Abstract
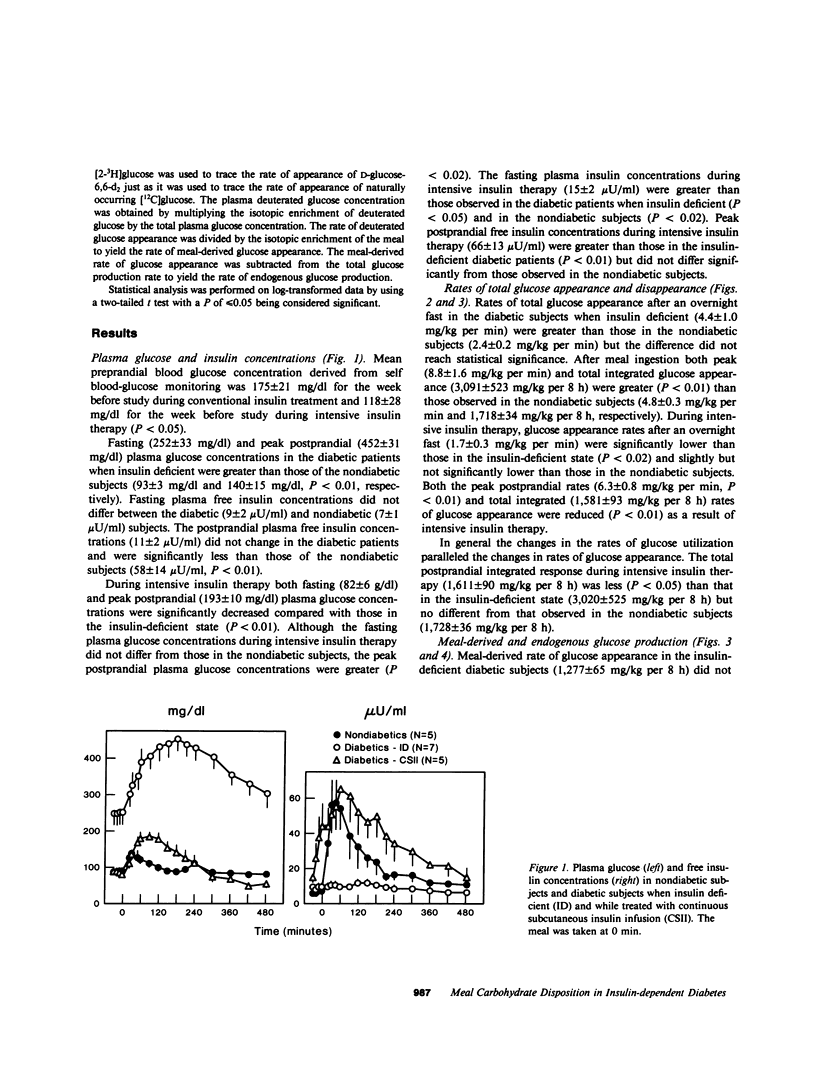
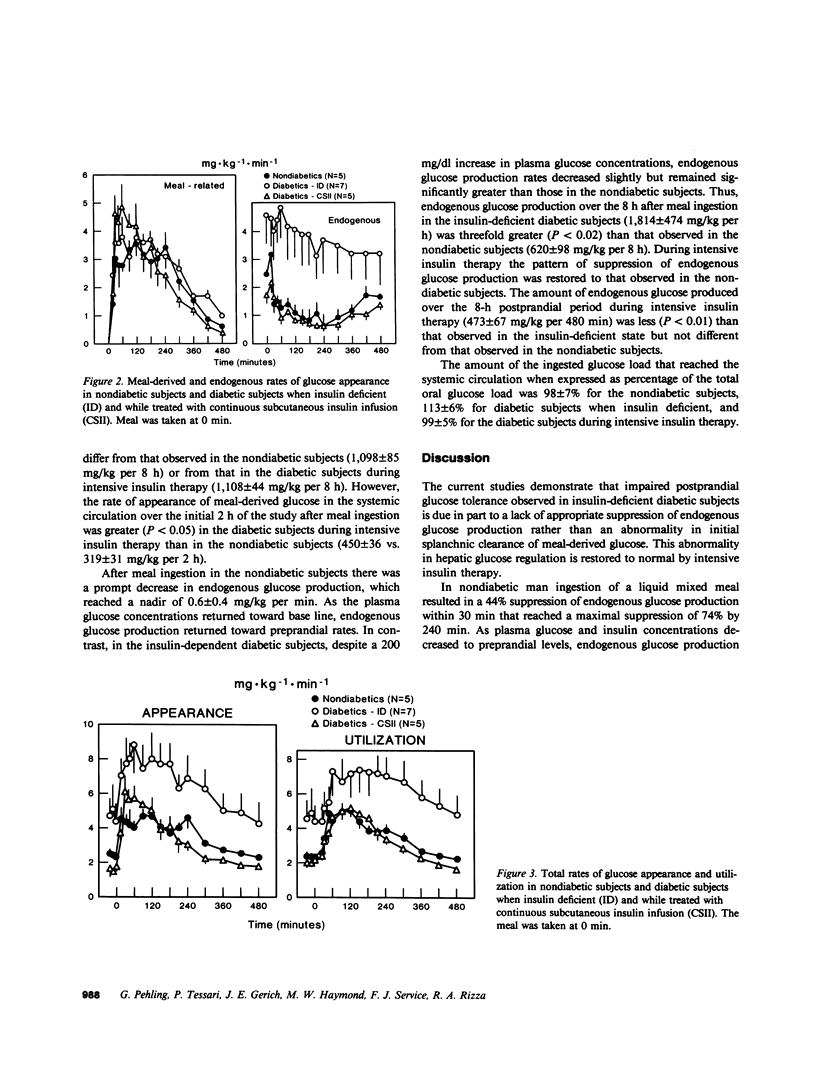
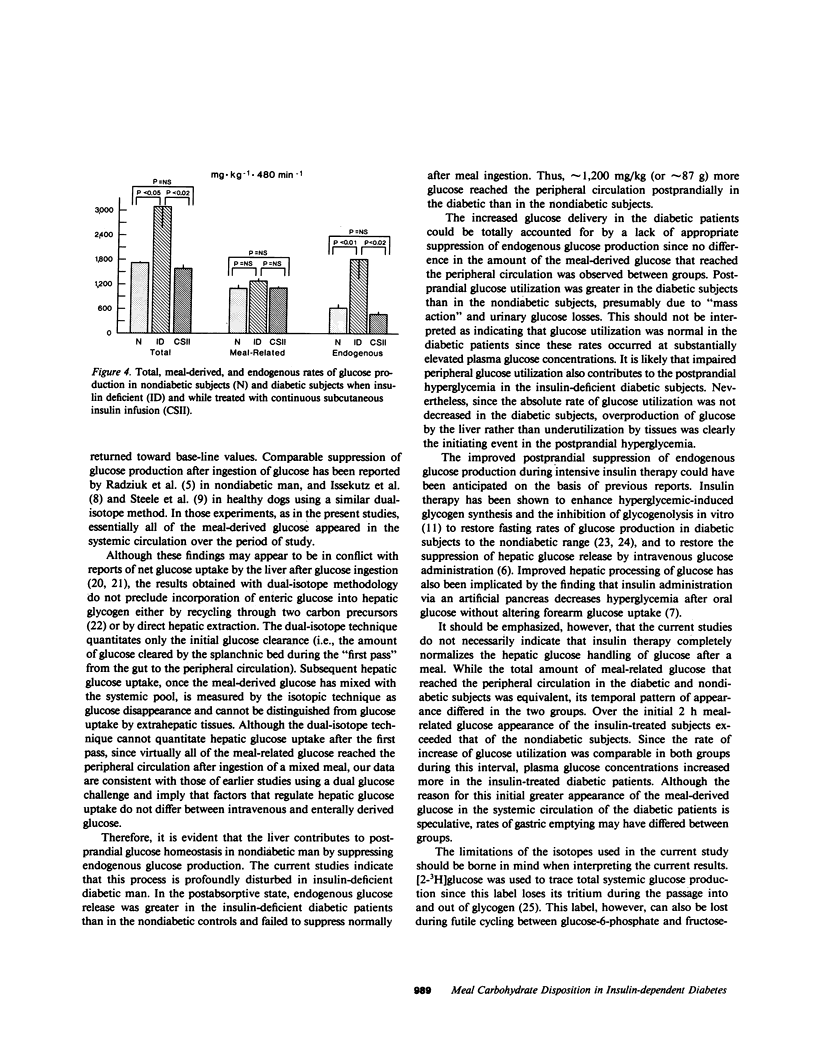
Postprandial hyperglycemia in insulin-deficient, insulin-dependent diabetic subjects may result from impaired suppression of endogenous glucose production and/or abnormal disposition of meal-derived glucose. To investigate the relative contributions of these processes and to determine whether 2 wk of near normoglycemia achieved by using intensive insulin therapy could restore the pattern of glucose disposal to normal, meal-related and endogenous rates of glucose appearance were measured isotopically after ingestion of a mixed meal that contained deuterated glucose in seven lean insulin-dependent and five lean nondiabetic subjects. Diabetic subjects were studied once when insulin deficient and again during intensive insulin therapy after 2 wk of near normoglycemia. Total glucose production was determined by using tritiated glucose and the contribution of meal-related glucose was determined by using the plasma enrichment of deuterated glucose. The elevated basal and peak postprandial plasma glucose concentrations (252 +/- 33 and 452 +/- 31 mg/dl) of diabetic subjects when insulin deficient were decreased by intensive insulin therapy to values (82 +/- 6 and 193 +/- 10 mg/dl, P less than 0.01) that approximated those of nondiabetic subjects (93 +/- 3 and 140 +/- 15 mg/dl, respectively). Total and endogenous rates of glucose appearance (3,091 +/- 523 and 1,814 +/- 474 mg/kg per 8 h) in the diabetic subjects were significantly (P less than 0.02) greater than those in non-diabetic subjects (1,718 +/- 34 and 620 +/- 98 mg/kg per 8 h, respectively), whereas meal-derived rates of glucose appearance did not differ. Intensive insulin therapy decreased (P less than 0.01) both total (1,581 +/- 98 mg/kg per 8 h) and endogenous (478 +/- 67 mg/kg per 8 h) glucose appearance to rates that approximated those observed in the nondiabetic subjects, but did not alter meal-related glucose appearance. Thus, excessive entry of glucose into the peripheral circulation in insulin-deficient diabetic patients after ingestion of a mixed meal resulted from a lack of appropriate suppression of endogenous glucose production rather than impairment of initial splanchnic glucose uptake. Intensive insulin therapy restored postprandial suppression of endogenous glucose production to rates observed in nondiabetic subjects.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bier D. M., Arnold K. J., Sherman W. R., Holland W. H., Holmes W. F., Kipnis D. M. In-vivo measurement of glucose and alanine metabolism with stable isotopic tracers. Diabetes. 1977 Nov;26(11):1005–1015. doi: 10.2337/diab.26.11.1005. [DOI] [PubMed] [Google Scholar]
- Cherrington A. D., Lacy W. W., Chiasson J. L. Effect of glucagon on glucose production during insulin deficiency in the dog. J Clin Invest. 1978 Sep;62(3):664–677. doi: 10.1172/JCI109174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEBODO R. C., STEELE R., ALTSZULER N., DUNN A., BISHOP J. S. ON THE HORMONAL REGULATION OF CARBOHYDRATE METABOLISM; STUDIES WITH C14 GLUCOSE. Recent Prog Horm Res. 1963;19:445–488. [PubMed] [Google Scholar]
- DeFronzo R. A., Ferrannini E., Hendler R., Wahren J., Felig P. Influence of hyperinsulinemia, hyperglycemia, and the route of glucose administration on splanchnic glucose exchange. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5173–5177. doi: 10.1073/pnas.75.10.5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn A., Katz J., Golden S., Chenoweth M. Estimation of glucose turnover and recycling in rabbits using various [3H, 14C]glucose labels. Am J Physiol. 1976 Apr;230(4):1159–1162. doi: 10.1152/ajplegacy.1976.230.4.1159. [DOI] [PubMed] [Google Scholar]
- Exton J. H., Harper S. C., Tucker A. L., Ho R. J. Effects of insulin on gluconeogenesis and cyclic AMP levels in perfused livers from diabetic rats. Biochim Biophys Acta. 1973 Nov 2;329(1):23–40. doi: 10.1016/0304-4165(73)90005-6. [DOI] [PubMed] [Google Scholar]
- Felig P., Wahren J., Hendler R. Influence of oral glucose ingestion on splanchnic glucose and gluconeogenic substrate metabolism in man. Diabetes. 1975 May;24(5):468–475. doi: 10.2337/diab.24.5.468. [DOI] [PubMed] [Google Scholar]
- Felig P., Wahren J. Influence of endogenous insulin secretion on splanchnic glucose and amino acid metabolism in man. J Clin Invest. 1971 Aug;50(8):1702–1711. doi: 10.1172/JCI106659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrannini E., Wahren J., Felig P., DeFronzo R. A. The role of fractional glucose extraction in the regulation of splanchnic glucose metabolism in normal and diabetic man. Metabolism. 1980 Jan;29(1):28–35. doi: 10.1016/0026-0495(80)90094-3. [DOI] [PubMed] [Google Scholar]
- Hall S. E., Saunders J., Sönksen P. H. Glucose and free fatty acid turnover in normal subjects and in diabetic patients before and after insulin treatment. Diabetologia. 1979 May;16(5):297–306. doi: 10.1007/BF01223618. [DOI] [PubMed] [Google Scholar]
- Hue L. The role of futile cycles in the regulation of carbohydrate metabolism in the liver. Adv Enzymol Relat Areas Mol Biol. 1981;52:247–331. doi: 10.1002/9780470122976.ch4. [DOI] [PubMed] [Google Scholar]
- Issekutz B., Jr, Issekutz T. B., Elahi D. Glucose kinetics during oral glucose tolerance test in normal, methylprednisolone-treated and alloxan diabetic dogs. Diabetes. 1974 Aug;23(8):645–650. doi: 10.2337/diab.23.8.645. [DOI] [PubMed] [Google Scholar]
- Issekutz T. B., Issekutz B., Jr, Elahi D. Estimation of hepatic glucose output in non-steady state. The simultaneous use of 2-3H-glucose and 14C-glucose in the dog. Can J Physiol Pharmacol. 1974 Apr;52(2):215–224. doi: 10.1139/y74-029. [DOI] [PubMed] [Google Scholar]
- Jackson R. A., Peters N., Advani U., Perry G., Rogers J., Brough W. H., Pilkington T. R. Forearm glucose uptake during the oral glucose tolerance test in normal subjects. Diabetes. 1973 Jun;22(6):442–458. doi: 10.2337/diab.22.6.442. [DOI] [PubMed] [Google Scholar]
- Katz J., Dunn A. Glucose-2-t as a tracer for glucose metabolism. Biochemistry. 1967 Jan;6(1):1–5. doi: 10.1021/bi00853a001. [DOI] [PubMed] [Google Scholar]
- Katz L. D., Glickman M. G., Rapoport S., Ferrannini E., DeFronzo R. A. Splanchnic and peripheral disposal of oral glucose in man. Diabetes. 1983 Jul;32(7):675–679. doi: 10.2337/diab.32.7.675. [DOI] [PubMed] [Google Scholar]
- Nakagawa S., Nakayama H., Sasaki T., Yoshino K., Yu Y. Y. A simple method for the determination of serum free insulin levels in insulin-treated patients. Diabetes. 1973 Aug;22(8):590–600. doi: 10.2337/diab.22.8.590. [DOI] [PubMed] [Google Scholar]
- Newgard C. B., Hirsch L. J., Foster D. W., McGarry J. D. Studies on the mechanism by which exogenous glucose is converted into liver glycogen in the rat. A direct or an indirect pathway? J Biol Chem. 1983 Jul 10;258(13):8046–8052. [PubMed] [Google Scholar]
- Perley M. J., Kipnis D. M. Plasma insulin responses to oral and intravenous glucose: studies in normal and diabetic sujbjects. J Clin Invest. 1967 Dec;46(12):1954–1962. doi: 10.1172/JCI105685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radziuk J., McDonald T. J., Rubenstein D., Dupre J. Initial splanchnic extraction of ingested glucose in normal man. Metabolism. 1978 Jun;27(6):657–669. doi: 10.1016/0026-0495(78)90003-3. [DOI] [PubMed] [Google Scholar]
- Rizza R. A., Cryer P. E., Gerich J. E. Role of glucagon, catecholamines, and growth hormone in human glucose counterregulation. Effects of somatostatin and combined alpha- and beta-adrenergic blockade on plasma glucose recovery and glucose flux rates after insulin-induced hypoglycemia. J Clin Invest. 1979 Jul;64(1):62–71. doi: 10.1172/JCI109464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizza R. A., Mandarino L. J., Gerich J. E. Dose-response characteristics for effects of insulin on production and utilization of glucose in man. Am J Physiol. 1981 Jun;240(6):E630–E639. doi: 10.1152/ajpendo.1981.240.6.E630. [DOI] [PubMed] [Google Scholar]
- Rizza R., Verdonk C., Miles J., Service F. J., Gerich J. Effect of intermittent endogenous hyperglucagonemia on glucose homeostasis in normal and diabetic man. J Clin Invest. 1979 Jun;63(6):1119–1123. doi: 10.1172/JCI109404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEELE R., WALL J. S., DE BODO R. C., ALTSZULER N. Measurement of size and turnover rate of body glucose pool by the isotope dilution method. Am J Physiol. 1956 Sep;187(1):15–24. doi: 10.1152/ajplegacy.1956.187.1.15. [DOI] [PubMed] [Google Scholar]
- Saccà L., Cicala M., Trimarco B., Ungaro B., Vigorito C. Differential effects of insulin on splanchnic and peripheral glucose disposal after an intravenous glucose load in man. J Clin Invest. 1982 Jul;70(1):117–126. doi: 10.1172/JCI110583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Service F. J., Molnar G. D., Rosevear J. W., Ackerman E., Gatewood L. C., Taylor W. F. Mean amplitude of glycemic excursions, a measure of diabetic instability. Diabetes. 1970 Sep;19(9):644–655. doi: 10.2337/diab.19.9.644. [DOI] [PubMed] [Google Scholar]
- Steele R., Bjerknes C., Rathgeb I., Altszuler N. Glucose uptake and production during the oral glucose tolerance test. Diabetes. 1968 Jul;17(7):415–421. doi: 10.2337/diab.17.7.415. [DOI] [PubMed] [Google Scholar]



