Abstract
Background & Aims
The gut microbiota is a complex and densely populated community in a dynamic environment determined by host physiology. We investigated how intestinal oxygen levels affect the composition of the fecal and mucosally adherent microbiota.
Methods
We used the phosphorescence quenching method and a specially designed intraluminal oxygen probe to dynamically quantify gut luminal oxygen levels in mice. 16S rRNA gene sequencing was used to characterize the microbiota in intestines of mice exposed to hyperbaric oxygen, human rectal biopsy and mucosal swab samples, and paired human stool samples.
Results
Average pO2 values in the lumen of the cecum were extremely low (<1 mmHg). In altering oxygenation of intestines of mice, we observed that oxygen diffused from intestinal tissue and established a radial gradient the extended from the tissue interface into the lumen. Increasing tissue oxygenation with hyperbaric oxygen altered the composition of the gut microbioita in mice. In humans, 16S rRNA gene analyses revealed an increased proportion of oxygen-tolerant organisms of the Proteobacteria and Actinobacteria phyla associated with the rectal mucosa, compared with the feces, indicating an effect of oxygenation on the microbiota. A consortium of asaccharolytic bacteria of the Firmicute and Bacteroidetes phyla, which primarily metabolize peptones and amino acids, was associated primarily with mucus. This could be due to the presence of proteinaceous substrates provided by mucus and the shedding of the intestinal epithelium.
Conclusions
In an analysis of intestinal microbiota of mice and humans, we observed a radial gradient of microbes linked to distribution of oxygen and nutrients provided by host tissue.
Keywords: aerobic, anaerobic, microbe, spatial gradient of oxygen
Introduction
The bacterial microbes that inhabit the intestinal tract form a complex community dominated by obligately anaerobic organisms from both the Firmicutes and Bacteroidetes Phyla.1 Carbohydrates are a major source of energy for the microbiota that relies heavily on fermentative metabolism in the anaerobic environment. Considerable attention has focused on the mechanisms by which the saccharolytic microbes digest glycans to produce short chain fatty acids that, in turn, influence host physiology.2 Although the gut microbiota in humans is generally stable in composition, alterations of the host induced, for example, by a change in diet3, 4 or intestinal inflammation,5 can alter both the microbiota composition and function.6 Within a healthy host, there are differences in the gut microbiota along the longitudinal axis of the gut7 as well as between different regions within a single fecal sample.8 In this report, we examined the radial distribution of the gut microbiota and attempted to identify host-derived factors in the intestinal environment that could influence its composition. Identification of such factors could improve our understanding of the taxonomic differences between the mucosally-associated and fecal microbiota in health as well as the development of the “dysbiotic” microbiota associated with intestinal inflammation.
The intestinal niche is largely devoid of oxygen. Several lines of evidence implicate both aerobic and facultative anaerobic bacteria in the development of the anaerobic environment.9 Culture-dependent surveys of the newborn microbiota suggest that the initial community of aerobic and facultative anaerobic bacteria might consume oxygen in the intestine, allowing the population of obligate anaerobes to develop.10, 11 This observation is supported by more recent studies, showing predominance of oxygen tolerant Gamma-Proteobacteria in the gut microbiota of newborns.12 In addition, analysis of flatus composition in humans reveals a mixture of gases produced by microbial metabolism, namely hydrogen, carbon dioxide, methane, and hydrogen sulfide. Abundant levels of nitrogen are also present, but oxygen levels are very low.13 However, direct evidence for the role of the gut microbiota in regulating oxygen levels in the intestinal lumen is still lacking.
The mechanism responsible for maintaining the anaerobic environment of the gut lumen is unclear, due in part to the difficulty in quantifying intestinal oxygen levels. Although there are several reports on measurements of oxygenation of the intestinal tissue,14, 15 very few describe measurements of oxygen directly in the lumen of the gut. Most experiments relied on Clark-type oxygen electrodes,16-18 showing that the pO2 (partial pressure of oxygen) in the lumen is less than 0.5 mmHg.16 However, use of electrodes is complicated by their invasiveness and potential leaks of oxygen into the measurement environment. Recently, EPR (Electron Paramagnetic Resonance) oximetry was applied to non-invasively image gut oxygen levels in mice.19 A spin probe (charcoal) was delivered orally, and the decay of the spin polarization was used to assess oxygen concentration.20 The measured pO2 levels decreased from 58 mmHg in the stomach to 3 mmHg near the distal sigmoid colon, significantly exceeding those measured by electrodes.16, 18. One possible source of the discrepancy is that the charcoal probe was calibrated using aqueous suspensions, while the measurements were performed when the probe was in viscous fecal material. Large differences between the rates of oxygen diffusion in these two environments may have caused errors, since the signal in EPR oximetry (EPR line width) is a function of both oxygen concentration and oxygen diffusion coefficient.
In the present study we adapted the phosphorescence quenching method21 to measure oxygen levels in the intestinal lumen. Using a newly developed probe, we quantified oxygen levels in the intestinal lumen, gaining evidence that bacteria in the gut consume oxygen delivered by the colonic tissue. Furthermore, we demonstrate enrichment of oxygen-tolerant bacteria in the vicinity of the rectal mucosa in healthy human subjects as well as an unanticipated signature of asaccharolytic bacteria, which are dependent upon the metabolism of proteinacious substrates in the rectal mucus. Together, the compendium of bacteria exhibiting aerotolerance and protein-based metabolism distinguish the mucosally-associated microbiota from the feces. These bacterial taxa may play a role in the development of the dysbiotic microbiota associated with the inflammatory response in Crohn’s disease and ulcerative colitis.5
Materials and Methods
Molecular oxygen (O2) quenches phosphorescence originating from excited triplet electronic states of molecules. The dependence of the phosphorescence lifetime (τ) on the pO2 in the environment throughout the range of biological oxygen concentrations follows the Stern-Volmer model (Eq. 1): 1/τ=1/τ0+kq×pO2, where τ is the phosphorescence lifetime, and τ0 and kq are probe-specific parameters. By exciting an object containing a probe with a pulse of light and measuring the phosphorescence decay, pO2 in the environment can be quantified. The measurements have millisecond response, high specificity and are independent of the probe distribution throughout the environment. The synthesis of the oxygen probe, the details of in vivo phosphorescence measurements as well as the methods for acquisition of human samples, storage, and sequencing can be found in the Supporting Information.
Animals
C57/B6 mice 8-12 weeks of age were used. All had free access to chow (AIN76, Research Diets). Anesthesia was induced through a nose cone via inhalation of isoflurane 2.5% mixed with air, after which isoflurane proportion was decreased to 1.5%. Low levels of isoflurane (<3%) do not cause significant changes in oxygenation22. Animals were anesthetized, and a laparotomy performed in some cases (see Discussion for details). At the end of the experiments, the mice were euthanized according to guidelines established by the American Veterinary Medical Association Panel on Euthanasia. Experimental protocols were approved by the Institutional Animal Care and Use Committee at the University of Pennsylvania.
Results
Oxygen measurements
The synthetic phosphorescent probes for tissue oxygen measurements 23-26 do not interact with proteins or other endogenous molecules, and the calibration parameters of these probes remain unchanged in any aqueous environment, ensuring absolute oxygen quantification.27 In the present work, we used one such probe, Oxyphor G4,24 to measure pO2 in the intestinal tissue. The probe was injected into the tail vein in mice, and measurements were performed in reflectance-type geometry (Fig. 1A). Excitation photons (λex=635 nm) are able to diffuse several cm into the tissue,24, 28 while excitation efficiency decreases exponentially with depth. The largest contribution to the signal is from the probe-containing layers closest to the fiber tip; however, in mice phosphorescence could be considered averaging over the thickness of the entire intestinal wall, which is ~300 μm thick.
Figure 1.
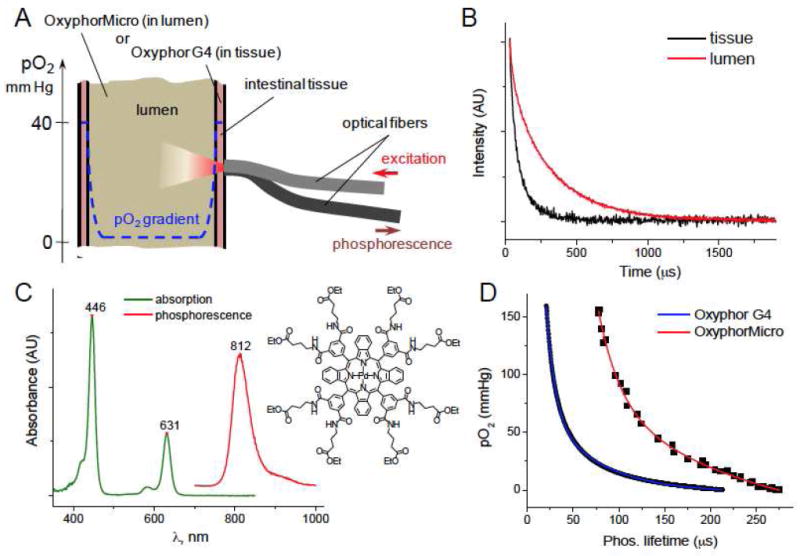
Application of phosphorescence oximetry to intestinal oxygen measurements. A) Intestinal oxygen measurements by phosphorescence quenching (the scheme does not represent correct proportions of physical dimensions). B) Phosphorescence decays typically observed in tissue (Oxyphor G4) and intraluminal (OxyphorMicro) oxygen measurements. C) Structure, optical absorption, and phosphorescence spectra of Pt tetrabenzoporphyrin used as an oxygen sensing element in both Oxyphor G4 and OxyphorMicro. D) Calibration curves for Oxyphor G4 in physiological saline and OxyphorMicro directly in mouse fecal material at 36.5°C. Data for OxyphorMicro are a superposition of several animal samples.
For quantitative oxygen measurements, the probe constants have to be determined in the same medium as encountered by the probe during measurements. In particular, quenching constant kq depends on the rate of oxygen diffusion that differs between viscous intraluminal substances and water. Thus, calibration parameters obtained in solutions/suspensions are not suitable for measurements in the lumen.19 Furthermore, properties of molecular probes, such as Oxyphor G4, may be affected during their transit through the intestinal tract, e.g. due to actions of gut enzymes, bile acids and/or interactions with processed food.
The quenching constant kq is a function of the viscosity of the medium through which oxygen must diffuse during the probes’ excited triplet lifetime (τ0=200-300 μs for probes like Oxyphor G4). We reasoned that probe molecules dispersed in a large particle would predominantly encounter oxygen that traveled only within that particle. For example, in a 20 μm particle (~108 probe molecules at 50 μM concentration) less than 1% of the probe will experience collisions with oxygen entering from the outside, assuming oxygen diffusion coefficient in the particle is 2×10-7 cm2s-1 (i.e. 100x lower than that in water, a typical value for solid polymers).29, 30 Hence, calibration of such a particle should be minimally affected by the properties of the environment, while oxygen equilibrium between the inside and outside of the particle would be established in milliseconds.
Based on the above, a probe was prepared, abbreviated OxyphorMicro, comprising polymethylmetacrylate (PMMA) particles (~10-20 μm in diameter) containing co-dissolved phosphorescent Pd tetrabenzoporphyrin (PdTBP)24 (Fig. 1C). The oxygen diffusion coefficient in PMMA is 4×10-8 cm2s-1 at 25°C.30 OxyphorMicro was calibrated in aqueous suspensions as well as directly in fecal material extracted from the mouse cecum (see SI). The resulting plots (Fig. 1D) revealed good animal-to-animal reproducibility and almost no alterations between environments of different viscosity, suggesting that phosphorescence quenching is minimally affected by the medium and can be a robust indicator of luminal oxygenation.
OxyphorMicro was mixed with chow, and after ingestion it remained only in the intestinal tract due to its hydrophobicity and inability to diffuse into the tissue. Indeed, large PMMA particles cannot be endocytosed by cells at the mucosal interface and/or distributed throughout the tissue by the lymph flow. At the same time, hydrophobic PdTBP dye cannot leach from the nanoparticles into a hydrophilic environment. Measurements in the gut lumen were performed in the same fashion as in tissue (Fig. 1A). After laparotomy the fiber tip was positioned directly by the organ of interest. Furthermore, because the probe phosphorescence was very strong, measurements could also be accomplished in intact animals by placing the fiber against the shaved abdomen. In this configuration, excitation light diffuses through the abdominal wall and intestinal tissue before reaching the probe. As a result, origin of the signal could not be attributed to any specific part of the intestine. However, there was very little variation in the measured oxygen values regardless of the fiber position (vide infra). All values revealed the luminal pO2 levels below 1 mmHg. The pO2 in laparotomized mice was always somewhat higher, probably due to diffusion of oxygen from the air after surgery (Fig. 2B,C).
Figure 2.
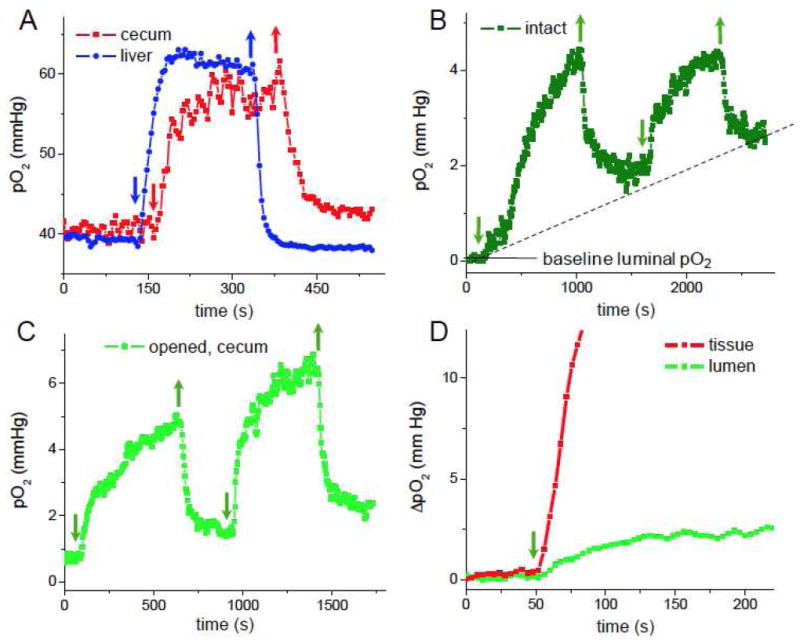
Effect of altering oxygen concentration in the inhaled gas mixture on host tissue and gut luminal oxygen levels. Arrows indicate switching pure O2 on (↓) and returning to ambient air (↑). A) Changes in intestinal tissue pO2 in the cecum. Data for the liver tissue are shown for comparison. Changes in intestinal luminal pO2 using trans-abdominal measurements (B) and in the cecum after laparotomy (C). D) Relative time-dependent changes in tissue and luminal (cecum) pO2’s: ΔpO2=pO2(t)-pO2(0), where pO2(t) and pO2(0) are the pO2 values measured at time t and in the beginning of the experiment (time zero), respectively.
Phosphorescence oximetry reveals exchange of oxygen between the host and gut microbiota
Intestinal tissue measurements in the cecum revealed baseline pO2 levels of approximately 40 mmHg (Fig. 2A), which is in good agreement with earlier measurements by oxygen electrodes.15 Inhalation of pure oxygen led to a rapid increase in tissue pO2, which reached a new steady state and, as expected, decreased to the baseline once the mouse was returned to ambient air. Similar changes in tissue pO2 upon inhalation of pure oxygen have been observed previously in other tissues.31
Remarkably, inhalation of pure oxygen also led to an increase in the luminal pO2, which dropped upon return to ambient air. Similar changes were observed using either a trans-abdominal (Fig. 2B) or after-laparotomy measurements (Fig. 2C). The increase in luminal oxygenation due to the inhalation of pure oxygen is slower than the concurrent increase in the tissue pO2 (Fig. 2D), and a steady state is not reached, as oxygen continues to diffuse slowly into the lumen over more than 500s (Figs. 2B,C). Furthermore, upon return to ambient air, luminal oxygen levels failed to reach the baseline (Fig. 2B, dotted line). This behavior may be rationalized considering that diffusion of oxygen through the fecal substrate is significantly slower than through liquid. Based on the diffusion theory, if the oxygen diffusion coefficient in the intraluminal medium is 2×10-6 cm2s-1 (only 10 times lower than in water), complete tissuelumen equilibrium should take ~30-50 min to establish in an intestinal tube 2-3 mm in diameter.
At the initial state, the oxygen gradient extends from the tissue throughout the mucosal interface into the lumen (Fig. 1A), whereby microorganisms adjacent to the tissue consume most of the available oxygen, keeping the bulk of the lumen deeply anaerobic. The fact that phosphorescence measurements in intact animals show overall very low baseline intraluminal pO2 (Figs. 2B,C) suggests that the oxygen-containing layer, which is the closest to the tissue and to the fiber tip, is very thin and contributes minimally to the overall signal. Therefore, we conclude that the oxygen gradient in the lumen must be very steep. Additional studies will be needed to quantify the dynamics of oxygen consumption at the mucosal interface. Overall, these results demonstrate that changes in host oxygenation alter oxygen content of the luminal environment, raising the question of whether oxygen levels may thereby modulate the composition of the gut microbiota.
Hyperbaric Oxygen Therapy (HBOT) results in alterations in gut microbiota composition
Having demonstrated that enhanced host oxygenation alters luminal oxygenation in the gut, we examined the effect of HBOT on the composition of the gut microbiota in mice. HBOT (100% O2 at 2.0 atm) was delivered to mice for 2 h daily for 9 days, similar to clinical protocols.32, 33 Under these conditions, HBOT increases tissue oxygenation by up to 5-fold.32, 33 Control animals were also placed into HBOT chambers for the same period of time, but ambient oxygen pressure was maintained. Fecal pellets were collected at days 1 (baseline), 4, 6, and 9, and DNA was extracted and prepared for 16S rRNA gene sequencing. Sequence data from control and HBOT mice were compared using UniFrac. Abundance-weighted analysis showed no differences among groups. However, a non-weighted analysis of community membership showed differences after HBOT treatment [p=0.043 (day 4), p=0.008 (day 6), p=0.021 (day 9); day 6 data in Fig. 3A], but no significant difference at day 1 (p=0.17). A second experiment (data not shown) also demonstrated a difference in the gut microbiome after HBOT. Analysis of bacterial abundance using a generalized linear mixed effects model (Fig. S3) showed that 28 lineages changed detectably after HBOT, most with complex temporal behavior. An analysis of community membership showed that one lineage--Anaerostipes, a catalase-negative obligately anaerobic Firmicute--was detected in fewer samples after HBOT (p<10e-10), consistent with selective loss of an oxygen-intolerant lineage (Fig 3B). These results demonstrate that host oxygenation can alter the composition of the gut microbiota, but the short-term effects are both modest and complex.
Figure 3.
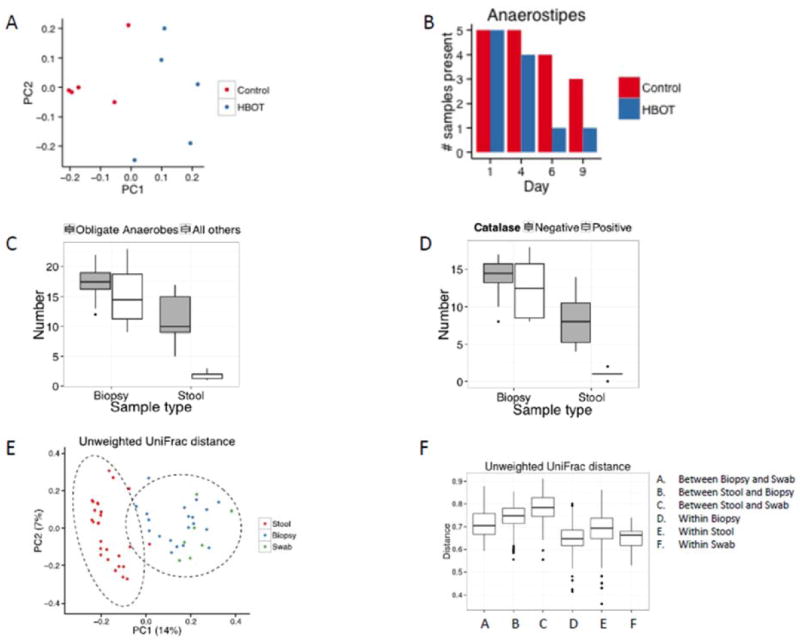
Differences in oxygen tolerance and community membership between the mucosallyassociated and fecal microbiota. Bacterial communities were profiled using 16S rRNA gene sequencing. A) Separation of murine bacterial communities, analyzed using 16S rRNA gene sequencing of V1V2 tags, after day 6 of Hyperbaric Oxygen Therapy (HBOT). An unweighted UniFrac plot is shown (p=0.008; permanova). B) Number of samples with OTUs annotating as Anaerostipes. The difference achieves p<10e-10 (logistic mixed-effects model). C) Number of bacterial genera classified as obligate anaerobes vs. more oxygen-tolerant “all others” in paired human rectal biopsy and stool samples. D) Number of bacterial genera classified as catalase positive vs. negative in paired human rectal biopsy and stool samples. E) Relationship between mucosally- and stool-associated microbiota using an unweighted Unifrac PCoA ordination (Ellipses represent 95% confidence intervals for a multivariate normal distribution). F) Pairwise distance in microbiota composition between stool, rectal biopsy, and rectal swab samples.
The gut microbiota in rectal biopsies are enriched for oxygen tolerant organisms with catalase activity
Our results suggest that a fraction of the gut microbiota consumes oxygen, leading to extremely low oxygen levels at the baseline and causing a decrease in luminal pO2 after an oxygenation burst (Figs. 2B,C). Using samples collected from a controlled feeding experiment in ten healthy human subjects over a ten-day inpatient stay,3 we searched for such a bacterial population at the mucosal interface. Fecal samples were collected daily, and an unprepped flexible sigmoidoscopy was performed on days 1 and 10 of the study to obtain rectal biopsies. Although the composition of the fecal microbiota reached a new steady state within 24 h of the dietary intervention, there were no consistent taxonomic alterations associated with this dietary intervention, i.e. intersubject variability remained the largest source of variance.3 However, the mucosally-associated microbiota in the rectal biopsies was found to be distinct (Fig. 3E). We quantified the unassigned reads in each rank in taxonomy based on sample type to exclude the possibility that this difference was due to a bias in reads assigned to the different groups (Fig. S4).
Based the notion of the host-gut microbiota oxygen gradient, we hypothesized that the mucosally-adherent microbiota would have a higher representation of oxygen tolerant bacterial taxa when compared to the bacteria residing in feces. Bacteria can be classified into five groups by their ability to utilize oxygen for cellular functions: obligate aerobes, microaerophiles, facultative bacteria, aerotolerant bacteria, and obligate anaerobes. Based on the literature,34-36 obligate anaerobic bacteria can be classified at the genus level,37 and each of the 70 bacterial genera in the stool and biopsy samples with proportion greater than 0.002, were classified into one of these categories (Table S1). For each participant, we then determined whether the predominant organisms in each stool sample and biopsy specimen were obligate anaerobes or “all others” based on their averaged abundance on days 1 and 10. For this analysis, we classified bacterial described as “anaerobic” in the literature as being “obligate anaerobes” because they were also catalase negative. For each participant, we computed an odds ratio of obligate anaerobic bacteria being more abundant in the stool sample than in the biopsy sample. The one-sample Wilcoxon signed-rank test was used to test for the null hypothesis of no enrichment, that is, population median odds ratio equals one. This analysis, shown in Fig. 3C, revealed that bacteria that are capable of tolerating oxygen were greatly enriched in the mucosal biopsy samples relative to the stool (p=0.0001).
Survival of oxygen tolerant bacteria requires the expression of ROS (reactive oxygen species) detoxifying enzymes, such as catalases, peroxidases and superoxide dismutases.38 Catalase activity in the 52 genera (Table S1) was found to be more commonly expressed in the mucosally-associated microbiota than in the microbes residing in the stool (Fig. 3D, p=0.002). In total, these results demonstrate that the mucosally adherent microbiota is better adapted for residence in an oxygen-containing environment relative to the microbiota in the feces.
Aerotolerant and asaccharolytic protein metabolizing bacterial taxa distinguish rectal mucosally-associated microbiota from feces
Mucosally-adherent bacteria reside primarily within the outer intestinal mucus layer39 where many of the bacterial taxa are saccharolytic, i.e. capable of harvesting energy through the digestion of carbohydrates.2, 40 To compare mucus-associated gut microbiota relative to the stool, we collected rectal mucus using a swab inserted through the anus, in parallel with stool from several healthy human subjects and performed 454 16S gene sequencing from the isolated DNA. PCoA of a non-weighted Unifrac analysis examining the presence vs. absence of bacterial taxa revealed that microbiota obtained by rectal swab and biopsy were more similar to each other than to the microbiota of the stool (Fig. 3E). PERMANOVA with permutation revealed significant differences between groups (p=0.0001) with differences between swab and stool (p=0.009) and biopsy and stool (p=0.001), dominating the difference between the swab and biopsy samples (Fig. 3F). The similarity between the biopsy and swab specimens predominated despite the strong contribution of intersubject variability.
Taxa from these three sampling sources are visualized in a heat map (Fig. 4). The greater similarity between the rectal swab and biopsies relative to the feces on the unweighted Unifrac analysis (Figs. 3E,F) is due the consistent presence of nine low-abundance bacterial taxa in the mucosal samples that are absent in most stool samples (Fig. 4). These included four genera from cluster XIII of the Clostridium in the Firmicutes phylum (Anaerococcus, Finegoldia, Murdochiella, and Peptoniphilus),41 one Bacteroidetes (Porphyromonas), two Proteobacteria (Campylobacter and Enterobacteriaceae) and one Actinobacteria (Corynebacterium) (Table S2). Despite their divergence in phylogeny, these six genera of the Firmicutes, Bacteroidetes, and Proteobacteria phyla contain asaccharolytic organisms that require proteinaceous substrates as carbon and energy sources.41-45 In addition, four taxa in the Actinobacteria and Proteobacteria phyla are either aerobic, facultatively anaerobic, or microaerophilic organisms that exhibit tolerance to oxygen. These results show that the mucosally-adherent microbiota can be distinguished from those in the stool by the presence of a consortium of asaccharolytic and aerotolerant bacteria.
Figure 4.
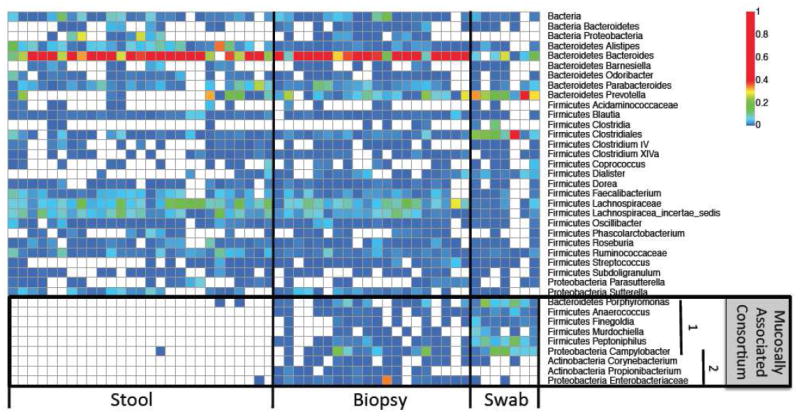
Heatmap of the microbiota in stool, biopsy, and rectal swab samples from 16S rRNA gene sequencing. In the “Mucosally Associated Consortium” 1=Asaccharolytic bacteria and 2=Aerobic or facultative anaerobic bacteria.
Genes for carbohydrate metabolism are decreased in abundance in the mucosally-adherent microbiota
Analysis of gene representation using Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt), which infers full gene composition from 16S rRNA sequence data,46 allowed us to perform metabolic reconstruction of the stool and mucosally-associated microbiome.47 A heatmap of gene abundance assigned to major metabolic pathways revealed a lower abundance of carbohydrate metabolism genes in the microbiome obtained by swab (Fig. S5). Although the percent of genes associated with carbohydrate metabolism was 10-12% in the stool and paired biopsy specimens, the percentage obtained by rectal swab was significantly lower (Fig. 5, p=0.016 by exact binomial test) consistent with the maximal separation between rectal swab and the other two sample sources (Fig. 3F). This observation supports the idea that asaccharolytic bacteria are associated with the rectal mucus.
Figure 5.
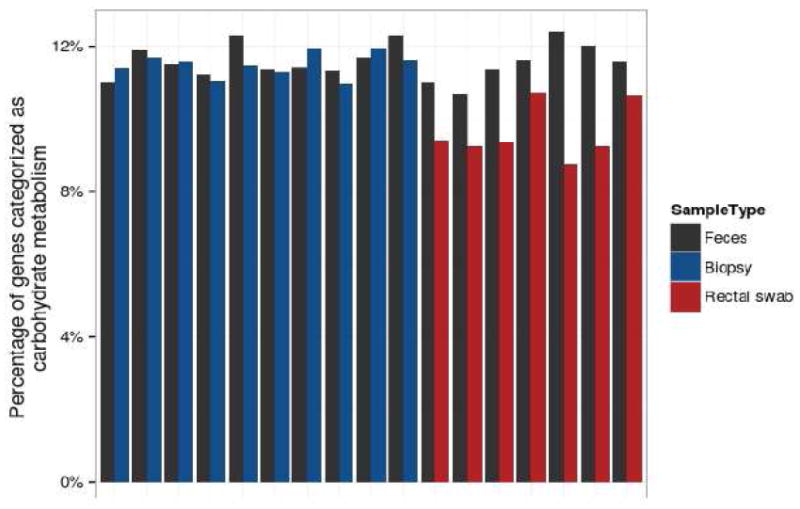
Gene content in gut bacteria inferred from 16S rRNA gene data. Gene content was inferred by using 16S rRNA gene tag data to access whole genome sequences of bacterial relatives, and this information was used to infer potential gene content in the bacterial communities studied.
Discussion
Previous studies established that the composition of the gut microbiota differs along the longitudinal axis of the gut. Here we report that the gut microbiota is also segregated radially and correlated with radial oxygen gradient and distribution of the tissue-associated mucus, which provides a nutrient source. Using the phosphorescence quenching method, we provide the first direct evidence that oxygenation of the host influences gut luminal oxygenation. Luminal oxygenation increased upon increase in host oxygenation, demonstrating diffusion of oxygen from the host tissue into the intestinal lumen and suggesting the presence of a spatial oxygen gradient. The decrease in the luminal pO2 upon return of the host tissue to normoxia supports the notion that oxygen is being consumed by the gut microbiota residing close to the mucosal interface. Our data show an increase in the abundance of oxygen-tolerant organisms adherent to the mucosal surface, consistent with this idea. A luminal oxygen gradient might play a role in “aerotaxis” of motile bacteria, through bacterial energy sensing pathways48, into the mucus layer where they are able to survive and proliferate. This resembles the ecology of environmental microoxygenic zones, such as activated sewage sludge,49 marine snow and soil aggregates that develop oxygen gradients, whereby microbes juxtaposed to the oxygenated environment consume oxygen before it reaches the interior of the microbial community.50 The presence of a spatial gradient of oxygen in the lumen provides an explanation for the enrichment of aerobic and facultative anaerobic organisms from the Proteobacteria and Actinobacteria phyla in rectal biopsy and swab samples.
The results of our studies of HBOT in mice show that alteration of host oxygenation can modify the composition of the gut microbiota. These results are in agreement with previous culture-based studies on the effect of HBOT in a model of intestinal obstruction51. Nevertheless, the results suggest that the effects, at least in the short-term, may be modest and complex. Increased tissue oxygenation could directly influence microbes, such as reducing Anaerostipes, but could also have an effect on host immune system,33 that could indirectly influence the gut microbiota composition. In addition, alterations in the host oxygenation might alter mucosallyadherent bacterial populations differently than those in the feces.
In addition to the presence of mucosally-associated oxygen tolerant taxa, there were six additional taxa found exclusively in biopsies and rectal swab samples, but not in the feces. Remarkably, all six represent assacharolytic bacteria that utilize proteinaceous substances rather than carbohydrates. Because all but one were also found in rectal swab samples, we infer that these assacharolytic organisms reside in the intestinal mucus layer, which is a richer source of proteinaceous substrates than feces. The protein source might be shedding of the intestinal epithelium into the mucus,52 or the protein may come from the mucus itself, since mucus is composed of glycoproteins.2, 52, 53 The ability of saccharolytic bacteria to digest glycans in mucus2, 40 may help polypeptides to become more accessible for assacharolytic bacteria, enhancing the syntrophic interactions between Bacteroides and Clostridial genera.2 Taken together these results highlight both mutualistic and syntrophic interactions between bacteria that play a role in the coaggregation of taxa residing together in a spatially-defined architecture such as have been described for biofilms.39, 52 Such interactions have been recently suggested by dense metabolomic and taxonomic profiling of endoscopic mucosal lavage samples from the colon.54
The observed enrichment of Actinobacteria and Proteobacteria in the mucosally-associated microbiota in healthy human subjects may also be relevant to intestinal disease states. Both of these phyla contain taxa, such as enterobacteriaceae, that are consistently observed in the dysbiotic microbiota associated with inflammatory bowel disease.55 This supports the notion that dysbiosis is the result of the oxidative nature of the host inflammatory response.47, 56 These aerotolerant organisms, normally residing at low levels in intestinal mucus8 in a healthy host, could serve as keystone communities that expand during intestinal inflammation facilitated by the greater flux of oxidative metabolites and oxygen into more highly hydrated feces with the development of diarrhea.55 In this manner, the oxidative nature of the intestinal inflammatory response may affect the community membership, gene expression, and function of the gut microbiota47, 50, 57, 58 in a way that perpetuates the inflammatory condition.
Supplementary Material
Acknowledgments
Grant Support: This work was supported by NASPGHAN Fellow to Faculty Transition Award in IBD Research (LA); Project NIH UH2/3 DK083981 (GDW, FDB, JDL), NIH R01 GM103591 (GDW, SAV); Office of Naval Research Grant N000141310613 (SRT); The Molecular Biology and Molecular Pathology and Imaging Cores of the Penn Center for the Molecular Studies in Digestive and Liver Diseases (P30 DK050306); The Joint Penn-CHOP Center for Digestive, Liver, and Pancreatic Medicine.
Abbreviations
- pO2
partial pressure of oxygen
- EPR
Electron Paramagnetic Resonance
- SNR
signal-to-noise ratio
- PMMA
polymethylmetacrylate
- PdTBP
Pd tetrabenzoporphyrin
- ROS
reactive oxygen species
- PICRUSt
Phylogenetic Investigation of Communities by Reconstruction of Unobserved States
- HBOT
hyperbaric oxygen therapy
Footnotes
Author Contributions:
All authors contributed to study design, analysis, drafting, and finalization of the manuscript, in particular:
Study concept and design: Albenberg, Thom, Vinogradov, Wu
Synthesis and calibration of oxygen probe: Esipova, Vinogradov
Acquisition of data: Albenberg, Esipova, Judge, Vinogradov, Wu
Analysis and interpretation of data: Albenberg, Esipova, Judge, Bittinger, Baldassano, Lewis, Thom, Bushman, Vinogradov, Wu
Statistical analysis: Bittinger, Chen, Li
Drafting of the manuscript: Albenberg, Bittinger, Thom, Bushman, Vinogradov, Wu
Revision of the manuscript: Esipova, Judge, Baldassano, Lewis
Technical support: Judge, Grunberg, Laughlin
Disclosures: None of the authors have disclosures related to this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Qin J, Li R, Raes J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fischbach MA, Sonnenburg JL. Eating for two: how metabolism establishes interspecies interactions in the gut. Cell Host Microbe. 2011;10:336–47. doi: 10.1016/j.chom.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–8. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2013 doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frank DN, St Amand AL, Feldman RA, et al. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A. 2007;104:13780–5. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koeth RA, Wang Z, Levison BS, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–85. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belzer C, de Vos WM. Microbes inside--from diversity to function: the case of Akkermansia. ISME J. 2012;6:1449–58. doi: 10.1038/ismej.2012.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swidsinski A, Loening-Baucke V, Verstraelen H, et al. Biostructure of fecal microbiota in healthy subjects and patients with chronic idiopathic diarrhea. Gastroenterology. 2008;135:568–79. doi: 10.1053/j.gastro.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 9.Espey MG. Role of oxygen gradients in shaping redox relationships between the human intestine and its microbiota. Free Radical Biology and Medicine. 2013;55:130–140. doi: 10.1016/j.freeradbiomed.2012.10.554. [DOI] [PubMed] [Google Scholar]
- 10.Fanaro S, Chierici R, Guerrini P, et al. Intestinal microflora in early infancy: composition and development. Acta Paediatr Suppl. 2003;91:48–55. doi: 10.1111/j.1651-2227.2003.tb00646.x. [DOI] [PubMed] [Google Scholar]
- 11.Favier CF, Vaughan EE, De Vos WM, et al. Molecular monitoring of succession of bacterial communities in human neonates. Appl Environ Microbiol. 2002;68:219–26. doi: 10.1128/AEM.68.1.219-226.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palmer C, Bik EM, DiGiulio DB, et al. Development of the human infant intestinal microbiota. PLoS Biol. 2007;5:e177. doi: 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suarez F, Furne J, Springfield J, et al. Insights into human colonic physiology obtained from the study of flatus composition. Am J Physiol. 1997;272:G1028–33. doi: 10.1152/ajpgi.1997.272.5.G1028. [DOI] [PubMed] [Google Scholar]
- 14.Dawson AM, Trenchar D, Guz A. SMALL BOWEL TONOMETRY - ASSESSMENT OF SMALL GUT MUCOSAL OXYGEN TENSION IN DOG AND MAN. Nature. 1965;206:943–&. doi: 10.1038/206943b0. [DOI] [PubMed] [Google Scholar]
- 15.Sheridan WG, Lowndes RH, Young HL. INTRAOPERATIVE TISSUE OXIMETRY IN THE HUMAN GASTROINTESTINAL-TRACT. American Journal of Surgery. 1990;159:314–319. doi: 10.1016/s0002-9610(05)81226-7. [DOI] [PubMed] [Google Scholar]
- 16.Crompton DW, Shrimpto Dh, Silver IA. MEASUREMENTS OF OXYGEN TENSION IN LUMEN OF SMALL INTESTINE OF DOMESTIC DUCK. Journal of Experimental Biology. 1965;43:473–&. doi: 10.1242/jeb.43.3.473. [DOI] [PubMed] [Google Scholar]
- 17.Brune A, Emerson D, Breznak JA. THE TERMITE GUT MICROFLORA AS AN OXYGEN SINK - MICROELECTRODE DETERMINATION OF OXYGEN AND PH GRADIENTS IN GUTS OF LOWER AND HIGHER TERMITES. Applied and Environmental Microbiology. 1995;61:2681–2687. doi: 10.1128/aem.61.7.2681-2687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Due VL, Bonde J, Kann T, et al. Extremely low oxygen tension in the rectal lumen of human subjects. Acta Anaesthesiologica Scandinavica. 2003;47:372–373. doi: 10.1034/j.1399-6576.2003.00542.x. [DOI] [PubMed] [Google Scholar]
- 19.He G, Shankar RA, Chzhan M, et al. Noninvasive measurement of anatomic structure and intraluminal oxygenation in the gastrointestinal tract of living mice with spatial and spectral EPR imaging. Proc Natl Acad Sci U S A. 1999;96:4586–91. doi: 10.1073/pnas.96.8.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swartz HM, Clarkson RB. The measurement of oxygen in vivo using EPR techniques. Phys Med Biol. 1998;43:1957–1975. doi: 10.1088/0031-9155/43/7/017. [DOI] [PubMed] [Google Scholar]
- 21.Rumsey WL, Vanderkooi JM, Wilson DF. Imaging of phosphorescence: A novel method for measuring the distribution of oxygen in perfused tissue. Science. 1988;241:1649–1651. doi: 10.1126/science.241.4873.1649. [DOI] [PubMed] [Google Scholar]
- 22.Wilson DF, Lee WMF, Makonnen S, et al. Oxygen pressures in the interstitial space and their relationship to those in the blood plasma in resting skeletal muscle. Journal of Applied Physiology. 2006;101:1648–1656. doi: 10.1152/japplphysiol.00394.2006. [DOI] [PubMed] [Google Scholar]
- 23.Lebedev AY, Cheprakov AV, Sakadzic S, et al. Dendritic phosphorescent probes for oxygen imaging in biological systems. ACS Applied Materials & Interfaces. 2009;1:1292–1304. doi: 10.1021/am9001698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Esipova TV, Karagodov A, Miller J, et al. Two new “protected” oxyphors for biological oximetry: properties and application in tumor imaging. Analytical Chemistry. 2011;83:8756–8765. doi: 10.1021/ac2022234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vinogradov SA, Wilson DF. Porphyrin-dendrimers as biological oxygen sensors. In: Capagna S, Ceroni P, editors. Designing Dendrimers. New York: Wiley; 2012. [Google Scholar]
- 26.Wilson DF. Quantifying the role of oxygen pressure in tissue function. American Journal of Physiology-Heart and Circulatory Physiology. 2008;294:H11–H13. doi: 10.1152/ajpheart.01293.2007. [DOI] [PubMed] [Google Scholar]
- 27.Ceroni P, Lebedev AY, Marchi E, et al. Evaluation of phototoxicity of dendritic porphyrinbased phosphorescent oxygen probes: an in vitro study. Photochemical & Photobiological Sciences. 2011;10:1056–1065. doi: 10.1039/c0pp00356e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Apreleva SV, Wilson DF, Vinogradov SA. Tomographic imaging of oxygen by phosphorescence lifetime. Applied Optics. 2006;45:8547–8559. doi: 10.1364/ao.45.008547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsushima M, Tokuda K, Ohsaka T. Use of hydrodynamic chronocoulometry for simultaneous determination of diffusion coefficients and concentrations of dioxygen in various media. Analytical Chemistry. 1994;66:4551–4556. [Google Scholar]
- 30.Poulsen L, Ogilby PR. Oxygen diffusion in glassy polymer films: Effects of other gases and changes in pressure. Journal of Physical Chemistry A. 2000;104:2573–2580. [Google Scholar]
- 31.Vinogradov SA, Lo L-W, Jenkins WT, et al. Noninvasive Imaging of the Distribution in Oxygen in Tissue In Vivo Using Near-Infrared Phosphors. Biophysical Journal. 1996;70:1609–1617. doi: 10.1016/S0006-3495(96)79764-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tibbles PM, Edelsberg JS. Hyperbaric-oxygen therapy. N Engl J Med. 1996;334:1642–8. doi: 10.1056/NEJM199606203342506. [DOI] [PubMed] [Google Scholar]
- 33.Thom SR. Hyperbaric oxygen: its mechanisms and efficacy. Plast Reconstr Surg. 2011;127(Suppl 1):131S–141S. doi: 10.1097/PRS.0b013e3181fbe2bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bergey D. Bergey’s Manual of Systematic Bacteriology. New York: Springer; 2001. [Google Scholar]
- 35.Collins MD, Hoyles L, Tornqvist E, et al. Characterization of some strains from human clinical sources which resemble “Leptotrichia sanguinegens”: description of Sneathia sanguinegens sp. nov., gen. nov. Syst Appl Microbiol. 2001;24:358–61. doi: 10.1078/0723-2020-00047. [DOI] [PubMed] [Google Scholar]
- 36.Wexler HM, Reeves D, Summanen PH, et al. Sutterella wadsworthensis gen. nov., sp. nov., bile-resistant microaerophilic Campylobacter gracilis-like clinical isolates. Int J Syst Bacteriol. 1996;46:252–8. doi: 10.1099/00207713-46-1-252. [DOI] [PubMed] [Google Scholar]
- 37.ra S, r D, u D, et al. The Anaerobic Way of Life. In: Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, editors. The Prokaryotes. Vol. 2. Springer; 2006. pp. 86–101. [Google Scholar]
- 38.Imlay JA. Cellular defenses against superoxide and hydrogen peroxide. Annu Rev Biochem. 2008;77:755–76. doi: 10.1146/annurev.biochem.77.061606.161055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johansson ME, Larsson JM, Hansson GC. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4659–65. doi: 10.1073/pnas.1006451107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sonnenburg JL, Xu J, Leip DD, et al. Glycan foraging in vivo by an intestine-adapted bacterial symbiont. Science. 2005;307:1955–9. doi: 10.1126/science.1109051. [DOI] [PubMed] [Google Scholar]
- 41.Ulger-Toprak N, Liu C, Summanen PH, et al. Murdochiella asaccharolytica gen. nov., sp. nov., a Gram-stain-positive, anaerobic coccus isolated from human wound specimens. Int J Syst Evol Microbiol. 2010;60:1013–6. doi: 10.1099/ijs.0.015909-0. [DOI] [PubMed] [Google Scholar]
- 42.Ohara-Nemoto Y, Shimoyama Y, Kimura S, et al. Asp- and Glu-specific novel dipeptidyl peptidase 11 of Porphyromonas gingivalis ensures utilization of proteinaceous energy sources. J Biol Chem. 2011;286:38115–27. doi: 10.1074/jbc.M111.278572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mayrand D, Holt SC. Biology of asaccharolytic black-pigmented Bacteroides species. Microbiol Rev. 1988;52:134–52. doi: 10.1128/mr.52.1.134-152.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stahl M, Butcher J, Stintzi A. Nutrient acquisition and metabolism by Campylobacter jejuni. Front Cell Infect Microbiol. 2012;2:5. doi: 10.3389/fcimb.2012.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ezaki T, Kawamura Y, Li N, et al. Proposal of the genera Anaerococcus gen. nov., Peptoniphilus gen. nov. and Gallicola gen. nov. for members of the genus Peptostreptococcus. Int J Syst Evol Microbiol. 2001;51:1521–8. doi: 10.1099/00207713-51-4-1521. [DOI] [PubMed] [Google Scholar]
- 46.Langille MG, Zaneveld J, Caporaso JG, et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol. 2013;31:814–21. doi: 10.1038/nbt.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morgan XC, Tickle TL, Sokol H, et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012;13:R79. doi: 10.1186/gb-2012-13-9-r79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taylor BL, Zhulin IB, Johnson MS. Aerotaxis and other energy-sensing behavior in bacteria. Annu Rev Microbiol. 1999;53:103–28. doi: 10.1146/annurev.micro.53.1.103. [DOI] [PubMed] [Google Scholar]
- 49.Chiu ZC, Chen MY, Lee DJ, et al. Oxygen diffusion and consumption in active aerobic granules of heterogeneous structure. Appl Microbiol Biotechnol. 2007;75:685–91. doi: 10.1007/s00253-007-0847-6. [DOI] [PubMed] [Google Scholar]
- 50.Morris RL, Schmidt TM. Shallow breathing: bacterial life at low O(2) Nat Rev Microbiol. 2013;11:205–12. doi: 10.1038/nrmicro2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Akin ML, Uluutku H, Erenoglu C, et al. Hyperbaric oxygen ameliorates bacterial translocation in rats with mechanical intestinal obstruction. Dis Colon Rectum. 2002;45:967–72. doi: 10.1007/s10350-004-6337-3. [DOI] [PubMed] [Google Scholar]
- 52.Rickard AH, Gilbert P, High NJ, et al. Bacterial coaggregation: an integral process in the development of multi-species biofilms. Trends Microbiol. 2003;11:94–100. doi: 10.1016/s0966-842x(02)00034-3. [DOI] [PubMed] [Google Scholar]
- 53.Koropatkin NM, Cameron EA, Martens EC. How glycan metabolism shapes the human gut microbiota. Nat Rev Microbiol. 2012;10:323–35. doi: 10.1038/nrmicro2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McHardy IH, Goudarzi M, Tong M, et al. Integrative analysis of the microbiome and metabolome of the human intestinal mucosal surface reveals exquisite interrelationships. Microbiome. 2013;1:17. doi: 10.1186/2049-2618-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nagalingam NA, Lynch SV. Role of the microbiota in inflammatory bowel diseases. Inflamm Bowel Dis. 2012;18:968–84. doi: 10.1002/ibd.21866. [DOI] [PubMed] [Google Scholar]
- 56.Winter SE, Lopez CA, Baumler AJ. The dynamics of gut-associated microbial communities during inflammation. EMBO Rep. 2013;14:319–27. doi: 10.1038/embor.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marteyn B, West NP, Browning DF, et al. Modulation of Shigella virulence in response to available oxygen in vivo. Nature. 2010;465:355–8. doi: 10.1038/nature08970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Patwa LG, Fan TJ, Tchaptchet S, et al. Chronic intestinal inflammation induces stress-response genes in commensal Escherichia coli. Gastroenterology. 2011;141:1842–51. e1–10. doi: 10.1053/j.gastro.2011.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


