Abstract
Cells change their shape and mechanics dramatically during development and tissue healing in response to morphogens, cell-cell contact, adhesion to extracellular matrix, and more. Several regulatory links have been described between cell shape, cytoskeletal tension, matrix adhesiveness and stiffness, and recent studies have begun to uncover how these mechanotransduction pathways can impact transcriptional signaling and cell fate decision. The integrated mechanisms linking cell forces, form and fate are likely critical for driving normal morphogenesis, tissue development, and healing. Dysregulation of these mechanisms may also tip the scale from normal to diseased states. Here, we highlight mechanisms that alter cell shape and mechanics, and the pathways affected by these changes.
Introduction
The development of complex multicellular organisms, organs, and tissues involves carefully orchestrated rearrangements in the organization of cells resulting from changes in cell shape and polarity, cell migration, as well as cell-generated contractile forces [1]. A critical feature of these multicellular specializations is that the structural and mechanical events are tightly associated with the cellular differentiation programs [2].
Classically, the progression of differentiation to specific cell types results in the expression of specialized cytoskeletal, adhesive, and extracellular matrix proteins that can change the overall shape, organization, and contractile apparatus of cells (for review on forces in morphogenesis, refer to [3]). In the earliest stages of embryogenesis, for example, the establishment of mesoderm results in a mesenchymal population that invades basally to give rise to new compartments. Differentiation of into specialized cells results in unique shape and structural characteristics associated with their differentiated functions, for example, adipocytes adopt a round morphology critical for lipid storage, requiring decreased adhesion and the disassembly of actin stress fibers during adipocyte differentiation [4]. With the growing body of literature defining scaffolding and polarity proteins that define cellular architecture, we may soon be able to define the molecular basis for how cells organize.
The regulatory link between cell fate and structure, however, is not unidirectional. For example, the degree of cell spreading against an extracellular matrix has been shown to drive changes in cell signaling, proliferation, survival, and stem cell differentiation [5]. Similarly, direct modulation of cellular contractility by non-muscle myosin activity can regulate cell fate [6,7]. Here, we integrate recent literature to describe the current paradigm for how the local physical microenvironment can modulate cell shape and mechanics, and how these changes in cellular form and forces are transduced to drive changes in cellular signaling and fate (Fig 1). These regulatory mechanisms are not limited to development and physiology, and emerging experimental models of altered microenvironments during disease will provide a better understanding of the role of structure-function mechanisms in pathological states.
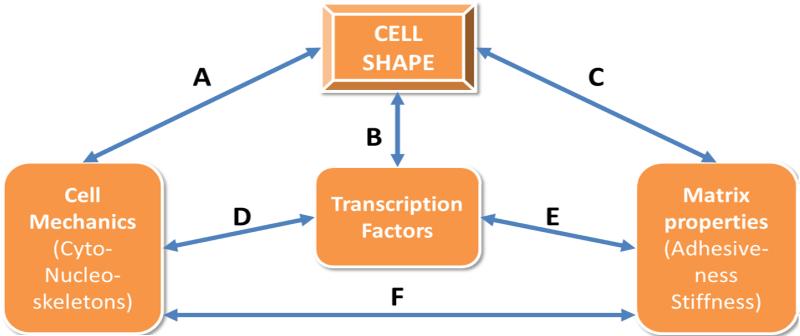
Figure 1. Cell shape dynamics as a regulator of cell fate.
Regulation of cell shape is a complex and dynamic process. Classically, in vitro cell shape was thought to be the output of variables such as adhesive ligands or more recently substrate stiffness, while the field of clinical pathology uses cell shape as a histological marker of normal versus diseased cells. During development, morphogenic cues, alignment and tension drive cell shape changes to create new tissues and organs. Using engineering approaches, such as limiting adhesion or altering stiffness, we can modulate cell shape to alter the cell’s mechanics (arrows A or C) for example via Rho mediated tension or actin reorganization, which in turn can regulate transcriptional activity to drive cell fate (D). Alternatively, changes to cell’s environment during disease or healing changes cell shape (C), possibly exacerbating initial pathology (C - E). It is also possible to imagine that transcriptional changes alter the cell’s mechanics (D - F - C), stiffening the local environment, leading to cell shape changes.
Cell shape and mechanics as an integrated mechanochemical regulator of cell function
The density of cells in culture has long been recognized as a major regulator of cell proliferation and differentiation [8-10], but how the increase in cell density exerts these effects was largely thought to be via increased juxtacrine and paracrine signaling [11,12]. Folkman and Moscona [13] were the first to suggest an alternative, that the crowding-induced decrease in cell spreading and flattening against the underlying substrate could contribute to growth arrest, and Ingber [14] showed that decreasing matrix ligand availability could phenocopy the decreased spreading and proliferation in the absence of any cell-cell contacts. Using micropatterned substrates to directly control cell shape without the confounding effects of altering matrix density demonstrated that the area of cell spreading could drive changes in cell proliferation and survival [15]. Using bone marrow-derived mesenchymal stem cells as a model for multi-lineage differentiation, we further showed that the degree of cell spreading could switch their commitment between lineage fates, in which well spread cells undergo osteogenesis while less spread cells undergo adipogenesis [7]. While the area of cell spreading appears to be a major determinant for cell fate signaling, more recent studies have shown that changes in cell aspect ratio, given the same area of cell spreading, can also modulate fate choices [16,17]. Studies re-introducing cell-cell contacts in micropatterned contexts showed that in addition to crowding, the presence of neighboring cells via engagement of cadherins can modulate cell spreading via changes in Rac and Rho GTPase signaling [18-21] (for review on cell-cell contact adhesion signaling refer to [22]). Together, these studies suggested that changes in cell density and cell-cell contact, matrix adhesiveness, and the geometric presentation of matrix could each drive changes in cell shape, and that these cell shape changes were themselves involved in regulating cell signaling and fate.
Cell spreading appears to regulate fate signaling at least in part through its effects on cytoskeletal contractility by activation of non-muscle myosin II. Increasing cell spreading in mesenchymal stem cells upregulates RhoA activity, ROCK activity, myosin phosphorylation, and cell-generated traction forces against underlying matrix leading to osteogenesis and exogenous upregulation of RhoA or ROCK activity triggered osteogenesis while blocking RhoA-mediated contractility induced adipogenesis [7,23,24]. Because RhoA-mediated traction forces are known to be required for the maturation of focal adhesions [21,25], and the degree of focal adhesion assembly directly correlates with the degree of cell spreading [26], it has largely been presumed that the mechanism by which forces are transduced into a fate signal resides within the adhesions. Yet, although some studies suggest the involvement of focal adhesion kinase (FAK) in these proliferation and differentiation responses [27,28], a clear mechanism implicating adhesions remains to be reported.
More recently, substrate stiffness has been shown to also drive changes in cell proliferation and differentiation [6,29-31]. Seeding cells on acrylamide gels of decreasing stiffness led to growth arrest[31], and differentiation changes in a number of different stem cell types, including changes in mesenchymal stem cell lineage commitment [6,32-35]. Interestingly, it was reported that the same ranges of stiffness that altered cell fates also were associated with changes in cell spreading against the substrate [6,36]. By measuring the spread area, traction forces, and focal adhesion assembly of single cells within a population cultured on substrates of different stiffness, we found that traction forces and focal adhesion assembly correlated highly with cell spreading and secondarily with substrate stiffness, suggesting that the effect of substrate stiffness on lineage commitment is driven through stiffness-mediated changes in cell shape, though this sequence has not been directly demonstrated [24]. An important note is that when cell spreading is held constant, cells are still able to alter their mechanics in response to changes in substrate stiffness [36]. Cells are able to undergo “stiffness matching” in where they reorganize their actin cytoskeleton to essentially match that stiffness of their substrate. Gilbert et al. [33] demonstrated this effect in the context of the muscle stem cell niche, maintaining isolated stem cells on a matrix with stiffness matching their in vivo niche yielded improved engraftment and healing when implanted. The implication of this result is that cell properties such as shape and cytoskeletal dynamics were unaltered during ex vivo culture such that upon implantation the cells could function appropriately. Recent follow-on studies suggest that the approach could be used to heal older muscles, where culturing muscle stem cells from aged mice on soft hydrogels before re-implantation, improves engraftment and regeneration [37].
As with muscle, many native stem cell niches are soft relative to standard tissue culture plates. Dixon et al., [38] exploited this knowledge to preserve stem-ness of pluripotent stem cells. Using a composite material, cells initially experienced a soft matrix and were poorly attached, remaining stem-like. When the softer material was leached out the cells experienced a stiffer matrix altering their shape to become more spread and began to undergo differentiation [38]. However, while cell shape was altered, the interactions with the surrounding matrix were as well. The finding that stem cells appear to proliferate and remain stem-like in soft settings while mature somatic cells appear to proliferate only on stiff matrices suggests that the mechanisms for transducing stiffness can be inverted during cell differentiation. How cells rewire these mechanotransduction pathways remains to be described.
Transduction of cell shape and mechanics into cellular responses
It has become increasingly evident that changes in cell shape and mechanics are critical drivers of cell signaling and function, yet the mechanisms by which structure and function are linked are only recently being uncovered. To identify mechanosensitive transcription pathways, Dupont et al [39] performed a gene expression screen on cells plated atop varying matrix stiffnesses and identified YAP (Yes-associated protein) and TAZ (transcriptional activator with PDZ-binding motif) as key, differentially expressed factors, which localize to the nucleus with increased stiffness. One might speculate that actin polymerization levels modulate YAP/TAZ shuttling and nuclear localization, similar to the MRTF-regulation of SRF (serum response factor) (for review on transcriptional control of actin dynamics refer to [40]). However, inhibition of Rho activity prevented YAP/TAZ nuclear localization while inhibiting F-actin polymerization did not. Thus YAP/TAZ only accumulates in the nucleus when the cell is actively able to generate tension, providing the first description of a transcriptional mechanism that appears to be dependent on force. As with the earlier studies on stiffness dependent lineage commitment [6,41], knockdown of YAP/TAZ activity on stiff surfaces increased adipogenic differentiation, while the depletion of YAP/TAZ on stiff surfaces prevented osteogenic differentiation. An analogous study, again based on differential whole genome gene expression datasets identified SRF as a cell shape-modulated transcriptional signal. While SRF activity can be regulated by RhoA via MRTFs [42,43], and cell spreading regulates RhoA [7,44], cell shape-stimulated SRF activity was surprisingly independent of Rho-mediated cell tension. Instead, cell shape regulated SRF activity via the classical MAPK/TCF pathway. Spread cells(i.e. high adhesion) increased JNK activation, Sap-1 promoter binding, and SRF-mediated transcription, while rounded cells (i.e. low adhesion) stimulated p38 dependent Net promoter binding and inhibition of transcription [45].
One more example of mechanoregulated transcription comes from studies of blood vessel formation, a concert of proliferation, migration and tube formation, requiring different levels of cytoskeletal organization and activity. GATA2 was found to act as a key Rho-mediated mechanosensitive regulator in endothelial cells, increasing VEGFR2 (vascular endothelial growth factor receptor 2) expression, with increased activity on stiff matrices [46] (for a more indepth review on mechanosensitive transcriptional factors refer to [47]). While inhibition of Rho mediated tension in endothelial cells by binding transcription factor TFII-1, inhibits VEGFR2 expression. GATA2 and TFII-1 antagonize each other controlling the vessel formation [48]. The balance of stiffness sensing by GATA2 and VEGFR2 activity by TFII-1 allowed for the appropriate coordination of proliferation, migration and network formation both in in vitro and in vivo models.
While much effort has focused on focal adhesions as a potential early mechanosensor that responds to changes in substrate stiffness and cell shape, the nucleoskeleton has recently been implicated as well. It was observed that cells on a soft matrix have a wrinkled nucleus, in contrast to cells on a stiff matrix which have a smoother, flattened appearance, and that this change resulted from a change in the expression of lamin-A, a class of intermediate filaments of the nucleoskeleton that stabilize the nucleus[49] (for review on Lamins refer to [50]). Cells residing in soft tissue depots expressed low levels of nuclear lamin-A in contrast to cells in stiffer tissues which expressed high levels of nuclear lamin-A. Importantly, directly changing lamin-A levels modulated the differentiation of mesenchymal stem cells. A possible mechanism for this effect is that Lamin-A interacts with nuclear actin to regulate serum response factor (SRF) and also stabilizes YAP nuclear localization.
On the horizon: Dimensionality and dynamic regulation of cell shape and fate
One limitation to the generalizability of these aforementioned systems is their use of flat culture substrates. While some cells are found on flat surfaces in the body, such as with epidermal and epithelial linings, many cells are surrounded by a 3-dimensional (3D) matrix (for review on 3D microenvironments refer to [51]). An example of this shortcoming is illustrated by recent findings suggesting that cells remain rounded and do [51]. Cells are not spread in stiff 3D matrices as they would on stiff 2D substrates. Khetan et al. [52] encapsulated stem cells within hyaluronic acid hydrogels and subjected the matrix to sequential crosslinking with a degradable peptide. Sequential crosslinking directs cells to remain rounded, while in non-crosslinked gels cells become spread. When placed in bipotent media (1:1 mix of adipogenic to osteogenic media) rounded cells favored adipogenesis while spread favored osteogenesis. However, if crosslinking was delayed or when myosin activity was chemically inhibited cells remained spread and underwent adipogenesis. These results demonstrate that the relationship between matrix stiffness, cell shape, cytoskeleton tension, and differentiation signaling can be substantially more complex in a 3D environment.
Importantly, while mechanotransduction studies have largely focused on maintaining uniform adhesive and mechanical conditions throughout a study, the dynamics of changes in cell adhesion, shape and mechanics could be important to how they impact cell function. One study has recently examined whether cells develop a “memory” of their previous mechanical state [53]. Using a novel biomaterial system where substrate stiffness can be changed over time, it was found that YAP activity persisted the longer a cell is cultured on a stiff matrix and biased mesenchymal stem cells towards osteogenesis. The longer a cell was on a stiff matrix before that matrix was softened led to a more elongated phenotype, while cells only experiencing a soft matrix were more rounded. More surprising was that YAP persisted on stiff matrices even after actin stress fibers were chemically abolished. These findings demonstrate a need to consider temporal control in understanding how these signals are interpreted.
Although mechanotransduction studies have focused mainly on normal cell function, the field will inevitably need to address how these regulatory mechanisms contribute to abnormal phenotype in pathologic conditions. In fact, the field of clinical pathology is based on evaluation of the cell’s shape, using morphology parameters as metrics. Therefore understanding cell shape not only in the context of morphogenesis and homeostasis but also of disease is of great interest. For example, in epidermal differentiation, cytoskeletal reorganization is a critical part of the process. Here, a loss of SRF leads to the inability of cytoskeletal reorganization to drive polarization, and as such cells cannot undergo division, differentiation and stratification [54]. In cutaneous wounding, cells undergo a shape change as they become more fibro-proliferative when migrating to close the wound. Mechanical strain increases cytoskeletal tension through focal adhesions inducing a signaling cascade leading to inflammation and fibrosis. Inhibiting focal adhesion formation prevents cells from converting this mechanical signal into eventual scarring [55]. Cell shape dynamics have also been described in the cancer field, in the context of transitions that lead to extravasation from a tumor to metastasize at a secondary site. In one study, hypoxia inducible factor (HIF) directly regulated Rho activity, leading to increased cell motility via increased cytoskeletal reorganization, with a further increase when placed on stiffer substrates to mimic tumor stiffness [56]. Inhibition of focal adhesions or HIF activity blocked Rho-mediated cell motility. These illustrate the need to understand how the cell integrates signals to mediate cell shape dynamics (or cytoskeletal reorganization) to ultimately regulate pathological states [57].
Conclusion
Early seminal studies stressed that cell shape can have profound effects on a cell’s behavior. These early studies used simple tools to manipulate cell adhesion, shape, and mechanics, and set the stage, for more recent studies that have begun to identify molecular mechanisms for how such cues are regulating signaling. As the focus intensifies on a more complete understanding of these mechanisms, advances in biomaterials engineering has allowed the examination of cells within 3D matrices, revealing a complex interaction between matrix stiffness, cell shape and mechanics, and cell fate. Cytoskeletal and nucleoskeletal signaling pathways have been identified that connect changes in cell shape and mechanics to downstream effects. Further contributing to the complexity of these pathways, soluble factors also can alter cell shape and tension. To continue to further our integrated understanding of the links between cellular forces, form and function, biologists and engineers need to continue to work together to develop experimental models to uncover the dynamic regulation and feedback of these processes.
Acknowledgments
We wish to thank Jeroen Eyckmans, William Polacheck for helpful discussions and reviewing the manuscript. We gratefully acknowledge funding sources T32 EB005583 (E.B.) and EB00262 and GM74048 (C.S.C.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Leptin M, Grunewald B. Cell shape changes during gastrulation in Drosophila. Development. 1990;110:73–84. doi: 10.1242/dev.110.1.73. [DOI] [PubMed] [Google Scholar]
- 2.Thompson DW. On growth and form. University Press; 1942. [Google Scholar]
- 3.Heisenberg C-P, Bellaïche Y. Forces in tissue morphogenesis and patterning. Cell. 2013;153:948–62. doi: 10.1016/j.cell.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 4.Spiegelman BM, Farmer SR. Decreases in tubulin and actin gene expression prior to morphological differentiation of 3T3 adipocytes. Cell. 1982;29:53–60. doi: 10.1016/0092-8674(82)90089-7. [DOI] [PubMed] [Google Scholar]
- 5.Ingber D. Extracellular matrix and cell shape: potential control points for inhibition of angiogenesis. J. Cell. Biochem. 1991;47:236–41. doi: 10.1002/jcb.240470309. [DOI] [PubMed] [Google Scholar]
- 6.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006:126677. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 7.McBeath R, Pirone D, Nelson C. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 8.Abercrombie M, Heaysman JE. Observations on the social behaviour of cells in tissue culture. II. Monolayering of fibroblasts. Exp. Cell Res. 1954;6:293–306. doi: 10.1016/0014-4827(54)90176-7. [DOI] [PubMed] [Google Scholar]
- 9.Abercrombie M, Dunn GA. Adhesions of fibroblasts to substratum during contact inhibition observed by interference reflection microscopy. Exp. Cell Res. 1975;92:57–62. doi: 10.1016/0014-4827(75)90636-9. [DOI] [PubMed] [Google Scholar]
- 10.Abbott J. The Loss of Phenotypic Traits by Differentiated Cells: III. The Reversible Behavior of Chondrocytes in Primary Cultures. J. Cell Biol. 1966;28:473–487. doi: 10.1083/jcb.28.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ross SE, Hemati N, Longo KA, Bennett CN, Lucas PC, Erickson RL, MacDougald OA. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289:950–3. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- 12.Cadigan KM, Nusse R. Wnt signaling: a common theme in animal development. Genes Dev. 1997;11:3286–305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- 13.Folkman J, Moscona A. Role of cell shape in growth control. Nature. 1978;273:345–9. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- 14.Ingber DE, Folkman J. Mechanochemical switching between growth and differentiation during fibroblast growth factor-stimulated angiogenesis in vitro: role of extracellular matrix. J. Cell Biol. 1989;109:317–30. doi: 10.1083/jcb.109.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276:1425–8. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 16.Ruiz SA, Chen CS. Emergence of patterned stem cell differentiation within multicellular structures. Stem Cells. 2008;26:2921–7. doi: 10.1634/stemcells.2008-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kilian KA, Bugarija B, Lahn BT, Mrksich M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc. Natl. Acad. Sci. U. S. A. 2010;107:4872–7. doi: 10.1073/pnas.0903269107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Nieuw Amerongen GP, Beckers CML, Achekar ID, Zeeman S, Musters RJP, van Hinsbergh VWM. Involvement of Rho kinase in endothelial barrier maintenance. Arterioscler. Thromb. Vasc. Biol. 2007;27:2332–9. doi: 10.1161/ATVBAHA.107.152322. [DOI] [PubMed] [Google Scholar]
- 19.Braga VM, Machesky LM, Hall A, Hotchin NA. The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell-cell contacts. J. Cell Biol. 1997;137:1421–31. doi: 10.1083/jcb.137.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nelson CM, Pirone DM, Tan JL, Chen CS. Vascular endothelial-cadherin regulates cytoskeletal tension, cell spreading, and focal adhesions by stimulating RhoA. Mol. Biol. Cell. 2004;15:2943–53. doi: 10.1091/mbc.E03-10-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu WF, Nelson CM, Pirone DM, Chen CS. E-cadherin engagement stimulates proliferation via Rac1. J. Cell Biol. 2006;173:431–41. doi: 10.1083/jcb.200510087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Braga VM. Cell-cell adhesion and signalling. Curr. Opin. Cell Biol. 2002;14:546–556. doi: 10.1016/s0955-0674(02)00373-3. [DOI] [PubMed] [Google Scholar]
- 23.Ren XD, Kiosses WB, Schwartz MA. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 1999;18:578–85. doi: 10.1093/emboj/18.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu J, Wang Y-K, Yang MT, Desai R a, Yu X, Liu Z, Chen CS. Mechanical regulation of cell function with geometrically modulated elastomeric substrates. Nat. Methods. 2010;7:733–6. doi: 10.1038/nmeth.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riveline D, Zamir E, Balaban NQ, Schwarz US, Ishizaki T, Narumiya S, Kam Z, Geiger B, Bershadsky AD. Focal Contacts as Mechanosensors: Externally Applied Local Mechanical Force Induces Growth of Focal Contacts by an Mdia1-Dependent and Rock-Independent Mechanism. J. Cell Biol. 2001;153:1175–1186. doi: 10.1083/jcb.153.6.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen CS, Alonso JL, Ostuni E, Whitesides GM, Ingber DE. Cell shape provides global control of focal adhesion assembly. Biochem. Biophys. Res. Commun. 2003;307:355–361. doi: 10.1016/s0006-291x(03)01165-3. [DOI] [PubMed] [Google Scholar]
- 27.Pirone DM, Liu WF, Ruiz SA, Gao L, Raghavan S, Lemmon CA, Romer LH, Chen CS. An inhibitory role for FAK in regulating proliferation: a link between limited adhesion and RhoA-ROCK signaling. J. Cell Biol. 2006;174:277–88. doi: 10.1083/jcb.200510062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schober M, Raghavan S, Nikolova M, Polak L, Pasolli HA, Beggs HE, Reichardt LF, Fuchs E. Focal adhesion kinase modulates tension signaling to control actin and focal adhesion dynamics. J. Cell Biol. 2007;176:667–80. doi: 10.1083/jcb.200608010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Young DA, Choi YS, Engler AJ, Christman KL. Stimulation of adipogenesis of adult adipose-derived stem cells using substrates that mimic the stiffness of adipose tissue. Biomaterials. 2013;34:8581–8. doi: 10.1016/j.biomaterials.2013.07.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bae YH, Mui KL, Hsu BY, Liu S-L, Cretu A, Razinia Z, Xu T, Puré E, Assoian RK. A FAK-Cas-Rac-Lamellipodin Signaling Module Transduces Extracellular Matrix Stiffness into Mechanosensitive Cell Cycling. Sci. Signal. 2014;7:ra57. doi: 10.1126/scisignal.2004838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klein EA, Yin L, Kothapalli D, Castagnino P, Byfield FJ, Xu T, Levental I, Hawthorne E, Janmey PA, Assoian RK. Cell-cycle control by physiological matrix elasticity and in vivo tissue stiffening. Curr. Biol. 2009;19:1511–8. doi: 10.1016/j.cub.2009.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trappmann B, Gautrot JE, Connelly JT, Strange DGT, Li Y, Oyen ML, Cohen Stuart MA, Boehm H, Li B, Vogel V, et al. Extracellular-matrix tethering regulates stem-cell fate. Nat. Mater. 2012;11:642–9. doi: 10.1038/nmat3339. [DOI] [PubMed] [Google Scholar]
- 33.Gilbert PM, Havenstrite KL, Magnusson KEG, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP, Blau HM. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329:1078–81. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holst J, Watson S, Lord MS, Eamegdool SS, Bax DV, Nivison-Smith LB, Kondyurin A, Ma L, Oberhauser AF, Weiss AS, et al. Substrate elasticity provides mechanical signals for the expansion of hemopoietic stem and progenitor cells. Nat. Biotechnol. 2010;28:1123–8. doi: 10.1038/nbt.1687. [DOI] [PubMed] [Google Scholar]
- 35.Chowdhury F, Na S, Li D, Poh Y-C, Tanaka TS, Wang F, Wang N. Material properties of the cell dictate stress-induced spreading and differentiation in embryonic stem cells. Nat. Mater. 2010;9:82–8. doi: 10.1038/nmat2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tee S-Y, Fu J, Chen CS, Janmey PA. Cell shape and substrate rigidity both regulate cell stiffness. Biophys. J. 2011;100:L25–7. doi: 10.1016/j.bpj.2010.12.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cosgrove BD, Gilbert PM, Porpiglia E, Mourkioti F, Lee SP, Corbel SY, Llewellyn ME, Delp SL, Blau HM. Rejuvenation of the muscle stem cell population restores strength to injured aged muscles. Nat. Med. 2014;20:255–64. doi: 10.1038/nm.3464. ** This study builds on “stiffness matching” i.e. the idea that stem cell niches tend to be soft and therefore culturing stem cells on a substrate that matches that of their niche perserves stem-ness. This work elegantly extends to healing injured and aged muscle. This has great implications for improving muscle regeneration in our increasingly aging population
- 38.Dixon JE, Shah DA, Rogers C, Hall S, Weston N, Parmenter CDJ, McNally D, Denning C, Shakesheff KM. Combined hydrogels that switch human pluripotent stem cells from self-renewal to differentiation. Proc. Natl. Acad. Sci. 2014 doi: 10.1073/pnas.1319685111. doi:10.1073/pnas.1319685111. * The authors demonstrate a simple biomaterials based approach to maintain stemness in human pluripotent stem cells without exogenous factors. This highlights the cells’ preference to remain stem-like in soft matrices, while “switching” to a stiffer matrix primes the cells to undergo differentiation.
- 39.Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–83. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 40.Olson EN, Nordheim A. Linking actin dynamics and gene transcription to drive cellular motile functions. Nat. Rev. Mol. Cell Biol. 2010;11:353–65. doi: 10.1038/nrm2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hong J-H, Hwang ES, McManus MT, Amsterdam A, Tian Y, Kalmukova R, Mueller E, Benjamin T, Spiegelman BM, Sharp PA, et al. TAZ, a transcriptional modulator of mesenchymal stem cell differentiation. Science. 2005;309:1074–8. doi: 10.1126/science.1110955. [DOI] [PubMed] [Google Scholar]
- 42.Wang D-Z, Li S, Hockemeyer D, Sutherland L, Wang Z, Schratt G, Richardson JA, Nordheim A, Olson EN. Potentiation of serum response factor activity by a family of myocardin-related transcription factors. Proc. Natl. Acad. Sci. U. S. A. 2002;99:14855–60. doi: 10.1073/pnas.222561499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuwahara K, Barrientos T, Pipes GCT, Li S, Olson EN. Muscle-specific signaling mechanism that links actin dynamics to serum response factor. Mol. Cell. Biol. 2005;25:3173–81. doi: 10.1128/MCB.25.8.3173-3181.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tan JL, Tien J, Pirone DM, Gray DS, Bhadriraju K, Chen CS. Cells lying on a bed of microneedles: an approach to isolate mechanical force. Proc. Natl. Acad. Sci. U. S. A. 2003;100:1484–9. doi: 10.1073/pnas.0235407100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wozniak Ma, Cheng CQ, Shen CJ, Gao L, Olarerin-George AO, Won K-J, Hogenesch JB, Chen CS. Adhesion regulates MAP kinase/ternary complex factor exchange to control a proliferative transcriptional switch. Curr. Biol. 2012;22:2017–26. doi: 10.1016/j.cub.2012.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mammoto A, Huang S, Ingber DE. Filamin links cell shape and cytoskeletal structure to Rho regulation by controlling accumulation of p190RhoGAP in lipid rafts. J. Cell Sci. 2007;120:456–67. doi: 10.1242/jcs.03353. [DOI] [PubMed] [Google Scholar]
- 47.Mammoto A, Mammoto T, Ingber DE. Mechanosensitive mechanisms in transcriptional regulation. J. Cell Sci. 2012;125:3061–73. doi: 10.1242/jcs.093005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mammoto A, Connor KM, Mammoto T, Yung CW, Huh D, Aderman CM, Mostoslavsky G, Smith LEH, Ingber DE. A mechanosensitive transcriptional mechanism that controls angiogenesis. Nature. 2009;457:1103–8. doi: 10.1038/nature07765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Swift J, Ivanovska IL, Buxboim A, Harada T, Dingal PCDP, Pinter J, Pajerowski JD, Spinler KR, Shin J-W, Tewari M, et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science. 2013;341:1240104. doi: 10.1126/science.1240104. * This work begins to unravel the mystery of mechanosensory machinery of the nucleus adding another layer of stiffness sensing to the cell
- 50.Ho CY, Lammerding J. Lamins at a glance. J. Cell Sci. 2012;125:2087–93. doi: 10.1242/jcs.087288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baker BM, Chen CS. Deconstructing the third dimension: how 3D culture microenvironments alter cellular cues. J. Cell Sci. 2012;125:3015–24. doi: 10.1242/jcs.079509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khetan S, Guvendiren M, Legant WR, Cohen DM, Chen CS, Burdick JA. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat. Mater. 2013;12:458–65. doi: 10.1038/nmat3586. ** The authors developed a biomaterial based 3D system for studying the effects of traction on directing stem cell fate. More importantly, they demonstrated that in 3D environments, spread area alone is not responsible for determining cell fate, cells must be able to generate traction, achieved through matrix degradation, to undergo lineage commitment
- 53.Yang C, Tibbitt MW, Basta L, Anseth KS. Mechanical memory and dosing influence stem cell fate. 2014 doi: 10.1038/nmat3889. doi:10.1038/NMAT3889. ** This is the first study to connect transcriptional regulation to a cell’s “memory” of its previous mechanical state. It may provide insight for phenomena such as “stiffness matching” refered to in refs 33, 38
- 54.Luxenburg C, Pasolli HA, Williams SE, Fuchs E. Developmental roles for Srf, cortical cytoskeleton and cell shape in epidermal spindle orientation. Nat. Cell Biol. 2011;13:203–14. doi: 10.1038/ncb2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wong VW, Rustad KC, Akaishi S, Sorkin M, Glotzbach JP, Januszyk M, Nelson ER, Levi K, Paterno J, Vial IN, et al. Focal adhesion kinase links mechanical force to skin fibrosis via inflammatory signaling. Nat. Med. 2012;18:148–52. doi: 10.1038/nm.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gilkes DM, Xiang L, Lee SJ, Chaturvedi P, Hubbi ME, Wirtz D, Semenza GL. Hypoxia-inducible factors mediate coordinated RhoA-ROCK1 expression and signaling in breast cancer cells. Proc. Natl. Acad. Sci. U. S. A. 2014;111:E384–93. doi: 10.1073/pnas.1321510111. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 57.Burnette DT, Shao L, Ott C, Pasapera AM, Fischer RS, Baird M a, Der Loughian C, Delanoe-Ayari H, Paszek MJ, Davidson MW, et al. A contractile and counterbalancing adhesion system controls the 3D shape of crawling cells. J. Cell Biol. 2014;205:83–96. doi: 10.1083/jcb.201311104. [DOI] [PMC free article] [PubMed] [Google Scholar]