Abstract
High-density lipoproteins (HDL) are a heterogeneous group of lipoproteins composed of various lipids and proteins. HDL is formed both in the systemic circulation and in the brain. In addition to being a crucial player in the reverse cholesterol transport pathway, HDL possesses a wide range of other functions including anti-oxidation, anti-inflammation, pro-endothelial function, anti-thrombosis, and modulation of immune function. It has been firmly established that high plasma levels of HDL protect against cardiovascular disease. Accumulating evidence indicates that the beneficial role of HDL extends to many other systems including the central nervous system. Cognition is a complex brain function that includes all aspects of perception, thought, and memory. Cognitive function often declines during aging and this decline manifests as cognitive impairment/dementia in age-related and progressive neurodegenerative disorders such as Alzheimer's disease, Parkinson's disease, Huntington's disease, and amyotrophic lateral sclerosis. A growing concern is that no effective therapy is currently available to prevent or treat these devastating diseases. Emerging evidence suggests that HDL may play a pivotal role in preserving cognitive function under normal and pathological conditions. This review attempts to summarize recent genetic, clinical and experimental evidence for the impact of HDL on cognition in aging and in neurodegenerative disorders as well as the potential of HDL-enhancing approaches to improve cognitive function.
Keywords: High-density lipoproteins, Apolipoproteins, Cognition, Alzheimer's disease, Parkinson's disease, Huntington's disease, Amyotrophic lateral sclerosis, HDL-enhancing pharmacotherapy
Introduction
Lipoproteins are the complexes of various lipids and proteins (Vance and Vance, 2008). They are formed in extracellular space and circulate as soluble subcellular-sized particles in body fluids. The general structure of lipoprotein particles includes a hydrophobic core surrounded by a hydrophilic shell. The hydrophobic core contains neutral lipids, predominantly triglycerides (TG) and cholesterol esters (CE). The hydrophilic shell consists of primarily phospholipids (PL), unesterified free cholesterol (FC), and various apolipoproteins (apo), which mediate interactions with a variety of other molecules including enzymes, transporters, and receptors through a dynamic process. The main function of lipoproteins is facilitating the delivery and clearance of lipids and lipid-soluble or lipid-associating molecules throughout the body. Lipoproteins can be characterized by their size, density, electrophoretic mobility, and composition. The most commonly used classification of lipoproteins is by density. Due to the dynamic nature of lipoproteins, each class of lipoproteins can be divided into several subclasses. The focus of this review is on high-density lipoproteins (HDL), a heterogeneous group of lipoprotein particles with a density of 1.063 – 1.210 g/mL and size of approximately 7-20 nm. They are formed both in the systemic circulation and in the brain. Plasma HDL has been studied extensively because of its well established protective role in the cardiovascular system. Recent studies strongly suggest that the benefits of HDL extend to many other systems including the central nervous system (CNS). Mounting evidence indicates that HDL modulates cognitive function in aging and age-related neurodegenerative disorders.
Major neurodegenerative disorders include Alzheimer's disease (AD), Parkinson's disease (PD), Huntington's disease (HD), and amyotrophic lateral sclerosis (ALS). In these diseases, specific areas of the CNS deteriorate progressively, resulting in devastating neurological dysfunction. AD is a cognitive disorder that causes the most common form of dementia, accounting for 50-60% of all cases (Alzheimer's Association, 2013). Although PD, HD, and ALS are considered primarily as movement disorders, cognitive impairment/dementia is found in substantial groups of patients with these diseases (Goldstein and Abrahams, 2013; Svenningsson et al., 2012; Walker, 2007). Much progress has been made on the etiology and pathogenic mechanisms of these neurodegenerative disorders; however, currently there are still no effective measures to prevent or treat these debilitating diseases. Emerging evidence suggests that HDL modulates cognitive function under normal and pathological conditions. This review provides an overview of HDL function in the systemic circulation and in the brain, summarizes recent genetic, clinical and experimental evidence for the impact of HDL on cognition in aging and in neurodegenerative disorders, and explores the potential of HDL-enhancing approaches to improve cognitive function.
HDL Metabolism in the Systemic Circulation
Although HDL is often referred to as HDL cholesterol (HDL-C), apoA-I is the major protein component of HDL in the plasma and determines most of its functions (Segrest et al., 2000). In addition to apoA-I, plasma HDL also contains many other apos. Mature human apoA-I is a 243-residue polypeptide produced predominantly by the liver and intestine. The lipid-associating domain (residues 44-243) of human apoA-I contains tandem repeats of amphipathic α-helixes (Segrest et al., 1994). HDL biogenesis starts with the interaction between lipid-poor apoA-I and ATP-binding cassette transporter A1 (ABCA1) on the cell membrane of peripheral tissues, resulting in the formation of nascent discoidal HDL particles from cell membrane-derived PL and FC (Oram and Heinecke, 2005). Of note, other apos can also act as lipid acceptors for ABCA1. Importantly, this is the first step of reverse cholesterol transport (RCT), a process that removes excess cholesterol from peripheral tissues to the liver for excretion in the bile. Once the discoidal particles reach plasma, apoA-I activates lecithin cholesterol acyltransferase (LCAT), forming mature, spherical, CE-rich HDL particles (Fig. 1).
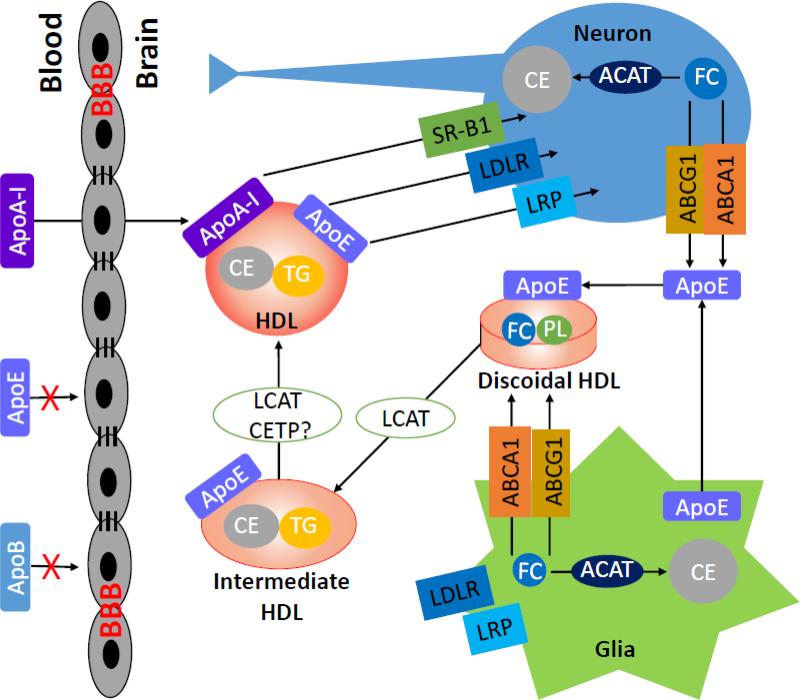
Fig. 1. Schematic of HDL metabolism in the systemic circulation.
Formation of the nascent discoidal HDL through apoA-I and ABCA1 is the first step in reverse cholesterol transport (RCT), a process that removes excess cholesterol from peripheral tissues to the liver for excretion. In the plasma, apoA-I activates LCAT, which converts discoidal HDL to mature, spherical, CE-rich HDL particles. HDL interacts with other lipoprotein particles and cells through multiple receptors, transporters, and enzymes. Mature HDL removes cholesterol from peripheral cells through other ABC transporters such as ABCG1. Lipid-rich HDL selectively delivers CE to hepatocytes and steroidogenic cells through SR-B1. HDL-bound CETP mediates the exchange of CE and TG between HDL and non-HDL particles. ABC, ATP-binding cassette transporter; Apo, apolipoprotein; CE, cholesteryl esters; CETP, cholesteryl ester transfer protein; FC, unesterified free cholesterol; HDL, high-density lipoproteins; LCAT, lecithin cholesterol acyltransferase; LDL, low-density lipoprotein; LDLR, low-density lipoprotein receptor; PL, phospholipids; SR-B1, scavenger receptor B1; TG, triglycerides.
In the plasma, HDL interacts with cells and other lipoprotein particles through multiple receptors, transporters, and enzymes. Mature HDL can remove cholesterol from peripheral cells through other ABC transporters such as ABCG1 and ABCG4, further promoting RCT. Lipid-rich HDL selectively delivers CE to hepatocytes and steroidogenic cells through scavenge receptor B1 (SR-B1), regenerating lipid-poor apoA-I/HDL particles for further interaction with ABCA1. HDL-bound cholesteryl ester transfer protein (CETP) mediates the exchange of CE from HDL to non-HDL particles and the transfer of TG from TG-rich lipoproteins to HDL, resulting in a decrease of HDL-C levels. Thus, CETP inhibitors have been developed to raise HDL levels (discussed below). Other major HDL-interacting proteins include phospholipid transfer protein (PLTP), endothelial lipase, and hepatic lipase (Vance and Vance, 2008).
It is well established that plasma levels of apoA-I/HDL are negatively correlated with the incidence of coronary heart disease in humans (Davidson and Toth, 2007). The mechanisms by which apoA-I/HDL protects against atherosclerosis are not fully understood at present. One of the major mechanisms is related to the role of apoA-I/HDL in RCT (Oram and Heinecke, 2005). The initial cholesterol efflux involving the interaction of apoA-I and ABCA1 is a critical step in RCT. Mutations/polymorphisms on ABCA1 cause a significant reduction in HDL levels (familial hypoalphalipoproteinemia), to the point of near absence as reported in patients with Tangier disease (Oram and Heinecke, 2005).
In addition to its role in RCT, apoA-I/HDL exerts a wide range of other functions including anti-oxidation (Navab et al., 2000), anti-inflammation (Cockerill et al., 1995), proendothelial function (O'Connell and Genest, 2001), anti-thrombosis (Barter et al., 2004), and modulation of immune function (Barter et al., 2004). The multi-functionality of HDL contributes to its cardioprotective role. With the advance of modern technologies, recent proteomic and lipidomic analyses have revealed that approximately 188 proteins and over 200 lipid species are associated with plasma HDL (Toth et al., 2013). In addition, microRNAs (miRNAs) have also been found in human plasma HDL, and remarkably, HDL could deliver miRNAs to recipient cells through the SR-B1-depedent pathway (Vickers et al., 2011). Clearly, the complexity of HDL composition and function presents both the challenge and opportunity to develop HDL-based biomarkers and therapies for a number of diseases.
HDL Metabolism in the Central Nervous System
While lipoprotein metabolism in the systemic circulation has been studied extensively, interest in lipoprotein metabolism in the brain has only increased in recent years because of connections between apoE and the development of several neurological disorders (discussed below). The brain is highly enriched in cholesterol. It contains ~25% of total body cholesterol despite the fact that the brain accounts for only 2% of total body mass (Dietschy and Turley, 2001). It is generally thought that there is no net transfer of cholesterol from the periphery into the CNS because the blood-brain barrier (BBB) restricts the movement of plasma lipoproteins into the brain. Thus, essentially all cholesterol in the brain comes from de novo synthesis. In adults, the rate of cholesterol synthesis exceeds the need for forming new structures. One of the excretory pathways involves the formation of 24S-hydroxycholesterol that crosses the BBB into the plasma (Dietschy and Turley, 2001).
The major apolipoprotein in the brain is apoE, primarily produced by glial cells. In humans, there are three isoforms of apoE coded by three alleles: APOE-ε2, APOE-ε3, and APOE-ε4, with an allele frequency of 7, 78, and 15%, respectively (Strittmatter and Roses, 1996). ApoE has received tremendous attention due to its genetic association with AD. While the APOE-ε2 allele confers some protection against AD (Corder et al., 1994), the APOE-ε4 allele is associated with an increased risk of AD (Corder et al., 1993; Poirier et al., 1993). The brain also expresses lipoprotein receptors (e.g., LDLR, LRP, and SR-B1), enzymes (e.g., LCAT and lipases), transfer proteins (e.g., PLTP and CETP), and ABC transporters (e.g., ABCA1 and ABCG1), although the presence of CETP in the brain is controversial (Albers et al., 1992; Demeester et al., 2000; Yamada et al., 1995). Because these proteins have well-established roles in cholesterol metabolism in the periphery, they are thought to play similar functions in the brain (Fig. 2). HDL-like lipoprotein particles are found in the CSF and contain mainly apoE and apoA-I (Koch et al., 2001; Pitas et al., 1987). While the source of apoE is clearly from glia as plasma apoE cannot cross the BBB (Linton et al., 1991), the origin of apoA-I in the CSF is uncertain. It is generally thought that the brain does not produce apoA-I and that apoA-I in the brain comes from the circulation (Dietschy and Turley, 2001). However, porcine cerebral endothelial cells have been shown to produce apoA-I (Mockel et al., 1994). Notably, the concentration of apoA-I in the CSF is comparable to that of apoE (Koch et al., 2001). In addition, plasma and CSF HDL cholesterol and apoA-I levels are correlated, suggesting that plasma apoA-I/HDL levels can influence brain apoA-I/HDL levels (Fagan et al., 2000). While the role of apoE in brain cholesterol metabolism and other pathways is well established (Yu et al., 2010), the neurobiological role of apoA-I has not been well studied. Experimental evidence has shown that rat astrocytes interact with both human apoE and apoA-I and generate HDL-like particles with distinct properties: apoE-HDL particles are cholesterol-rich whereas apoA-I-HDL particles are phospholipid-rich (Ito et al., 1999). Human CSF lipoproteins are capable of inducing a significant cholesterol efflux from rat astrocytes (Demeester et al., 2000). The efflux ability of CSF lipoproteins is correlated more with the concentration of apoA-I in the CSF than that of apoE (Demeester et al., 2000). Also, exogenous human apoA-I is able to initiate a signal transduction pathway of intracellular cholesterol trafficking involving the activation of protein kinase C (PKC) in rat astrocytes for HDL biogenesis (Ito et al., 2004; Ito et al., 2002). In addition, apoA-I and apoE-containing HDL in the CSF go through different remodeling in response to traumatic brain injury in human (Kay et al., 2003). These findings, together with other evidence discussed below, suggest that apoA-I-containing HDL may have important functions in the brain under physiological and pathological conditions.
Fig. 2. Schematic of HDL metabolism in the brain.
Similar to peripheral tissues, the brain expresses various lipoprotein receptors (e.g., LDLR, LRP, and SR-B1), enzymes (e.g., LCAT and lipases), transfer proteins (e.g., PLTP and CETP), and ABC transporters (e.g., ABCA1 and ABCG1). However, the presence of CETP in the brain is controversial. ApoE, synthesized primarily by glia, and apoA-I from the blood generate HDL particles and mediate cholesterol efflux through interactions with ABCA1 and ABCG1. LCAT converts the discoidal HDL to mature HDL particles. The HDL particles are remodeled by the interactions of apoE and apoA-I with various lipoprotein receptors on neurons and glia. ABC, ATP-binding cassette transporter; ACAT, acyl-coenzyme A:cholesterol acyltransferase; Apo, apolipoprotein; BBB, blood-brain barrier; CE, cholesteryl esters; CETP, cholesteryl ester transfer protein; FC, unesterified free cholesterol; HDL, high-density lipoproteins; LCAT, lecithin cholesterol acyltransferase; LDL, low-density lipoprotein; LDLR, low-density lipoprotein receptor; LRP, low-density lipoprotein receptor-related protein; PL, phospholipids; SR-B1, scavenger receptor B1; TG, triglycerides.
Genetic, Clinical and Experimental Evidence for Protective Effects of HDL on Cognitive Function
HDL and age-related cognitive decline
While many genetic and environmental factors contribute to a healthy aging process, recent studies indicate that HDL may play a significant role in maintaining cognitive function during aging. A study with a group of 139 centenarians (Ashkenazi Jews older than 95 years) showed that plasma HDL levels were highly and positively correlated with cognitive function (Atzmon et al., 2002). Consistent with the HDL levels, increased plasma apoA-I and decreased plasma triglyceride levels were also correlated with a significantly superior cognitive function. Another study in 158 Ashkenazi Jews with exceptional longevity (average age 99 years) also found that high levels of HDL were associated with less age-related cognitive impairment and improved memory (Barzilai et al., 2006). In agreement, the Leiden 85-plus study with 561 subjects also reported that low HDL was associated with cognitive impairment independent of atherosclerotic disease (van Exel et al., 2002). A recent population-based study, the Longitudinal Aging Study Amsterdam, further showed that high HDL was associated with better memory performance in people aged 65 years and older (van den Kommer et al., 2012). Consistently, low HDL levels have been found to be associated with poor memory and decline in memory in middle-aged adults and cognitively normal elderly individuals in the Whitehall II study and the Sydney Memory and Aging study, respectively (Singh-Manoux et al., 2008; Song et al., 2012). These findings underscore the protective effects of increased plasma HDL and its role in maintaining superior cognition during aging.
Notably, known as a major genetic determinant for AD, the genotype of apoE also affects cognitive decline in normal aging. Carriers of the APOE-ε4 allele showed decline in memory before the age of 60 years and exhibited greater acceleration than non-carriers (Caselli et al., 2009). A recent study also showed that in aged individuals without dementia, possession of the APOE-ε4 allele predicted a higher rate of cognitive decline in the ninth decade of life (Schiepers et al., 2012). It is also worth noting that carriers of APOE-ε4 have a more proatherogenic profile with lower HDL and higher VLDL and TG levels in the plasma than non-carriers. A recent study suggests that the high lipid affinity of apoE4 is responsible for such a lipid profile (Li et al., 2013). These findings suggest that besides the direct influence of apoE4 on brain function, systemic effects of apoE4 may also contribute to the compromised cognitive performance in carriers.
In addition to APOE-ε4 allele, recent gene association studies provide further evidence for the beneficial effects of HDL and/or apoA-I on cognitive function in aging. Functional polymorphisms in the gene for cholesteryl ester transfer protein (CETP), which cause lower levels of CETP and higher levels of HDL, are associated with slower cognitive decline during aging (Barzilai et al., 2006; Izaks et al., 2012; Sanders et al., 2010), although some inconsistency exists (Yu et al., 2012). Furthermore, genetic variants in apoC-III, which cause lower levels of TG and higher levels of HDL, are associated with exceptional longevity (Atzmon et al., 2006) and cardioprotection (Jorgensen et al., 2014; Pollin et al., 2008; The et al., 2014), whereas the apoC-III variants that raise TG and lower HDL are associated with impaired cognition (Smith et al., 2009). Whole-genome sequence-based analysis suggests that common variation contributes more to heritability of HDL levels than rare variation (Morrison et al., 2013). Whether all HDL-regulating genetic variations affect cognitive function awaits further investigation.
HDL and Alzheimer's disease
Alzheimer's disease (AD) is a progressive neurodegenerative disorder. Approximately 11% of people aged 65 years and older are afflicted with the disease and it is the most common cause of dementia in the elderly (Alzheimer's Association, 2013). AD is characterized clinically by progressive cognitive impairment. Pathological hallmarks of the AD brain include intracellular neurofibrillary tangles and deposits of amyloid-β protein (Aβ) in neuritic plaques and cerebral vessels. The pathogenesis of AD, particularly the sporadic form of AD, is not fully understood. While aging itself is the biggest risk factor for AD, the APOE-ε4 allele is a major genetic risk factor for sporadic AD (Corder et al., 1993). The role of apoE in AD has been well studied. For a more extensive coverage of the literature, the interested reader is referred to excellent recent reviews (Holtzman et al., 2012; Kanekiyo et al., 2014; Mahley and Huang, 2012). In addition to APOE, recent large genome-wide association studies have identified over 20 loci that contribute to the risk of sporadic AD (reviewed in (Reitz, 2012; Rosenthal and Kamboh, 2014)). Several loci, such as CLU (clusterin or apoJ) and ABCA7, are closely involved in the cholesterol metabolism pathway. However, both CLU and ABCA7 also have roles in the innate immunity. Whether CLU and ABCA7 variants associated with the AD risk influence brain or plasma HDL levels or functions is unknown. This section focuses on the relationship between plasma apoA-I-containing HDL and AD.
Clinical studies in different ethnic populations have shown that high levels of plasma HDL were associated with a decreased risk for AD, although there have been a few exceptions (Launer et al., 2001; Reitz et al., 2004; Vollbach et al., 2005). An early study with a group of 45 Japanese patients with AD found that plasma levels of apoA-I and apoA-II were markedly decreased compared to 79 controls (Kawano et al., 1995). Consistently, a study with a cohort of 98 French AD patients and 59 controls showed that decreased HDL cholesterol and serum apoA-I concentrations were highly correlated with the severity of AD (Merched et al., 2000). Another study with 334 elderly French subjects found that high HDL cholesterol levels were associated with a significantly decreased risk of AD (Bonarek et al., 2000). Furthermore, the Honolulu-Asia aging study with 929 men indicated that the levels of apoA-I and HDL cholesterol were inversely associated with the risk of AD (Saczynski et al., 2007). More recently, the Manhattan cognitive study with 1,130 individuals also showed that high levels of HDL cholesterol were associated with a decreased risk of both probable and possible AD (Reitz et al., 2010). Consistently, the InChianti study with 1,051 Italians older than 65 years of age reported that low HDL cholesterol levels were associated with dementia (Zuliani et al., 2010). In addition, another recent study with 664 subjects from the Sydney Memory and Aging study reported that elderly individuals with mild cognitive impairment (MCI) had abnormal plasma levels of HDL-associated apos. MCI subjects had lower levels of apoA-I, apoA-II and apoH, and higher level of apoE and apoJ. Lower apoA-I, apoA-II and apoH levels increased the risk of cognitive decline over two years. Intriguingly, among the apos, apoA-I was the most significant predictor of cognitive decline (Song et al., 2012).
Further support for a protective role of HDL in AD comes from studies in animal models. Generally, mice are not an ideal animal model for studying human lipoprotein metabolism and AD due to physiological differences between the species. However, many different lines of transgenic mouse models have been developed to better emulate relevant human physiology (Getz and Reardon, 2012; LaFerla and Green, 2012). Multiple laboratories have consistently shown that genetic and pharmacological manipulation of important players in HDL biogenesis-related pathways, such as ABCA1 and liver X receptors (LXR), modifies the development of AD-like pathology and cognitive impairment in mouse models of AD (Burns et al., 2006; Donkin et al., 2010; Fitz et al., 2010; Hirsch-Reinshagen et al., 2005; Jiang et al., 2008; Koldamova et al., 2005a; Koldamova et al., 2005b; Riddell et al., 2007; Vanmierlo et al., 2011; Wahrle et al., 2005; Wesson et al., 2011; Zelcer et al., 2007). Furthermore, genetic overexpression of human apoA-I and accompanied increase of functional HDL prevented the development of age-related cognitive deficits in the APP/PS1 mouse model of AD (Lewis et al., 2010). Consistently, lack of apoA-I exacerbated cognitive deficits in APP/PS1 mice (Lefterov et al., 2010). Intriguingly, genetic manipulation of apoA-I does not affect total brain parenchymal Aβ deposition (Fagan et al., 2004; Lefterov et al., 2010; Lewis et al., 2010) but significantly changes the dynamics of cerebrovascular Aβ deposition; apoA-I overexpression attenuates whereas apoA-I deficiency exacerbates cerebral amyloid angiopathy (CAA) in AD mice (Lefterov et al., 2010; Lewis et al., 2010). Notably, HDL deficiency could be particularly detrimental in APOE-ε4 carriers as a recent study showed that ABCA1 deficiency worsened AD-like cognitive impairment and Aβ deposition in human apoE4 but not in apoE3-targeted replacement mice (Fitz et al., 2012). In apoE4 mice, plasma HDL and Aβ levels were significantly decreased and the plasma HDL level was negatively correlated with amyloid plaques in the brain, suggesting a role of plasma HDL in Aβ clearance. Taken together, these findings provide compelling evidence that HDL and associated apolipoproteins play a pivotal role in modulating the pathogenesis of AD.
HDL and Parkinson's disease
Parkinson's disease (PD) is the second most common neurodegenerative disorder. The prevalence of PD increases steeply with age; the risk of PD is about 0.05% before 50 years and rises to 0.3%, 0.9%, and 1.8% before 60, 70, and 80 years, respectively (Elbaz et al., 2002). Although PD is primarily a movement disorder, cognitive impairment is common in PD and is associated with increased morbidity and mortality (Svenningsson et al., 2012). Pathological features of PD include loss of dopaminergic neurons and depigmentation of the substantia nigra and the presence of Lewy bodies (proteinaceous intracellular inclusions) in the brain stem (Lees et al., 2009). The main component of Lewy bodies is the protein α-synuclein. Like AD, the etiology of PD is complex and multifactorial. The reader is referred to recent reviews (Lees et al., 2009; Svenningsson et al., 2012).
The influence of apoE genotypes on PD has been extensively investigated and the results have been inconsistent. While some studies showed that the presence of APOE-ε4 or APOE-ε2 increases the risk of PD (Huang et al., 2004; Lopez et al., 2007; Pulkes et al., 2011), others showed either a negative or no relationship between apoE genotypes and PD (Gao et al., 2011; Kurz et al., 2009; Williams-Gray et al., 2009). Notably, a large study with 3,465 case and control samples from the NINDS Neurogenetics repository revealed no association between apoE and PD (Federoff et al., 2012). However, others argue that despite a lack of association of apoE genotypes with the incidence of PD, the APOE-ε4 allele modulates the progression of cognitive impairment/dementia in PD (Buchanan et al., 2007; Morley et al., 2012; Pankratz et al., 2006; Tsuang et al., 2013). These observations suggest that PD dementia and AD may share a component of etiology.
In regard to other aspects of lipoprotein metabolism, few studies have focused on HDL. Several reports indicated that PD patients have a favorable cardiometabolic risk profile but it could be a result of reduced sympathetic activity and levodopa therapy (Cereda et al., 2012; Scigliano et al., 2006; Scigliano et al., 2009). One study showed that PD patients had increased levels of HDL cholesterol but did not include age- and sex-matched normal controls (Cassani et al., 2013). Another study found that only APOE-ε3/3 homozygous PD patients have increased levels of HDL cholesterol (Gregorio et al., 2013). Intriguingly, robust association between apoA-I levels and PD has been reported. Except one small study with 8 patients (Wang et al., 2010), several studies showed that the levels of apoA-I in the CSF or in the plasma were significantly decreased in PD patients (Qiang et al., 2013; Yin et al., 2009; Zhang et al., 2012). It was demonstrated that the expression level of apoA-I was decreased particularly in the early stage of PD (Zhang et al., 2012). In the most recent study with two independent cohorts of 152 and 187 PD patients, respectively, apoA-I was found to be the most promising candidate biomarker for PD, with lower apoA-I levels corresponding to earlier age at disease onset (Qiang et al., 2013). The association between plasma apoA-I levels and age at PD onset was not affected by medications or degree of motoric impairment. In addition, in a group of 150 asymptomatic individuals at risk for PD, lower levels of plasma apoA-I were associated with greater dopaminergic deficits, whereas individuals with high dopaminergic system integrity have apoA-I levels similar to those of normal controls not at risk for PD (Qiang et al., 2013). Furthermore, in symptomatic PD patients, plasma apoA-I levels correlate with the degree of motoric impairment. These findings indicate that high apoA-I levels both protect against the development of PD and slow its progression, suggesting that apoA-I may not merely serve as a biomarker for PD but could play a direct role in its pathogenesis. Supporting this notion, the activity of paraoxonase 1 (PON1), an apoA-I/HDL associated enzyme that exerts anti-oxidative and anti-inflammatory effects (Aviram and Rosenblat, 2004), has been shown to be decreased in PD (Ikeda et al., 2011). Several studies have also reported that individuals with polymorphisms in the PON1 gene associated with decreased enzyme activity have an increased risk of developing PD (Akhmedova et al., 2001; Kondo and Yamamoto, 1998; Zintzaras and Hadjigeorgiou, 2004). It is also noteworthy that similar to apoA-I, α-synuclein adopts a unique amphipathic helical structure that mediates reversible lipid binding (Bussell and Eliezer, 2003). Since apoA-I is present in the CSF, it is possible that apoA-I and α-synuclein may interact directly in the brain. Further research in this area is warranted.
HDL and Huntington's disease
Huntington's disease (HD) is an autosomal-dominant, progressive neurodegenerative disorder. The prevalence of HD is approximately 6 per 100,000 individuals (Pringsheim et al., 2012). Patients with HD exhibit distinct symptoms, including chorea and dystonia, incoordination, cognitive decline, and behavioral difficulties (Walker, 2007). Cognitive dysfunction may be present in the early stages of the disease, even before the onset of motor symptoms. HD is a monogenetic disorder. The underlying genetic cause of HD is a trinucleotide (CAG) repeat expansion in the gene encoding a protein called huntingtin on chromosome 4 (Gusella et al., 1983). Although the cellular functions of huntingtin are still not completely understood, accumulating evidence suggest that the expanded polyglutamine segment confers a dominant toxic “gain of function” to the protein, leading to selective neuronal dysfunction and ultimately neurodegeneration (Ross and Tabrizi, 2011; Walker, 2007). HD can occur at any age. The age of onset is mainly determined by the number of CAG repeats (inverse correlation) in the huntingtin gene (Snell et al., 1993). However, other modifying genes and environmental factors have been shown to influence the age of onset for HD (Gusella and MacDonald, 2009; Wexler et al., 2004). Earlier studies indicated that the APOE-ε4 allele is associated with a delayed age of onset in HD (Kehoe et al., 1999; Panas et al., 1999), but a later study found that apoE genotypes did not influence the age of onset for the disease (Saft et al., 2004).
Intriguingly, convincing evidence indicates that cholesterol metabolism is disrupted in HD (Karasinska and Hayden, 2011; Valenza and Cattaneo, 2011). It was found that cholesterol biosynthesis was reduced in cultured human HD cells, and total cholesterol mass was significantly decreased in the brain of HD mice and in brain-derived cells expressing mutant huntingtin (Valenza et al., 2005). The primary mechanism by which mutant huntingtin compromised cellular cholesterol biosynthesis was found to be through the inhibition of sterol regulatory element binding proteins (SREBPs) (Valenza et al., 2005). Recently, cholesterol defect was demonstrated in multiple rodent models of HD and it was manifested in astrocytes (Valenza et al., 2010). Consistent with the reduction in cholesterol biosynthesis in the brain, brain cholesterol turnover, shown by the level of 24S-hydroxycholesterol, was also reduced in the brain of HD mice (Valenza et al., 2010). Furthermore, the PREDICT-HD study showed that the plasma level of 24S-hydroxycholesterol was decreased in HD patients and correlated with the progression of the disease (Leoni et al., 2013). These findings suggest that modulation of cholesterol metabolism could be a potential therapeutic strategy for HD. Whether HDL-enhancing therapies provide benefits in HD has not been investigated. Interestingly, sirtuins (SIRT) are potential therapeutic targets for HD. Some evidence indicates that SIRT1 protects against whereas SIRT2 promotes neurodegeneration (Donmez and Outeiro, 2013). Resveratrol, a natural SIRT1 activator, provided neuroprotection in multiple models of HD (Maher et al., 2011; Pasinetti et al., 2011). Although not without controversy (Escola-Gil et al., 2013), resveratrol treatment has been shown to increase HDL cholesterol levels likely through activation of SIRT1, which upregulates the LXR signaling pathway and increases RCT (Li et al., 2007; Ramprasath and Jones, 2010). Also, genetic variations of SIRT1 have been found to be associated with increased HDL levels (Inamori et al., 2013). Thus, HDL-enhancing therapies could potentially help alleviate HD.
HDL and amyotrophic lateral sclerosis
Amyotrophic lateral sclerosis (ALS) is also known as Lou Gehrig's disease. ALS is the most common form of motor neuron disorders. The prevalence is approximately 6 per 100,000 persons (Mitchell and Borasio, 2007). ALS affects both men and women (ratio of males to females 1.6:1), and the risk of disease increases with age. The clinical features of ALS include weakness of the limbs and face as well as difficulties with speech, swallowing, and breathing. In the past, it was thought that cognitive function is spared in ALS; however, recent research indicates that cognitive impairment occurs in a substantial group of patients with ALS, some reaching criteria for diagnosis of frontotemporal dementia (FTD) (Goldstein and Abrahams, 2013). The clinical progression of ALS is one of the fastest of the neurodegenerative diseases, with death (often from respiratory failure) typically occurring within 3 to 5 years after onset.
Approximately 90% of ALS cases are sporadic and 10% are familial forms, resulting from highly penetrant, monogenic mutations that cause the disease. Mutations in multiple genes have been identified to be causal (recently reviewed in (Chen et al., 2013)). The major ones include superoxide dismutase 1 (SOD1), C9ORF72, and TAR DNA-Binding Protein (TARDBP). SOD1 was the first gene discovered to be linked to ALS; mutations in SOD1 account for 20% of familial and 1-4% of sporadic cases (Chen et al., 2013). Hexanucleotide repeat expansions in C9ORF72 have recently become the most common genetic cause for ALS, accounting for 41% of familial and 5% of sporadic cases (Byrne et al., 2012; DeJesus-Hernandez et al., 2011). Mutations in TARDBP occur in 4-5% of familial and 2% of sporadic cases, leading to the formation of pathological, ubiquitinated TARDBP protein, TDP-43 (Sreedharan et al., 2008). Intracellular inclusions containing TDP-43 are found not only in ALS but also in other neurodegenerative disorders including FTD, AD, PD, and HD.
The cause for sporadic ALS is largely unknown. In addition to the aforementioned genetic mutations, a number of other genetic mutations and polymorphisms have been identified to be associated with sporadic ALS (Chen et al., 2013). Studies on the influence of apoE genotype on ALS have provided conflicting results (Jawaid et al., 2011; Praline et al., 2011). A recent study in a large cohort of French patients with sporadic ALS (n=1,482) and matched controls (n=955) found that APOE-ε4 allele was associated with an increased risk of bulbar-onset ALS in men (Praline et al., 2011). In regard to plasma HDL, one particularly relevant gene is paraoxonase (PON). In a large North American Caucasian family-based and case-control cohort (n = 1,891), variants in the PON gene cluster were significantly associated with sporadic ALS (Saeed et al., 2006). Another study examined 20 single nucleotide polymorphisms (SNPs) across the PON gene cluster in a group of patients from France (480 cases, 475 controls), Quebec (159 cases, 95 controls), and Sweden (558 cases, 506 controls) (Valdmanis et al., 2008). Consistently, the results showed that a haplotype of SNPs rather than individual SNPs was significantly associated with sporadic ALS in these populations. Furthermore, in genomic DNA from individuals with familial and sporadic ALS, seven mutations in the PON gene were identified which are predicted to affect PON function (Ticozzi et al., 2010). These findings underscore the importance of PON function in the development of ALS, implicating that enhancement of functional HDL may help mitigate ALS. However, contrary to the studies described above, a recent study in the Dutch population did not find significant differences in mutational burden for rare variants or in allele frequencies of polymorphisms in PON between ALS patients and control subjects (van Blitterswijk et al., 2012). Thus, it is possible that PON may have population-specific effects on ALS. Since oxidative stress plays a central role in the pathogenic process of sporadic ALS (D'Amico et al., 2013), anti-oxidative measures could effectively modulate the progression of the disease. Further studies on the relationship between PON and ALS are warranted as it may lead to a potential PON/HDL therapy for ALS.
Several studies have also investigated the relationship between plasma lipid levels and survival of patients with ALS. However, the results are not consistent. One study showed that patients with ALS had high cholesterol levels, particularly a high LDL/HDL ratio, compared to the controls (Dupuis et al., 2008), whereas another study reported that patients with ALS had a lower LDL/HDL ratio (Sutedja et al., 2011). Interestingly, both studies showed that within the ALS group, a higher LDL/HDL ratio correlated with increased survival. In contrast, two recent studies found no significant association of hyperlipidemia with survival in sporadic ALS patients (Dedic et al., 2012; Paganoni et al., 2011). Clearly, further research is needed to clarify the relationship between plasma lipid levels and the status of ALS.
Potential mechanisms by which HDL modulates cognitive function
Although the evidence for the protective role of HDL in cognition is substantial, the underlying mechanisms by which HDL modulates cognitive function are poorly understood. Clearly, multiple functions of HDL are involved under different conditions. To simplify the discussion, AD is used to illustrate potential mechanisms of action for apoA-I to modulate the disease process (Fig. 3). Since the systemic effects of HDL are well established (Davidson and Toth, 2007) and the cerebrovascular function of HDL in AD has been summarized recently by an excellent review (Stukas et al., 2014), this section focuses on the potential direct role of apoA-I in the brain.
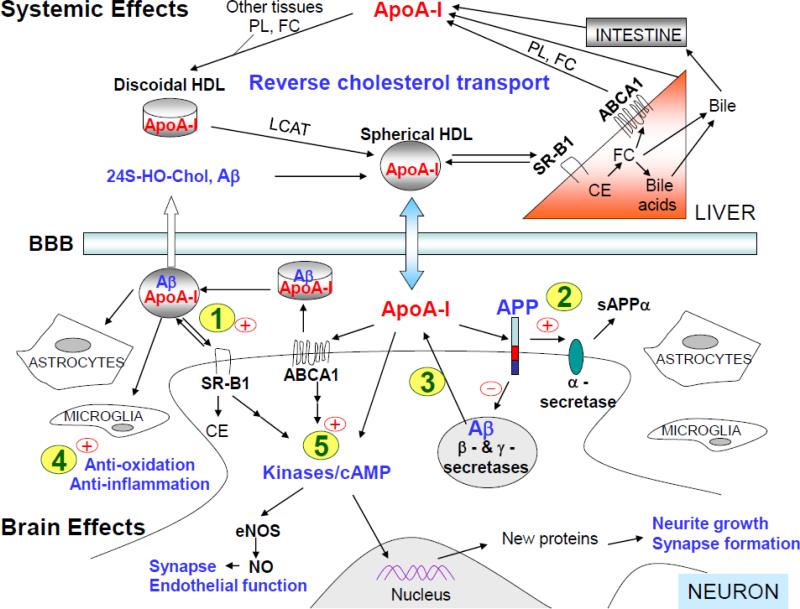
Fig. 3. Schematic of potential mechanisms by which apoA-I/HDL modulates the disease process related to AD.
ApoA-I is hypothesized to act on five major pathways to exert its neuroprotective effects pertinent to AD. (1) Cholesterol efflux pathway: ApoA-I in the brain promotes the cellular cholesterol efflux through ABCA1 and forms HDL-like particles. These particles are cleared by interacting with receptors such as SR-B1 by cells in the brain or through the BBB to peripheral circulation. (2) APP trafficking and processing pathway: ApoA-I-mediated increase in membrane fluidity may enhance α-secretase cleavage of APP at the cell membrane. Also, apoA-I binds to the extracellular domain of APP, which may prevent APP from undergoing the endocytic process, thereby inhibiting the access of β- and γ-secretases to APP and reducing the generation of Aβ. (3) Aβ clearance pathway: ApoA-I binds to Aβ and inhibits Aβ aggregation. ApoA-I/HDL in the brain can mediate the clearance of Aβ by local cells (e.g., astrocytes and microglia) through the scavenger receptor (e.g., SR-B1) and/or by crossing the BBB to the systemic circulation. (4) Anti-oxidation and anti-inflammation: ApoA-I/HDL possesses anti-oxidant and anti-inflammatory properties that are neuroprotective. (5) Signal transduction and synaptic plasticity: ApoA-I/HDL activates several kinases and increases the level of cAMP directly or indirectly through ABCA1 or SR-B1. These molecules play important roles in signaling pathways pertinent to synaptic function and memory formation. ABC, ATP-binding cassette transporter; ApoA-I, apolipoprotein A-I; Aβ, amyloid-β protein; APP, amyloid-β precursor protein; BBB, blood-brain barrier; cAMP, cyclic AMP; CE, cholesterol esters; eNOS, endothelial nitric oxide synthase; FC, unesterified free cholesterol; HDL, high-density lipoproteins; LCAT, lecithin cholesterol acyltransferase; PL, phospholipids; NO, nitric oxide; sAPPα, soluble N-terminal fragment of APP produced by α-secretase cleavage; 24S-HO-Chol, 24S-hydroxycholesterol; SR-B1, scavenger receptor B1.
Cholesterol efflux pathway
It has been shown in vitro and in vivo that, as in the periphery, apoA-I in the brain promotes the cellular cholesterol efflux through ABCA1 and forms discoidal HDL-like particles (Ito et al., 1999; Wahrle et al., 2004). With the activation of LCAT by apoA-I, FC is converted to CE, resulting in the formation of spheroidal HDL-like particles. These particles are cleared by interacting with receptors such as SR-B1 by cells in the brain or through the BBB to peripheral circulation (Panzenboeck et al., 2002). They also function to deliver cholesterol to sites for growth or healing (Kay et al., 2003). While it is true that most apolipoproteins can act as cholesterol acceptors in ABCA1-mediated cholesterol efflux, they exhibit differential efficacy and produce particles with distinct properties (Ito et al., 1999). It has been shown that apoA-I in the CSF is more efficient than apoE for mediating cholesterol efflux (Demeester et al., 2000).
APP trafficking and processing pathway
APP trafficking and processing are modulated by a number of mechanisms (Cam and Bu, 2006; Haass et al., 2012; Small and Gandy, 2006). One of the mechanisms is cell membrane fluidity, regulated mainly by the cholesterol content. While the non-amyloidogenic cleavage of APP by α-secretase occurs in cholesterol-poor and phospholipid-rich domains, the amyloidogenic cleavages by β- and γ-secretases are preferred in the cholesterol-rich domains (lipid rafts) (Wolozin, 2001). Another controlling mechanism for APP processing is the distinct localization of secretases. The α-secretase activity is located primarily at the cell surface, whereas β- and γ-secretase activities are found mainly in membranous compartments (e.g., endosomes) inside the cell (Cam and Bu, 2006; Haass et al., 2012; Small and Gandy, 2006). Therefore, apoA-I/HDL in the brain may affect the APP processing pathways through both of the following mechanisms: a) apoA-I mediates efficient cellular cholesterol efflux (Demeester et al., 2000); the resultant increase in membrane fluidity could enhance α-secretase cleavage of APP at the cell membrane and b) apoA-I binds to APP at the cell surface (Koldamova et al., 2001); thereby it may prevent APP from undergoing the endocytic process, which is necessary for β- and γ-secretases to access APP. Thus, the final consequence of these effects would be reduced generation of Aβ.
Aβ clearance pathway
Overproduction of Aβ in the brain causes familial AD, but impaired Aβ clearance from the brain is implicated in sporadic AD (Castellano et al., 2011; Mawuenyega et al., 2010; Scheuner et al., 1996). ApoA-I binds to Aβ and inhibits Aβ aggregation and cytotoxicity in vitro (Koldamova et al., 2001). In addition, the binding affinity of human apoA-I for Aβ is higher than that of human apoE (Koldamova et al., 2001). Therefore, the apoA-I/HDL in the brain is expected to be more effective in binding Aβ and mediates the clearance of Aβ by local cells (e.g., astrocytes and microglia) through the scavenger receptor (e.g., SR-B1) and/or by crossing the BBB to the systemic circulation (Sagare et al., 2012). Supporting this notion, studies in AD mice have demonstrated that lack of apoA-I exacerbates whereas overexpression of human A-I ameliorates cerebrovascular deposition of Aβ (Lefterov et al., 2010; Lewis et al., 2010). Additionally, a recent study has shown that apoE has minimal direct interaction with Aβ and competes with Aβ for the same clearance pathways within the brain (Verghese et al., 2013). These intriguing results suggest that upregulation of apoA-I and/or inhibition of apoE competition with Aβ for cellular uptake in the brain might be an effective means to enhance Aβ clearance.
Anti-oxidation and anti-inflammation
Oxidative stress and inflammation contribute to the etiology of AD (Keeney et al., 2013; Schrag et al., 2013; Wyss-Coray and Rogers, 2012). Anti-oxidant and anti-inflammatory properties of apoA-I/HDL have been shown to play significant roles in protecting against cardiovascular disease (Barter et al., 2004). These same mechanisms may play a significant role in neuroprotection. Previous studies support this hypothesis: a) the level of CSF apoA-I is increased significantly after infection in macaques (Saito et al., 1997); b) CSF apoA-I-containing lipoproteins remodel after traumatic brain injury in humans (Kay et al., 2003); c) reconstituted human apoA-I-containing HDL reduces neuronal damage in rat models of stroke, via an anti-oxidative mechanism (Paterno et al., 2004); d) an apoA-I mimetic peptide inhibits inflammation in the brain and improves cognitive performance in mice (Buga et al., 2006; Handattu et al., 2009); and e) overexpression of human apoA-I attenuates neuroinflammation in AD mice (Lewis et al., 2010).
Signal transduction and synaptic plasticity
Aβ-induced synaptic dysfunction is thought to be the underlying cause for cognitive impairment in AD (Selkoe, 2002). ApoA-I/HDL has been shown to activate several kinases (e.g. PKA, PKC, PI3K, MAPK, and Akt) and increase the level of cAMP directly or indirectly through ABCA1 or SR-B1 in peripheral cells and in astrocytes (Haidar et al., 2004; Ito et al., 2004; Mineo et al., 2003; Yamauchi et al., 2003). These molecules play important roles in signaling pathways pertinent to synaptic function and memory formation. ApoA-I may directly modulate synaptic plasticity through interactions with these signaling molecules.
HDL-Enhancing Pharmacotherapies to Improve Cognitive Function
As discussed in previous sections, compelling evidence indicates that functional HDL is crucial for the protection of cardiovascular, cerebrovascular, and cognitive functions. Thus, therapeutic approaches that enhance HDL functions will benefit both peripheral and central nervous systems. Although exercise, diet and other lifestyle measures are the most favorable ways to raise HDL levels, adherence to these measures might be difficult to achieve. Furthermore, there are genetic conditions in which lifestyle change alone may not be sufficient to modulate the level and function of HDL. In these scenarios, therapeutic intervention is needed. This section summarizes HDL-enhancing pharmacotherapies currently available or under investigation.
Niacin and niacin receptor agonists
Niacin, also known as vitamin B3 or nicotinic acid, is an important precursor for the coenzymes NAD and NADP, which are essential for proper tissue catabolism and anabolism. GPR109A (PUMA-G/HM74A) was identified as the receptor for niacin (Tunaru et al., 2003). GRP109A is a G-protein coupled receptor expressed in adipocytes, spleen, and immune cells. When activated, GRP109A reduces intracellular cAMP and inhibits lipolysis.
Niacin has been used for over 50 years to raise HDL-C levels (Carlson, 2005). At present, niacin is the most effective HDL-raising agent available clinically. It also lowers the level of TG, lipoprotein(a), and LDL-C (Toth et al., 2013). A recent clinical trial (AIM-HIGH) showed that in patients with cardiovascular disease and low HDL-C levels, treatment with extended-release niacin, 1500 to 2000 mg per day, significantly increased HDL-C (25%) while decreasing TG (29%) and LDL-C (16%) (Investigators et al., 2011). Further analysis also showed that niacin treatment modestly increased apoA-1 (7%), decreased apoB (13%), decreased the apoB/apoA-I ratio (19%), and decreased Lipoprotein(a) (21%) (Albers et al., 2013). However, these favorable changes in lipoprotein profiles did not lead to the reduction of cardiovascular events. It is worth noting that the patients in this trial were receiving intensive statin therapy and their baseline LDL-C was very low (74 mg/dL) (Investigators, 2011). Thus, it is possible that no additional benefits from niacin treatment can be achieved in patients with very low LDL-C levels. This possibility is supported by another recent clinical trial (HPS2-THRIVE) (Group, 2013). In this study, participants were treated with extended-release niacin combined with laropiprant, a prostaglandin-D2 receptor-1 inhibitor, to alleviate niacin-induced facial flushing. Subgroup analysis from this study showed that in participants with LDL-C lower than 78mg/dL no benefit was found with niacin/laropiprant treatment, but in participants with LDL-C higher than 78 mg/dL benefit was observed with the treatment (Group, 2013). It is worth noting that the formulation of niacin influences the side effects observed. Standard immediate-release niacin causes a high frequency of flushing and long-acting niacin causes less flushing but increases the risk of hepatotoxicity, whereas extended-release niacin causes fewer of both types of adverse effects (McKenney, 2003). Thus, a proper formulation of niacin should be selected to reduce potential side effects.
In addition to niacin, synthetic GRP109A agonists, such as MK-1903, have been developed. MK-1903 has been evaluated in phase I and II studies to treat dyslipidemia. MK-1903 treatment produced a significant decrease in plasma free fatty acids. However, MK-1903 had a smaller effect on serum lipid levels compared with niacin, suggesting that niacin may act on a GRP109A-independent pathway (Boatman et al., 2012). Further studies in animal models and humans confirmed that GPR109A receptor does not mediate niacin's lipid efficacy (Lauring et al., 2012), opening the door for identifying new molecular target(s) of niacin and developing novel approaches to raise HDL.
PPARα agonists – Fibrates
There are four commonly prescribed drugs in the fibrate family: bezafibrate, ciprofibrate, gemfibrozil, and fenofibrate. Fibrates mainly work by activating the peroxisome proliferator-activated receptor α (PPARα). Activation of PPARα induces the transcription of genes that promote lipoprotein lipolysis, decrease TG production, facilitate LDL clearance, reduce CE and TG exchange between VLDL and HDL, and increase HDL/apoA-I production (Staels et al., 1998). Thus, fibrates are used in patients with low HDL-C or high TG levels. However, mixed results have been reported from clinical trials with fibrates for cardiovascular diseases (reviewed in (Toth et al., 2013)). Post hoc analyses of multiple trials suggest that fibrates produce significant benefits only in subgroups of patients with low HDL-C and high TG levels. Interestingly, in a group of 22 elderly hypertriglyceridemic patients, 600mg of gemfibrozil daily resulted in a significant decrease in serum TG levels. Patients treated with gemfibrozil maintained better cerebral perfusion and scored better on cognitive performance measures than untreated controls (Rogers et al., 1989). Cognitive benefits of fibrates needs to be confirmed in further clinical studies.
In addition, fibrates are commonly used in combination therapy with stains. It is important to note that the combination of gemfibrozil and statin significantly increases the risk of rhabdomyolysis (Pierce et al., 1990; Staffa et al., 2002), due to partial inhibition of gemfibrozil on the metabolism of statins (Prueksaritanont et al., 2002). In contrast to gemfibrozil, fenofibrate does not increase the concentrations of statins (Bergman et al., 2004). The combination of fenofibrate and statin has been used in large, long-term clinical trials and there was no evidence for an increased risk of myositis or rhabdomyolysis compared to statin monotherapy (Farnier et al., 2011; Group et al., 2010). A recent meta-analysis on the safety of the coadministration of statin with fenofibrate also concluded that statin-fenofibrate combination therapy was tolerated as well as statin monotherapy (Guo et al., 2012).
ApoA-I infusion
The strong negative correlation between plasma apoA-I levels and cardiovascular disease and consistent experimental results in animal models have led to direct infusion of apoA-I in human clinical trials. Nissen et al. infused human patients with a recombinant apoA-IMilano, a form of apoA-I that is associated with lower risk for cardiovascular disease (Franceschini et al., 1980), and observed a significant regression of coronary atherosclerosis (Nissen et al., 2003). Another group performed a randomized human trial to test the infusion of apoA-I incorporated into recombinant HDL (rHDL). The study determined that short-term infusion of rHDL produced a significant reduction of atheroma volume and improved plaque characterization index and coronary score (Tardif et al., 2007). More evidence of atheroprotection was obtained with the infusion of apoA-I or apoA-IMilano in both animal models and human clinical trials. Remarkably, a single infusion was shown to be enough to significantly reduce atherosclerosis and infer positive effects on plaque characterization (Tardif, 2010). Additionally, researchers infused rabbits with either lipid free apoA-I or apoA-I in rHDL. The infusions markedly inhibited vascular inflammation in the rabbits (Patel et al., 2010). A recent study found that infusion of apoA-I produced an increase in cholesterol efflux from macrophages, favorably remodeled HDL and reduced cytokine secretion in both rabbits and human blood (Diditchenko et al., 2013).
Whether apoA-I infusion has any effect on cognition has not been investigated. As discussed in previous sections, low levels of apoA-I have been associated with poor cognitive function in aging and in neurodegenerative diseases. Experimentally, apoA-I overexpression in the periphery was shown to reduce neuroinflammation, attenuate cerebral amyloid angiopathy and inhibit cognitive decline in a mouse model of AD (Lewis et al., 2010). Thus, a beneficial effect of apoA-I infusion on cognitive function is an intriguing possibility, which merits further research.
ApoA-I/HDL mimetic peptides
A major obstacle in the path of using native apoA-I as a therapeutic is its lack of oral bioavailability. Additional concern stems from the high cost and relative difficulty of mass-producing full-length apoA-I. Thus, orally bioavailable small peptides, which retain the atheroprotective effects of apoA-I, have been developed. These small peptides are described as apoA-I mimetics. The general design of the apoA-I mimetics is an amphipathic peptide, which adopts an alpha helical secondary structure similar to that seen in the full-length apoA-I (Segrest et al., 1992). Mimetics can be synthesized from D-amino acids and thus have higher oral bioavailability. Of note, in addition to apoA-I mimetics, peptides derived from other HDL-associated apos including apoE and apoJ have also been created. Readers interested in gaining a more comprehensive understanding of HDL mimetic peptides are encouraged to refer to excellent recent reviews (Getz et al., 2010; Leman et al., 2013).
Research into the development of apoA-I mimetic peptides began in an effort to design therapeutics for atherosclerosis. In line with that goal, a number of mimetic peptides were created and tested for therapeutic benefit in mice and cell culture. In order to preserve lipid-binding and anti-atherosclerotic activity, an 18 amino acid peptide was designed with structural but not sequence homology to apoA-I. The peptide, called 18A, formed an amphipathic alpha helical secondary structure and was shown to have a similar lipid-binding capacity as full-length apoA-I (Anantharamaiah et al., 1985).
Modifications were made to 18A, wherein a number of non-polar residues were replaced with phenylalanines (F) in an attempt to bolster its atheroprotective affects, and the most successful of these modified peptides was called 4F (Datta et al., 2001). The oral bioavailability of 4F in the plasma was quite low; and thus its enantiomer, D-4F, was created and shown to remain in the plasma for much longer after oral gavage (Navab et al., 2005a). The atheroprotective and anti-inflammatory efficacy of 4F has been described in vitro, in animal models and in human clinical trials. D-4F has been shown to inhibit atherosclerotic lesion development and also to reduce inflammation in mice and rabbits (Navab et al., 2005a; Van Lenten et al., 2007). The mimetic peptide remodels HDL into lipid-poor HDL, promotes RCT, induces functional changes in macrophage activity, and reduces lipid oxidation in vascular plaques (Navab et al., 2005a; Smythies et al., 2010). Furthermore, a single dose of D-4F was well tolerated and improved the HDL anti-inflammatory profile of human patients with cardiovascular disease (Bloedon et al., 2008). These data make further studies on D4F a particularly intriguing objective.
Due to the known correlation between vascular risk factors and cognitive decline, HDL mimetic peptides have been tested for efficacy in improving mental health. In fact, D-4F has been shown to have effects on cognitive capacity. In LDL receptor-null mice, D-4F was shown to reduce inflammation in the vasculature of the brain and improve cognitive performance without influencing plasma lipid levels (Buga et al., 2006). Additionally, D-4F in combination with pravastatin was shown to inhibit Aβ plaque formation and improve cognitive function by inducing an anti-inflammatory effect in the brain without affecting plasma HDL-C levels (Handattu et al., 2009), suggesting that D-4F improves the quality not the quantity of HDL and/or directly modulates disease-related processes in the brain. Further studies on 4F and other HDL mimetic peptides are needed to illuminate their potential use as therapeutics in neurological disorders.
CETP inhibitors
Based on several lines of evidence that CETP deficiency/inhibition is associated with an elevated level of HDL and a decreased risk for cardiovascular disease, CETP inhibitors have been developed and tested in clinical trials. Torcetrapib was the first CETP inhibitor tested. In ILLUMINATE trial (Barter et al., 2007), torcetrapib significantly increased the level of HDL-C in treated patients but failed to show a clinical benefit. In fact, torcetrapib was associated with an increase in cardiovascular events due to unexpected off-target adverse effects resulting in hypertension. Dalcetrapib was the second CETP inhibitor to undergo clinical trials. In the dal-OUTCOMES trial (Schwartz et al., 2012), dalcetrapib successfully increased HDL-C levels but did not reduce recurrent cardiovascular events. Dalcetrapib was safe and the reason for its failure is not clear. It has been suggested that dalcetrapib-induced increase in HDL-C levels might not have been sufficient or it was not accompanied by an enhancement of the protective properties of HDL (Rader and deGoma, 2014; Toth et al., 2013). Two new CETP inhibitors, anacetrapib and evacetrapib, are much more potent than dalcetrapib and do not have the off-target adverse effects of torcetrapib (Gotto et al., 2014; Nicholls et al., 2011). Outcomes from ongoing large clinical trials with anacetrapib (ClinicalTrials.gov number NCT01252953) and evacetrapib (ClinicalTrials.gov number NCT01687998) are eagerly awaited with high expectations.
Reverse cholesterol transport (RCT) enhancers - Liver X receptor agonists and retinoid X receptor agonists
As RCT is thought to be the most relevant cardioprotective mechanism mediated by HDL, much effort has been made to develop agents that promote RCT. Liver X receptors (LXRα and LXRß) are oxysterol activated nuclear receptors. Together with retinoid X receptors (RXRs), LXRs regulate the expression of a variety of target genes that control lipid and glucose homeostasis, steroidogenesis and inflammatory responses. Activation of LXRs has been shown to promote RCT though ABCA1 and ABCG1 and increase intestinal HDL generation (Brunham et al., 2006; Costet et al., 2000).
Several synthetic LXR agonists, including T0901317, GW3965 and LXR-623, are currently undergoing experimental testing for the treatment of dyslipidemia and atherosclerosis. Recently, accumulating preclinical evidence indicates the therapeutic potential of LXR agonists for AD. Studies in multiple laboratories have shown that LXR agonists improve cognitive functions either with or without reducing Aβ levels in the brain of AD mice (Donkin et al., 2010; Fitz et al., 2010; Jiang et al., 2008; Riddell et al., 2007; Vanmierlo et al., 2011; Wesson et al., 2011). Specifically, in the APP23 mouse model of AD, T0901317 treatment ameliorated amyloid pathology and memory deficits (Fitz et al., 2010). It was shown that T0901317 treatment resulted in a decrease in Aβ levels in the interstitial fluid of the hippocampus, supporting the role of LXR agonists in facilitating Aβ clearance. In vitro experiments demonstrated that ABCA1 was essential for lipidation of apoE and mediated the effects of T0901317 on Aβ degradation by microglia (Fitz et al., 2010). The specific role of ABCA1 in mediating benefits of LXR agonists in AD mice was further confirmed by another study with GW3965 in the APP/PS1 mouse model of AD (Donkin et al., 2010). These findings indicate that LXR agonists exert neurological benefits through the ABCA1/apoE-HDL pathway. Interestingly, a recent study showed that GW3965 treatment dramatically increased the level of apoA-I in the brain of APP/PS1 mice independent of ABCA1 (Stukas et al., 2012). Therefore, increase of apoA-I/HDL may also contribute to the beneficial effects of LXR agonists in AD mice.
In addition to LXR agonists, emerging evidence indicates that RXR agonists may also possess the therapeutic potential for AD. In a highly publicized report, acute treatment with a RXR agonist, bexarotene, a drug currently approved for the treatment of cutaneous T-cell lymphoma, rapidly and dramatically decreased Aβ levels/plaques in the brain of AD mice (Cramer et al., 2012). Bexarotene treatment lowered soluble Aβ levels in mouse interstitial fluid by 25% within 24 hours and reduced Aβ plague area by more than 50% within 72 hours. It was shown that bexarotene increased Aβ clearance via an apoE-dependent mechanism as the treatment promoted the expression of apoE, ABCA1, and ABCG1 in the brain. Remarkably, bexarotene rescued cognitive function in a mouse model of AD after as few as 7 days of treatment (Cramer et al., 2012). However, the effectiveness of bexarotene in AD mice has been questioned by subsequent studies as the reduction of Aβ plaques in treated mice could not be reproduced (Fitz et al., 2013; Price et al., 2013; Tesseur et al., 2013; Veeraraghavalu et al., 2013). The discrepancy observed in these studies might result from differences in drug formulations and mouse models (Landreth et al., 2013). Nevertheless, some studies replicated the decrease in soluble Aβ levels (Fitz et al., 2013; Veeraraghavalu et al., 2013) and the improvement of cognitive function (Fitz et al., 2013; Tesseur et al., 2013) in bexarotene-treated AD mice. Importantly, bexarotene increased Aβ clearance and rescued cognitive function in APP/PS1 mice expressing either human apoE3 or apoE4 isoform (Fitz et al., 2013). In contrast, a more recent study did not find any changes in Aβ plaques or cognitive deficits in bexarotene-treated APP/PS1 mice (LaClair et al., 2013). Thus, further studies are required to clarify the effects of bexarotene on AD-related processes.
ApoA-I upregulators – RVX-208
RVX-208 is a novel small molecule that stimulates apoA-I gene expression leading to an increase in HDL levels and functionality (Bailey et al., 2010). Recent studies showed that RVX-208 is a specific inhibitor for BET bromodomains that regulate expression of a variety of genes including apoA-I (McLure et al., 2013; Picaud et al., 2013). Early testing in African green monkeys demonstrated that a 63-day RVX-208 treatment markedly increased serum apoA-I (60%) and HDL-C levels (97%), accompanied by the enhancement of ABCA1, ABCG1, and SR-B1-mediated cholesterol efflux (Bailey et al., 2010).
Positive results from animal models and early human clinical trials have led to further human clinical trials. The Phase IIb SUSTAIN trial (NCT01423188) was designed to evaluate the lipid efficacy, safety and tolerability of RVX-208, and the ASSURE trial (NCT01067820) was designed to evaluate the effect of RVX-208 on atherosclerotic plaque burden using intravascular ultrasound (IVUS) imaging (Nicholls et al., 2012). Findings from these clinical trials suggest that RVX-208 has the potential for the treatment of cardiovascular disease.
In addition, RVX-208 may also have the therapeutic potential for diabetes and AD. A Phase II clinical trial of RVX-208 in pre-diabetic patients is ongoing (NCT01728467). On AD, a pilot Phase Ia study showed a trend of increase in the level of Aβ40 in the plasma of patients treated with RVX-208 for 7 days compared to controls (Resverlogix 2008) (http://www.resverlogix.com/upload/latest_news/81/01/2008-11-10_alzheimers_program_final.pdf). This preliminary result was confirmed in a more recent Phase II ASSERT trial, in which 12-week treatment with RVX-208 significantly increased the plasma Aβ40 level compared to baseline or the level of placebo-treated controls (Resverlogix 2011) (http://www.resverlogix.com/media/press-release.html?id=451). These intriguing observations are consistent with the findings from genetic upregulation of apoA-I in AD mice (Lewis et al., 2010) and support the notion that elevating apoA-I levels in the systemic circulation enhances Aβ clearance from the brain. Further clinical trials of RXV-208 in AD or pre-AD patients will be needed to determine whether RVX-208 can modulate the progression of AD.
Herbal medicine
In traditional herbal medicine, numerous plants have been used to treat age-related cognitive disorders. The exploration of modern medical science into the detailed mechanism has found that the natural products, as individual phytochemicals (e.g. galantamine from Galanthus woronowii) or as plant extracts (e.g. EGb761 from leaf extract of Ginkgo biloba), are potentially neuroprotective and may improve cognition. The successful development of two cholinesterase (ChE) inhibitors (galantamine and rivastigmine) for dementia from natural products has led to extensive research on other plant-derived drugs. Plants and their constituents with pharmacological activities relevant to the treatment of cognitive disorders, including anticholinesterase (anti-ChE), anti-inflammatory, anti-oxidant and estrogenic effects, have recently been reviewed (Perry and Howes, 2011). Listed in this section are the plants and their active constituents that have been used in traditional practices of medicine for their reputed cognition-enhancing effects through potential HDL-related mechanisms (Table1). Apparently, a variety of plants can modulate HDL metabolism and function via multiple mechanisms, including activation of LXR/RXR and PPAR, increase in ABCA1 and apoA-I expression, and regulation of PON1 activity. Therefore, these phytochemicals and plant extracts offer an alternative and potentially effective approach to enhance both HDL and cognitive function.
Table 1.
Herbal medicines that enhance cognition through HDL-related mechanisms
| Compound | Plant | Mechanism of action | Model System | Reference |
|---|---|---|---|---|
| Purified phytochemical | ||||
| Curcumin | Rhizoma zingibers | ↑ LXR-β/RXR-α ↑ ABCA1 and apoA-I |
Chronic cerebral hypoperfusion rats | (Tian et al., 2013) |
| Berberine | Coptis chinensis Franch | ↓ Aβ deposition, gliosis, and cognitive impairment | AD mice | (Durairajan et al., 2012) |
| ↑ LXRα and PPARα ↓ SREBPs |
Diabetic hamster | (Liu et al., 2010) | ||
| ↑ LXRα-ABCAI-dependent cholesterol efflux | Macrophages | (Lee et al., 2010) | ||
| Glabridin | Glycyrrhiza glabra (licorice) | ↓ Brain cholinesterase activity | Scopolamine induced amnesia mice | (Cui et al., 2008) |
| Preserve learning and memory | Diabetic rats | (Hasanein, 2011) | ||
| Bind and protect PON1 activity | In vitro modeling | (Atrahimovich et al., 2012) | ||
| Interact with PON1 hydrophobic groove | In vitro modeling | (Atrahimovich et al., 2013) | ||
| Crocin | Crocus sativus and Gardenia jasminoides | ↑ HDL ↓ Learning deficits |
Diabetic and chronic cerebral hypoperfusion rats | (Shirali et al., 2013; Tashakori-Sabzevar et al., 2013) |
| Plant extract | ||||
| EGb761 | Ginkgo biloba | ↑ HDL-C ↓ Lipid peroxidation |
Ethanol-fed rats | (Yao et al., 2007) |
| ↑ ABCA1 stability ↓ Foam cell formation |
Macrophages | (Tsai et al., 2010) | ||
| ↑ Cognition | Human clinical trial | (Herrschaft et al., 2012; Weinmann et al., 2010) | ||
| Ginseng | Panax ginseng | Dual FXR/LXRα agonist | Hyperlipidemic rats | (Ji and Gong, 2007) |
| PPARα modulation | Mice | (Yoon et al., 2003) | ||
| ↑ HDL ↓ TC, TG, LDL, MDA |
Humans | (Kim and Park, 2003) | ||
| Sage weed extract | Salvia officinalis and S. lavandulifolia | ↑ Memory | AD patients clinical trial | (Akhondzadeh et al., 2003) |
| ↑ ABCA1 and ABCG1 | Lipid-laden foam cells | (Park et al., 2012) | ||
| Lemon balm extract | Melissa officinalis | ↑ PPARs | Insulin-resistant high-fat diet-fed C57BL/6 mice | (Weidner et al., 2013) |
Concluding Remarks/Perspectives
In the past 20 years, much progress has been made on understanding the symptoms, etiology and pathogenic mechanisms of AD, PD, HD, and ALS. However, to date there is no effective prevention or treatment for these debilitating diseases. It is clear that cognitive impairment occurs in all of these disorders. Compelling evidence suggests that HDL could be a converging target for developing therapeutic strategies to mitigate cognitive deficits in these devastating disorders. However, several important issues need to be addressed. First, the level of plasma HDL-C does not always represent the level and function of HDL. The concentration of apoA-I is a more accurate measurement of HDL levels. Second, not all HDL is equal. It has been shown that HDL can be anti-inflammatory or pro-inflammatory (Navab et al., 2005b). This may explain some of the discrepancies regarding the association of HDL levels with disease status in clinical studies. Thus, in addition to the quantity of HDL, a reliable and practical assay needs to be developed to measure the quality of HDL. Third, it is not clear if apoA-I has to be present in the brain to exert beneficial effects. Further studies are needed to dissect systemic and local effects of apoA-I/HDL on cognitive function. Lastly, the exact mechanism of action for HDL to modulate cognitive function has not been elucidated. Since HDL is a modifiable target, more studies are urgently needed in this regard. A small increase in functional HDL levels may have a profound capacity to prevent, delay and/or halt the progression of the diseases.
Our society is aging at an unprecedented pace, mainly due to longer life spans and the aging of the baby boomer generation. Aging itself remains the strongest risk factor for all of the most prevalent chronic diseases including neurodegenerative disorders. Thus, the increasingly aged population will inevitably have a large impact on health care systems and national economies along with emotional and financial burden on the patients and their families. Consequently, therapeutic interventions aimed to increase the quality of life at advanced age are in high demand, both at the level of individuals and society. Safe and effective HDL-enhancing therapies may fulfil this demand.
Highlights.
Multifunctional HDL particles are formed both in the periphery and in the brain.
HDL metabolism pathways modulate the progress of neurodegenerative disorders.
Elevated levels of HDL preserve cognition in aging and neurodegenerative disorders.
HDL-enhancing therapies may be applied to improve cognitive function.
Acknowledgements
This work was supported in part by grants from the National Institutes of Health (AG031846), the Alzheimer's Drug Discovery Foundation (#20131002), and the College of Pharmacy (Engebretson/Bighley Drug Design and Development Program) and the Academic Health Center of the University of Minnesota to LL.
Abbreviations
- ABC
ATP-binding cassette transporter
- AD
Alzheimer's disease
- Akt
protein kinase B
- ALS
amyotrophic lateral sclerosis
- Apo
apolipoprotein
- Aβ
amyloid-β protein
- BBB
blood-brain barrier
- CAA
cerebral amyloid angiopathy
- CE
cholesterol esters
- CETP
cholesteryl ester transfer protein
- ChE
cholinesterase
- CLU
clusterin/apoJ
- CNS
central nervous system
- CSF
cerebrospinal fluid
- FC
unesterified free cholesterol
- FTD
frontotemporal dementia
- HD
Huntington's disease
- HDL
high-density lipoproteins
- HDL-C
HDL cholesterol
- LCAT
lecithin cholesterol acyltransferase
- LDL
low-density lipoprotein
- LDLR
low-density lipoprotein receptor
- LRP
low-density lipoprotein receptor-related protein
- LXR
liver X receptors
- MAPK
mitogen-activated protein kinases
- MCI
mild cognitive impairment
- miRNAs
microRNAs
- PD
Parkinson's disease
- PI3K
phosphatidylinositol-4,5-bisphosphate 3-kinase
- PKA
protein kinase A
- PKC
protein kinase C
- PL
phospholipids
- PLTP
phospholipid transfer protein
- PON
paraoxonase
- PPAR
peroxisome proliferator-activated receptor
- rHDL
recombinant HDL
- RCT
reverse cholesterol transport
- SIRT
sirtuins
- SOD1
superoxide dismutase 1
- SR-B1
scavenger receptor B1
- SREBPs
sterol regulatory element binding proteins
- TARDBP
TAR DNA-binding protein
- TG
triglycerides
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akhmedova SN, et al. Paraoxonase 1 Met--Leu 54 polymorphism is associated with Parkinson's disease. J Neurol Sci. 2001;184:179–82. doi: 10.1016/s0022-510x(01)00439-7. [DOI] [PubMed] [Google Scholar]
- Akhondzadeh S, et al. Salvia officinalis extract in the treatment of patients with mild to moderate Alzheimer's disease: a double blind, randomized and placebo-controlled trial. J Clin Pharm Ther. 2003;28:53–9. doi: 10.1046/j.1365-2710.2003.00463.x. [DOI] [PubMed] [Google Scholar]
- Albers JJ, et al. Relationship of apolipoproteins A-1 and B, and lipoprotein(a) to cardiovascular outcomes: the AIM-HIGH trial (Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglyceride and Impact on Global Health Outcomes). J Am Coll Cardiol. 2013;62:1575–9. doi: 10.1016/j.jacc.2013.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers JJ, et al. Cholesteryl ester transfer protein in human brain. Int J Clin Lab Res. 1992;21:264–6. doi: 10.1007/BF02591657. [DOI] [PubMed] [Google Scholar]
- Alzheimer's Association 2013 Alzheimer's disease facts and figures. Alzheimers Dement. 2013;9:208–45. doi: 10.1016/j.jalz.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Anantharamaiah GM, et al. Studies of synthetic peptide analogs of the amphipathic helix. Structure of complexes with dimyristoyl phosphatidylcholine. J Biol Chem. 1985;260:10248–55. [PubMed] [Google Scholar]
- Atrahimovich D, et al. The effects and mechanism of flavonoid-rePON1 interactions. Structure-activity relationship study. Bioorg Med Chem. 2013;21:3348–55. doi: 10.1016/j.bmc.2013.02.055. [DOI] [PubMed] [Google Scholar]
- Atrahimovich D, et al. Glabridin protects paraoxonase 1 from linoleic acid hydroperoxide inhibition via specific interaction: a fluorescence-quenching study. J Agric Food Chem. 2012;60:3679–85. doi: 10.1021/jf2046009. [DOI] [PubMed] [Google Scholar]
- Atzmon G, et al. Plasma HDL levels highly correlate with cognitive function in exceptional longevity. J Gerontol A Biol Sci Med Sci. 2002;57:M712–5. doi: 10.1093/gerona/57.11.m712. [DOI] [PubMed] [Google Scholar]
- Atzmon G, et al. Lipoprotein genotype and conserved pathway for exceptional longevity in humans. PLoS Biol. 2006;4:e113. doi: 10.1371/journal.pbio.0040113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviram M, Rosenblat M. Paraoxonases 1, 2, and 3, oxidative stress, and macrophage foam cell formation during atherosclerosis development. Free Radic Biol Med. 2004;37:1304–16. doi: 10.1016/j.freeradbiomed.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Bailey D, et al. RVX-208: a small molecule that increases apolipoprotein A-I and high-density lipoprotein cholesterol in vitro and in vivo. J Am Coll Cardiol. 2010;55:2580–9. doi: 10.1016/j.jacc.2010.02.035. [DOI] [PubMed] [Google Scholar]
- Barter PJ, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–22. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- Barter PJ, et al. Antiinflammatory properties of HDL. Circ Res. 2004;95:764–72. doi: 10.1161/01.RES.0000146094.59640.13. [DOI] [PubMed] [Google Scholar]
- Barzilai N, et al. A genotype of exceptional longevity is associated with preservation of cognitive function. Neurology. 2006;67:2170–5. doi: 10.1212/01.wnl.0000249116.50854.65. [DOI] [PubMed] [Google Scholar]
- Bergman AJ, et al. Simvastatin does not have a clinically significant pharmacokinetic interaction with fenofibrate in humans. J Clin Pharmacol. 2004;44:1054–62. doi: 10.1177/0091270004268044. [DOI] [PubMed] [Google Scholar]
- Bloedon LT, et al. Safety, pharmacokinetics, and pharmacodynamics of oral apoA-I mimetic peptide D-4F in high-risk cardiovascular patients. J Lipid Res. 2008;49:1344–52. doi: 10.1194/jlr.P800003-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boatman PD, et al. (1aR,5aR)1a,3,5,5a-Tetrahydro-1H-2,3-diaza cyclopropa[a]pentalene-4-carboxylic acid (MK-1903): a potent GPR109a agonist that lowers free fatty acids in humans. J Med Chem. 2012;55:3644–66. doi: 10.1021/jm2010964. [DOI] [PubMed] [Google Scholar]
- Bonarek M, et al. Relationships between cholesterol, apolipoprotein E polymorphism and dementia: a cross-sectional analysis from the PAQUID study. Neuroepidemiology. 2000;19:141–8. doi: 10.1159/000026249. [DOI] [PubMed] [Google Scholar]
- Brunham LR, et al. Intestinal ABCA1 directly contributes to HDL biogenesis in vivo. J Clin Invest. 2006;116:1052–62. doi: 10.1172/JCI27352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan DD, et al. Association of APOE with Parkinson disease age-at-onset in women. Neurosci Lett. 2007;411:185–8. doi: 10.1016/j.neulet.2006.07.080. [DOI] [PubMed] [Google Scholar]
- Buga GM, et al. D-4F decreases brain arteriole inflammation and improves cognitive performance in LDL receptor-null mice on a Western diet. J Lipid Res. 2006;47:2148–60. doi: 10.1194/jlr.M600214-JLR200. [DOI] [PubMed] [Google Scholar]
- Burns MP, et al. The effects of ABCA1 on cholesterol efflux and Abeta levels in vitro and in vivo. J Neurochem. 2006;98:792–800. doi: 10.1111/j.1471-4159.2006.03925.x. [DOI] [PubMed] [Google Scholar]
- Bussell R, Jr., Eliezer D. A structural and functional role for 11-mer repeats in alpha-synuclein and other exchangeable lipid binding proteins. J Mol Biol. 2003;329:763–78. doi: 10.1016/s0022-2836(03)00520-5. [DOI] [PubMed] [Google Scholar]
- Byrne S, et al. Cognitive and clinical characteristics of patients with amyotrophic lateral sclerosis carrying a C9orf72 repeat expansion: a population-based cohort study. Lancet Neurol. 2012;11:232–40. doi: 10.1016/S1474-4422(12)70014-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cam JA, Bu G. Modulation of beta-amyloid precursor protein trafficking and processing by the low density lipoprotein receptor family. Mol Neurodegener. 2006;1:8. doi: 10.1186/1750-1326-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson LA. Nicotinic acid: the broad-spectrum lipid drug. A 50th anniversary review. J Intern Med. 2005;258:94–114. doi: 10.1111/j.1365-2796.2005.01528.x. [DOI] [PubMed] [Google Scholar]
- Caselli RJ, et al. Longitudinal modeling of age-related memory decline and the APOE epsilon4 effect. N Engl J Med. 2009;361:255–63. doi: 10.1056/NEJMoa0809437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassani E, et al. Cardiometabolic factors and disease duration in patients with Parkinson's disease. Nutrition. 2013;29:1331–5. doi: 10.1016/j.nut.2013.04.013. [DOI] [PubMed] [Google Scholar]
- Castellano JM, et al. Human apoE isoforms differentially regulate brain amyloid-beta peptide clearance. Sci Transl Med. 2011;3:89ra57. doi: 10.1126/scitranslmed.3002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereda E, et al. Low cardiometabolic risk in Parkinson's disease is independent of nutritional status, body composition and fat distribution. Clin Nutr. 2012;31:699–704. doi: 10.1016/j.clnu.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Chen S, et al. Genetics of amyotrophic lateral sclerosis: an update. Mol Neurodegener. 2013;8:28. doi: 10.1186/1750-1326-8-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockerill GW, et al. High-density lipoproteins inhibit cytokine-induced expression of endothelial cell adhesion molecules. Arterioscler Thromb Vasc Biol. 1995;15:1987–94. doi: 10.1161/01.atv.15.11.1987. [DOI] [PubMed] [Google Scholar]
- Corder EH, et al. Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat Genet. 1994;7:180–4. doi: 10.1038/ng0694-180. [DOI] [PubMed] [Google Scholar]
- Corder EH, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–3. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Costet P, et al. Sterol-dependent transactivation of the ABC1 promoter by the liver X receptor/retinoid X receptor. J Biol Chem. 2000;275:28240–5. doi: 10.1074/jbc.M003337200. [DOI] [PubMed] [Google Scholar]
- Cramer PE, et al. ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models. Science. 2012;335:1503–6. doi: 10.1126/science.1217697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui YM, et al. Effect of glabridin from Glycyrrhiza glabra on learning and memory in mice. Planta Med. 2008;74:377–80. doi: 10.1055/s-2008-1034319. [DOI] [PubMed] [Google Scholar]
- D'Amico E, et al. Clinical perspective on oxidative stress in sporadic amyotrophic lateral sclerosis. Free Radic Biol Med. 2013;65:509–27. doi: 10.1016/j.freeradbiomed.2013.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta G, et al. Effects of increasing hydrophobicity on the physical-chemical and biological properties of a class A amphipathic helical peptide. J Lipid Res. 2001;42:1096–104. [PubMed] [Google Scholar]
- Davidson MH, Toth PP. High-density lipoprotein metabolism: potential therapeutic targets. Am J Cardiol. 2007;100:n32–40. doi: 10.1016/j.amjcard.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Dedic SI, et al. Is hyperlipidemia correlated with longer survival in patients with amyotrophic lateral sclerosis? Neurol Res. 2012;34:576–80. doi: 10.1179/1743132812Y.0000000049. [DOI] [PubMed] [Google Scholar]
- DeJesus-Hernandez M, et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72:245–56. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeester N, et al. Characterization and functional studies of lipoproteins, lipid transfer proteins, and lecithin:cholesterol acyltransferase in CSF of normal individuals and patients with Alzheimer's disease. J Lipid Res. 2000;41:963–74. [PubMed] [Google Scholar]
- Diditchenko S, et al. Novel formulation of a reconstituted high-density lipoprotein (CSL112) dramatically enhances ABCA1-dependent cholesterol efflux. Arterioscler Thromb Vasc Biol. 2013;33:2202–11. doi: 10.1161/ATVBAHA.113.301981. [DOI] [PubMed] [Google Scholar]
- Dietschy JM, Turley SD. Cholesterol metabolism in the brain. Curr Opin Lipidol. 2001;12:105–12. doi: 10.1097/00041433-200104000-00003. [DOI] [PubMed] [Google Scholar]
- Donkin JJ, et al. ATP-binding cassette transporter A1 mediates the beneficial effects of the liver X receptor agonist GW3965 on object recognition memory and amyloid burden in amyloid precursor protein/presenilin 1 mice. J Biol Chem. 2010;285:34144–54. doi: 10.1074/jbc.M110.108100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donmez G, Outeiro TF. SIRT1 and SIRT2: emerging targets in neurodegeneration. EMBO Mol Med. 2013;5:344–52. doi: 10.1002/emmm.201302451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuis L, et al. Dyslipidemia is a protective factor in amyotrophic lateral sclerosis. Neurology. 2008;70:1004–9. doi: 10.1212/01.wnl.0000285080.70324.27. [DOI] [PubMed] [Google Scholar]
- Durairajan SS, et al. Berberine ameliorates beta-amyloid pathology, gliosis, and cognitive impairment in an Alzheimer's disease transgenic mouse model. Neurobiol Aging. 2012;33:2903–19. doi: 10.1016/j.neurobiolaging.2012.02.016. [DOI] [PubMed] [Google Scholar]
- Elbaz A, et al. Risk tables for parkinsonism and Parkinson's disease. J Clin Epidemiol. 2002;55:25–31. doi: 10.1016/s0895-4356(01)00425-5. [DOI] [PubMed] [Google Scholar]
- Escola-Gil JC, et al. Resveratrol administration or SIRT1 overexpression does not increase LXR signaling and macrophage-to-feces reverse cholesterol transport in vivo. Transl Res. 2013;161:110–7. doi: 10.1016/j.trsl.2012.10.008. [DOI] [PubMed] [Google Scholar]
- Fagan AM, et al. ApoAI deficiency results in marked reductions in plasma cholesterol but no alterations in amyloid-beta pathology in a mouse model of Alzheimer's disease-like cerebral amyloidosis. Am J Pathol. 2004;165:1413–22. doi: 10.1016/s0002-9440(10)63399-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan AM, et al. Differences in the Abeta40/Abeta42 ratio associated with cerebrospinal fluid lipoproteins as a function of apolipoprotein E genotype. Ann Neurol. 2000;48:201–10. [PubMed] [Google Scholar]
- Farnier M, et al. Long-term safety and efficacy of fenofibrate/pravastatin combination therapy in high risk patients with mixed hyperlipidemia not controlled by pravastatin monotherapy. Curr Med Res Opin. 2011;27:2165–73. doi: 10.1185/03007995.2011.626398. [DOI] [PubMed] [Google Scholar]
- Federoff M, et al. A large study reveals no association between APOE and Parkinson's disease. Neurobiol Dis. 2012;46:389–92. doi: 10.1016/j.nbd.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitz NF, et al. Liver X receptor agonist treatment ameliorates amyloid pathology and memory deficits caused by high-fat diet in APP23 mice. J Neurosci. 2010;30:6862–72. doi: 10.1523/JNEUROSCI.1051-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitz NF, et al. Comment on “ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models”. Science. 2013;340:924–c. doi: 10.1126/science.1235809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitz NF, et al. Abca1 deficiency affects Alzheimer's disease-like phenotype in human ApoE4 but not in ApoE3-targeted replacement mice. J Neurosci. 2012;32:13125–36. doi: 10.1523/JNEUROSCI.1937-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschini G, et al. A-IMilano apoprotein. Decreased high density lipoprotein cholesterol levels with significant lipoprotein modifications and without clinical atherosclerosis in an Italian family. J Clin Invest. 1980;66:892–900. doi: 10.1172/JCI109956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, et al. Apolipoprotein E genotypes and the risk of Parkinson disease. Neurobiol Aging. 2011;32:2106, e1–6. doi: 10.1016/j.neurobiolaging.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getz GS, Reardon CA. Animal models of atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:1104–15. doi: 10.1161/ATVBAHA.111.237693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getz GS, et al. HDL apolipoprotein-related peptides in the treatment of atherosclerosis and other inflammatory disorders. Curr Pharm Des. 2010;16:3173–84. doi: 10.2174/138161210793292492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein LH, Abrahams S. Changes in cognition and behaviour in amyotrophic lateral sclerosis: nature of impairment and implications for assessment. Lancet Neurol. 2013;12:368–80. doi: 10.1016/S1474-4422(13)70026-7. [DOI] [PubMed] [Google Scholar]
- Gotto AM, Jr., et al. Evaluation of lipids, drug concentration, and safety parameters following cessation of treatment with the cholesteryl ester transfer protein inhibitor anacetrapib in patients with or at high risk for coronary heart disease. Am J Cardiol. 2014;113:76–83. doi: 10.1016/j.amjcard.2013.08.041. [DOI] [PubMed] [Google Scholar]
- Gregorio ML, et al. Impact of genetic variants of apolipoprotein E on lipid profile in patients with Parkinson's disease. Biomed Res Int. 2013;2013:641515. doi: 10.1155/2013/641515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Group AS, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563–74. doi: 10.1056/NEJMoa1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Group HTC. HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur Heart J. 2013;34:1279–91. doi: 10.1093/eurheartj/eht055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, et al. Meta-analysis of safety of the coadministration of statin with fenofibrate in patients with combined hyperlipidemia. Am J Cardiol. 2012;110:1296–301. doi: 10.1016/j.amjcard.2012.06.050. [DOI] [PubMed] [Google Scholar]
- Gusella JF, MacDonald ME. Huntington's disease: the case for genetic modifiers. Genome Med. 2009;1:80. doi: 10.1186/gm80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusella JF, et al. A polymorphic DNA marker genetically linked to Huntington's disease. Nature. 1983;306:234–8. doi: 10.1038/306234a0. [DOI] [PubMed] [Google Scholar]
- Haass C, et al. Trafficking and proteolytic processing of APP. Cold Spring Harb Perspect Med. 2012;2:a006270. doi: 10.1101/cshperspect.a006270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haidar B, et al. Apolipoprotein A-I activates cellular cAMP signaling through the ABCA1 transporter. J Biol Chem. 2004;279:9963–9. doi: 10.1074/jbc.M313487200. [DOI] [PubMed] [Google Scholar]
- Handattu SP, et al. Oral apolipoprotein A-I mimetic peptide improves cognitive function and reduces amyloid burden in a mouse model of Alzheimer's disease. Neurobiol Dis. 2009;34:525–34. doi: 10.1016/j.nbd.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasanein P. Glabridin as a major active isoflavan from Glycyrrhiza glabra (licorice) reverses learning and memory deficits in diabetic rats. Acta Physiol Hung. 2011;98:221–30. doi: 10.1556/APhysiol.98.2011.2.14. [DOI] [PubMed] [Google Scholar]
- Herrschaft H, et al. Ginkgo biloba extract EGb 761(R) in dementia with neuropsychiatric features: a randomised, placebo-controlled trial to confirm the efficacy and safety of a daily dose of 240 mg. J Psychiatr Res. 2012;46:716–23. doi: 10.1016/j.jpsychires.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Hirsch-Reinshagen V, et al. The absence of ABCA1 decreases soluble ApoE levels but does not diminish amyloid deposition in two murine models of Alzheimer disease. J Biol Chem. 2005;280:43243–56. doi: 10.1074/jbc.M508781200. [DOI] [PubMed] [Google Scholar]
- Holtzman DM, et al. Apolipoprotein E and apolipoprotein E receptors: normal biology and roles in Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2:a006312. doi: 10.1101/cshperspect.a006312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, et al. APOE-[epsilon]2 allele associated with higher prevalence of sporadic Parkinson disease. Neurology. 2004;62:2198–202. doi: 10.1212/01.wnl.0000130159.28215.6a. [DOI] [PubMed] [Google Scholar]
- Ikeda K, et al. Serological profiles of urate, paraoxonase-1, ferritin and lipid in Parkinson's disease: changes linked to disease progression. Neurodegener Dis. 2011;8:252–8. doi: 10.1159/000323265. [DOI] [PubMed] [Google Scholar]
- Inamori T, et al. The combined effects of genetic variation in the SIRT1 gene and dietary intake of n-3 and n-6 polyunsaturated fatty acids on serum LDL-C and HDL-C levels: a population based study. Lipids Health Dis. 2013;12:4. doi: 10.1186/1476-511X-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Investigators A-H. The role of niacin in raising high-density lipoprotein cholesterol to reduce cardiovascular events in patients with atherosclerotic cardiovascular disease and optimally treated low-density lipoprotein cholesterol: baseline characteristics of study participants. The Atherothrombosis Intervention in Metabolic syndrome with low HDL/high triglycerides: impact on Global Health outcomes (AIM-HIGH) trial. Am Heart J. 2011;161:538–43. doi: 10.1016/j.ahj.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Investigators A-H, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–67. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- Ito J, et al. Apolipoprotein A-I induces translocation of protein kinase C[alpha] to a cytosolic lipid-protein particle in astrocytes. J Lipid Res. 2004;45:2269–76. doi: 10.1194/jlr.M400222-JLR200. [DOI] [PubMed] [Google Scholar]
- Ito J, et al. Apolipoprotein A-I induces translocation of cholesterol, phospholipid, and caveolin-1 to cytosol in rat astrocytes. J Biol Chem. 2002;277:7929–35. doi: 10.1074/jbc.M103878200. [DOI] [PubMed] [Google Scholar]
- Ito J, et al. Differential generation of high-density lipoprotein by endogenous and exogenous apolipoproteins in cultured fetal rat astrocytes. J Neurochem. 1999;72:2362–9. doi: 10.1046/j.1471-4159.1999.0722362.x. [DOI] [PubMed] [Google Scholar]
- Izaks GJ, et al. Cholesteryl Ester Transfer Protein (CETP) genotype and cognitive function in persons aged 35 years or older. Neurobiol Aging. 2012;33:1851, e7–16. doi: 10.1016/j.neurobiolaging.2012.02.022. [DOI] [PubMed] [Google Scholar]
- Jawaid A, et al. Does apolipoprotein E genotype modify the clinical expression of ALS? Eur J Neurol. 2011;18:618–24. doi: 10.1111/j.1468-1331.2010.03225.x. [DOI] [PubMed] [Google Scholar]
- Ji W, Gong BQ. Hypolipidemic effects and mechanisms of Panax notoginseng on lipid profile in hyperlipidemic rats. J Ethnopharmacol. 2007;113:318–24. doi: 10.1016/j.jep.2007.06.022. [DOI] [PubMed] [Google Scholar]
- Jiang Q, et al. ApoE promotes the proteolytic degradation of Abeta. Neuron. 2008;58:681–93. doi: 10.1016/j.neuron.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen AB, et al. Loss-of-Function Mutations in APOC3 and Risk of Ischemic Vascular Disease. N Engl J Med. 2014 doi: 10.1056/NEJMoa1308027. [DOI] [PubMed] [Google Scholar]
- Kanekiyo T, et al. ApoE and Abeta in Alzheimer's Disease: Accidental Encounters or Partners? Neuron. 2014;81:740–754. doi: 10.1016/j.neuron.2014.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasinska JM, Hayden MR. Cholesterol metabolism in Huntington disease. Nat Rev Neurol. 2011;7:561–72. doi: 10.1038/nrneurol.2011.132. [DOI] [PubMed] [Google Scholar]
- Kawano M, et al. Marked decrease of plasma apolipoprotein AI and AII in Japanese patients with late-onset non-familial Alzheimer's disease. Clin Chim Acta. 1995;239:209–11. doi: 10.1016/0009-8981(95)06115-t. [DOI] [PubMed] [Google Scholar]
- Kay AD, et al. Remodeling of cerebrospinal fluid lipoprotein particles after human traumatic brain injury. J Neurotrauma. 2003;20:717–23. doi: 10.1089/089771503767869953. [DOI] [PubMed] [Google Scholar]
- Keeney JT, et al. Apolipoprotein A-I: insights from redox proteomics for its role in neurodegeneration. Proteomics Clin Appl. 2013;7:109–22. doi: 10.1002/prca.201200087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe P, et al. Age of onset in Huntington disease: sex specific influence of apolipoprotein E genotype and normal CAG repeat length. J Med Genet. 1999;36:108–11. [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Park KS. Effects of Panax ginseng extract on lipid metabolism in humans. Pharmacol Res. 2003;48:511–3. doi: 10.1016/s1043-6618(03)00189-0. [DOI] [PubMed] [Google Scholar]
- Koch S, et al. Characterization of four lipoprotein classes in human cerebrospinal fluid. J Lipid Res. 2001;42:1143–51. [PubMed] [Google Scholar]
- Koldamova R, et al. Lack of ABCA1 considerably decreases brain ApoE level and increases amyloid deposition in APP23 mice. J Biol Chem. 2005a;280:43224–35. doi: 10.1074/jbc.M504513200. [DOI] [PubMed] [Google Scholar]
- Koldamova RP, et al. Apolipoprotein A-I directly interacts with amyloid precursor protein and inhibits A beta aggregation and toxicity. Biochemistry. 2001;40:3553–60. doi: 10.1021/bi002186k. [DOI] [PubMed] [Google Scholar]
- Koldamova RP, et al. The liver X receptor ligand T0901317 decreases amyloid beta production in vitro and in a mouse model of Alzheimer's disease. J Biol Chem. 2005b;280:4079–88. doi: 10.1074/jbc.M411420200. [DOI] [PubMed] [Google Scholar]
- Kondo I, Yamamoto M. Genetic polymorphism of paraoxonase 1 (PON1) and susceptibility to Parkinson's disease. Brain Res. 1998;806:271–3. doi: 10.1016/s0006-8993(98)00586-1. [DOI] [PubMed] [Google Scholar]
- Kurz MW, et al. APOE alleles in Parkinson disease and their relationship to cognitive decline: a population-based, longitudinal study. J Geriatr Psychiatry Neurol. 2009;22:166–70. doi: 10.1177/0891988709332945. [DOI] [PubMed] [Google Scholar]
- LaClair KD, et al. Treatment with bexarotene, a compound that increases apolipoprotein-E, provides no cognitive benefit in mutant APP/PS1 mice. Mol Neurodegener. 2013;8:18. doi: 10.1186/1750-1326-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFerla FM, Green KN. Animal models of Alzheimer disease. Cold Spring Harb Perspect Med. 2012:2. doi: 10.1101/cshperspect.a006320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landreth GE, et al. Response to comments on “ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models”. Science. 2013;340:924–g. doi: 10.1126/science.1234114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launer LJ, et al. Cholesterol and neuropathologic markers of AD: a population-based autopsy study. Neurology. 2001;57:1447–52. doi: 10.1212/wnl.57.8.1447. [DOI] [PubMed] [Google Scholar]
- Lauring B, et al. Niacin lipid efficacy is independent of both the niacin receptor GPR109A and free fatty acid suppression. Sci Transl Med. 2012;4:148ra115. doi: 10.1126/scitranslmed.3003877. [DOI] [PubMed] [Google Scholar]
- Lee TS, et al. Anti-atherogenic effect of berberine on LXRalpha-ABCA1-dependent cholesterol efflux in macrophages. J Cell Biochem. 2010;111:104–10. doi: 10.1002/jcb.22667. [DOI] [PubMed] [Google Scholar]
- Lees AJ, et al. Parkinson's disease. Lancet. 2009;373:2055–66. doi: 10.1016/S0140-6736(09)60492-X. [DOI] [PubMed] [Google Scholar]
- Lefterov I, et al. Apolipoprotein A-I deficiency increases cerebral amyloid angiopathy and cognitive deficits in APP/PS1DeltaE9 mice. J Biol Chem. 2010;285:36945–57. doi: 10.1074/jbc.M110.127738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leman LJ, et al. Molecules That Mimic Apolipoprotein A-I: Potential Agents for Treating Atherosclerosis. J Med Chem. 2013 doi: 10.1021/jm4005847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leoni V, et al. Plasma 24S-hydroxycholesterol correlation with markers of Huntington disease progression. Neurobiol Dis. 2013;55:37–43. doi: 10.1016/j.nbd.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis TL, et al. Overexpression of human apolipoprotein A-I preserves cognitive function and attenuates neuroinflammation and cerebral amyloid angiopathy in a mouse model of Alzheimer disease. J Biol Chem. 2010;285:36958–68. doi: 10.1074/jbc.M110.127829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, et al. Molecular mechanisms responsible for the differential effects of apoE3 and apoE4 on plasma lipoprotein-cholesterol levels. Arterioscler Thromb Vasc Biol. 2013;33:687–93. doi: 10.1161/ATVBAHA.112.301193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, et al. SIRT1 deacetylates and positively regulates the nuclear receptor LXR. Mol Cell. 2007;28:91–106. doi: 10.1016/j.molcel.2007.07.032. [DOI] [PubMed] [Google Scholar]
- Linton MF, et al. Phenotypes of apolipoprotein B and apolipoprotein E after liver transplantation. J Clin Invest. 1991;88:270–81. doi: 10.1172/JCI115288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, et al. Beneficial effect of berberine on hepatic insulin resistance in diabetic hamsters possibly involves in SREBPs, LXRalpha and PPARalpha transcriptional programs. Endocr J. 2010;57:881–93. doi: 10.1507/endocrj.k10e-043. [DOI] [PubMed] [Google Scholar]
- Lopez M, et al. Apolipoprotein E epsilon4 allele is associated with Parkinson disease risk in a Mexican Mestizo population. Mov Disord. 2007;22:417–20. doi: 10.1002/mds.21340. [DOI] [PubMed] [Google Scholar]
- Maher P, et al. ERK activation by the polyphenols fisetin and resveratrol provides neuroprotection in multiple models of Huntington's disease. Hum Mol Genet. 2011;20:261–70. doi: 10.1093/hmg/ddq460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley RW, Huang Y. Apolipoprotein e sets the stage: response to injury triggers neuropathology. Neuron. 2012;76:871–85. doi: 10.1016/j.neuron.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawuenyega KG, et al. Decreased clearance of CNS beta-amyloid in Alzheimer's disease. Science. 2010;330:1774. doi: 10.1126/science.1197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenney J. Niacin for dyslipidemia: considerations in product selection. Am J Health Syst Pharm. 2003;60:995–1005. doi: 10.1093/ajhp/60.10.995. [DOI] [PubMed] [Google Scholar]
- McLure KG, et al. RVX-208, an inducer of ApoA-I in humans, is a BET bromodomain antagonist. PLoS One. 2013;8:e83190. doi: 10.1371/journal.pone.0083190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merched A, et al. Decreased high-density lipoprotein cholesterol and serum apolipoprotein AI concentrations are highly correlated with the severity of Alzheimer's disease. Neurobiol Aging. 2000;21:27–30. doi: 10.1016/s0197-4580(99)00103-7. [DOI] [PubMed] [Google Scholar]
- Mineo C, et al. High density lipoprotein-induced endothelial nitric-oxide synthase activation is mediated by Akt and MAP kinases. J Biol Chem. 2003;278:9142–9. doi: 10.1074/jbc.M211394200. [DOI] [PubMed] [Google Scholar]
- Mitchell JD, Borasio GD. Amyotrophic lateral sclerosis. Lancet. 2007;369:2031–41. doi: 10.1016/S0140-6736(07)60944-1. [DOI] [PubMed] [Google Scholar]
- Mockel B, et al. Expression of apolipoprotein A-I in porcine brain endothelium in vitro. J Neurochem. 1994;62:788–98. doi: 10.1046/j.1471-4159.1994.62020788.x. [DOI] [PubMed] [Google Scholar]
- Morley JF, et al. Genetic influences on cognitive decline in Parkinson's disease. Mov Disord. 2012;27:512–8. doi: 10.1002/mds.24946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison AC, et al. Whole-genome sequence-based analysis of high-density lipoprotein cholesterol. Nat Genet. 2013;45:899–901. doi: 10.1038/ng.2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navab M, et al. Apolipoprotein A-I mimetic peptides. Arterioscler Thromb Vasc Biol. 2005a;25:1325–31. doi: 10.1161/01.ATV.0000165694.39518.95. [DOI] [PubMed] [Google Scholar]
- Navab M, et al. The double jeopardy of HDL. Ann Med. 2005b;37:173–8. doi: 10.1080/07853890510007322. [DOI] [PubMed] [Google Scholar]
- Navab M, et al. Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: step 1. J Lipid Res. 2000;41:1481–94. [PubMed] [Google Scholar]
- Nicholls SJ, et al. Effects of the CETP inhibitor evacetrapib administered as monotherapy or in combination with statins on HDL and LDL cholesterol: a randomized controlled trial. Jama. 2011;306:2099–109. doi: 10.1001/jama.2011.1649. [DOI] [PubMed] [Google Scholar]
- Nicholls SJ, et al. ApoA-I induction as a potential cardioprotective strategy: rationale for the SUSTAIN and ASSURE studies. Cardiovasc Drugs Ther. 2012;26:181–7. doi: 10.1007/s10557-012-6373-5. [DOI] [PubMed] [Google Scholar]
- Nissen SE, et al. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. Jama. 2003;290:2292–300. doi: 10.1001/jama.290.17.2292. [DOI] [PubMed] [Google Scholar]
- O'Connell BJ, Genest J., Jr. High-density lipoproteins and endothelial function. Circulation. 2001;104:1978–83. doi: 10.1161/hc3901.096667. [DOI] [PubMed] [Google Scholar]
- Oram JF, Heinecke JW. ATP-binding cassette transporter A1: a cell cholesterol exporter that protects against cardiovascular disease. Physiol Rev. 2005;85:1343–72. doi: 10.1152/physrev.00005.2005. [DOI] [PubMed] [Google Scholar]
- Paganoni S, et al. Body mass index, not dyslipidemia, is an independent predictor of survival in amyotrophic lateral sclerosis. Muscle Nerve. 2011;44:20–4. doi: 10.1002/mus.22114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panas M, et al. Apolipoprotein E and presenilin-1 genotypes in Huntington's disease. J Neurol. 1999;246:574–7. doi: 10.1007/s004150050406. [DOI] [PubMed] [Google Scholar]
- Pankratz N, et al. Presence of an APOE4 allele results in significantly earlier onset of Parkinson's disease and a higher risk with dementia. Mov Disord. 2006;21:45–9. doi: 10.1002/mds.20663. [DOI] [PubMed] [Google Scholar]
- Panzenboeck U, et al. ABCA1 and scavenger receptor class B, type I, are modulators of reverse sterol transport at an in vitro blood-brain barrier constituted of porcine brain capillary endothelial cells. J Biol Chem. 2002;277:42781–9. doi: 10.1074/jbc.M207601200. [DOI] [PubMed] [Google Scholar]
- Park SH, et al. Sage weed (Salvia plebeia) extract antagonizes foam cell formation and promotes cholesterol efflux in murine macrophages. Int J Mol Med. 2012;30:1105–12. doi: 10.3892/ijmm.2012.1103. [DOI] [PubMed] [Google Scholar]
- Pasinetti GM, et al. Neuroprotective and metabolic effects of resveratrol: therapeutic implications for Huntington's disease and other neurodegenerative disorders. Exp Neurol. 2011;232:1–6. doi: 10.1016/j.expneurol.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, et al. Anti-inflammatory effects of apolipoprotein A-I in the rabbit. Atherosclerosis. 2010;212:392–7. doi: 10.1016/j.atherosclerosis.2010.05.035. [DOI] [PubMed] [Google Scholar]
- Paterno R, et al. Reconstituted high-density lipoprotein exhibits neuroprotection in two rat models of stroke. Cerebrovasc Dis. 2004;17:204–11. doi: 10.1159/000075792. [DOI] [PubMed] [Google Scholar]
- Perry E, Howes MJ. Medicinal plants and dementia therapy: herbal hopes for brain aging? CNS Neurosci Ther. 2011;17:683–98. doi: 10.1111/j.1755-5949.2010.00202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picaud S, et al. RVX-208, an inhibitor of BET transcriptional regulators with selectivity for the second bromodomain. Proc Natl Acad Sci U S A. 2013;110:19754–9. doi: 10.1073/pnas.1310658110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce LR, et al. Myopathy and rhabdomyolysis associated with lovastatin-gemfibrozil combination therapy. Jama. 1990;264:71–5. [PubMed] [Google Scholar]
- Pitas RE, et al. Lipoproteins and their receptors in the central nervous system. Characterization of the lipoproteins in cerebrospinal fluid and identification of apolipoprotein B,E(LDL) receptors in the brain. J Biol Chem. 1987;262:14352–60. [PubMed] [Google Scholar]
- Poirier J, et al. Apolipoprotein E polymorphism and Alzheimer's disease. Lancet. 1993;342:697–9. doi: 10.1016/0140-6736(93)91705-q. [DOI] [PubMed] [Google Scholar]
- Pollin TI, et al. A null mutation in human APOC3 confers a favorable plasma lipid profile and apparent cardioprotection. Science. 2008;322:1702–5. doi: 10.1126/science.1161524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praline J, et al. APOE epsilon4 allele is associated with an increased risk of bulbar-onset amyotrophic lateral sclerosis in men. Eur J Neurol. 2011;18:1046–52. doi: 10.1111/j.1468-1331.2010.03330.x. [DOI] [PubMed] [Google Scholar]
- Price AR, et al. Comment on “ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models”. Science. 2013;340:924–d. doi: 10.1126/science.1234089. [DOI] [PubMed] [Google Scholar]
- Pringsheim T, et al. The incidence and prevalence of Huntington's disease: a systematic review and meta-analysis. Mov Disord. 2012;27:1083–91. doi: 10.1002/mds.25075. [DOI] [PubMed] [Google Scholar]
- Prueksaritanont T, et al. Mechanistic studies on metabolic interactions between gemfibrozil and statins. J Pharmacol Exp Ther. 2002;301:1042–51. doi: 10.1124/jpet.301.3.1042. [DOI] [PubMed] [Google Scholar]
- Pulkes T, et al. Association between apolipoprotein E genotypes and Parkinson's disease. J Clin Neurosci. 2011;18:1333–5. doi: 10.1016/j.jocn.2011.01.028. [DOI] [PubMed] [Google Scholar]
- Qiang JK, et al. Plasma apolipoprotein A1 as a biomarker for Parkinson disease. Ann Neurol. 2013;74:119–27. doi: 10.1002/ana.23872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rader DJ, deGoma EM. Future of cholesteryl ester transfer protein inhibitors. Annu Rev Med. 2014;65:385–403. doi: 10.1146/annurev-med-050311-163305. [DOI] [PubMed] [Google Scholar]
- Ramprasath VR, Jones PJ. Anti-atherogenic effects of resveratrol. Eur J Clin Nutr. 2010;64:660–8. doi: 10.1038/ejcn.2010.77. [DOI] [PubMed] [Google Scholar]
- Reitz C. Dyslipidemia and dementia: current epidemiology, genetic evidence, and mechanisms behind the associations. J Alzheimers Dis. 2012;30(Suppl 2):S127–45. doi: 10.3233/JAD-2011-110599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitz C, et al. Relation of plasma lipids to Alzheimer disease and vascular dementia. Arch Neurol. 2004;61:705–14. doi: 10.1001/archneur.61.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitz C, et al. Association of higher levels of high-density lipoprotein cholesterol in elderly individuals and lower risk of late-onset Alzheimer disease. Arch Neurol. 2010;67:1491–7. doi: 10.1001/archneurol.2010.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddell DR, et al. The LXR agonist TO901317 selectively lowers hippocampal Abeta42 and improves memory in the Tg2576 mouse model of Alzheimer's disease. Mol Cell Neurosci. 2007;34:621–8. doi: 10.1016/j.mcn.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Rogers RL, et al. Reducing hypertriglyceridemia in elderly patients with cerebrovascular disease stabilizes or improves cognition and cerebral perfusion. Angiology. 1989;40:260–9. [PubMed] [Google Scholar]
- Rosenthal SL, Kamboh MI. Late-Onset Alzheimer's Disease Genes and the Potentially Implicated Pathways. Curr Genet Med Rep. 2014;2:85–101. doi: 10.1007/s40142-014-0034-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross CA, Tabrizi SJ. Huntington's disease: from molecular pathogenesis to clinical treatment. Lancet Neurol. 2011;10:83–98. doi: 10.1016/S1474-4422(10)70245-3. [DOI] [PubMed] [Google Scholar]
- Saczynski JS, et al. The relation between apolipoprotein A-I and dementia: the Honolulu-Asia aging study. Am J Epidemiol. 2007;165:985–92. doi: 10.1093/aje/kwm027. [DOI] [PubMed] [Google Scholar]
- Saeed M, et al. Paraoxonase cluster polymorphisms are associated with sporadic ALS. Neurology. 2006;67:771–6. doi: 10.1212/01.wnl.0000227187.52002.88. [DOI] [PubMed] [Google Scholar]
- Saft C, et al. Apolipoprotein E genotypes do not influence the age of onset in Huntington's disease. J Neurol Neurosurg Psychiatry. 2004;75:1692–6. doi: 10.1136/jnnp.2003.022756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagare AP, et al. Neurovascular dysfunction and faulty amyloid beta-peptide clearance in Alzheimer disease. Cold Spring Harb Perspect Med. 2012:2. doi: 10.1101/cshperspect.a011452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K, et al. Marked increases in concentrations of apolipoprotein in the cerebrospinal fluid of poliovirus-infected macaques: relations between apolipoprotein concentrations and severity of brain injury. Biochem J. 1997;321(Pt 1):145–9. doi: 10.1042/bj3210145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders AE, et al. Association of a functional polymorphism in the cholesteryl ester transfer protein (CETP) gene with memory decline and incidence of dementia. Jama. 2010;303:150–8. doi: 10.1001/jama.2009.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuner D, et al. Secreted amyloid beta-protein similar to that in the senile plaques of Alzheimer's disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer's disease. Nat Med. 1996;2:864–70. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- Schiepers OJ, et al. APOE E4 status predicts age-related cognitive decline in the ninth decade: longitudinal follow-up of the Lothian Birth Cohort 1921. Mol Psychiatry. 2012;17:315–24. doi: 10.1038/mp.2010.137. [DOI] [PubMed] [Google Scholar]
- Schrag M, et al. Oxidative stress in blood in Alzheimer's disease and mild cognitive impairment: a meta-analysis. Neurobiol Dis. 2013;59:100–10. doi: 10.1016/j.nbd.2013.07.005. [DOI] [PubMed] [Google Scholar]
- Schwartz GG, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367:2089–99. doi: 10.1056/NEJMoa1206797. [DOI] [PubMed] [Google Scholar]
- Scigliano G, et al. Reduced risk factors for vascular disorders in Parkinson disease patients: a case-control study. Stroke. 2006;37:1184–8. doi: 10.1161/01.STR.0000217384.03237.9c. [DOI] [PubMed] [Google Scholar]
- Scigliano G, et al. Sympathetic modulation by levodopa reduces vascular risk factors in Parkinson disease. Parkinsonism Relat Disord. 2009;15:138–43. doi: 10.1016/j.parkreldis.2008.04.036. [DOI] [PubMed] [Google Scholar]
- Segrest JP, et al. The amphipathic alpha helix: a multifunctional structural motif in plasma apolipoproteins. Adv Protein Chem. 1994;45:303–69. doi: 10.1016/s0065-3233(08)60643-9. [DOI] [PubMed] [Google Scholar]
- Segrest JP, et al. The amphipathic helix in the exchangeable apolipoproteins: a review of secondary structure and function. J Lipid Res. 1992;33:141–66. [PubMed] [Google Scholar]
- Segrest JP, et al. Structure and function of apolipoprotein A-I and high-density lipoprotein. Curr Opin Lipidol. 2000;11:105–15. doi: 10.1097/00041433-200004000-00002. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–91. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- Shirali S, et al. Effect of crocin on the insulin resistance and lipid profile of streptozotocin-induced diabetic rats. Phytother Res. 2013;27:1042–7. doi: 10.1002/ptr.4836. [DOI] [PubMed] [Google Scholar]
- Singh-Manoux A, et al. Low HDL cholesterol is a risk factor for deficit and decline in memory in midlife: the Whitehall II study. Arterioscler Thromb Vasc Biol. 2008;28:1556–62. doi: 10.1161/ATVBAHA.108.163998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small SA, Gandy S. Sorting through the cell biology of Alzheimer's disease: intracellular pathways to pathogenesis. Neuron. 2006;52:15–31. doi: 10.1016/j.neuron.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CE, et al. Apolipoprotein C3 polymorphisms, cognitive function and diabetes in Caribbean origin Hispanics. PLoS One. 2009;4:e5465. doi: 10.1371/journal.pone.0005465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smythies LE, et al. Apolipoprotein A-I mimetic 4F alters the function of human monocyte-derived macrophages. Am J Physiol Cell Physiol. 2010;298:C1538–48. doi: 10.1152/ajpcell.00467.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell RG, et al. Relationship between trinucleotide repeat expansion and phenotypic variation in Huntington's disease. Nat Genet. 1993;4:393–7. doi: 10.1038/ng0893-393. [DOI] [PubMed] [Google Scholar]
- Song F, et al. Plasma apolipoprotein levels are associated with cognitive status and decline in a community cohort of older individuals. PLoS One. 2012;7:e34078. doi: 10.1371/journal.pone.0034078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreedharan J, et al. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science. 2008;319:1668–72. doi: 10.1126/science.1154584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staels B, et al. Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation. 1998;98:2088–93. doi: 10.1161/01.cir.98.19.2088. [DOI] [PubMed] [Google Scholar]
- Staffa JA, et al. Cerivastatin and reports of fatal rhabdomyolysis. N Engl J Med. 2002;346:539–40. doi: 10.1056/NEJM200202143460721. [DOI] [PubMed] [Google Scholar]
- Strittmatter WJ, Roses AD. Apolipoprotein E and Alzheimer's disease. Annu Rev Neurosci. 1996;19:53–77. doi: 10.1146/annurev.ne.19.030196.000413. [DOI] [PubMed] [Google Scholar]
- Stukas S, et al. The LXR agonist GW3965 increases apoA-I protein levels in the central nervous system independent of ABCA1. Biochim Biophys Acta. 2012;1821:536–46. doi: 10.1016/j.bbalip.2011.08.014. [DOI] [PubMed] [Google Scholar]
- Stukas S, et al. High-Density Lipoproteins and Cerebrovascular Integrity in Alzheimer's Disease. Cell Metab. 2014 doi: 10.1016/j.cmet.2014.01.003. [DOI] [PubMed] [Google Scholar]
- Sutedja NA, et al. Beneficial vascular risk profile is associated with amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2011;82:638–42. doi: 10.1136/jnnp.2010.236752. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, et al. Cognitive impairment in patients with Parkinson's disease: diagnosis, biomarkers, and treatment. Lancet Neurol. 2012;11:697–707. doi: 10.1016/S1474-4422(12)70152-7. [DOI] [PubMed] [Google Scholar]
- Tardif JC. Emerging high-density lipoprotein infusion therapies: fulfilling the promise of epidemiology? J Clin Lipidol. 2010;4:399–404. doi: 10.1016/j.jacl.2010.08.018. [DOI] [PubMed] [Google Scholar]
- Tardif JC, et al. Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: a randomized controlled trial. Jama. 2007;297:1675–82. doi: 10.1001/jama.297.15.jpc70004. [DOI] [PubMed] [Google Scholar]
- Tashakori-Sabzevar F, et al. Crocetin attenuates spatial learning dysfunction and hippocampal injury in a model of vascular dementia. Curr Neurovasc Res. 2013;10:325–34. doi: 10.2174/15672026113109990032. [DOI] [PubMed] [Google Scholar]
- Tesseur I, et al. Comment on “ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models”. Science. 2013;340:924–e. doi: 10.1126/science.1233937. [DOI] [PubMed] [Google Scholar]
- The TG, et al. Loss-of-Function Mutations in APOC3, Triglycerides, and Coronary Disease. N Engl J Med. 2014 doi: 10.1056/NEJMoa1307095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian M, et al. Curcumin induces ABCA1 expression and apolipoprotein A-I-mediated cholesterol transmembrane in the chronic cerebral hypoperfusion aging rats. Am J Chin Med. 2013;41:1027–42. doi: 10.1142/S0192415X13500699. [DOI] [PubMed] [Google Scholar]
- Ticozzi N, et al. Paraoxonase gene mutations in amyotrophic lateral sclerosis. Ann Neurol. 2010;68:102–7. doi: 10.1002/ana.21993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth PP, et al. High-density lipoproteins: a consensus statement from the National Lipid Association. J Clin Lipidol. 2013;7:484–525. doi: 10.1016/j.jacl.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Tsai JY, et al. EGb761 ameliorates the formation of foam cells by regulating the expression of SR-A and ABCA1: role of haem oxygenase-1. Cardiovasc Res. 2010;88:415–23. doi: 10.1093/cvr/cvq226. [DOI] [PubMed] [Google Scholar]
- Tsuang D, et al. APOE epsilon4 increases risk for dementia in pure synucleinopathies. JAMA Neurol. 2013;70:223–8. doi: 10.1001/jamaneurol.2013.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunaru S, et al. PUMA-G and HM74 are receptors for nicotinic acid and mediate its anti-lipolytic effect. Nat Med. 2003;9:352–5. doi: 10.1038/nm824. [DOI] [PubMed] [Google Scholar]
- Valdmanis PN, et al. Association of paraoxonase gene cluster polymorphisms with ALS in France, Quebec, and Sweden. Neurology. 2008;71:514–20. doi: 10.1212/01.wnl.0000324997.21272.0c. [DOI] [PubMed] [Google Scholar]
- Valenza M, Cattaneo E. Emerging roles for cholesterol in Huntington's disease. Trends Neurosci. 2011;34:474–86. doi: 10.1016/j.tins.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Valenza M, et al. Cholesterol defect is marked across multiple rodent models of Huntington's disease and is manifest in astrocytes. J Neurosci. 2010;30:10844–50. doi: 10.1523/JNEUROSCI.0917-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenza M, et al. Dysfunction of the cholesterol biosynthetic pathway in Huntington's disease. J Neurosci. 2005;25:9932–9. doi: 10.1523/JNEUROSCI.3355-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Blitterswijk M, et al. Rare and common paraoxonase gene variants in amyotrophic lateral sclerosis patients. Neurobiol Aging. 2012;33:1845, e1–3. doi: 10.1016/j.neurobiolaging.2012.01.007. [DOI] [PubMed] [Google Scholar]
- van den Kommer TN, et al. Role of lipoproteins and inflammation in cognitive decline: do they interact? Neurobiol Aging. 2012;33:196, e1–12. doi: 10.1016/j.neurobiolaging.2010.05.024. [DOI] [PubMed] [Google Scholar]
- van Exel E, et al. Association between high-density lipoprotein and cognitive impairment in the oldest old. Ann Neurol. 2002;51:716–21. doi: 10.1002/ana.10220. [DOI] [PubMed] [Google Scholar]
- Van Lenten BJ, et al. Lipoprotein inflammatory properties and serum amyloid A levels but not cholesterol levels predict lesion area in cholesterol-fed rabbits. J Lipid Res. 2007;48:2344–53. doi: 10.1194/jlr.M700138-JLR200. [DOI] [PubMed] [Google Scholar]
- Vance DE, Vance JE. Biochemistry of lipids, lipoproteins and membranes. Elsevier; Amsterdam ; Boston: 2008. [Google Scholar]
- Vanmierlo T, et al. Liver X receptor activation restores memory in aged AD mice without reducing amyloid. Neurobiol Aging. 2011;32:1262–72. doi: 10.1016/j.neurobiolaging.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Veeraraghavalu K, et al. Comment on “ApoE therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models”. Science. 2013;340:924–f. doi: 10.1126/science.1235505. [DOI] [PubMed] [Google Scholar]
- Verghese PB, et al. ApoE influences amyloid-beta (Abeta) clearance despite minimal apoE/Abeta association in physiological conditions. Proc Natl Acad Sci U S A. 2013;110:E1807–16. doi: 10.1073/pnas.1220484110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers KC, et al. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. 2011;13:423–33. doi: 10.1038/ncb2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollbach H, et al. APOA1 polymorphism influences risk for early-onset nonfamiliar AD. Ann Neurol. 2005;58:436–41. doi: 10.1002/ana.20593. [DOI] [PubMed] [Google Scholar]
- Wahrle SE, et al. Deletion of Abca1 increases Abeta deposition in the PDAPP transgenic mouse model of Alzheimer disease. J Biol Chem. 2005;280:43236–42. doi: 10.1074/jbc.M508780200. [DOI] [PubMed] [Google Scholar]
- Wahrle SE, et al. ABCA1 is required for normal central nervous system ApoE levels and for lipidation of astrocyte-secreted apoE. J Biol Chem. 2004;279:40987–93. doi: 10.1074/jbc.M407963200. [DOI] [PubMed] [Google Scholar]
- Walker FO. Huntington's disease. Lancet. 2007;369:218–28. doi: 10.1016/S0140-6736(07)60111-1. [DOI] [PubMed] [Google Scholar]
- Wang ES, et al. Tetranectin and apolipoprotein A-I in cerebrospinal fluid as potential biomarkers for Parkinson's disease. Acta Neurol Scand. 2010;122:350–9. doi: 10.1111/j.1600-0404.2009.01318.x. [DOI] [PubMed] [Google Scholar]
- Weidner C, et al. Lemon balm extract causes potent antihyperglycemic and antihyperlipidemic effects in insulin-resistant obese mice. Mol Nutr Food Res. 2013 doi: 10.1002/mnfr.201300477. [DOI] [PubMed] [Google Scholar]
- Weinmann S, et al. Effects of Ginkgo biloba in dementia: systematic review and meta-analysis. BMC Geriatr. 2010;10:14. doi: 10.1186/1471-2318-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesson DW, et al. Sensory network dysfunction, behavioral impairments, and their reversibility in an Alzheimer's beta-amyloidosis mouse model. J Neurosci. 2011;31:15962–71. doi: 10.1523/JNEUROSCI.2085-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexler NS, et al. Venezuelan kindreds reveal that genetic and environmental factors modulate Huntington's disease age of onset. Proc Natl Acad Sci U S A. 2004;101:3498–503. doi: 10.1073/pnas.0308679101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams-Gray CH, et al. Apolipoprotein E genotype as a risk factor for susceptibility to and dementia in Parkinson's disease. J Neurol. 2009;256:493–8. doi: 10.1007/s00415-009-0119-8. [DOI] [PubMed] [Google Scholar]
- Wolozin B. A fluid connection: cholesterol and Abeta. Proc Natl Acad Sci U S A. 2001;98:5371–3. doi: 10.1073/pnas.101123198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray T, Rogers J. Inflammation in Alzheimer disease-a brief review of the basic science and clinical literature. Cold Spring Harb Perspect Med. 2012;2:a006346. doi: 10.1101/cshperspect.a006346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T, et al. Astroglial localization of cholesteryl ester transfer protein in normal and Alzheimer's disease brain tissues. Acta Neuropathol. 1995;90:633–6. doi: 10.1007/BF00318577. [DOI] [PubMed] [Google Scholar]
- Yamauchi Y, et al. Apolipoprotein A-I activates protein kinase C alpha signaling to phosphorylate and stabilize ATP binding cassette transporter A1 for the high density lipoprotein assembly. J Biol Chem. 2003;278:47890–7. doi: 10.1074/jbc.M306258200. [DOI] [PubMed] [Google Scholar]
- Yao P, et al. Ginkgo biloba extract prevents ethanol induced dyslipidemia. Am J Chin Med. 2007;35:643–52. doi: 10.1142/S0192415X07005132. [DOI] [PubMed] [Google Scholar]
- Yin GN, et al. Neuronal pentraxin receptor in cerebrospinal fluid as a potential biomarker for neurodegenerative diseases. Brain Res. 2009;1265:158–70. doi: 10.1016/j.brainres.2009.01.058. [DOI] [PubMed] [Google Scholar]
- Yoon M, et al. Peroxisome proliferator-activated receptor alpha is involved in the regulation of lipid metabolism by ginseng. Br J Pharmacol. 2003;138:1295–302. doi: 10.1038/sj.bjp.0705169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, et al. Proposed mechanism for lipoprotein remodelling in the brain. Biochim Biophys Acta. 2010;1801:819–23. doi: 10.1016/j.bbalip.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, et al. The CETP I405V polymorphism is associated with an increased risk of Alzheimer's disease. Aging Cell. 2012;11:228–33. doi: 10.1111/j.1474-9726.2011.00777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelcer N, et al. Attenuation of neuroinflammation and Alzheimer's disease pathology by liver x receptors. Proc Natl Acad Sci U S A. 2007;104:10601–6. doi: 10.1073/pnas.0701096104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, et al. Quantitative proteomic analysis of serum proteins in patients with Parkinson's disease using an isobaric tag for relative and absolute quantification labeling, two-dimensional liquid chromatography, and tandem mass spectrometry. Analyst. 2012;137:490–5. doi: 10.1039/c1an15551b. [DOI] [PubMed] [Google Scholar]
- Zintzaras E, Hadjigeorgiou GM. Association of paraoxonase 1 gene polymorphisms with risk of Parkinson's disease: a meta-analysis. J Hum Genet. 2004;49:474–81. doi: 10.1007/s10038-004-0176-x. [DOI] [PubMed] [Google Scholar]
- Zuliani G, et al. Relationship between low levels of high-density lipoprotein cholesterol and dementia in the elderly. The InChianti study. J Gerontol A Biol Sci Med Sci. 2010;65:559–64. doi: 10.1093/gerona/glq026. [DOI] [PMC free article] [PubMed] [Google Scholar]