentiated teratocarcinoma cells [8]. Structurally, Cripto-1 is a cell membrane-associated protein containing signal sequences for extracellular secretion, a modified EGF-like domain, a conserved cysteine-rich domain (CFC-motif) and a short hydrophobic carboxy-terminus, which contains sequences for glycosylphosphatidylinositol (GPI) modification [8–13]. Removal of the GPI anchor by GPI-phospholipase D creates a soluble form of biologically active Cripto-1 [14] (Fig 1). Although several studies have shown the presence of both Cripto-1 forms in multiple cell lines and in vivo, biological activities that are differentially regulated by the soluble versus cell-associated form are not yet clearly delineated [11, 15].
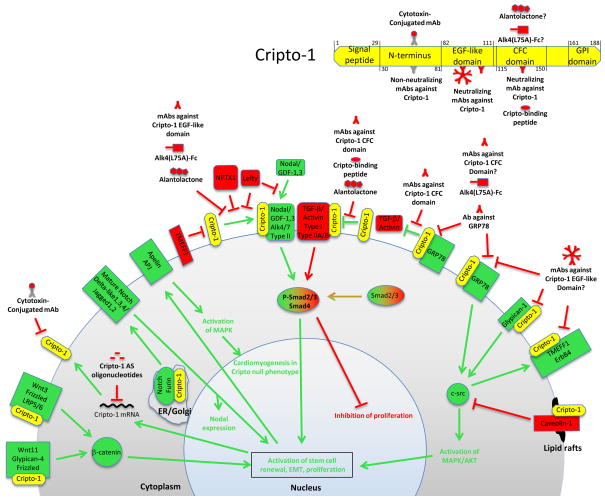
Figure 1.
The multi-faceted Cripto-1-interacting hub. Cripto-1, in yellow, interacts with various components of multiple signaling pathways to drive processes including stem cell renewal, EMT, proliferation and oncogenesis (green). Inhibitors and cellular components that counteract the oncogenic properties of Cripto-1 are shown in red. Above right, the domains of Cripto-1 are shown with therapeutic interactions. Interactions that are hypothesized, but not yet experimentally shown, are indicated by a “?”. Numbers represent the amino acid numbers at the beginning and end of each domain/region.
Mechanisms that directly regulate CR-1 expression during embryogenesis and tumorigenesis are incompletely defined. However, our group has previously shown that the promoter region of the CR-1 gene contains Smad-binding elements and T-cell factor/lymphoid enhancer factor (Tcf/Lef)-binding elements [16, 17]. In addition, during cardiac differentiation, hypoxia directly enhances CR-1 expression through the binding of the trancriptonal factor HIF-1α to hypoxia-responsive elements within the CR-1 promoter [18]. CR-1 has also been identified as a primary target gene of the Wnt/β-catenin signaling pathway in colon carcinoma cells [19]. Furthermore, Behrens and colleagues demonstrated that the CR-1 promoter contains Nkx2-5 binding elements and that Nkx2-5 transcriptionally activates the CR-1 gene in early cardiac progenitors thereby regulating their maintenance and differentiation [20].
CR-1 is directly repressed by the orphan nuclear receptor germ cell nuclear factor (GCNF) during retinoic acid-induced differentiation of human embryonal carcinoma cells following binding of GCNF to a DR0 motif in the human CR-1 promoter region [21]. Bianco and colleagues found that LRH-1 orphan nuclear receptor binds to the CR-1 promoter and positively regulates CR-1 promoter luciferase activity in NTERA-2 cells and CR-1 gene expression in human embryonal and breast carcinoma cell lines as this relates to the methylation status of the CR-1 gene [22]. Interestingly, in Xenopus embryos, although xCR1 mRNA is equally distributed in all cells, xCR1 protein expression is restricted to the cells of the animal hemisphere [23]. This cell-specific translational repression mechanism is regulated through a specific element in the xCR1 mRNA 3′UTR called the TCE (translational control element) which binds Bicaudal-C RNA binding protein [24]. Recently, Chen and colleagues reported that in NSCLC (non-small cell lung cancer) tumors CR-1 is negatively regulated by the miR-15a/16 cluster [25]. Their results indicated that miR-15a-16 can repress CR-1 expression and luciferase activity through the wild-type CR-1 3′UTR which possesses a miR15a/16 binding element.
3. Role of Cripto-1 in embryogenesis and stem cell maintenance
During embryonic development in the mouse, Cr-1 is initially detected prior to gastrulation, in the inner cell mass and in extraembryonic trophoblast cells in the 4-day blastocyst. The highest Cr-1 expression is detected in epiblast cells undergoing EMT that are migrating and that give rise to the mesoderm and endoderm. Cr-1 and Cryptic signaling are involved in regulating the formation of the primitive streak, patterning of the anterior/posterior axis, specification of mesoderm and endoderm during gastrulation, and establishment of left/right (L/R) asymmetry of developing organs [26, 27]. Mouse embryos that lack the Cr-1 gene (Cr-1−/− mice) die at day 7.5 of embryogenesis due to defects in mesoderm formation and axial organization [27, 28]. After day 8 of embryogenesis, Cr-1 expression is restricted to the developing heart. Interestingly, genetic studies in humans have shown the involvement of CR-1 in the pathogenesis of ventricular septal defects, which is one of the most common congenital heart defects [29]. In adults, Cr-1 expression is significantly reduced and is probably sequestered to the stem cell compartment of adult tissues [30].
Cripto-1 is an established regulator of embryonic stem (ES) cells and iPSCs. Together with Nanog, Oct4 and connexin 43, Cripto-1 has been recognized as a potential stem cell marker [30]. Cripto-1 was found as a direct downstream target gene of Oct-4 and Nanog [31]. Reciprocally, Cripto-1, Nodal and Activin are essential in initiating and maintaining the expression of Nanog and Oct-4 [32]. Cripto-1 re-expression was detected with other ES cell genes during reprogramming in iPSCs that are derived from adult differentiated cells [33, 34]. Furthermore, Cripto-1 is involved in a molecular mechanism by which ES cells specify the neural lineage. Boles and colleagues identified a novel binding partner of Cr-1, the neuronal pentraxin 1 (NPTX-1), a secreted protein that is transiently released from differentiating ES cells and that is critical for neural induction [35]. NPTX-1 directly binds to Cripto-1 and inhibits both Nodal and BMP signaling. In the same context, Cr-1−/− embryoid bodies spontaneously differentiate toward neurons, which opens new possibilities for cell replacement therapy in neurodegenerative diseases such as Parkinson’s disease [36]. Cr-1 was also identified as a key factor in the hematopoietic stem cell niche. Together with the cofactor GRP78, Cr-1 has an essential role in the maintenance of dormant ES cells under hypoxia by regulating the HIF-1 complex [37] and in the maintenance of mammary stem cells ex vivo [38].
4. Cripto-1 interacting partners in cellular signaling
Cripto-1 has multiple binding partners and can modulate a variety of intracellular signaling pathways implicated in embryogenesis and oncogenic transformation (Fig 1). During embryogenesis, Cripto-1 functions primarily as a coreceptor for the TGF-β family ligands Nodal and growth and differentiation factors (GDFs) 1 and 3, leading to the activation of type I (Alk4/Alk7) serine-threonine kinase receptors and the Activin type II receptor complex, which triggers both the phosphorylation and activation of the Smad-2/Smad-3 intracellular signaling pathway. Cripto-1 also interacts with other TGF-β family members such as TGF-β1 and Activins A and B, interfering with the binding of these ligands to their cognate receptors and attenuating TGF-β1 and Activin A and B signaling in multiple cell lines. In addition to functioning as a co-receptor for Nodal, Cripto-1 can also function as a ligand for Glypican-1 which is a GPI-anchored heparan sulfate proteoglycan tethered to the plasma membrane within lipid raft microdomains. Binding of Cripto-1 to Glypican-1 activates the c-src/mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-kinase (PI3K)/Protein Kinase B (Akt) signaling pathways that regulate cell proliferation, motility, and survival [39].
There may be considerable cross-talk between Cripto-1 and the Wnt signaling pathway. For instance, phosphorylated Smad-2 can activate the Wnt/β-catenin signaling pathway independently of Smad-4 through p300 [40]. It was recently discovered by Nagaoka et al., that in addition to being a transcriptional target of the canonical Wnt pathway, CR-1 modulates the canonical Wnt/β-catenin/Tcf pathway by binding to the Wnt co-receptors low-density lipoprotein receptor-related protein (LRP) 5 and LRP6, which then facilitates their binding to Wnt3a [41]. CR-1 thereby functionally enhances Wnt3a signaling through cytoplasmic stabilization of β-catenin and elevated β-catenin/Tcf transcriptional activation. Another pathway affected by CR-1 is the canonical Notch signaling pathway. The Notch-1/Cbf-1 pathway is required for Nodal expression around the node and for proper left-right determination in mouse and zebrafish [42, 43]. Notch4 can also regulate the expression of Nodal in malignant melanoma cells, indicating a cross-talk between the Nodal/Cripto-1 and Notch pathways during tumorigenesis [44]. Furthermore, CR-1 is also involved in the regulation of Notch receptor processing by enhancing the cleavage of the Notch1 precursor in the extracellular domain through a furin-l protease in the ER/Golgi complex thereby potentiating Notch signaling [45].
Another molecule that might play a role in Cripto-1 signaling is exemplified by Tomoregulin-1 [46]. Tomoregulin-1 can specifically bind with low affinity to the erbB4 receptor [47]. Since Cripto-1 can indirectly enhance the tyrosine phosphorylation of erbB4 in breast cancer cells, interaction between Cripto-1 and Tomoregulin-1 might be important for phosphorylation and activation of erbB4 in mammalian cells [48]. Furthermore, CR-1 has been shown to interact with Caveolin-1 (Cav-1) in a specialized type of lipid raft, caveolae. Cav-1 acts as an inhibitor of Cripto-1 function, interfering with Cripto-1 activation of c-src and MAPK signaling pathways and reducing migration, invasion, and formation of branching structures stimulated by Cripto-1 in mouse mammary epithelial cells [49]. Lefty is another known Cripto-1 binding partner that functions as a co-receptor antagonist by binding to Cripto-1 and preventing Nodal from binding to its receptor complex. Lefty, like Cripto-1 is highly enriched in stem cells [50, 51]. Interestingly, Lefty that is secreted from human ES cells can down regulate Nodal signaling in metastatic melanoma and breast carcinoma cells thereby reducing their tumorigenic phonotype [44, 49]. Cripto-1 can also regulate another pathway as was shown by D’Aniello and colleagues. They demonstrated that Cr-1 null ES cells, which are unable to differentiate into a cardiac lineage, can be rescued by Apelin [52]. Apelin is a peptide identified as the ligand of the orphan G protein-coupled receptor related to Angiotensin-type I receptor (APJ) and regulates body fluid homeostasis, cardiovascular function and angiogenesis. CR-1 was found to directly stimulate the expression of Apelin and APJ. Furthermore, the Nodal/Cripto-1/Smad-4 pathway is also involved in promoting the expression of the hypertrophic response gene atrial natriuretic peptide (ANP) by activating the expression of the PITX2C transcription factor thereby contributing to myocardial hypertrophy [53].
A protein-protein interaction screen using Cripto-1 as bait led to the identification of Glucose Regulated Protein 78 kDa (GRP78) as a cell surface Cripto-1 binding partner [54, 55] and signaling co-factor [54]. GRP78 is an HSP70 family member that regulates protein folding and the unfolded protein response in the ER and its levels are highly elevated in response to nutrient deprivation, hypoxia and ER stress [56]. A fraction of GRP78 can localize to the cell surface where it mediates signaling by several growth stimulatory and cytostatic/cytotoxic effectors [57, 58]. Cell surface levels of GRP78 are high in stem cells and tumor cells and the elevated GRP78 levels in human tumors are closely associated with promotion of tumor growth, malignancy and therapy resistance [56, 59, 60]. Cripto-1 binding to GRP78 may be generally required for Cripto-1 function since treatment with a GRP78-directed antibody (N-20) that competitively blocks Cripto-1/GRP78 binding, or GRP78 knockdown, blocks Cripto-1 effects on Activin A, Activin B, TGF-β1 and Nodal signaling and also inhibits soluble Cripto-1-dependent activation of c-src, Erk/MAPK and PI3K/Akt pathways [61]. More recent studies indicate that soluble Cripto-1 signals via cell surface GRP78 to promote maintenance of hematopoietic stem cells [37] and both fetal and adult mammary stem cells [38]. In both of these studies, soluble Cripto-1 was shown to selectively regulate cells expressing high levels of surface GRP78, to activate the PI3K/Akt pathway and to promote stem cell maintenance ex vivo. Furthermore, treatment of cells with the neutralizing GRP78 N-20 antibody or ALK4L75A-Fc, which acts as a soluble Cripto-1 “ligand trap” caused loss of the stem cell phenotype [37, 38]. These studies raise the possibility that Cripto-1 and GRP78 coordinately regulate stem cell function in other tissues and developmental contexts. Furthermore, since Cripto-1 and GRP78 are both highly expressed in a wide variety of human tumors, their cooperative promotion of stem cell properties in those settings, particularly in response to stresses that induce their expression, may promote tumor aggressiveness, plasticity and therapy resistance. In this regard, agents that disrupt Cripto-1/GRP78 signaling such as GRP78 neutralizing antibodies and ALK4L75A-Fc [38] provide attractive therapeutic strategies.
5. Cripto-1: EMT and cancer stem cells
Broadly defined, CSCs are subsets of cells in various tumors that exhibit enhanced tumorigenicity in experimental settings and which are able to reestablish the cellular heterogeneity of the original tumor [62, 63]. CSCs, also known as tumor initiating cells, share several characteristics that have been associated with normal tissue SCs [64]. CSCs were first described in tumors of hematopoietic origin [62, 63] and have now been identified in several types of solid tumors, including cancers arising in the breast [65], lung [66], prostate [67], colon [68, 69], brain [70], head and neck [71], pancreas [72] and skin [73]. Long-term self-renewal potential, quiescence and resistance to chemotherapy and radiotherapy are proprieties associated with CSCs [74]. The activation of an EMT program is a fundamental step for morphogenesis during embryonic development that has parallels during tumor progression and metastasis that has also been associated with CSCs [75]. EMT is marked by an alteration of tissue organization with the loss of epithelial properties and the acquisition of a mesenchymal phenotype allowing for a gain in cell motility which enables mesenchymal cells to invade adjacent tissues. The reverse process, termed MET, is believed to participate in the establishment of distant metastases by allowing cancer cells to regain epithelial features and to colonize and integrate into distant organs [76]. While it remains unclear whether cancer stem cells are strictly dependent on regulatory pathways governing normal tissue stem cells, a number of pathways and mechanisms common to normal stem cells, early embryonic development, organogenesis, wound healing, and various stages of tumor progression have been elucidated [77–80]. The coordinated actions of EMT, other types of cellular developmental plasticity and other properties of stemness might even lead to a more aggressive tumor phenotype [81–84].
CR-1 is an example of a gene that has been shown to play a role in normal stem cells and during EMT, and has also been found to be expressed in a CSC subpopulation contributing to early cancer progression [85, 86]. In the embryo, Cr-1 is detected at high levels during gastrulation, when epiblastic cells undergo EMT, facilitating their migration through the primitive streak and eventually giving rise to the mesoderm and endoderm [30]. CR-1 has also been shown to promote EMT, migration, invasion and branching morphogenesis in vitro in mouse mammary epithelial cells and in vivo in mammary gland hyperplasias and in tumors derived from MMTV-CR-1 transgenic mice [87–89]. Moreover, NMuMG mouse mammary epithelial cells that overexpress the transcription factor Msx2 undergo morphological and molecular modifications that are commonly associated with EMT. Interestingly, an increase in Cr-1 expression was detected in NMuMG Msx2-transfected cells suggesting that Cr-1 might promote EMT in these cells [90]. Furthermore, CR-1 is involved in tumor epithelial cell plasticity and may be an important EMT regulator in conjunction with Snail, Slug, Twist, and Six1 [91]. In this context, CR-1 can significantly enhance Snail expression in mammary epithelial cells [87]. Noteworthy, CR-1 is enriched in a subpopulation of cancer cells with stem-like characteristics. Recent evidence has demonstrated the presence of two distinct subpopulations of cells possessing high and low levels of CR-1 expression in human embryonal carcinoma (EC) cells [92],, pluripotent stem cells derived from germ cell teratocarcinomas. Interestingly, both subpopulations behaved differently showing distinct gene expression profiles and differences in vitro and in vivo with respect to oncogenic competency. The EC cell fraction containing high levels of CR-1 formed tumor spheres in a serum-free suspension culture with an efficiency significantly higher than the CR-1 low-expressing EC cells. In addition, when injected subcutaneously into nude mice, the CR-1 high-expressing EC cells were able to generate tumors that were larger in size and with a shorter tumor latency period compared with tumors derived from CR-1 low-expressing cells [92]. In the same context, components of the Nodal/CR-1 signaling pathway were found to be overexpressed in pancreatic stem cells which regulated self-renewal and in vivo tumorigenicity [93]. Blocking the Alk4/7 receptor reversed the chemoresistance of the pancreatic CSCs. Moreover, CR-1 has also been identified in a CSC population of hormone-responsive and refractory human prostate tumor cell lines having distinct patterns of androgen metabolism, supporting a potential role for this population in prostate oncogenesis and tumor progression [94]. Additionally, a small subpopulation of CR-1 expressing cells was isolated from metastatic melanoma cells and was identified as a marker for CSCs in melanoma [86]. Finally, a recent report described three signaling pathways namely canonical Wnt, non-canonical Wnt and TGF-β, which induce an EMT program and subsequently function in an autocrine manner to maintain the mesenchymal stem cell state [95]. Remarkably, CR-1, together with other TGF-β and Wnt family members and proangiogenic factors, was among the reported secreted proteins present in the culture medium of immortalized human mammary epithelial cells that had undergone EMT and expressed phenotypic properties of CSCs.
6. Cripto-1 in transformation, migration, invasion and angiogenesis
Reactivation of certain signaling pathways that are crucial during embryonic development might induce cellular transformation and tumor progression in adult tissues [96]. CR-1 is a typical example of an embryonic gene that is re-expressed during tumorigenesis, functioning as an oncogene and driving cellular proliferation, migration, and invasion, as well as stimulating tumor angiogenesis in vitro and in vivo [30, 97]. CR-1 was first demonstrated to induce cellular transformation in vitro in mouse mammary epithelial cells and mouse embryonic fibroblasts, which acquired a transformed phenotype after being transfected with a CR-1 expression vector, as assessed by their ability to grow in an anchorage-independent manner in soft agar [85]. Furthermore, the involvement of Cripto-1 in tumor progression was shown by its ability to enhance migration and invasion of a variety of normal mammary epithelial cells, MCF7 human breast cancer cells, and CaSki human cervical carcinoma cells. CR-1 was able to induce the expression of vimentin in CaSki cells suggesting that it may contribute to the invasive mesenchymal phenotype acquired by these cells. Interestingly, CR-1 expression was significantly increased in rat embryo fibroblasts or Fischer rat thyroid cells transformed by different oncogenes, such as c-Ha-ras or c-Ki-ras [85]. Futhermore, v-ras/Smad-7-transformed keratinocytes develop skin tumors that overexpress Cr-1 [98], suggesting that Smad-7-induced tumor formation may require upregulation of Cr-1 and other EGF-related peptides. Evidence also suggests that CR-1 might also modulate tumor angiogenesis, as demonstrated by Bianco and colleagues, where CR-1 was able to enhance the proliferation, migration and invasion of human umbilical endothelial cells, and stimulated their differentiation into vascular-like structures in Matrigel [99]. Similarly, overexpression of CR-1 in MCF-7 breast cancer cell xenografts enhanced tumor neovascularization in vivo [99]. It is possible that low oxygen levels trigger CR-1 expression within tumors, thereby inducing microvessel formation to sustain tumor growth. This in fact seems likely since, as alluded to above, it has been reported that hypoxic conditions can enhance CR-1 expression in human embryonal carcinoma cells that is mediated by the direct binding of HIF-1α to the CR-1 promoter [18].
CR-1 can also function as an oncogene in vivo through possible cross-talk with other signaling pathways to promote mammary tumorigenesis. For example, there is a significant increase in Cr-1 expression in mammary tumors derived from transgenic mice overexpressing the oncogenes, neu (erbB-2), TGF-α, Int-3, polyoma middle T (PyMT) or simian virus 40 large T antigens [100]. A human CR-1 transgene has also been shown to directly promote mammary hyperplasias and adenocarcinomas of the mammary gland in transgenic mouse models overexpressing the human CR-1 transgene in mouse mammary glands under the control of the mouse mammary tumor virus (MMTV) or the whey acidic protein (WAP) promoters [89, 101]. The majority of nulliparous MMTV-CR-1 transgenic mice exhibit enhanced ductal branching, intraductal hyperplasias and hyperplastic alveolar nodules, and at least 30% of multiparous females develop multifocal hyperplasias and papillary adenocarcinomas. The relatively long latency period of tumor formation implies that additional genetic alterations and/or cross-talk with other signaling pathways such as Wnt/β-catenin are necessary to induce mammary tumor formation. In fact, Strizzi and colleagues reported that the expression of the active form of β-catenin, dephosphorylated (DP)-β-catenin, was significantly increased in multiparous MMTV-CR-1 mammary tumors as compared to mammary tissue from control FVB/N mice [87]. They also found increased expression of phosphorylated (P)-c-src, P-focal adhesion kinase (FAK), P-Akt, P-glycogen synthase kinase 3β (GSK3β), and integrins α3, αv, β1, β3, and β4 in MMTV-CR-1 tumors, suggesting that CR-1 might play an important role in facilitating proliferation, migration and invasion of tumor cells in vivo. High levels of N-cadherin, vimentin, cyclin-D1, Snail, α-smooth muscle actin and fibronectin, and low levels of E-cadherin were also found in these CR-1 overexpressing tumors [87]. In addition to mammary tumors, 20% of MMTV-CR-1 females also developed uterine leiomyosarcomas after two years, and high levels of (P)-c-src, P-Akt, P-GSK3β and DP-β-catenin as well as nuclear β-catenin were found in these uterine tumors, when compared to uteri from control mice [102]. This evidence suggests that CR-1 can facilitate mammary and uterine tumorigenesis by either activating c-src/Akt and/or via cross-talk with the canonical Wnt/β-catenin signaling pathway. Similarly, almost 50% of aged nulliparous WAP-CR-1 mice develop multifocal intraductal hyperplasias, and more than a half of multiparous WAP-CR-1 females develop mammary tumors of mixed histological subtypes, representing glandular, papillary and undifferentiated carcinoma, myoepithelioma and adeno-squamous carcinomas [101]. Like the MMTV-CR-1 mice, hyperactivation of the canonical Wnt/β-catenin pathway was detected in WAP-CR-1 mammary tumors. As mentioned previously, activation of the Wnt/β-catenin pathway during early mouse embryogenesis and in human colon carcinoma cells can enhance CR-1 expression [16, 19].
7. Expression of Cripto-1 in human carcinomas and premalignant lesions
As previously discussed in this review, CR-1 is not significantly expressed at significant levels in adult somatic tissues, with the possible exception of the tissue SC compartment, and its re-expression can be observed during oncogenic transformation. In addition to functioning as an oncogene in vitro and in vivo, CR-1 overexpression is detected at the mRNA and protein levels in a wide variety of solid human tumors of non-neuronal origin, including those of the reproductive and gastrointestinal systems, and also lung, skin, nasopharinx and embryonal carcinomas [85]. Furthermore, soluble CR-1 levels are elevated in the plasma obtained from colon and breast carcinoma patients [103].
However, two studies have also recently detected CR-1 expression in brain cancer. In a study by Tysnes and colleagues, invasive and angiogenic xenograft samples obtained from patients with glioblastoma (GBM), showed elevated expression of CR-1 [104]. Additionally, patient samples from primary GBM showed significantly high levels of CR-1 protein as detected by immunohistochemistry, and the higher CR-1 scores were also associated with shorter survival in a subset of younger patients. In a more recent study, high CR-1 protein levels were detected in plasma from GBM cancer patients, which was significantly correlated with a shorter overall survival. Interestingly, CR-1 was detected in the perivascular areas of GBM cells, and also in endothelial cells [105]. For an extensive review on Cripto-1 expression in solid human tumors of non-neuronal origin, see [85, 97].
8. Cripto-1 as a therapeutic target in human cancer
Due to its intimate involvement in processes such as oncogenesis and EMT and minimal expression in adult tissues, CR-1 may be considered as an attractive target for therapeutic intervention. In particular, the association of CR-1 with CSCs is intriguing as this population of cells is intrinsically resistant to standard chemotherapy and radiotherapy [106]. Current strategies that can effectively target and neutralize the potential oncogenic effects of CR-1 include the use of antisense (AS) oligonucleotides, monoclonal antibodies (mAbs), inhibitory peptides, small molecule antagonists and antibodies directed against CR-1 binding partners (Fig 1). Antisense oligonucleotides targeting the expression of CR-1 have been effective in the inhibition of breast, colon and ovarian cancer cells in vitro alone and with greater effect when combined with AS oligonucleotides targeting other oncogenic growth factors, such as TGF-α and amphiregulin [107–110]. These same AS oligonucleotides have also shown efficacy in vivo by inhibiting the proliferation of colon cancer xenografts in nude mice alone and with higher efficacy when combined with conventional chemotherapy or radiotherapy [108].
A number of mAbs directed against CR-1 have been used in various model systems with significant effects on reducing cellular proliferation and inhibiting tumor growth. Biogen-Idec has produced several mouse mAbs that target various regions within CR-1. A mAb (A8.G3.5) generated against the CFC motif has shown efficacy by inhibiting cancer cell proliferation in vitro and tumor xenografts in vivo by relieving the CR-1-mediated blockade of Activin B signaling and growth inhibition [111]. Antibodies targeting the EGF-like motif have also proven to be effective in inhibiting Nodal signaling by disrupting the interaction of CR-1 with Nodal [112]. Xing and colleagues have produced rat monoclonal IgM antibodies (C4, C13) that also target the EGF-like motif of CR-1 and that have proven to be highly effective in inhibiting the growth of breast, colon, lung, prostate and leukemic cancer cell lines in vitro [113]. In addition, these antibodies can inhibit the xenograft tumor growth of colon cancer cells in vivo, enhance the cytotoxic effects of chemotherapeutic agents, and can induce apoptosis in these systems and in multi-drug resistant leukemia cells [113, 114]. An Fc chimera consisting of the Alk4 extracellular domain fused to the Fc domain of human IgG also has antitumor potential since it blocked CR-1-induced proliferation, migration and stem cell maintenance [38]. Antibodies produced against the N-terminus of CR-1 (B3.F6.1 and A10.B2.18) have shown strong binding without neutralization of the biological activity of CR-1 [112, 115] and a humanized version of B3.F6.1 conjugated to a cytotoxin (DM4) has been used in a recently concluded phase I clinical trial in relapsed/refractory solid tumors with no current plans to continue [112, 116]. CR-1 binding partners can also be targeted for therapeutic intervention, such as GRP78. Disruption of the CR-1/GRP78 complex with an anti-GRP78 antibody has been effective in abrogating Akt/MAPK signaling in NCCIT cells [61] and elucidating the role of CR-1 in the maintenance of hematopoietic stem cells [37].
Other approaches have been used to neutralize CR-1 binding to the Activin/TGF-β signaling complex. Alantolactone, a natural small molecule derived from several plants [117], has been shown to impair the CR-1-mediated blockade of Activin signaling by disrupting the association of CR-1 with the Activin receptor type IIA [118], mimicking the effects of mAbs targeting the CFC motif of CR-1. Recently, a non-natural tetrameric tripeptide that binds the CR-1 CFC motif was found to enhance differentiation of mouse ES cells in vitro and improve neurological function in an in vivo rat model of Parkinson’s disease [119]. This peptide has the potential to re-activate the Activin signaling complex in an oncogenic setting in a similar fashion as seen with alantolactone and CFC-targeting antibodies. Whether alone or in concert with other therapeutic regimens, the abrogation of CR-1 expression and binding to Activin/TGF-β signaling complex has significant therapeutic potential.
9. Conclusion and perspectives
The abnormal spatial and temporal reexpression of embryonic signaling genes at different stages of tumor development in a variety of human cancers is now a well-recognized fact. In particular, the subversion of these key regulatory genes in CSCs or transit amplifying progenitor cells in human cancers may be extremely deleterious for restricting tumor progression and for preventing the re-emergence of secondary cancer following the use of primary chemo- and/or radiotherapy. Therefore, the targeting of embryonic genes that drive the maintenance or self-renewal of CSCs/TICs becomes attractive therapeutically. Conventional cancer therapies generally attack more fully differentiated and/or rapidly cycling tumor cells without significantly impeding the relatively small and quiescent population of more undifferentiated CSCs. Therapies that deplete the bulk tumor population combined with novel therapies that disrupt singular or multiple embryonic signaling pathways in CSCs, the CSC niche or processes such as EMT that initiate the formation of CSCs seems to be warranted for successfully and permanently eradicating tumors. CR-1/TDGF-1 is an example of one such embryonic gene that is expressed at significant levels in a relatively high proportion of human cancers. CR-1 is functionally an important nexus point for several different embryonic signaling pathways such as Nodal, Notch and Wnt/β-catenin that have been implicated in regulating the etiology and progression of human tumors. The identification of upstream genes that regulate CR-1 expression and activity as well as downstream targets that are in turn regulated by CR-1 will significantly improve our understanding of the biology of this complex regulatory gene and hopefully expose other potential novel therapeutic targets in cancer.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pierce GB, Speers WC. Tumors as caricatures of the process of tissue renewal: prospects for therapy by directing differentiation. Cancer research. 1988;48:1996–2004. [PubMed] [Google Scholar]
- 2.Johnston RN, Pai SB, Pai RB. The origin of the cancer cell: oncogeny reverses phylogeny. Biochemistry and cell biology = Biochimie et biologie cellulaire. 1992;70:831–4. doi: 10.1139/o92-130. [DOI] [PubMed] [Google Scholar]
- 3.Dreesen O, Brivanlou AH. Signaling pathways in cancer and embryonic stem cells. Stem cell reviews. 2007;3:7–17. doi: 10.1007/s12015-007-0004-8. [DOI] [PubMed] [Google Scholar]
- 4.Abbott DE, Bailey CM, Postovit LM, Seftor EA, Margaryan N, Seftor RE, et al. The epigenetic influence of tumor and embryonic microenvironments: how different are they? Cancer microenvironment: official journal of the International Cancer Microenvironment Society. 2008;1:13–21. doi: 10.1007/s12307-008-0004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruno RD, Smith GH. Reprogramming non-mammary and cancer cells in the developing mouse mammary gland. Seminars in cell & developmental biology. 2012;23:591–8. doi: 10.1016/j.semcdb.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen L, Kasai T, Li Y, Sugii Y, Jin G, Okada M, et al. A model of cancer stem cells derived from mouse induced pluripotent stem cells. PloS one. 2012;7:e33544. doi: 10.1371/journal.pone.0033544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishi M, Sakai Y, Akutsu H, Nagashima Y, Quinn G, Masui S, et al. Induction of cells with cancer stem cell properties from nontumorigenic human mammary epithelial cells by defined reprogramming factors. Oncogene. 2014;33:643–52. doi: 10.1038/onc.2012.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciccodicola A, Dono R, Obici S, Simeone A, Zollo M, Persico MG. Molecular characterization of a gene of the ‘EGF family’ expressed in undifferentiated human NTERA2 teratocarcinoma cells. The EMBO journal. 1989;8:1987–91. doi: 10.1002/j.1460-2075.1989.tb03605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dono R, Scalera L, Pacifico F, Acampora D, Persico MG, Simeone A. The murine cripto gene: expression during mesoderm induction and early heart morphogenesis. Development. 1993;118:1157–68. doi: 10.1242/dev.118.4.1157. [DOI] [PubMed] [Google Scholar]
- 10.Gritsman K, Zhang J, Cheng S, Heckscher E, Talbot WS, Schier AF. The EGF-CFC protein one-eyed pinhead is essential for nodal signaling. Cell. 1999;97:121–32. doi: 10.1016/s0092-8674(00)80720-5. [DOI] [PubMed] [Google Scholar]
- 11.Minchiotti G, Manco G, Parisi S, Lago CT, Rosa F, Persico MG. Structure-function analysis of the EGF-CFC family member Cripto identifies residues essential for nodal signalling. Development. 2001;128:4501–10. doi: 10.1242/dev.128.22.4501. [DOI] [PubMed] [Google Scholar]
- 12.Shen MM, Wang H, Leder P. A differential display strategy identifies Cryptic, a novel EGF-related gene expressed in the axial and lateral mesoderm during mouse gastrulation. Development. 1997;124:429–42. doi: 10.1242/dev.124.2.429. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J, Talbot WS, Schier AF. Positional cloning identifies zebrafish one-eyed pinhead as a permissive EGF-related ligand required during gastrulation. Cell. 1998;92:241–51. doi: 10.1016/s0092-8674(00)80918-6. [DOI] [PubMed] [Google Scholar]
- 14.Watanabe K, Hamada S, Bianco C, Mancino M, Nagaoka T, Gonzales M, et al. Requirement of glycosylphosphatidylinositol anchor of Cripto-1 for trans activity as a Nodal co-receptor. J Biol Chem. 2007;282:35772–86. doi: 10.1074/jbc.M707351200. [DOI] [PubMed] [Google Scholar]
- 15.Yan YT, Liu JJ, Luo Y, EC, Haltiwanger RS, Abate-Shen C, et al. Dual roles of Cripto as a ligand and coreceptor in the nodal signaling pathway. Mol Cell Biol. 2002;22:4439–49. doi: 10.1128/MCB.22.13.4439-4449.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamada S, Watanabe K, Hirota M, Bianco C, Strizzi L, Mancino M, et al. beta-Catenin/TCF/LEF regulate expression of the short form human Cripto-1. Biochemical and biophysical research communications. 2007;355:240–4. doi: 10.1016/j.bbrc.2007.01.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mancino M, Strizzi L, Wechselberger C, Watanabe K, Gonzales M, Hamada S, et al. Regulation of human Cripto-1 gene expression by TGF-beta1 and BMP-4 in embryonal and colon cancer cells. J Cell Physiol. 2008;215:192–203. doi: 10.1002/jcp.21301. [DOI] [PubMed] [Google Scholar]
- 18.Bianco C, Cotten C, Lonardo E, Strizzi L, Baraty C, Mancino M, et al. Cripto-1 is required for hypoxia to induce cardiac differentiation of mouse embryonic stem cells. Am J Pathol. 2009;175:2146–58. doi: 10.2353/ajpath.2009.090218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morkel M, Huelsken J, Wakamiya M, Ding J, van de Wetering M, Clevers H, et al. Beta-catenin regulates Cripto- and Wnt3-dependent gene expression programs in mouse axis and mesoderm formation. Development. 2003;130:6283–94. doi: 10.1242/dev.00859. [DOI] [PubMed] [Google Scholar]
- 20.Behrens AN, Ren Y, Ferdous A, Garry DJ, Martin CM. Nkx2–5 Regulates Tdgf1 (Cripto) Early During Cardiac Development. Journal of clinical & experimental cardiology. 2012;(Suppl 11):1–4. doi: 10.4172/2155-9880.S11-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hentschke M, Kurth I, Borgmeyer U, Hubner CA. Germ cell nuclear factor is a repressor of CRIPTO-1 and CRIPTO-3. J Biol Chem. 2006;281:33497–504. doi: 10.1074/jbc.M606975200. [DOI] [PubMed] [Google Scholar]
- 22.Bianco C, Castro NP, Baraty C, Rollman K, Held N, Rangel MC, et al. Regulation of human Cripto-1 expression by nuclear receptors and DNA promoter methylation in human embryonal and breast cancer cells. J Cell Physiol. 2013;228:1174–88. doi: 10.1002/jcp.24271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Forinash KD, McGivern J, Fritz B, Dorey K, Sheets MD. Spatially restricted translation of the xCR1 mRNA in Xenopus embryos. Mol Cell Biol. 2009;29:3791–802. doi: 10.1128/MCB.01865-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y, Park S, Blaser S, Sheets MD. Determinants of RNA binding and translational repression by the Bicaudal-C regulatory protein. J Biol Chem. 2014;289:7497–504. doi: 10.1074/jbc.M113.526426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen F, Hou SK, Fan HJ, Liu YF. MiR-15a-16 represses Cripto and inhibits NSCLC cell progression. Molecular and cellular biochemistry. 2014 doi: 10.1007/s11010-014-1981-y. [DOI] [PubMed] [Google Scholar]
- 26.Meno C, Ito Y, Saijoh Y, Matsuda Y, Tashiro K, Kuhara S, et al. Two closely-related left-right asymmetrically expressed genes, lefty-1 and lefty-2: their distinct expression domains, chromosomal linkage and direct neuralizing activity in Xenopus embryos. Genes to cells: devoted to molecular & cellular mechanisms. 1997;2:513–24. doi: 10.1046/j.1365-2443.1997.1400338.x. [DOI] [PubMed] [Google Scholar]
- 27.Ding J, Yang L, Yan YT, Chen A, Desai N, Wynshaw-Boris A, et al. Cripto is required for correct orientation of the anterior-posterior axis in the mouse embryo. Nature. 1998;395:702–7. doi: 10.1038/27215. [DOI] [PubMed] [Google Scholar]
- 28.Xu C, Liguori G, Persico MG, Adamson ED. Abrogation of the Cripto gene in mouse leads to failure of postgastrulation morphogenesis and lack of differentiation of cardiomyocytes. Development. 1999;126:483–94. doi: 10.1242/dev.126.3.483. [DOI] [PubMed] [Google Scholar]
- 29.Wang B, Yan J, Peng Z, Wang J, Liu S, Xie X, et al. Teratocarcinoma-derived growth factor 1 (TDGF1) sequence variants in patients with congenital heart defect. Int J Cardiol. 2011;146:225–7. doi: 10.1016/j.ijcard.2009.08.046. [DOI] [PubMed] [Google Scholar]
- 30.Bianco C, Rangel MC, Castro NP, Nagaoka T, Rollman K, Gonzales M, et al. Role of Cripto-1 in stem cell maintenance and malignant progression. Am J Pathol. 2010;177:532–40. doi: 10.2353/ajpath.2010.100102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loh YH, Wu Q, Chew JL, Vega VB, Zhang W, Chen X, et al. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat Genet. 2006;38:431–40. doi: 10.1038/ng1760. [DOI] [PubMed] [Google Scholar]
- 32.Vallier L, Mendjan S, Brown S, Chng Z, Teo A, Smithers LE, et al. Activin/Nodal signalling maintains pluripotency by controlling Nanog expression. Development. 2009;136:1339–49. doi: 10.1242/dev.033951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aasen T, Raya A, Barrero MJ, Garreta E, Consiglio A, Gonzalez F, et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nature biotechnology. 2008;26:1276–84. doi: 10.1038/nbt.1503. [DOI] [PubMed] [Google Scholar]
- 34.Aoi T, Yae K, Nakagawa M, Ichisaka T, Okita K, Takahashi K, et al. Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science. 2008;321:699–702. doi: 10.1126/science.1154884. [DOI] [PubMed] [Google Scholar]
- 35.Boles NC, Hirsch SE, Le S, Corneo B, Najm F, Minotti AP, et al. NPTX1 regulates neural lineage specification from human pluripotent stem cells. Cell Rep. 2014;6:724–36. doi: 10.1016/j.celrep.2014.01.026. [DOI] [PubMed] [Google Scholar]
- 36.Liguori GL, Echevarria D, Bonilla S, D’Andrea D, Liguoro A, Persico MG, et al. Characterization of the functional properties of the neuroectoderm in mouse Cripto(−/−) embryos showing severe gastrulation defects. The International journal of developmental biology. 2009;53:549–57. doi: 10.1387/ijdb.082650gl. [DOI] [PubMed] [Google Scholar]
- 37.Miharada K, Karlsson G, Rehn M, Rorby E, Siva K, Cammenga J, et al. Cripto regulates hematopoietic stem cells as a hypoxic-niche-related factor through cell surface receptor GRP78. Cell stem cell. 2011;9:330–44. doi: 10.1016/j.stem.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 38.Spike BT, Kelber JA, Booker E, Kalathur M, Rodewald R, Lipianskaya J, et al. CRIPTO/GRP78 Signaling Maintains Fetal and Adult Mammary Stem Cells Ex Vivo. Stem cell reports. 2014;2:427–39. doi: 10.1016/j.stemcr.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bianco C, Strizzi L, Normanno N, Khan N, Salomon DS. Cripto-1: an oncofetal gene with many faces. Curr Top Dev Biol. 2005;67:85–133. doi: 10.1016/S0070-2153(05)67003-2. [DOI] [PubMed] [Google Scholar]
- 40.Hirota M, Watanabe K, Hamada S, Sun Y, Strizzi L, Mancino M, et al. Smad2 functions as a co-activator of canonical Wnt/beta-catenin signaling pathway independent of Smad4 through histone acetyltransferase activity of p300. Cell Signal. 2008;20:1632–41. doi: 10.1016/j.cellsig.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagaoka T, Karasawa H, Turbyville T, Rangel MC, Castro NP, Gonzales M, et al. Cripto-1 enhances the canonical Wnt/beta-catenin signaling pathway by binding to LRP5 and LRP6 co-receptors. Cell Signal. 2013;25:178–89. doi: 10.1016/j.cellsig.2012.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krebs LT, Iwai N, Nonaka S, Welsh IC, Lan Y, Jiang R, et al. Notch signaling regulates left-right asymmetry determination by inducing Nodal expression. Genes & development. 2003;17:1207–12. doi: 10.1101/gad.1084703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raya A, Kawakami Y, Rodriguez-Esteban C, Buscher D, Koth CM, Itoh T, et al. Notch activity induces Nodal expression and mediates the establishment of left-right asymmetry in vertebrate embryos. Genes & development. 2003;17:1213–8. doi: 10.1101/gad.1084403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Postovit LM, Seftor EA, Seftor RE, Hendrix MJ. Targeting Nodal in malignant melanoma cells. Expert opinion on therapeutic targets. 2007;11:497–505. doi: 10.1517/14728222.11.4.497. [DOI] [PubMed] [Google Scholar]
- 45.Watanabe K, Nagaoka T, Lee JM, Bianco C, Gonzales M, Castro NP, et al. Enhancement of Notch receptor maturation and signaling sensitivity by Cripto-1. The Journal of cell biology. 2009;187:343–53. doi: 10.1083/jcb.200905105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harms PW, Chang C. Tomoregulin-1 (TMEFF1) inhibits nodal signaling through direct binding to the nodal coreceptor Cripto. Genes & development. 2003;17:2624–9. doi: 10.1101/gad.1127703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Uchida T, Wada K, Akamatsu T, Yonezawa M, Noguchi H, Mizoguchi A, et al. A novel epidermal growth factor-like molecule containing two follistatin modules stimulates tyrosine phosphorylation of erbB-4 in MKN28 gastric cancer cells. Biochemical and biophysical research communications. 1999;266:593–602. doi: 10.1006/bbrc.1999.1873. [DOI] [PubMed] [Google Scholar]
- 48.Bianco C, Kannan S, De Santis M, Seno M, Tang CK, Martinez-Lacaci I, et al. Cripto-1 indirectly stimulates the tyrosine phosphorylation of erb B-4 through a novel receptor. J Biol Chem. 1999;274:8624–9. doi: 10.1074/jbc.274.13.8624. [DOI] [PubMed] [Google Scholar]
- 49.Bianco C, Strizzi L, Mancino M, Watanabe K, Gonzales M, Hamada S, et al. Regulation of Cripto-1 signaling and biological activity by caveolin-1 in mammary epithelial cells. Am J Pathol. 2008;172:345–57. doi: 10.2353/ajpath.2008.070696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tabibzadeh S, Hemmati-Brivanlou A. Lefty at the crossroads of “stemness” and differentiative events. Stem cells. 2006;24:1998–2006. doi: 10.1634/stemcells.2006-0075. [DOI] [PubMed] [Google Scholar]
- 51.Bendall SC, Hughes C, Campbell JL, Stewart MH, Pittock P, Liu S, et al. An enhanced mass spectrometry approach reveals human embryonic stem cell growth factors in culture. Mol Cell Proteomics. 2009;8:421–32. doi: 10.1074/mcp.M800190-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.D’Aniello C, Lonardo E, Iaconis S, Guardiola O, Liguoro AM, Liguori GL, et al. G protein-coupled receptor APJ and its ligand apelin act downstream of Cripto to specify embryonic stem cells toward the cardiac lineage through extracellular signal-regulated kinase/p70S6 kinase signaling pathway. Circulation research. 2009;105:231–8. doi: 10.1161/CIRCRESAHA.109.201186. [DOI] [PubMed] [Google Scholar]
- 53.Su D, Jing S, Guan L, Li Q, Zhang H, Gao X, et al. Role of Nodal-PITX2C signaling pathway in glucose-induced cardiomyocyte hypertrophy. Biochemistry and cell biology = Biochimie et biologie cellulaire. 2014 doi: 10.1139/bcb-2013-0124. [DOI] [PubMed] [Google Scholar]
- 54.Gray PC, Vale W. Cripto/GRP78 modulation of the TGF-beta pathway in development and oncogenesis. FEBS letters. 2012;586:1836–45. doi: 10.1016/j.febslet.2012.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shani G, Fischer WH, Justice NJ, Kelber JA, Vale W, Gray PC. GRP78 and Cripto form a complex at the cell surface and collaborate to inhibit transforming growth factor beta signaling and enhance cell growth. Mol Cell Biol. 2008;28:666–77. doi: 10.1128/MCB.01716-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee AS. Glucose-regulated proteins in cancer: molecular mechanisms and therapeutic potential. Nature reviews Cancer. 2014;14:263–76. doi: 10.1038/nrc3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ni M, Zhang Y, Lee AS. Beyond the endoplasmic reticulum: atypical GRP78 in cell viability, signalling and therapeutic targeting. Biochem J. 2011;434:181–8. doi: 10.1042/BJ20101569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ni M, Zhang Y, Lee AS. Beyond the endoplasmic reticulum: atypical GRP78 in cell viability, signalling and therapeutic targeting. The Biochemical journal. 2011;434:181–8. doi: 10.1042/BJ20101569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jamora C, Dennert G, Lee AS. Inhibition of tumor progression by suppression of stress protein GRP78/BiP induction in fibrosarcoma B/C10ME. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:7690–4. doi: 10.1073/pnas.93.15.7690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu R, Li X, Gao W, Zhou Y, Wey S, Mitra SK, et al. Monoclonal antibody against cell surface GRP78 as a novel agent in suppressing PI3K/AKT signaling, tumor growth, and metastasis. Clinical cancer research: an official journal of the American Association for Cancer Research. 2013;19:6802–11. doi: 10.1158/1078-0432.CCR-13-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kelber JA, Panopoulos AD, Shani G, Booker EC, Belmonte JC, Vale WW, et al. Blockade of Cripto binding to cell surface GRP78 inhibits oncogenic Cripto signaling via MAPK/PI3K and Smad2/3 pathways. Oncogene. 2009;28:2324–36. doi: 10.1038/onc.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nature medicine. 1997;3:730–7. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 63.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–8. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 64.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–11. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 65.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–35. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 67.Wang X, Kruithof-de Julio M, Economides KD, Walker D, Yu H, Halili MV, et al. A luminal epithelial stem cell that is a cell of origin for prostate cancer. Nature. 2009;461:495–500. doi: 10.1038/nature08361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–10. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 69.Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–5. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 70.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 71.Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:973–8. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell stem cell. 2007;1:313–23. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 73.Malanchi I, Peinado H, Kassen D, Hussenet T, Metzger D, Chambon P, et al. Cutaneous cancer stem cell maintenance is dependent on beta-catenin signalling. Nature. 2008;452:650–3. doi: 10.1038/nature06835. [DOI] [PubMed] [Google Scholar]
- 74.Kreso A, van Galen P, Pedley NM, Lima-Fernandes E, Frelin C, Davis T, et al. Self-renewal as a therapeutic target in human colorectal cancer. Nature medicine. 2014;20:29–36. doi: 10.1038/nm.3418. [DOI] [PubMed] [Google Scholar]
- 75.Micalizzi DS, Farabaugh SM, Ford HL. Epithelial-mesenchymal transition in cancer: parallels between normal development and tumor progression. J Mammary Gland Biol Neoplasia. 2010;15:117–34. doi: 10.1007/s10911-010-9178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Oskarsson T, Batlle E, Massague J. Metastatic stem cells: sources, niches, and vital pathways. Cell stem cell. 2014;14:306–21. doi: 10.1016/j.stem.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ben-Porath I, Thomson MW, Carey VJ, Ge R, Bell GW, Regev A, et al. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet. 2008;40:499–507. doi: 10.1038/ng.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Spike BT, Engle DD, Lin JC, Cheung SK, La J, Wahl GM. A mammary stem cell population identified and characterized in late embryogenesis reveals similarities to human breast cancer. Cell stem cell. 2012;10:183–97. doi: 10.1016/j.stem.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Youssef KK, Lapouge G, Bouvree K, Rorive S, Brohee S, Appelstein O, et al. Adult interfollicular tumour-initiating cells are reprogrammed into an embryonic hair follicle progenitor-like fate during basal cell carcinoma initiation. Nat Cell Biol. 2012;14:1282–94. doi: 10.1038/ncb2628. [DOI] [PubMed] [Google Scholar]
- 80.Chang HY, Sneddon JB, Alizadeh AA, Sood R, West RB, Montgomery K, et al. Gene expression signature of fibroblast serum response predicts human cancer progression: similarities between tumors and wounds. PLoS Biol. 2004;2:E7. doi: 10.1371/journal.pbio.0020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–15. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Scheel C, Weinberg RA. Cancer stem cells and epithelial-mesenchymal transition: concepts and molecular links. Seminars in cancer biology. 2012;22:396–403. doi: 10.1016/j.semcancer.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Meacham CE, Morrison SJ. Tumour heterogeneity and cancer cell plasticity. Nature. 2013;501:328–37. doi: 10.1038/nature12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Spike BT, Wahl GM. p53, Stem Cells, and Reprogramming: Tumor Suppression beyond Guarding the Genome. Genes Cancer. 2011;2:404–19. doi: 10.1177/1947601911410224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.de Castro NP, Rangel MC, Nagaoka T, Salomon DS, Bianco C. Cripto-1: an embryonic gene that promotes tumorigenesis. Future Oncol. 2010;6:1127–42. doi: 10.2217/fon.10.68. [DOI] [PubMed] [Google Scholar]
- 86.Strizzi L, Abbott DE, Salomon DS, Hendrix MJ. Potential for cripto-1 in defining stem cell-like characteristics in human malignant melanoma. Cell cycle. 2008;7:1931–5. doi: 10.4161/cc.7.13.6236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Strizzi L, Bianco C, Normanno N, Seno M, Wechselberger C, Wallace-Jones B, et al. Epithelial mesenchymal transition is a characteristic of hyperplasias and tumors in mammary gland from MMTV-Cripto-1 transgenic mice. J Cell Physiol. 2004;201:266–76. doi: 10.1002/jcp.20062. [DOI] [PubMed] [Google Scholar]
- 88.Wechselberger C, Ebert AD, Bianco C, Khan NI, Sun Y, Wallace-Jones B, et al. Cripto-1 enhances migration and branching morphogenesis of mouse mammary epithelial cells. Experimental cell research. 2001;266:95–105. doi: 10.1006/excr.2001.5195. [DOI] [PubMed] [Google Scholar]
- 89.Wechselberger C, Strizzi L, Kenney N, Hirota M, Sun Y, Ebert A, et al. Human Cripto-1 overexpression in the mouse mammary gland results in the development of hyperplasia and adenocarcinoma. Oncogene. 2005;24:4094–105. doi: 10.1038/sj.onc.1208417. [DOI] [PubMed] [Google Scholar]
- 90.di Bari MG, Ginsburg E, Plant J, Strizzi L, Salomon DS, Vonderhaar BK. Msx2 induces epithelial-mesenchymal transition in mouse mammary epithelial cells through upregulation of Cripto-1. J Cell Physiol. 2009;219:659–66. doi: 10.1002/jcp.21712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Micalizzi DS, Wang CA, Farabaugh SM, Schiemann WP, Ford HL. Homeoprotein Six1 increases TGF-beta type I receptor and converts TGF-beta signaling from suppressive to supportive for tumor growth. Cancer research. 2010;70:10371–80. doi: 10.1158/0008-5472.CAN-10-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Watanabe K, Meyer MJ, Strizzi L, Lee JM, Gonzales M, Bianco C, et al. Cripto-1 is a cell surface marker for a tumorigenic, undifferentiated subpopulation in human embryonal carcinoma cells. Stem cells. 2010;28:1303–14. doi: 10.1002/stem.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lonardo E, Hermann PC, Mueller MT, Huber S, Balic A, Miranda-Lorenzo I, et al. Nodal/Activin signaling drives self-renewal and tumorigenicity of pancreatic cancer stem cells and provides a target for combined drug therapy. Cell stem cell. 2011;9:433–46. doi: 10.1016/j.stem.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 94.Cocciadiferro L, Miceli V, Kang KS, Polito LM, Trosko JE, Carruba G. Profiling cancer stem cells in androgen-responsive and refractory human prostate tumor cell lines. Annals of the New York Academy of Sciences. 2009;1155:257–62. doi: 10.1111/j.1749-6632.2009.03696.x. [DOI] [PubMed] [Google Scholar]
- 95.Scheel C, Eaton EN, Li SH, Chaffer CL, Reinhardt F, Kah KJ, et al. Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in the breast. Cell. 2011;145:926–40. doi: 10.1016/j.cell.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nagaoka T, Karasawa H, Castro NP, Rangel MC, Salomon DS, Bianco C. An evolving web of signaling networks regulated by Cripto-1. Growth Factors. 2012;30:13–21. doi: 10.3109/08977194.2011.641962. [DOI] [PubMed] [Google Scholar]
- 97.Rangel MC, Karasawa H, Castro NP, Nagaoka T, Salomon DS, Bianco C. Role of Cripto-1 during epithelial-to-mesenchymal transition in development and cancer. Am J Pathol. 2012;180:2188–200. doi: 10.1016/j.ajpath.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu X, Lee J, Cooley M, Bhogte E, Hartley S, Glick A. Smad7 but not Smad6 cooperates with oncogenic ras to cause malignant conversion in a mouse model for squamous cell carcinoma. Cancer research. 2003;63:7760–8. [PubMed] [Google Scholar]
- 99.Bianco C, Strizzi L, Ebert A, Chang C, Rehman A, Normanno N, et al. Role of human cripto-1 in tumor angiogenesis. J Natl Cancer Inst. 2005;97:132–41. doi: 10.1093/jnci/dji011. [DOI] [PubMed] [Google Scholar]
- 100.Kenney NJ, Smith GH, Maroulakou IG, Green JH, Muller WJ, Callahan R, et al. Detection of amphiregulin and Cripto-1 in mammary tumors from transgenic mice. Mol Carcinog. 1996;15:44–56. doi: 10.1002/(SICI)1098-2744(199601)15:1<44::AID-MC7>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 101.Sun Y, Strizzi L, Raafat A, Hirota M, Bianco C, Feigenbaum L, et al. Overexpression of human Cripto-1 in transgenic mice delays mammary gland development and differentiation and induces mammary tumorigenesis. Am J Pathol. 2005;167:585–97. doi: 10.1016/S0002-9440(10)63000-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Strizzi L, Bianco C, Hirota M, Watanabe K, Mancino M, Hamada S, et al. Development of leiomyosarcoma of the uterus in MMTV-CR-1 transgenic mice. J Pathol. 2007;211:36–44. doi: 10.1002/path.2083. [DOI] [PubMed] [Google Scholar]
- 103.Bianco C, Strizzi L, Mancino M, Rehman A, Hamada S, Watanabe K, et al. Identification of cripto-1 as a novel serologic marker for breast and colon cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2006;12:5158–64. doi: 10.1158/1078-0432.CCR-06-0274. [DOI] [PubMed] [Google Scholar]
- 104.Tysnes BB, Satran HA, Mork SJ, Margaryan NV, Eide GE, Petersen K, et al. Age-Dependent Association between Protein Expression of the Embryonic Stem Cell Marker Cripto-1 and Survival of Glioblastoma Patients. Transl Oncol. 2013;6:732–41. doi: 10.1593/tlo.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pilgaard L, Mortensen JH, Henriksen M, Olesen P, Sorensen P, Laursen R, et al. Cripto-1 Expression in Glioblastoma Multiforme. Brain Pathol. 2014 doi: 10.1111/bpa.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sell S. Cancer and stem cell signaling: a guide to preventive and therapeutic strategies for cancer stem cells. Stem cell reviews. 2007;3:1–6. doi: 10.1007/s12015-007-0015-5. [DOI] [PubMed] [Google Scholar]
- 107.De Luca A, Casamassimi A, Selvam MP, Losito S, Ciardiello F, Agrawal S, et al. EGF-related peptides are involved in the proliferation and survival of MDA-MB-468 human breast carcinoma cells. International journal of cancer Journal international du cancer. 1999;80:589–94. doi: 10.1002/(sici)1097-0215(19990209)80:4<589::aid-ijc17>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 108.De Luca A, Arra C, D’Antonio A, Casamassimi A, Losito S, Ferraro P, et al. Simultaneous blockage of different EGF-like growth factors results in efficient growth inhibition of human colon carcinoma xenografts. Oncogene. 2000;19:5863–71. doi: 10.1038/sj.onc.1203979. [DOI] [PubMed] [Google Scholar]
- 109.Casamassimi A, De Luca A, Agrawal S, Stromberg K, Salomon DS, Normanno N. EGF-related antisense oligonucleotides inhibit the proliferation of human ovarian carcinoma cells. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO. 2000;11:319–25. doi: 10.1023/a:1008350811639. [DOI] [PubMed] [Google Scholar]
- 110.Normanno N, De Luca A, Maiello MR, Bianco C, Mancino M, Strizzi L, et al. CRIPTO-1: a novel target for therapeutic intervention in human carcinoma. International journal of oncology. 2004;25:1013–20. [PubMed] [Google Scholar]
- 111.Adkins HB, Bianco C, Schiffer SG, Rayhorn P, Zafari M, Cheung AE, et al. Antibody blockade of the Cripto CFC domain suppresses tumor cell growth in vivo. The Journal of clinical investigation. 2003;112:575–87. doi: 10.1172/JCI17788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Biogen-Idec. A Phase I Study of BIIB015 in Relapsed/Refractory Solid Tumors. 2011. [Google Scholar]
- 113.Xing PX, Hu XF, Pietersz GA, Hosick HL, McKenzie IF. Cripto: a novel target for antibody-based cancer immunotherapy. Cancer research. 2004;64:4018–23. doi: 10.1158/0008-5472.CAN-03-3888. [DOI] [PubMed] [Google Scholar]
- 114.Hu XF, Li J, Yang E, Vandervalk S, Xing PX. Anti-Cripto Mab inhibit tumour growth and overcome MDR in a human leukaemia MDR cell line by inhibition of Akt and activation of JNK/SAPK and bad death pathways. British journal of cancer. 2007;96:918–27. doi: 10.1038/sj.bjc.6603641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bianco C, Salomon DS. Targeting the embryonic gene Cripto-1 in cancer and beyond. Expert opinion on therapeutic patents. 2010;20:1739–49. doi: 10.1517/13543776.2010.530659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kelly RK, Olson DL, Sun Y, Wen D, Wortham KA, Antognetti G, et al. An antibody-cytotoxic conjugate, BIIB015, is a new targeted therapy for Cripto positive tumours. European journal of cancer (Oxford, England: 1990) 2011;47:1736–46. doi: 10.1016/j.ejca.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 117.Rasul A, Khan M, Ali M, Li J, Li X. Targeting apoptosis pathways in cancer with alantolactone and isoalantolactone. TheScientificWorldJournal. 2013;2013:248532. doi: 10.1155/2013/248532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shi Y, Bao YL, Wu Y, Yu CL, Huang YX, Sun Y, et al. Alantolactone inhibits cell proliferation by interrupting the interaction between Cripto-1 and activin receptor type II A in activin signaling pathway. Journal of biomolecular screening. 2011;16:525–35. doi: 10.1177/1087057111398486. [DOI] [PubMed] [Google Scholar]
- 119.Lonardo E, Parish CL, Ponticelli S, Marasco D, Ribeiro D, Ruvo M, et al. A small synthetic cripto blocking Peptide improves neural induction, dopaminergic differentiation, and functional integration of mouse embryonic stem cells in a rat model of Parkinson’s disease. Stem cells. 2010;28:1326–37. doi: 10.1002/stem.458. [DOI] [PubMed] [Google Scholar]