Abstract
Adolescence is a developmental period characterized by notable changes in behavior, physical attributes, and an increase in endogenous sex steroid hormones, which may impact cognitive functioning. Moreover, sex differences in brain structure are present, leading to differences in neural function and cognition. Here, we examine sex differences in performance and blood oxygen level-dependent (BOLD) activation in a sample of adolescents during a spatial working memory (SWM) task. We also examine whether endogenous testosterone levels mediate differential brain activity between the sexes. Adolescents between ages 10 and 16 completed a SWM functional magnetic resonance imaging (fMRI) task, and serum hormone levels were assessed within seven days of scanning. While there were no sex differences in task performance (accuracy and reaction time), differences in BOLD response between girls and boys emerged, with girls deactivating brain regions in the default mode network and boys showing increased response in SWM-related brain regions of the frontal cortex. These results suggest that adolescent boys and girls adopted distinct neural strategies, while maintaining spatial cognitive strategies that facilitated comparable cognitive performance of a SWM task. A nonparametric bootstrapping procedure revealed that testosterone did not mediate sex-specific brain activity, suggesting that sex differences in BOLD activation during SWM may be better explained by other factors, such as early organizational effects of sex steroids or environmental influences. Elucidating sex differences in neural function and the influence of gonadal hormones can serve as a basis of comparison for understanding sexually dimorphic neurodevelopment and inform sex-specific psychopathology that emerges in adolescence.
Keywords: adolescence, spatial working memory, sex differences, testosterone, fMRI
1. Introduction
Adolescence is a developmental period marked by a reactivation of sex hormones and reorganization of brain structure and function. As such, it affords a unique window into an important stage of neural and behavioral change. Research on anatomical development of the human brain suggests that frontal and parietal cortical maturation extends well past adolescence [1-3] and occurs at different rates for males and females, with girls peaking approximately two years earlier [4, 5]. These regions play a critical role in executive functioning at various stages of development [6-8]. Along the same lines, brain regions underlying spatial working memory (SWM) abilities mature throughout adolescence [7-9]. Despite our knowledge of neurodevelopmental sex differences during the adolescent years, differences in the neural mechanisms underlying SWM function in adolescent boys and girls and potential contributing factors, such as gonadal sex hormones, have been largely unexplored. Previous studies have reported positive associations between testosterone and gray and white matter volumes in adolescent boys [10, 11], and regional effects of androgen receptor function have been related to cortical thinning of frontal brain areas [12], highlighting the relevance of testosterone on brain maturation. Gaining a broader insight into the mechanisms that help differentiate adolescent brains from other stages of development and whether these differences are meaningfully explained by testosterone can further the understanding of healthy neurodevelopment through which deviations caused by psychopathology can be compared.
1.1. Fronto-parietal neurodevelopment underlies maturation of working memory
Working memory is defined as the active process of maintaining and manipulating information in the mind [13]. In the context of executive functions, working memory is only one type of higher order cognitive process, and often overlaps with other processes like attention and inhibition. This overlap is tightly linked to underlying cortical pathways and neurotransmitter innervations between the prefrontal and posterior parietal cortices that facilitate distributed processing [14]. Research examining anatomical development of the brain has shown that cortical development begins with primary sensorimotor cortices and frontal and occipital poles, and ends with cortical development of parietal and then frontal lobes [2]. Moreover, development of the cortex coincides with a progressive increase in cognitive abilities that require engagement of frontal and parietal brain regions [8].
In the context of functional magnetic resonance imaging (fMRI) research, SWM has been studied most extensively in healthy adults and to a lesser degree in children and adolescents. Studies consistently show that adults have activation of premotor, lateral prefrontal and posterior parietal cortices during SWM tasks [15-18]. Similar patterns of brain activation during SWM have been shown in younger populations [19, 20], with children having more widespread activation [21] and bilateral recruitment of prefrontal and parietal regions relative to adults [7-9]. During adolescence, activation of frontal and parietal brain regions increases relative to childhood, while the transition into adulthood involves specialized recruitment of prefrontal and posterior parietal brain areas [22].
1.2. Sex differences in spatial cognition and effects of gonadal sex steroids
Some evidence in human adults suggests that visuospatial functioning is superior in males relative to females [23-26]; however, the few adult studies that have examined behavioral sex differences in SWM directly have mixed results [27-30]. A few other studies have examined sex differences in brain response during other types of working memory, such as verbal or object working memory, but have also yielded mixed results [31-33]. Only one study, by Schweinsburg and colleagues, has examined sex differences in brain activation during SWM in healthy adolescents, and they reported sex differences in blood oxygen level-dependent (BOLD) activation that included frontopolar regions in brains of a healthy adolescent sample, with boys showing increased BOLD response, in comparison to girls [9]. As there were no sex differences in performance, differences in BOLD response could not be explained by task behavior. Given the significance of adolescence for the development of executive functions, it is important to examine sex-dependent effects of SWM BOLD activation in this context, and whether other sexspecific variables, such as testosterone, may impact patterns of neural activity.
Testosterone is a potential factor mediating adolescent sex differences in SWM because of its dramatic increase during adolescence, especially in males, and its purported role in spatial cognition [34-36]. Furthermore, increases in circulating testosterone are associated with brain structure [11, 12, 37-39] that impact cognitive functioning [40] and differentiate adolescence from other developmental periods. One fMRI study measuring the relationship between sex hormone levels and brain response during mental rotation (a visuospatial task) found a positive association between testosterone levels and activation of inferior frontal gyrus, ventromedial prefrontal cortex, inferior parietal lobe, and left supramarginal gyrus in both men and women [41]. This study demonstrated that testosterone can impact degree of activation in the brain in response to a cognitive task with a conferred male advantage. Moreover, rodent studies have also shown that testosterone has a positive effect on cognition in males [36, 42, 43]. Despite the growing body of literature relating individual variability in testosterone levels to BOLD response during cognitive processing, only a few studies have examined the effects of testosterone on BOLD response during adolescence [44, 45], but none have done so during a SWM task.
In summary, previous literature provides strong evidence that adolescence is an important period for the development of SWM function and its underlying neural substrates; however, this has only been studied in the context of sex differences and adolescence once before [9]. Furthermore, no studies have examined the relationship between SWM and testosterone, even though adolescence is marked by a substantial increase in sex hormones. This gap in knowledge is addressed in the present study. First, sex differences in SWM performance and brain response are compared, followed by an examination of the potentially mediating role of testosterone in sex-specific brain response.
1.3. Rationale and hypotheses
To date, no studies have examined the relationship between endogenous testosterone levels and SWM BOLD activation at any developmental stage or in the context of sex differences in neural activity. However, elucidating the relationship of testosterone and brain function during this developmental stage is important for establishing a benchmark of healthy neurodevelopment. Thus, the present cross-sectional study examined to what degree testosterone mediated BOLD activation in male and female adolescents during a SWM task. Based on previous adolescent research [9], it was hypothesized that there would be no significant sex differences in performance (accuracy and reaction time) during a SWM task. However, in line with previous findings [8, 9, 16], it was hypothesized that BOLD signal during SWM would be distinct between boys and girls, particularly in regions implicated in SWM functioning, possibly due to differential engagement of neural resources between the sexes. Specifically, it was predicted that boys would show increased BOLD activation in frontal regions and superior parietal cortex compared with girls, perhaps reflecting a compensatory approach due to delayed cortical maturation of fronto-parietal networks in boys compared to girls. Furthermore, testosterone plays a critical role in brain maturation and previous studies have demonstrated an association between testosterone and BOLD response during cognitive processing [41, 46-48]; therefore, it was hypothesized that testosterone would partially mediate sex differences in BOLD response. Particularly, we predicted increased mediation in brain regions with higher densities of sex hormone receptors or connectivity with hormone receptor-rich brain areas, such as the prefrontal cortex, perhaps representing a (partial) mechanism underlying sex differences in neural response. Although we did not anticipate sex differences in SWM performance, the neural correlates of task accuracy and reaction time were examined with exploratory analyses to provide further context about neural strategies and their utilization by sex.
2. Results
2.1. Participant characteristics
From the total number of scans collected (n=74), 25 participants were excluded for the following reasons: accuracy levels were below an 80% threshold for SWM and/or vigilance trials (9 girls, 6 boys), excessive head motion (root mean square (RMS) > 2.5 mm; 1 girl, 3 boys), large signal dropout in frontal brain areas (1 girl, 1 boy), birth control medications (2 girls), and abnormal neuropsychological testing scores (1 boy), reducing the total number of subjects to 49. In the final sample, boys and girls did not differ significantly by age, socioeconomic status (SES), race, or IQ (see Table 1). As expected, significant group differences were seen in puberty, with girls reporting higher Pubertal Development Scale (PDS) values than boys, reflecting advanced pubertal status [49] (see Table 1). Additionally, age and puberty were positively correlated for both boys (r = .76, p < .001) and girls (r = .70, p < .001). A PDS score was missing for one male subject, but due to valid fMRI and hormone data, he was not excluded from analyses.
Table 1. Demographics.
| Group | Female | Male | Statistic | |
|---|---|---|---|---|
| (n=49) | (n=23) | (n=26) | ||
| Age (years) | 13.22 ± 1.71 | 13.22 ± 1.79 | 13.21 ± 1.67 | t47 = .02, p > .05 |
| Race (% White) | 77.6 | 73.9 | 80.8 | X1,47 = .57, p > .05 |
| Socioeconomic statusa | 27.82 ±12.90 | 29.09 ± 12.99 | 26.69 ± 12.96 | t47 = .65, p > .05 |
| IQb | 119.0 ± 10.6 | 118.1 ± 10.2 | 119.8 ± 11.2 | t47 = .53, p > .05 |
| Pubertyc | 3.02 ± 1.12 | 3.48 ± 1.04 | 2.6 ± 1.04 | Z46 = 2.92, p = .004 |
| Testosterone (ng/dl)d | 107.1 ± 165.6 | 13.54 ± 6.04 | 193.16 ± 193.85 | t46 = 4.63, p < .001 |
| range (ng/dl) | 2.7 - 508.0 | 5.3 - 25.4 | 2.7 - 508.0 |
Hollingshead Index of Social Position; larger values indicate lower socioeconomic status (middle class corresponds to 32-47 range);
Wechsler Abbreviated Scale of Intelligence;
Crockett Pubertal Development Scale; Values range from 1-5, with larger values referring to more advanced pubertal development. Male puberty sample was n = 25.
Male testosterone sample was n = 25.
2.2. Performance
Accuracy and reaction time (RT) data from SWM task performance were available for all 49 subjects. Independent samples t-tests indicated that boys and girls did not differ on accuracy or RT in SWM and vigilance conditions (see Table 2). Collapsing across sex, accuracy was statistically different between conditions, with better scores on vigilance compared to SWM (t48 = 11.0, p < .001), and RT was significantly shorter during vigilance (t48 = 5.43, p < .001) compared to SWM. Comparing within sex, accuracy was still significantly better during vigilance compared to SWM for both girls (t22 = 5.88, p < .001) and boys (t26 = 10.27, p < .001). RT was also significantly shorter during vigilance for both girls (t22 = 4.43, p < .001) and boys (t26 = 3.38, p = .002). In addition, age was positively correlated with accuracy (r = .29, p = .045) on the SWM task and vigilance task (r = .45, p = .001), and negatively correlated with SWM RT (r = -.35, p = .01) and vigilance RT (r = -.39, p = .005). Responses on the Exit Questionnaire also revealed that boys and girls did not differ in their self-reported cognitive strategies during the task (X2=.28, p = .63), which was predominantly spatial rather than verbal.
Table 2. Task Performance.
| Group | Female | Male | Statistic | |
|---|---|---|---|---|
| (n = 49) | (n = 23) | (n = 26) | ||
| Spatial working memory accuracy (%) | 93.21 ± 3.86 | 94.02 ± 4.13 | 92.51 ± 3.52 | t47 = 1.38, p > .05 |
| Spatial working memory RT (ms) | 624.05 ± 145.46 | 631.86 ± 139.55 | 617.14 ± 152.90 | t47 = .35, p > .05 |
| Control Accuracy (%) | 98.86 ± 1.29 | 99.09 ± 1.06 | 98.66 ± 1.46 | t47 = 1.18, p > .05 |
| Control RT (ms) | 541.88 ± 75.77 | 543.63 ± 72.16 | 540.34 ±80.22 | t47 = .15, p > .05 |
| Movement (LogRMS)a | .23 ± .13 | .26 ± .14 | .20 ± .11 | t47 = 1.44, p > .05 |
Log transformation of the average root mean square of movement during scan
Motion, as indexed by RMS, was non-normally distributed and log transformed. Boys and girls did not have statistically different LogRMS values (see Table 2). Age was negatively correlated with average RMS (r = -.30, p = .03) at the group level, indicating less head movement in older participants. Head motion was also assessed and corrected with a strict frame-to-frame displacement (FD) method [50, 51]. Results of the original fMRI analysis of covariance (ANCOVA) were compared to results obtained using this method to determine if motion significantly impacted BOLD activation results. Results based on original motion and FD motion correction were determined to be similar. Moreover, boys and girls did not differ in FD values relative to each other during SWM or vigilance trials (see Appendix A: Supplementary Data), suggesting that sex differences in SWM are not due to motion differences.
2.3. Testosterone assessment
Testosterone levels were within normal range for both girls and boys, based on age and pubertal stage, with boys showing significantly higher levels of testosterone compared to girls (see Table 1 for details). Correlation analyses also showed that testosterone was positively related to age (r = .34, p = .02), but not PDS (r = .17, p > .05). Testosterone levels were missing for one male subject, and his data were excluded from hormone-based analyses.
2.4. fMRI
2.4.1. Sex differences in SWM BOLD response
The typical SWM BOLD response pattern, with increased activation of premotor and fronto-parietal networks, and deactivation of default mode network (DMN) brain regions, was present in both girls and boys (Fig. 1). A voxel-wise ANCOVA (covarying for age and PDS) comparing masked percent signal change in BOLD activation during the SWM task relative to vigilance condition resulted in significant sex differences in right rostral anterior cingulate cortex, right middle temporal gyrus, right precuneus, right middle frontal gyrus, left dorsolateral prefrontal cortex, and bilateral inferior frontal gyrus, which extended to anterior insula bilaterally, with boys showing increased activation relative to girls in all clusters (Fig. 2 and Table 3). Further, examination of SWM – baseline and Vigilance – baseline revealed more complex patterns of activation. In precuneus, anterior cingulate cortex, and middle frontal gyrus, girls were, in fact, deactivating these regions during SWM. To a lesser extent, boys also deactivated these regions during SWM, which explains why the main SWM-vigilance contrasts appear as boys having increased BOLD signal. However, in the middle temporal gyrus, boys increased activation during SWM, while girls only showed deactivation (Fig. 3).
Fig. 1.
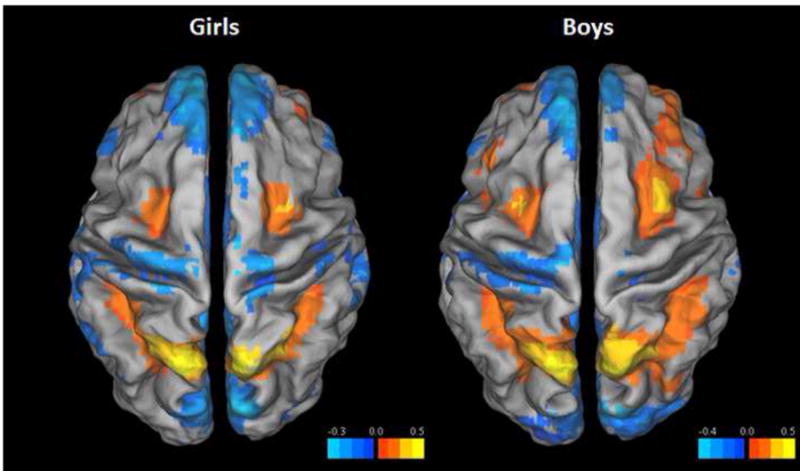
Statistical maps of female (left) and male (right) percent BOLD activation in spatial working memory - vigilance (SWM-Vig) contrast overlaid on a standard Talairach template. For visualization, both maps were voxel thresholded at p < .001.
Fig. 2.
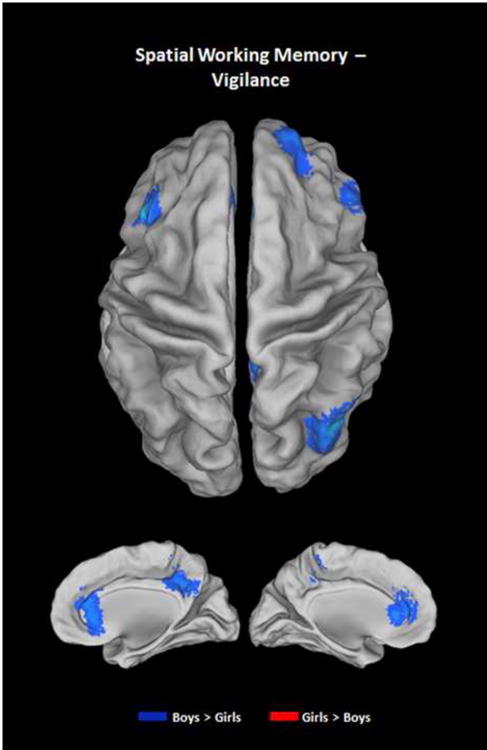
Statistical map of sex differences in percent BOLD activation in spatial working memory - vigilance (SWM-Vig) contrast (controlling for age and pubertal developmental status) overlaid on a standard Talairach template. In all significant clusters (p < .01 voxel and α < .05 cluster correction) including bilateral inferior frontal gyrus/anterior insula, rostral anterior cingulate cortex, precuneus, right middle frontal gyrus, left dorsolateral prefrontal cortex, and right middle temporal gyrus, boys show increased activation (blue) relative to girls.
Table 3. Sex Differences in Spatial Working Memory BOLD Activation.
| Brain Region | BA | Voxels | x | y | z |
|---|---|---|---|---|---|
| Boys > Girls | |||||
| R rostral anterior cingulate cortex | 32 | 157 | 5 | 23 | -7 |
| R inferior frontal gyrus/anterior insula | 47 | 141 | 41 | 20 | 4 |
| R middle temporal gyrus | 39 | 133 | 38 | -71 | 29 |
| L inferior frontal gyrus/anterior insula | 47 | 104 | -34 | 26 | -1 |
| R precuneus | 7 | 86 | 5 | -55 | 35 |
| R middle frontal gyrus | 9 | 79 | 20 | 56 | 29 |
| L dorsolateral prefrontal cortex | 9 | 56 | -52 | 23 | 35 |
| Girls > Boys | |||||
| --- | |||||
All voxels are significant at voxel-wise p < .01 and whole-brain corrected α < .05. Peak coordinates for the clusters are presented in standard Talairach space. L, left; R, right; BA, Brodmann area.
Fig. 3.
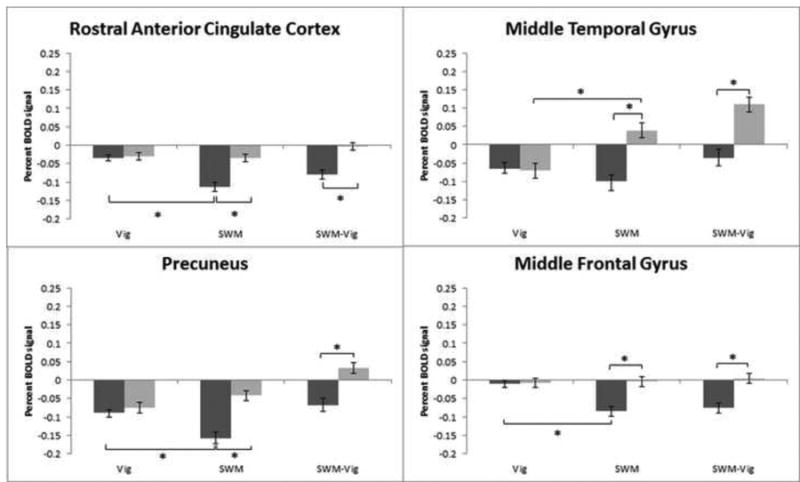
Default mode network-related brain activity patterns during spatial working memory and vigilance trials. Percent BOLD signal plotted against trial type: vigilance (Vig), spatial working memory (SWM), and SWM-Vig, including standard error of the mean. Boys are represented in light grey and girls in dark grey. BOLD percent signal change in right rostral anterior cingulate cortex, right middle temporal gyrus, right precuneus, and right middle frontal gyrus is depicted. Percent BOLD signal change refers to BOLD activity changes from baseline activation. Asterisks denote significant at p < .05.
In addition, boys actively recruited the dorsolateral prefrontal cortex during SWM, as reflected by increased percent BOLD signal change, relative to girls, who were not recruiting this region any differently than during vigilance. Initial comparisons of bilateral inferior frontal gyrus activation revealed that boys activated, while girls deactivated this region, during SWM (Fig. 4). However, since the bilateral inferior frontal gyrus clusters extended to the anterior insula, BOLD signal was compared separately for voxels that corresponded to inferior frontal gyrus and anterior insula. Boundaries for inferior frontal gyrus and anterior insula were determined using AFNI's whereami function. From these two clusters, signal was extracted and activation of SWM and Vigilance in relation to baseline was compared in manner similar to the original clusters. The pattern of activation revealed a dissociation between these regions. Boys showed greater bilateral anterior insula response during SWM, compared with girls, who had minimal activation in this region. In contrast, girls deactivated bilateral inferior frontal gyrus during SWM, while boys activated the same region relative to vigilance (Fig. 4). Therefore, the initial inspection of individual SWM – baseline and Vigilance – baseline contrasts of the entire inferior frontal gyrus/anterior insula cluster, which indicated increased activation in boys and deactivation in girls, was driven by activation in anterior insula and deactivation in inferior frontal gyrus, respectively. BOLD activity during the vigilance condition compared to baseline was not different between girls and boys in any of these regions.
Fig. 4.
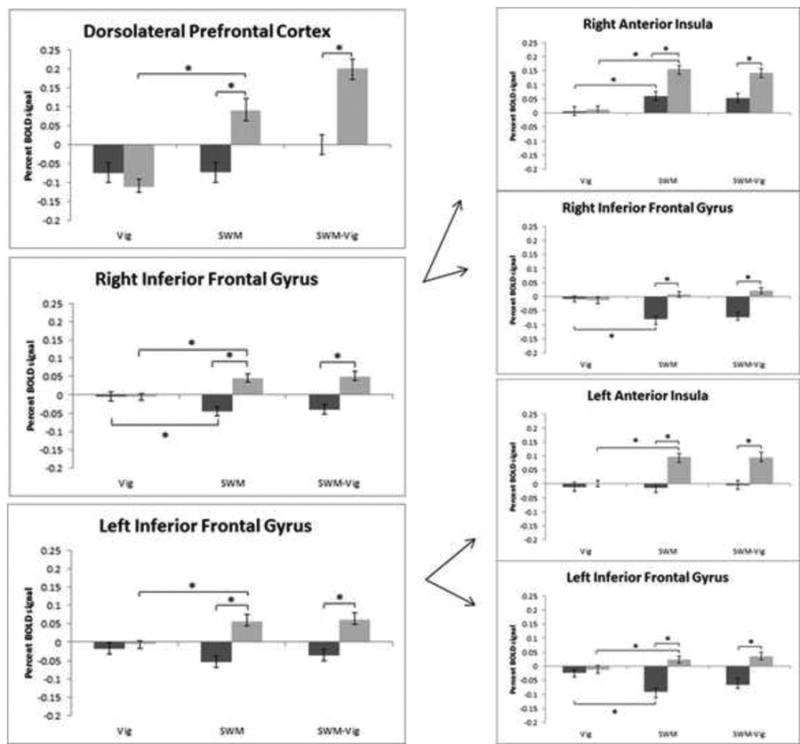
Cognitive control-related brain activity patterns during spatial working memory and vigilance trials. Percent BOLD signal plotted against trial type: vigilance (Vig), spatial working memory (SWM), and SWM-Vig, including standard error of the mean. Boys are represented in light grey and girls in dark grey. BOLD percent signal change in left dorsolateral prefrontal cortex, right inferior frontal gyrus/anterior insula, and left inferior frontal gyrus/anterior insula is depicted. Right and left inferior frontal gyrus/anterior insula clusters were further divided into inferior frontal gyrus and anterior insula components, and the corresponding percent BOLD signal is represented as right anterior insula, right inferior frontal gyrus, left anterior insula, and left inferior frontal gyrus. Percent BOLD signal change refers to BOLD activity changes from baseline activation. Asterisks denote significant at p < .05.
2.4.2. Testosterone did not mediate sex differences in SWM BOLD signal
Mean percent BOLD signal values extracted from these clusters were included in nonparametric tests of mediation as dependent variables, with testosterone added separately as a mediator variable to explain the effects of sex, the independent variable. The test of indirect effects makes no assumptions about the distribution of data or the initial relationship between the variables of interest. A significant indirect effect does not imply mediation or causation, but may explain a portion of the total effects of a dependent variable. As such, testosterone was highly associated with sex (p < .001); however, the test of mediation was non-significant (Table 4).
Table 4. Testosterone Mediation Analyses.
| Brain region | Testosterone (ng/dl) | |
|---|---|---|
| Indirect effect | 95% CI | |
| R rostral anterior cingulate cortex | -0.003 | [-.0211 - .0123] |
| R inferior frontal gyrus/anterior insula | -0.001 | [-.0201 - 0178] |
| R inferior frontal gyrus | 0.003 | [-.0188 - .0251] |
| R anterior insula | -0.006 | [-.0360 - .0250] |
| R middle temporal gyrus | -0.007 | [-.0617 - .0367] |
| L inferior frontal gyrus/anterior insula | < .0001 | [-.0295 - .0273] |
| L inferior frontal gyrus | -0.003 | [-.0344 - .0262] |
| L anterior insula | 0.003 | [-.0303 - .0358] |
| R precuneus | 0.011 | [-.0211 - .0466] |
| R middle frontal gyrus | -0.004 | [-.0318 - .0209] |
| L dorsolateral prefrontal cortex | -0.001 | [-.0543 - .0525] |
A nonparametric bootstrap analysis of the indirect effects of testosterone was conducted for each significant cluster (and sub-cluster) identified in a sex-differences ANCOVA of spatial working memory. There were no significant indirect or mediation effects of testosterone. CI = Confidence Interval, R=right, L=left.
2.4.3. Exploratory task performance regressions
Areas of the brain relevant for task accuracy and RT, specifically during SWM trials, were investigated with exploratory multiple regression analyses that included sex and the interaction of sex and task performance. In the accuracy model, one cluster in the right middle frontal gyrus (Talairach coordinates x, y, z: 26, 5, 59) was significantly related to SWM accuracy, such that increased accuracy was positively related to increased percent BOLD signal change across the whole sample (p < .01, α < .05). This effect was not significantly moderated by sex, as the interaction of accuracy and sex was not statistically significant. There were also no statistically significant effects of sex, controlling for accuracy and the interaction of sex and accuracy. A similar multiple regression model was constructed to examine RT-related brain activation (p < .01, α < .05). One cluster in the left middle occipital gyrus (Talairach coordinates x, y, z: -46, -76, 5) was significantly related to RT, in which faster RT was related to increased activation in this region. The left inferior parietal lobule (Talairach coordinates x, y, z: -31, -34, 38) was related to RT and was moderated by sex, as the interaction term was significantly related to this area. Specifically, girls with slower RT during SWM had increased BOLD response in the left inferior parietal lobule as compared to boys. No brain regions were statistically related to sex, controlling for RT and the interaction of sex and RT. Because activation of the left inferior parietal lobule was moderated by sex, percent BOLD signal values were extracted from this cluster and entered into a post-hoc linear regression with testosterone as the predictor. The results of this analysis were not statistically significant (t47=.50, p > .05).
3. Discussion
As hypothesized, sex-dependent patterns of brain activation during a SWM task were demonstrated in this healthy adolescent sample. Within task-related regions of BOLD activation, boys and girls had opposite patterns of activity in bilateral inferior frontal gyrus, with boys showing increased activation (driven by activity in the anterior insula), and girls deactivating (driven by deactivation in the inferior frontal gyrus) compared to the vigilance control task. Additionally in girls, rostral anterior cingulate cortex, precuneus and right middle frontal gyrus showed a deactivation of BOLD signal relative to vigilance, a finding not present in boys. In fact, boys had increased activation in right middle temporal gyrus and left dorsolateral prefrontal cortex, which was not seen in girls. Importantly, boys and girls had similar activation patterns during vigilance conditions, indicating that vigilance activation cannot explain sex differences in SWM task activation. As hypothesized, boys and girls did not differ in accuracy or reaction time during SWM or vigilance trials. Notably, the sexes did not differ in BOLD activation patterns related to task accuracy; however, girls with slower RT showed increased BOLD response in left inferior parietal lobule. Finally, testosterone did not mediate the effects of sex on SWM brain response, suggesting that the resurgence of testosterone during adolescence may not play a large role in sex-specific SWM neural response; rather, other sex hormones, early developmental hormonal effects, or environmental factors may be important factors for consideration.
3.1. Sex differences in activation of default mode network brain regions
The DMN is a neural network commonly identified with functional connectivity, independent components analysis, and task-induced deactivation in fMRI studies. It is one of many neural networks, but unique in that it typically deactivates during tasks requiring cognitive effort [52, 53]. Studies suggest that an inability to suppress the DMN during a cognitive task actually interferes with one's ability to perform at an optimal level [54, 55]. The DMN includes regions identified in this study (rostral anterior cingulate cortex [Talairach coordinates: -3, 39, -2], precuneus [Talairach coordinates: -2, -36, 37], and middle frontal gyrus [Talairach coordinates: 17, 37, 52]), where girls significantly deactivated relative to boys during SWM. Importantly, boys and girls did not differ in vigilance BOLD activation in these regions, suggesting that girls are more adept at disengaging regions of the DMN during more cognitively demanding tasks, which in turn facilitated optimal performance. Interestingly, girls also showed BOLD deactivation in bilateral inferior frontal gyrus and dorsolateral prefrontal cortex during the task, which could indicate that they did not need to recruit additional prefrontal regions to perform at an equivalent level to boys.
A recent coordinate-based meta-analysis of task-related deactivations identified consistent nodes of the DMN based on peak coordinates from over 1,500 publications [56]. They reported nine regions of convergence that included the precuneus, anterior cingulate cortex, left middle frontal gyrus, bilateral middle temporal gyrus, dorsomedial prefrontal cortex, bilateral inferior parietal lobules, and posterior cingulate cortex. These clusters correspond to regions where girls displayed patterns of deactivation, with the exception of the right middle frontal gyrus. However, the authors noted that negative findings of predicted regions, like the right middle frontal gyrus, might be due to high spatial variability of deactivations in this brain region. Also of note, in the current study there was a lack of significant deactivation in the right middle temporal gyrus in both girls and boys, which could signify that full disengagement of the DMN during a cognitively taxing task is not a mature process in the adolescent brain [57, 58]. In fact, boys demonstrated significant activation from baseline, which may reflect further immaturity of this network that is dependent on sex.
3.2. Sex differences in activation of WM-related brain regions
While girls disengaged regions of the DMN to achieve intact SWM performance, boys adopted a neural strategy, or a pattern of brain regions recruited, that involved a more active engagement of prefrontal regions already implicated in SWM functioning. Neural strategy is distinct from cognitive strategy, in that neural strategy may not necessarily reflect different cognitive states, but rather reflects an alternate use of neural resources. Specifically, boys increased BOLD activation in left dorsolateral prefrontal cortex, frequently implicated in working memory tasks [59-62], and anterior insular cortices, which have been linked to perceptual decision-making [63]. Specifically, research shows increased recruitment of anterior insula with increasing task difficulty that is reflective of more cognitive effort, whereby increasing sensory awareness leads to improved performance [63, 64]. Furthermore, the relationship between SWM accuracy and BOLD response in the middle frontal gyrus across the sexes reinforces the importance of fronto-cortical activation for effective SWM performance [18]. An alternative interpretation for increased recruitment of the middle temporal gyrus in the male participants could be that it reflects a compensatory process due to increased difficulty with the task. This notion is supported by a recent study that reported greater BOLD response in middle temporal gyrus in the high load condition of a spatial working memory task relative to the low load condition in a sample of healthy adults [64]. Importantly, despite differences in neural strategies, or patterns of brain response, girls and boys did not differ in their self-reported cognitive strategy, suggesting they understood task instructions and applied similar approaches.
Notably, contrary to our hypotheses, there were no sex differences in superior parietal cortex activation, suggesting that boys and girls did not recruit this brain region differently during SWM. The only other study examining sex differences during SWM in adolescents also did not find significant differences in superior parietal cortex. In fact, they reported sex differences that correspond well with the present study; specifically, they reported decreased activity in anterior cingulate in girls, and greater activity in frontopolar cortex in boys [9]. However, in the current study there was a significant interaction between sex and SWM RT in inferior parietal cortex, in which girls with slower reaction times displayed a stronger BOLD response. A previous study in male adults reported increased activation of the posterior parietal cortices with increasing reaction time during a verbal working memory task, demonstrating a nonspecific processing speed effect that might indicate these participants required more time to process the stimuli and, in turn, activate posterior parietal cortices more powerfully [65]. Although no sex differences in task accuracy emerged in the current sample, it is also possible that a subset of girls found the task more difficult leading to increased BOLD response in inferior parietal cortex. Middle occipital gyrus activation related to RT was not distinct by sex, which may indicate that visual attention and related processing speed demands were similar in boys and girls.
The neural activation pattern of adults includes frontal cortical recruitment and DMN deactivation [54], which is closely mirrored by the current sample of adolescents; although it is not fully mature in either boys or girls. Moreover, sex differences in BOLD activity during SWM may reflect transient divergent neural patterns of activation that emerge during adolescence, but given the scarcity of studies exploring sex differences in SWM BOLD activation in adults, it is not known whether these patterns of activation persist beyond adolescence. It is possible that these sex-specific adolescent brain patterns of activation during SWM are temporary and give way to neural strategies reflected in the adult population, wherein effective SWM performance reflects the interplay between efficient recruitment of fronto-parietal regions and deactivation of brain regions in the DMN. Alternatively, a distinct brain response during SWM may serve an adaptive purpose for each sex. A direct comparison of adolescent and adult brain response will be necessary to determine how sex differences in BOLD activity are distinguished in these periods of development. However, this comparison cannot definitively account for sex differences that may exist prior to adolescence, and no studies have examined sex difference in BOLD response during SWM in children; therefore longitudinal designs across the lifespan would be most informative.
The present data support an interpretation of distinct neural strategies in girls compared to boys, with girls showing less recruitment of the dorsolateral prefrontal cortex and increased suppression of DMN-related brain regions, while boys actively recruited frontal regions associated with working memory function and deactivated the DMN-related regions to a smaller extent. Moreover, the sex differences in (de)activation patterns were independent of age and pubertal status, and were not explained by trait levels of testosterone. Underlying brain structure, differences in neural connectivity, and/or other sexually dimorphic hormones may explain differences in BOLD signal between adolescent boys in girls and between adolescents and adults, more generally.
3.3. Potential mechanisms underlying sex differences in brain activation
Contrary to the hypothesis, testosterone did not mediate the effects of sex on brain response during SWM. Nonparametric tests of mediation revealed that testosterone did not explain significant variance in SWM BOLD response, above and beyond the effects of sex. However, these findings beg the question, if not testosterone, what explains adolescent sex differences in brain response during SWM? A rise in testosterone is a key feature of adolescence, specifically in boys, making it a strong candidate for examination. Estradiol levels also increase significantly in adolescents, predominantly in girls; however, blood samples were collected during a truncated window in the female sample to control for the effects of monthly variation of sex hormone levels. As a consequence, differences in estradiol levels between the sexes were statistically equivocal and were not examined in this study. However, it is important to mention that estradiol may still be an important mediator of the reported sex differences. Additionally, the effects of testosterone may also partially operate through conversion to estradiol by the enzyme, aromatase. Alternatively, the sex differences in SWM-related BOLD activity observed in this study may reflect early perinatal, rather than pubertal, organizational effects of sex hormones, or perhaps environmental factors such as prenatal exposure to toxins, early life stress, and social expectations [66].
The process of sexual differentiation begins with sex determination and proceeds through different stages of neonatal development to establish a male or female phenotype [67]. Sexual differentiation largely depends on sex hormones and their metabolites [67, 68], but also on chromosomal factors; in fact, sex differences exist before the presence of sex hormones. Specifically, around the sixth week of development, the SRY gene on the Y chromosome stimulates fetal gonads to develop into testes. Masculinization and de-feminization of an organism occurs weeks later when testicular hormones, such as testosterone, are produced [69]. The course of sexual differentiation demonstrates that chromosomal products have direct effects on sex dimorphism, and they can occur independently of sex hormones.
Sex chromosome genes may affect a neural phenotype directly and in a sex-specific fashion through several mechanisms. The first is through specific genes present in the Y chromosome that females would not have access to. Secondly, differences in sex chromosome dosage between XX and XY individuals can also lead to distinct phenotypes, even after inactivation of an X chromosome in females [70]. Finally, sex differences in X chromosome imprinting, with females receiving X chromosomes imprinted by both mother and father and males receiving a single maternally imprinted X chromosome, can impact phenotypic expression in the brain [70]. The effects of sex hormones and sex chromosome genes on sexual differentiation of the brain in utero cannot be measured with the design of the current study. We can only speculate that sex chromosome genes and/or organizational effects of sex hormones and their metabolites underlie the sex differences in BOLD response seen here. Additionally, environmental factors like prenatal exposure to toxins (including alcohol) and childhood maltreatment may affect brain development in a sexually dimorphic manner that may impact subsequent brain function [71, 72]. Although prenatal exposure to alcohol was an exclusionary criterion for this study, childhood maltreatment was not assessed. Furthermore, environmental exposure to toxins during development, or trauma during childhood, can affect the development of the brain and subsequent neural function distinctly in boys and girls, and we cannot rule out existing sex differences induced by unaccounted environmental influences.
3.4. Considerations and future directions
There are notable strengths of this study that deserve mention. First, the SWM task was designed to facilitate optimal performance and to discourage verbal encoding of spatial stimuli, and although not all youth reported the use of a spatial strategy, the majority did. Instructions for the task were less difficult than other working memory tasks, which appropriately accommodated the study age range. Second, due to the aims of the study, data were collected from female subjects during the early follicular phase of the menstrual cycle, which accounted for a large portion of endogenous hormonal variation in adolescent girls. Additionally, subjects taking endocrine disruptive medications, such as hormonal contraception, were excluded.
Although suitable for an fMRI study, the sample size of this study may have lacked the power to detect the effects of sex hormones across the sexes. This issue is complicated by the fact that testosterone assays can be unreliable due to the lack of a standard method of measurement. However, to circumvent this problem, samples were batched for analysis, and all assays were performed in the same location. Furthermore, while collection of plasma testosterone was restricted to the week of the scan during the same time of day for all subjects, more robust effects may have been detected if collected on the day of scanning. However, due to the design of the study and practical obstacles with participant scheduling, samples could not be collected the day of scanning, limiting our ability to speculate about the non-genomic actions of sex hormones. In future work, it will be important to continue accounting for endogenous hormonal variation across and within subjects. Finally, to make definitive statements about degree of network activation, in contrast to regions that comprise neural networks, during the SWM, it is important to conduct functional connectivity analyses. The present data suggest potential sex differences in engagement of fronto-parietal and default mode neural networks during SWM; however, these conclusions can only be confirmed through direct assessment of functional connectivity. Lastly, although the aim of the current study was not to capture developmental effects, a limitation of this study was the lack of comparison with children and adult samples. Future work should consider that developmental effects are not static and can be obscured with the use of cross-sectional data analysis; by using longitudinal analyses, this issue can be avoided.
3.5. Conclusions
Brain regions associated with working memory function and the DMN were differentially activated during a SWM task in a sample of healthy adolescents. Boys demonstrated a larger increase in percent BOLD activity relative to girls in working memory-related brain regions, while girls more strongly deactivated regions implicated in the DMN. Both neural activation patterns facilitated comparable cognitive performance; however a fully mature pattern of activation requires integration of both neural strategies. Sex differences in brain activation were not paralleled by performance (accuracy and reaction time), suggesting that adolescent boys and girls adopt distinct neural strategies resulting in similar performance, with boys engaging in greater recruitment of cognitive control regions. Further, sex differences in neural activation were not mediated by testosterone, which may indicate that the organizational effects of testosterone and other sex hormones early in development, or environmental influences play a larger role in sexually-dimorphic SWM brain function during adolescence. Sex differences in neural strategies likely evolve throughout adolescence and young adulthood, but longitudinal studies assessing working memory-related sex differences in these populations are needed to confirm the current results. Lastly, understanding healthy neurodevelopment during this critical stage of maturation may provide the foundation to distinguish aberrant neural function emergent in adolescence.
4. Experimental Procedure
4.1. Participants
Seventy-four healthy adolescents (males = 37) between the ages of 10 and 16 completed the experimental procedure. All subjects underwent comprehensive structured interviews by trained research assistants as part of a larger study on adolescent neurodevelopment. First, written assent and consent from children and their parents, respectively, were obtained in accordance with Oregon Health & Science University's Institutional Review Board. Youth and parents completed separate structured telephone interviews that included the Diagnostic Interview Schedule for Children Predictive Scales (DISC-PS-4.32b) [73], the Family History Assessment Module (FHAM) [74], and the Brief Lifetime version of the Customary Drinking and Drug Use Record [75]. Exclusionary criteria included lifetime history of diagnosed DSM-IV disorders, significant substance use (> 10 lifetime alcoholic drinks or > 2 drinks/occasion, > 5 uses of marijuana, any other drug use, or > 4 cigarettes per day), neurological illness, significant head trauma (loss of consciousness > 2 minutes), serious medical problems, prenatal exposure to drugs or alcohol, reported history of psychotic disorders in biological parents (i.e. schizophrenia or bipolar I), current medication that may affect neural function (e.g. psychoactive medication) or endocrine function (e.g. hormonal contraception), the inability of a parent to provide family history information, left-handedness (Edinburgh Handedness Inventory, [76]), pregnancy, and general MRI contraindications (e.g. braces, irremovable ferrous material).
After determining eligibility, all youth were administered the Wechsler Abbreviated Scale of Intelligence [77] to obtain an estimate of intellectual functioning. The Hollingshead Index of Social Position was administered to parents as part of the structured telephone interview to determine socioeconomic status (SES), which is based on occupation and education attainment of each parent [78]. Pubertal maturation was assessed with the self-rated Pubertal Development Scale (PDS) [79]. Self-reports on this scale have been shown to correlate highly with other measures of pubertal status, such as Tanner's Sexual Maturation Scale self-ratings [80].
4.2. Sex hormone assays
To determine serum levels of testosterone, 4 ml of blood was collected via venipuncture from all subjects at the Oregon Clinical and Translational Research Institute the same week of scanning. To reduce diurnal heterogeneity of hormone levels, blood was collected between 7:00 and 10:00 AM. In addition, samples from post-menarche girls were drawn during the follicular phase of the menstrual cycle (days 1-10) to further minimize variability, as well as interactions with progesterone, since progesterone levels are lowest at this phase [49, 81]. Menstrual cycle phase was determined by self-report. Testosterone levels were determined by Coat-A-Count radioimmunoassay (Diagnostic Product Corp., Los Angeles, CA). The intra-assay and inter-assay CVs were 7.0% and 7.4%, respectively, with a lower level of detection of 10 ng/dl. Hormone levels were examined to ensure none exceeded expected levels, as determined by age, sex, and pubertal status. Normal levels of testosterone range from 2 - 40 and 2 - 800 ng/dl, in pubertal girls and boys, respectively [49].
4.3. MRI data acquisition
Adolescents were scanned on a 3 Tesla Siemens Magnetom Tim Trio system (Siemens Medical Solutions, Erlangen, Germany) with a twelve-channel head coil at the Advanced Imaging Research Center at Oregon Health & Science University. Lying in a supine position in the scanner, pads were placed around each participant's head inside the coil to minimize head motion. All participants were instructed on the importance of remaining as still as possible throughout the entire scan session. Earplugs and noise-cancelling headphones were given to the youth to use during scanning in an effort to reduce their exposure to scanner noise. A 4-button opto-isolated button box designed for use in an MRI scanner was placed in every subject's right hand. Task stimuli were presented from a laptop computer through a data projector to a screen at the rear of the MRI bore. Participants viewed stimuli through a mirror mounted on the head coil and made responses using the button box.
4.3.1. Functional MRI
Functional images were collected in the axial plane oblique to the anterior – posterior commissure, using a high-angular resolution T2*-weighted echo-planar BOLD sequence (TR = 2000 ms, TE = 30 ms, FOV = 240 mm, flip angle = 90°, 33 slices, no gap, slice thickness = 3.8 mm, 166 repetitions). During fMRI data acquisition, the subjects performed a modified blocked design spatial and verbal working memory task [82] (Fig. 5). The task included 6 blocks alternating between SWM, verbal working memory and vigilance conditions with brief presentations of a fixation between block conditions. The verbal working memory data were not included in the final analysis, but have been described in a previous study [83]. Task conditions were differentiated with task instructions presented for 3500 ms followed by a 1500 ms interval before task stimuli appeared. During the SWM blocks, the stimuli consisted of capitalized, white, phonemically similar letters (e.g., D, G, P, etc.) that were presented one at a time in various spatial locations. Phonemic similarity between the letters was chosen to increase the difficulty of verbal rehearsal. Stimuli were arranged in various spatial locations on the screen to minimize the likelihood of verbal encoding strategies during the spatial working-memory tasks. In the SWM condition, the goal was to respond with a button press each time a stimulus appeared in a repeat location two stimuli prior, regardless of the stimulus content. During the verbal working memory condition, the respondent pressed a button each time the same letter was repeated two stimuli prior, regardless of spatial location. A vigilance condition was included to act as a control for motor response and visual and attentional processing of stimuli. During vigilance, white and gray dots were presented in the same spatial locations as letter stimuli, and youth were asked to make a response when a gray dot was presented. The entire task consisted of two runs and six blocks (two verbal WM and two spatial WM, and two vigilance) counterbalanced per run, and 16 trials per block for a total of 192 trials. Stimuli were presented on the screen for 500 ms, with an intertrial interval of 1500 ms during which subjects viewed a crosshair at the center of the screen. The total duration of the task was 11 minutes and 4 seconds. The task was displayed with Presentation software (Version 0.70, www.neurobs.com). Accuracy and reaction time (for correct trials) were collected for all blocks. Following fMRI data acquisition, youth completed an Exit Questionnaire that included questions regarding self-reported use of cognitive strategies (i.e. verbal versus spatial) during the task.
Fig. 5.
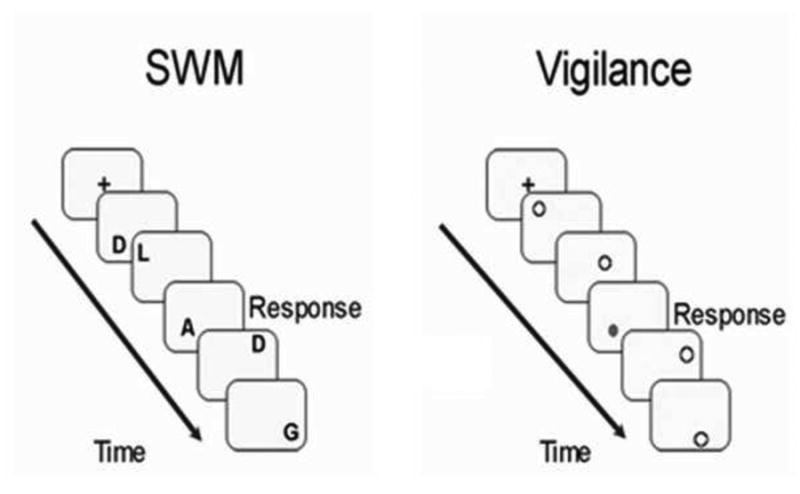
Spatial working memory (SWM) and vigilance conditions were included in the fMRI analysis. In SWM trials, participants were instructed to press a button each time a letter appeared in the same location two letters prior, regardless of the letter. During vigilance trials, participants were asked to press a button any time a gray dot appeared on the screen.
4.3.2. Anatomical MRI
A whole-brain, high-resolution structural image series was collected in the sagittal plane using a T1-weighted MPRAGE scanning sequence (TI = 900 ms, flip angle = 10°, TE = 3.58 ms, TR = 2300 ms, acquisition matrix = 256 x 240, FOV = 256mm, slice thickness = 1 mm, 33 slices) for co-registration to functional data.
4.4. Data analysis
4.4.1. Demographic and task performance
Due to the block design of the task, an accuracy threshold (>80% accuracy on SWM and vigilance trials) was used to minimize the effect of error signaling on the BOLD response. Demographic and task performance data were examined for normality and occurrence of outliers using SPSS Statistics 20 (Armonk, NY: IBM Corp.). Normality of data was examined within the whole group and by sex. Non-normal variables with absolute skew/kurtosis values exceeding 2.0 were log transformed. Whole group demographic characteristics were normally distributed, with the exception of pubertal status and testosterone levels. Task performance variables were also normally distributed, with the exception of reaction time in the vigilance condition. Additionally, IQ was non-normally distributed in males only. Sex differences for self-reported race were assessed with chi-square analysis, while differences in PDS were determined with a Mann-Whitney U analysis. All other variables were examined with independent samples t-tests. Within-group task comparisons were assessed with paired samples t-tests.
4.4.2. fMRI data processing
Imaging data were processed and analyzed using Analysis of Functional NeuroImages (AFNI) [84]. First, time series data were co-registered by aligning each acquisition to a selected repetition with an iterated least squares algorithm to estimate three rotational and three displacement parameters for each participant. Repetitions that showed greater than 2.5 mm or degrees in any of these parameters were removed from further analyses. A total of eight and seven repetitions were censored in the female and male datasets, respectively, which was not statistically different (t47 = 1.97, p > .05). Additionally, an average root mean square (RMS) value was calculated for within-run motion, across these six motion parameters for each subject to compare movement across groups. Any subject with an RMS value exceeding 1.5 mm was excluded from analyses. Given the sensitivity of the BOLD signal to head motion, movement was assessed with an additional method based on frame-wise displacement [50, 51] to confirm results (see Appendix A: Supplementary Data).
Functional masks were created to mask out non-brain areas and time series data were normalized, resulting in images scaled by percent signal change. Then, the time series data were correlated using a vector representing task design for the SWM, verbal working memory, and vigilance blocks, while covarying for motion and linear trends through the use of a deconvolution process. The percent signal change derived from fitting the time series data to the model represents the BOLD response. BOLD signal was contrasted between spatial and vigilance conditions, as well as compared to baseline, for each voxel in the brain. Finally, functional data sets were resampled into 3 mm3 voxels and were transformed into standard Talairach coordinates [85] for anatomical localization and between-subjects comparisons. Data obtained during verbal working memory conditions also underwent processing, but were not included in the current analyses.
4.5. fMRI data analysis
Task-specific activation was examined separately for each group using a one-sample t-test for the SWM-Vigilance contrast. Individual group masks of task-related activity for both males and females were thresholded at p < .05 and combined to form a map of task-related brain activity representative of the whole sample. Between-group analyses consisted of an analysis of covariance (ANCOVA) for the SWM-Vigilance contrast. Pubertal status was significantly different between boys and girls (t46 = 2.92, p = .005), so it was included as a covariate. In addition, age has been shown to affect brain response during SWM in a sex-specific fashion during adolescence [9], warranting its inclusion as a covariate. To correct for multiple comparisons, AlphaSim [84] was used to perform Monte Carlo simulations using both voxel and cluster thresholding . A voxel-wise threshold of p < .01 and whole-brain cluster threshold of α < .05 were used, which corresponded to 52 contiguous 3 mm3 voxels (or 1404 microliters). Because the group analysis determined sex differences of a contract (i.e. SWM-Vigilance), it was necessary to visually examine each trial against baseline. This was done by extracting percent BOLD signal change values from significant clusters and plotting the data against baseline by trial type (i.e. SWM, Vigilance) and sex. This allowed us to ascertain if group differences were driven by changes in BOLD signal in one trial or both for each sex.
Values of percent BOLD signal change from significant clusters from the ANCOVA were extracted and included in a nonparametric bootstrapping procedure [86] to assess mediation by testosterone. This method of hypothesis testing makes no assumptions about the shape of the distribution of the variables of interest, which included sex (independent variable), testosterone (mediator variable), and percent BOLD signal change from significant clusters (dependent variables). This approach provided the estimate of indirect effects of mediator variables, their estimated standard error, and 95% confidence intervals by using sampling with replacement with 5,000 re-samples.
To examine potential behavioral performance correlates, two multiple regressions were conducted using the same map of task-related brain activity that was used in the ANCOVA. The first regression included a measure of accuracy (percent correct responses during SWM trials), sex and sex-by-accuracy terms as independent variables. A second model included RT (during correct SWM trials), sex, and sex-by-RT as independent variables. Correction for multiple comparisons included Monte Carlo simulation using both voxel and cluster thresholding. A voxel-wise threshold of p < .01 and whole-brain cluster threshold of α < .05 were used, which corresponded to 51 contiguous 3 mm3 voxels (or 1377 microliters). Percent BOLD signal change values from clusters with a significant interaction of sex and a performance variable (accuracy or RT) were extracted and plotted to visually examine the nature of the interaction.
Supplementary Material
Spatial working memory (SWM) BOLD activity differs in adolescent boys and girls.
Boys recruit traditional SWM-related brain regions more robustly than girls.
Girls more effectively disengage default mode-related brain regions than boys.
Testosterone does not mediate sex differences in SWM BOLD response.
Acknowledgments
Members of the Developmental Brain Imaging Lab at Oregon Health & Science University are thanked for their efforts in data collection. Financial support for data collection was provided by the National Institute of Neurological Disorders and Stroke (K08 NS05052147). Data analysis and preparation of this article was supported by the National Institute on Alcohol Abuse and Alcoholism (R01 AA017664 & T32 AA007468-24) and the National Institute of Mental Health (R01 MH096773 & R00 MH091238).
Footnotes
Conflict of interest: The authors report no conflicts of interest.
Appendix A. Supplementary data: Supplementary methodology and data associated with this article can be found in the online version of the text.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Sciences. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- 2.Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8174–9. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koolschijn PC, Peper JS, Crone EA. The influence of sex steroids on structural brain maturation in adolescence. PloS one. 2014;9:e83929. doi: 10.1371/journal.pone.0083929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36:1065–73. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bramen JE, Hranilovich JA, Dahl RE, Chen J, Rosso C, Forbes EE, et al. Sex matters during adolescence: testosterone-related cortical thickness maturation differs between boys and girls. PloS one. 2012;7:e33850. doi: 10.1371/journal.pone.0033850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casey BJ, Cohen JD, Jezzard P, Turner R, Noll DC, Trainor RJ, et al. Activation of prefrontal cortex in children during a nonspatial working memory task with functional MRI. NeuroImage. 1995;2:221–9. doi: 10.1006/nimg.1995.1029. [DOI] [PubMed] [Google Scholar]
- 7.Klingberg T, Forssberg H, Westerberg H. Increased brain activity in frontal and parietal cortex underlies the development of visuospatial working memory capacity during childhood. Journal of cognitive neuroscience. 2002;14:1–10. doi: 10.1162/089892902317205276. [DOI] [PubMed] [Google Scholar]
- 8.Kwon H, Reiss AL, Menon V. Neural basis of protracted developmental changes in visuospatial working memory. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:13336–41. doi: 10.1073/pnas.162486399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schweinsburg AD, Nagel BJ, Tapert SF. fMRI reveals alteration of spatial working memory networks across adolescence. J Int Neuropsychol Soc. 2005;11:631–44. doi: 10.1017/S1355617705050757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peper JS, Brouwer RM, Schnack HG, van Baal GC, van Leeuwen M, van den Berg SM, et al. Sex steroids and brain structure in pubertal boys and girls. Psychoneuroendocrinology. 2009;34:332–42. doi: 10.1016/j.psyneuen.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Perrin JS, Herve PY, Leonard G, Perron M, Pike GB, Pitiot A, et al. Growth of white matter in the adolescent brain: role of testosterone and androgen receptor. J Neurosci. 2008;28:9519–24. doi: 10.1523/JNEUROSCI.1212-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raznahan A, Lee Y, Stidd R, Long R, Greenstein D, Clasen L, et al. Longitudinally mapping the influence of sex and androgen signaling on the dynamics of human cortical maturation in adolescence. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:16988–93. doi: 10.1073/pnas.1006025107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baddeley A. Working memory. Science. 1992;255:556–9. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- 14.Katsuki F, Constantinidis C. Unique and shared roles of the posterior parietal and dorsolateral prefrontal cortex in cognitive functions. Frontiers in integrative neuroscience. 2012;6:17. doi: 10.3389/fnint.2012.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Owen AM. The functional organization of working memory processes within human lateral frontal cortex: the contribution of functional neuroimaging. The European journal of neuroscience. 1997;9:1329–39. doi: 10.1111/j.1460-9568.1997.tb01487.x. [DOI] [PubMed] [Google Scholar]
- 16.Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Human brain mapping. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fletcher PC, Henson RN. Frontal lobes and human memory: insights from functional neuroimaging. Brain : a journal of neurology. 2001;124:849–81. doi: 10.1093/brain/124.5.849. [DOI] [PubMed] [Google Scholar]
- 18.Rypma B, Prabhakaran V, Desmond JE, Glover GH, Gabrieli JD. Load-dependent roles of frontal brain regions in the maintenance of working memory. NeuroImage. 1999;9:216–26. doi: 10.1006/nimg.1998.0404. [DOI] [PubMed] [Google Scholar]
- 19.Thomas KM, King SW, Franzen PL, Welsh TF, Berkowitz AL, Noll DC, et al. A developmental functional MRI study of spatial working memory. NeuroImage. 1999;10:327–38. doi: 10.1006/nimg.1999.0466. [DOI] [PubMed] [Google Scholar]
- 20.Thomason ME, Race E, Burrows B, Whitfield-Gabrieli S, Glover GH, Gabrieli JD. Development of spatial and verbal working memory capacity in the human brain. Journal of cognitive neuroscience. 2009;21:316–32. doi: 10.1162/jocn.2008.21028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geier CF, Garver K, Terwilliger R, Luna B. Development of working memory maintenance. Journal of neurophysiology. 2009;101:84–99. doi: 10.1152/jn.90562.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scherf KS, Sweeney JA, Luna B. Brain basis of developmental change in visuospatial working memory. Journal of cognitive neuroscience. 2006;18:1045–58. doi: 10.1162/jocn.2006.18.7.1045. [DOI] [PubMed] [Google Scholar]
- 23.Voyer D, Voyer S, Bryden MP. Magnitude of sex differences in spatial abilities: a metaanalysis and consideration of critical variables. Psychological bulletin. 1995;117:250–70. doi: 10.1037/0033-2909.117.2.250. [DOI] [PubMed] [Google Scholar]
- 24.Vecchi T, Girelli L. Gender differences in visuo-spatial processing: the importance of distinguishing between passive storage and active manipulation. Acta psychologica. 1998;99:1–16. doi: 10.1016/s0001-6918(97)00052-8. [DOI] [PubMed] [Google Scholar]
- 25.Astur RS, Ortiz ML, Sutherland RJ. A characterization of performance by men and women in a virtual Morris water task: a large and reliable sex difference. Behavioural brain research. 1998;93:185–90. doi: 10.1016/s0166-4328(98)00019-9. [DOI] [PubMed] [Google Scholar]
- 26.Rizk-Jackson AM, Acevedo SF, Inman D, Howieson D, Benice TS, Raber J. Effects of sex on object recognition and spatial navigation in humans. Behavioural brain research. 2006;173:181–90. doi: 10.1016/j.bbr.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 27.Janowsky JS, Chavez B, Orwoll E. Sex steroids modify working memory. Journal of cognitive neuroscience. 2000;12:407–14. doi: 10.1162/089892900562228. [DOI] [PubMed] [Google Scholar]
- 28.Minor K, Park S. Spatial working memory: absence of gender differences in schizophrenia patients and healthy control subjects. Biological psychiatry. 1999;46:1003–5. doi: 10.1016/s0006-3223(99)00149-3. [DOI] [PubMed] [Google Scholar]
- 29.Duff SJ, Hampson E. A sex difference on a novel spatial working memory task in humans. Brain and cognition. 2001;47:470–93. doi: 10.1006/brcg.2001.1326. [DOI] [PubMed] [Google Scholar]
- 30.Lejbak L, Crossley M, Vrbancic M. A male advantage for spatial and object but not verbal working memory using the n-back task. Brain and cognition. 2011;76:191–6. doi: 10.1016/j.bandc.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt H, Jogia J, Fast K, Christodoulou T, Haldane M, Kumari V, et al. No gender differences in brain activation during the N-back task: an fMRI study in healthy individuals. Human brain mapping. 2009;30:3609–15. doi: 10.1002/hbm.20783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bell EC, Willson MC, Wilman AH, Dave S, Silverstone PH. Males and females differ in brain activation during cognitive tasks. NeuroImage. 2006;30:529–38. doi: 10.1016/j.neuroimage.2005.09.049. [DOI] [PubMed] [Google Scholar]
- 33.Frings L, Wagner K, Unterrainer J, Spreer J, Halsband U, Schulze-Bonhage A. Genderrelated differences in lateralization of hippocampal activation and cognitive strategy. Neuroreport. 2006;17:417–21. doi: 10.1097/01.wnr.0000203623.02082.e3. [DOI] [PubMed] [Google Scholar]
- 34.Moffat SD, Hampson E. A curvilinear relationship between testosterone and spatial cognition in humans: possible influence of hand preference. Psychoneuroendocrinology. 1996;21:323–37. doi: 10.1016/0306-4530(95)00051-8. [DOI] [PubMed] [Google Scholar]
- 35.Janowsky JS, Oviatt SK, Orwoll ES. Testosterone influences spatial cognition in older men. Behavioral neuroscience. 1994;108:325–32. doi: 10.1037//0735-7044.108.2.325. [DOI] [PubMed] [Google Scholar]
- 36.Hawley WR, Grissom EM, Martin RC, Halmos MB, Bart CL, Dohanich GP. Testosterone modulates spatial recognition memory in male rats. Hormones and behavior. 2013;63:559–65. doi: 10.1016/j.yhbeh.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 37.Witte AV, Savli M, Holik A, Kasper S, Lanzenberger R. Regional sex differences in grey matter volume are associated with sex hormones in the young adult human brain. NeuroImage. 2010;49:1205–12. doi: 10.1016/j.neuroimage.2009.09.046. [DOI] [PubMed] [Google Scholar]
- 38.Peper JS, Hulshoff Pol HE, Crone EA, van Honk J. Sex steroids and brain structure in pubertal boys and girls: a mini-review of neuroimaging studies. Neuroscience. 2011;191:28–37. doi: 10.1016/j.neuroscience.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 39.Neufang S, Specht K, Hausmann M, Gunturkun O, Herpertz-Dahlmann B, Fink GR, et al. Sex differences and the impact of steroid hormones on the developing human brain. Cereb Cortex. 2009;19:464–73. doi: 10.1093/cercor/bhn100. [DOI] [PubMed] [Google Scholar]
- 40.Mueller SC, Merke DP, Leschek EW, Fromm S, Grillon C, Cornwell BR, et al. Grey matter volume correlates with virtual water maze task performance in boys with androgen excess. Neuroscience. 2011;197:225–32. doi: 10.1016/j.neuroscience.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schoning S, Engelien A, Kugel H, Schafer S, Schiffbauer H, Zwitserlood P, et al. Functional anatomy of visuo-spatial working memory during mental rotation is influenced by sex, menstrual cycle, and sex steroid hormones. Neuropsychologia. 2007;45:3203–14. doi: 10.1016/j.neuropsychologia.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 42.Meyer K, Korz V. Age dependent differences in the regulation of hippocampal steroid hormones and receptor genes: relations to motivation and cognition in male rats. Hormones and behavior. 2013;63:376–84. doi: 10.1016/j.yhbeh.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 43.Lagunas N, Calmarza-Font I, Grassi D, Garcia-Segura LM. Estrogen receptor ligands counteract cognitive deficits caused by androgen deprivation in male rats. Hormones and behavior. 2011;59:581–4. doi: 10.1016/j.yhbeh.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 44.Goddings AL, Burnett Heyes S, Bird G, Viner RM, Blakemore SJ. The relationship between puberty and social emotion processing. Developmental science. 2012;15:801–11. doi: 10.1111/j.1467-7687.2012.01174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klapwijk ET, Goddings AL, Burnett Heyes S, Bird G, Viner RM, Blakemore SJ. Increased functional connectivity with puberty in the mentalising network involved in social emotion processing. Hormones and behavior. 2013;64:314–22. doi: 10.1016/j.yhbeh.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Denson TF, Ronay R, von Hippel W, Schira MM. Endogenous testosterone and cortisol modulate neural responses during induced anger control. Social neuroscience. 2012 doi: 10.1080/17470919.2012.655425. [DOI] [PubMed] [Google Scholar]
- 47.Stanton SJ, Wirth MM, Waugh CE, Schultheiss OC. Endogenous testosterone levels are associated with amygdala and ventromedial prefrontal cortex responses to anger faces in men but not women. Biological psychology. 2009;81:118–22. doi: 10.1016/j.biopsycho.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moffat SD, Resnick SM. Long-term measures of free testosterone predict regional cerebral blood flow patterns in elderly men. Neurobiology of aging. 2007;28:914–20. doi: 10.1016/j.neurobiolaging.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 49.Wallach JB. Interpretation of diagnostic tests. 7th. Philadelphia, PA: Lippincott Williams and Wilkins; 2000. [Google Scholar]
- 50.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59:2142–54. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fair DA, Nigg JT, Iyer S, Bathula D, Mills KL, Dosenbach NU, et al. Distinct neural signatures detected for ADHD subtypes after controlling for micro-movements in resting state functional connectivity MRI data. Frontiers in systems neuroscience. 2012;6:80. doi: 10.3389/fnsys.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9673–8. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anticevic A, Repovs G, Shulman GL, Barch DM. When less is more: TPJ and default network deactivation during encoding predicts working memory performance. NeuroImage. 2010;49:2638–48. doi: 10.1016/j.neuroimage.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anticevic A, Cole MW, Murray JD, Corlett PR, Wang XJ, Krystal JH. The role of default network deactivation in cognition and disease. Trends in cognitive sciences. 2012;16:584–92. doi: 10.1016/j.tics.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Laird AR, Eickhoff SB, Li K, Robin DA, Glahn DC, Fox PT. Investigating the functional heterogeneity of the default mode network using coordinate-based meta-analytic modeling. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:14496–505. doi: 10.1523/JNEUROSCI.4004-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fair DA, Cohen AL, Dosenbach NU, Church JA, Miezin FM, Barch DM, et al. The maturing architecture of the brain's default network. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:4028–32. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Keulers EH, Goulas A, Jolles J, Stiers P. Maturation of task-induced brain activation and long range functional connectivity in adolescence revealed by multivariate pattern classification. NeuroImage. 2012;60:1250–65. doi: 10.1016/j.neuroimage.2011.12.079. [DOI] [PubMed] [Google Scholar]
- 59.McCarthy G, Puce A, Constable RT, Krystal JH, Gore JC, Goldman-Rakic P. Activation of human prefrontal cortex during spatial and nonspatial working memory tasks measured by functional MRI. Cereb Cortex. 1996;6:600–11. doi: 10.1093/cercor/6.4.600. [DOI] [PubMed] [Google Scholar]
- 60.D'Esposito M, Aguirre GK, Zarahn E, Ballard D, Shin RK, Lease J. Functional MRI studies of spatial and nonspatial working memory. Brain research Cognitive brain research. 1998;7:1–13. doi: 10.1016/s0926-6410(98)00004-4. [DOI] [PubMed] [Google Scholar]
- 61.Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283:1657–61. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- 62.Goldman-Rakic PS. Development of cortical circuitry and cognitive function. Child development. 1987;58:601–22. [PubMed] [Google Scholar]
- 63.Sterzer P, Kleinschmidt A. Anterior insula activations in perceptual paradigms: often observed but barely understood. Brain structure & function. 2010;214:611–22. doi: 10.1007/s00429-010-0252-2. [DOI] [PubMed] [Google Scholar]
- 64.Raabe M, Fischer V, Bernhardt D, Greenlee MW. Neural correlates of spatial working memory load in a delayed match-to-sample saccade task. NeuroImage. 2013 doi: 10.1016/j.neuroimage.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 65.Honey GD, Bullmore ET, Sharma T. Prolonged reaction time to a verbal working memory task predicts increased power of posterior parietal cortical activation. NeuroImage. 2000;12:495–503. doi: 10.1006/nimg.2000.0624. [DOI] [PubMed] [Google Scholar]
- 66.Blakemore SJ. Imaging brain development: the adolescent brain. NeuroImage. 2012;61:397–406. doi: 10.1016/j.neuroimage.2011.11.080. [DOI] [PubMed] [Google Scholar]
- 67.Arnold AP, Breedlove SM. Organizational and activational effects of sex steroids on brain and behavior: a reanalysis. Hormones and behavior. 1985;19:469–98. doi: 10.1016/0018-506x(85)90042-x. [DOI] [PubMed] [Google Scholar]
- 68.McCarthy MM, Arnold AP. Reframing sexual differentiation of the brain. Nature neuroscience. 2011;14:677–83. doi: 10.1038/nn.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koopman P. Sry and Sox9: mammalian testis-determining genes. Cellular and molecular life sciences : CMLS. 1999;55:839–56. doi: 10.1007/PL00013200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Davies W, Wilkinson LS. It is not all hormones: alternative explanations for sexual differentiation of the brain. Brain research. 2006;1126:36–45. doi: 10.1016/j.brainres.2006.09.105. [DOI] [PubMed] [Google Scholar]
- 71.Lebel C, Mattson SN, Riley EP, Jones KL, Adnams CM, May PA, et al. A longitudinal study of the long-term consequences of drinking during pregnancy: heavy in utero alcohol exposure disrupts the normal processes of brain development. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:15243–51. doi: 10.1523/JNEUROSCI.1161-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Elton A, Tripathi SP, Mletzko T, Young J, Cisler JM, James GA, et al. Childhood maltreatment is associated with a sex-dependent functional reorganization of a brain inhibitory control network. Human brain mapping. 2013 doi: 10.1002/hbm.22280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lucas CP, Zhang H, Fisher PW, Shaffer D, Regier DA, Narrow WE, et al. The DISC Predictive Scales (DPS): efficiently screening for diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:443–9. doi: 10.1097/00004583-200104000-00013. [DOI] [PubMed] [Google Scholar]
- 74.Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, et al. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcoholism, clinical and experimental research. 1995;19:1018–23. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- 75.Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): a measure of adolescent alcohol and drug involvement. Journal of studies on alcohol. 1998;59:427–38. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- 76.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 77.Weschler D. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio, TX: Harcourt Assessment; 1999. [Google Scholar]
- 78.Hollingshead AA. Four-factor index of social status. 1975 [Google Scholar]
- 79.Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17:117–33. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- 80.Albertsson-Wikland K, Rosberg S, Lannering B, Dunkel I, Selstam G, Norjavaara E. Twenty-four-hour profiles of luteinizing hormone, follicle-stimulating hormone, testosterone, and estradiol levels: a semilongitudinal study throughout puberty in healthy boys. The Journal of Clinical Endocrinology & Metabolism. 1997;82:541–9. doi: 10.1210/jcem.82.2.3778. [DOI] [PubMed] [Google Scholar]
- 81.Gillies GE, McArthur S. Estrogen actions in the brain and the basis for differential action in men and women: a case for sex-specific medicines. Pharmacological reviews. 2010;62:155–98. doi: 10.1124/pr.109.002071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nagel BJ, Ohannessian A, Cummins K. Performance dissociation during verbal and spatial working memory tasks. Perceptual and motor skills. 2007;105:243–50. doi: 10.2466/pms.105.1.243-250. [DOI] [PubMed] [Google Scholar]
- 83.Cservenka A, Herting MM, Nagel BJ. Atypical frontal lobe activity during verbal working memory in youth with a family history of alcoholism. Drug and alcohol dependence. 2012;123:98–104. doi: 10.1016/j.drugalcdep.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 85.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain : 3-dimensional proportional system : an approach to cerebral imaging. Stuttgart; New York: Georg Thieme; 1988. [Google Scholar]
- 86.Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behavior research methods, instruments, & computers : a journal of the Psychonomic Society, Inc. 2004;36:717–31. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


