Abstract
Arginine-vasopressin (AVP) and oxytocin (OT) and their receptors are very similar in structure. As a result, at least some of the effects of these peptides may be the result of crosstalk between their canonical receptors. The present study investigated this hypothesis by determining whether the induction of flank marking, a form of social communication in Syrian hamsters, by OT is mediated by the OT receptor or the AVP V1a receptor. Intracerebroventricular (ICV) injections of OT or AVP induced flank marking in a dose-dependent manner although the effects of AVP were approximately 100 times greater than those of OT. Injections of highly selective V1a receptor agonists but not OT receptor agonists induced flank marking, and V1a receptor antagonists but not OT receptor antagonists significantly inhibited the ability of OT to induce flank marking. Lastly, injection of alpha-melanocyte-stimulating hormone (α-MSH), a peptide that stimulates OT but not AVP release, significantly increased odor-induced flank marking, and these effects were blocked by a V1a receptor antagonist. These data demonstrate that OT induces flank marking by activating AVP V1a and not OT receptors, suggesting that the V1a receptor should be considered to be an OT receptor as well as an AVP receptor.
Keywords: flank marking, aggression, prosocial behavior, α-MSH, social behavior, social recognition
1. Introduction
Oxytocin (OT) and arginine-vasopressin (AVP) are evolutionarily conserved mammalian neuropeptides that play a major role in many complex social behaviors (Albers, 2012; Lee et al., 2009). OT and AVP are structurally very similar, differing only in two of nine amino acids (Caldwell and Young, 2006; Light and Du Vigneaud, 1958; Stoop, 2012). Receptors for OT (OTR) and for AVP (V1aR and V1bR) in the central nervous system (CNS) belong to the G-protein coupled receptor family (Gimpl and Fahrenholz, 2001), and they are also very similar in structure with approximately 85% homology (Neumann and Landgraf, 2012; Sala et al., 2011). Because of the structural similarities among these peptides and their receptors, it is not surprising that OT and AVP can reciprocally activate each other's receptors. In fact, in rats and mice OT and AVP display little selectivity for OT, V1a or V1b receptors (Manning et al., 2012). Taken together, these data suggest the hypothesis that the central effects of both OT and AVP can be mediated by OT, V1a and/or V1b receptors.
Perhaps the most robust effect of AVP on behavior is its ability to induce high levels of flank marking in Syrian hamsters following its injection into the brain (Bamshad and Albers, 1996; Ferris et al., 1984). Flank marking is a form of social communication in which hamsters deposit flank gland secretions by rubbing their flank glands against objects in the environment (Johnston and Brenner, 1982; Johnston and Lee, 1976). Studies of flank marking have not only provided an important window into the mechanisms underlying the control of social behavior by AVP (Ferris et al., 2013), they have also proven to be a powerful bioassay for the behavioral effects of AVP/OT agonists and antagonists in the brain (Albers et al., 1986; Ferris et al., 1988). In the following studies we tested the hypothesis that OT can influence flank marking by acting on V1a receptors.
2. Materials and Methods
2.1 Animals
Adult male Syrian hamsters (Harlan Laboratories Inc., Prattville, AL, and Charles River Laboratories Inc., Wilmington, MA, USA), 8-10 weeks old, weighing between 110-130g were used for all experiments. Hamsters were individually housed in polycarbonate cages (23x43x20 cm) upon arrival and were kept in a 14:10 light/dark cycle with food and water available ad libitum. All experimental procedures were in accordance with the National Institutes of Health Guidelines for the Use of Animals and were approved by the Georgia State University Animal Care and Use Committee.
2.2 Surgery, microinjections, and histology
Hamsters were deeply anesthetized with 5% isoflurane in an induction chamber and maintained under anesthesia with 3.75% isoflurane throughout all surgical procedures. All animals were implanted with a 4mm, 26-gauge cannula guide aimed at the lateral ventricle. The stereotaxic coordinates were +0.8mm anterior to bregma, −1.1mm from the midline, and −2.2mm below dura. Hamsters were allowed one week to recover from surgery before any behavioral testing.
Microinjections were administered over the course of 1 minute into the lateral ventricle using an infusion pump (Harvard Apparatus), a 5μl Hamilton syringe, and a 14mm, 32-gauge microinjection needle. The volume of all microinjections was 1μl. The needle was left in place in the cannula guide for an additional minute following the injection to allow drug diffusion into the ventricle. Hamsters were sacrificed by lethal injection of sodium pentobarbital after testing and were injected with ink to verify the injection sites.
2.3 Drugs
The following drugs were used in ICV injections: OT (Bachem, CA, USA) and AVP (Fisher scientific, TX, USA) both at concentrations of 0.09μM, 0.9μM, 9μM, 90μM, and 900μM; [Thr4,Gly7]OT (TGOT, a highly selective OT receptor agonist, a gift of Dr. Maurice Manning) at concentrations of 270μM and 2700μM; [Phe2]OVT (a highly selective V1a receptor agonist, a gift of Dr. Maurice Manning) at concentrations of 0.23μM, 2.3μM, and 23μM; desGly-NH2-d(CH ) [D-Tyr2Thr4}OVT (a selective OTR antagonist, OTA, a gift of Dr. Maurice Manning) and d(CH2)5[Tyr(Me)2]AVP (a selective V1aR antagonist known as Manning Compound, a gift of Dr. Maurice Manning) both at concentrations of 18μM, 45μM, or 90μM in cocktail solutions mixed with 90μM OT or at 90μM when used alone; α-MSH at 360μM (Tocris, UK). The concentrations of OT and AVP administered were based on the concentrations used in previous studies that were found effective in modulating flank marking, aggression, and sexual receptivity in hamsters (Albers et al., 1986; Ferris et al., 1988; Whitman and Albers, 1995). The concentrations of the OT and AVP agonists were based on their relative efficacies compared to those of OT and AVP in binding to OTR and V1aR in rats, respectively (Manning et al., 2012). The concentration of α-MSH used in these studies was based on those used to stimulate OT release in rats (Sabatier et al., 2003). All control animals were given a 1μl injection of saline.
2.4 Behavioral Testing
Hamsters were handled daily for four days before any behavioral testing. In Experiment 1, flank marking behavior was recorded in each hamster's home cage for 5 minutes immediately following ICV injection of OT or AVP (0.09μM, 0.9μM, 9μM, 90μM, and 900μM). Each hamster was injected with OT and AVP at one of the above five concentrations in a counterbalanced order at 48 hour intervals (n=5 for doses 0.09μM, 0.9μM, and 9μM; n=4 for doses 90μM and 900μM). Flank marking was scored when a hamster pressed its flank gland region against a cage wall and moved forward (Ferris et al., 1984).
In Experiments 2 & 3, flank marking was recorded in each hamster's home cage for five minutes immediately after ICV injection. In Experiment 2, animals received either 90μM OT (n=5), low or high doses of selective OTR agonist (n=9 and n=5, respectively), low, intermediate, or high doses of selective V1aR agonist (n=6, n=7, and n=7, respectively). In Experiment 3, animals were given 90μM OT (n=5), mixed solutions of 90μM OT with OTA (n=5, n=9, n=7 for OT mixed with 90μM OTA, OT mixed with 45μM OTA, and OT with 18μM OTA, respectively), 90μM OT with V1aA (n=6, n=6, n=8 for OT mixed with 90μM V1aA, OT with 45μM V1aA, and OT with 18μM V1aA, respectively), OTA alone (n=6), and V1aA alone (n=7).
Experiment 4 was designed to examine whether α-MSH, which has been shown to be a potent releaser of endogenous OT in rats (Sabatier et al., 2003), would stimulate flank marking. Because ICV injections of α-MSH did not stimulate flank marking in hamsters tested in their home cages, the following experiments examined whether α-MSH would influence flank marking in hamsters tested in cages containing the flank gland scent of other hamsters (i.e., odor-stimulated flank marking). Immediately following ICV injections, hamsters were placed in a testing arena (23x43x20 cm) that contained flank odors from another male hamster, and flank marks were recorded for 10 minutes (n=9 for both α-MSH and saline). The flank odors were freshly deposited in the arena by a donor that had been injected ICV with AVP and allowed to mark the arena 50 times (Gutzler et al., 2011). In Experiment 4B, the V1aR (90μM) antagonist was injected ICV 1 hour before an injection of α-MSH or saline. Within subject design was used in the experiment and the order of α-MSH or saline injections was counter-balanced (n=5). All the experiments took place during the first three hours of the dark phase of the daily light/dark cycle. All behavioral testing was videotaped and scored by an individual blind to the experimental conditions.
2.5 Data Analysis and Statistics
SPSS v21 was used to analyze all the data. The data are presented as mean ± standard error of the mean. Independent samples or paired t-tests were used for two-group comparisons and Analysis of Variance (ANOVA) was performed for comparisons among more than two groups. All comparisons were determined a priori; planned contrasts were performed following significant differences found in ANOVA tests. All tests were two tailed and differences were considered significant at p≤0.05.
3. Results
3.1 Dose-dependent effects of OT and AVP on Flank Marking
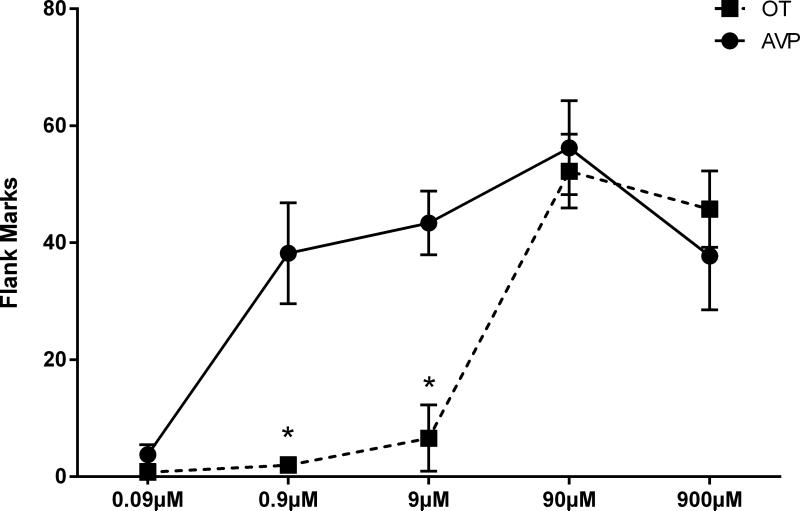
In order to examine the efficacies of OT and AVP in inducing flank marking, we microinjected OT and AVP ICV in a wide range of concentrations (0.09μM, 0.9μM, 9μM, 90μM, and 900μM). There was a significant drug by dose interaction (F(1,4)=7.848, p<0.05). Therefore, analyses were conducted to examine the effects of dose on the OT and AVP groups separately. There was a significant main effect of dose on the number of flank marks recorded following microinjections of OT and AVP (OT groups: F(4,18)=30.210, p<0.0001; AVP groups: F(4,18)=8.109, p<0.001; Fig.1). Hamsters injected with 0.09μM AVP displayed significantly fewer flank marks than those injected with concentrations of 0.9μM, 9μM, 90μM, or 900μM AVP (p<0.005 for all comparisons). Hamsters injected with 0.09μM, 0.9μM, or 9μM OT, flank marked less than those injected with either 90μM or 900μM OT (p<0.0001 for all comparisons). Concentrations of 0.9μM and 9μM OT induced fewer flank marks compared to the same concentrations of AVP when injected into the same animals (paired t-tests: t(4)=4.691, p<0.01; t(4)=10.977, p<0.001; respectively).
Figure 1.
Dose-dependent effects of AVP and OT on flank marking. There was a significant main effect of dose on the number of flank marks that OT or AVP induced. For the doses of 0.9μM and 9μM, OT induced fewer flank marks compared to AVP when injected into the same animals. Note: * indicates a significant difference between OT and AVP.
3.2 Effects of OTR and V1aR Agonists on Flank Marking
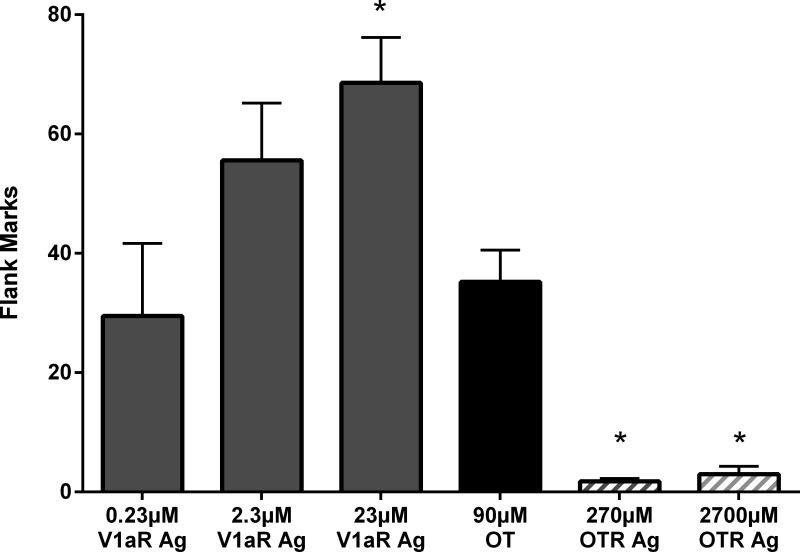
This experiment employed highly selective OTR and V1aR agonists to investigate whether flank marking was induced by activation of OT or V1a receptors. There was a significant main effect of drug treatment on flank marking (F(5,33)=15.010, p<0.0001, Fig.2). Hamsters injected with low or high concentrations of the selective OTR agonist displayed significantly fewer flank marks than those injected with 90μM OT (p<0.005 and p<0.01, respectively). In fact, 10 of the 14 animals injected with either low or high concentrations of the OTR agonist marked less than three times during the five minute test. The numbers of flank marks induced by the low and intermediate concentrations of the selective V1aR agonist did not significantly differ from that induced by OT (p=0.616 and p=0.07 for low and intermediate, respectively). Hamsters injected with the high concentration of V1aR agonist flank marked significantly more than did those animals injected with OT (p<0.005).
Figure 2.
Effects of OTR and V1aR agonists on flank marking. There was a significant main effect of drug treatment among the groups. V1aR Agonist (Ag); OTR Agonist (Ag). Note: * indicates a significant difference compared to OT.
3.3 Effects of OTR and V1aR Antagonists on OT-induced Flank Marking
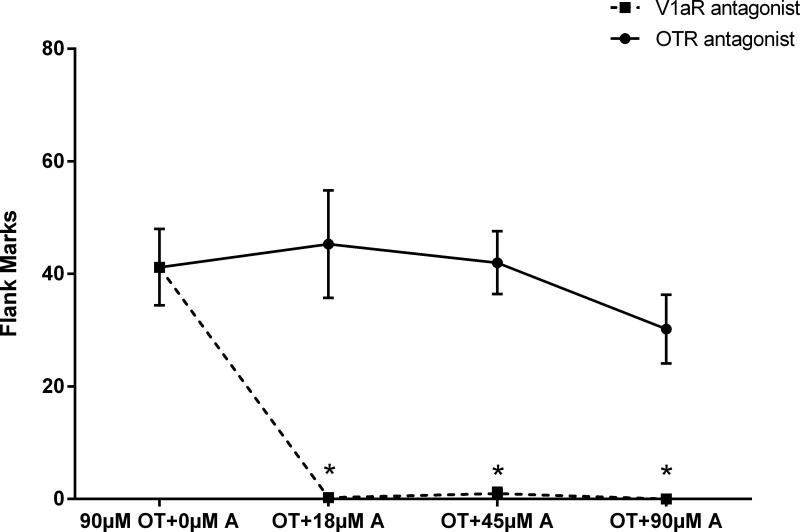
Experiment 3 examined whether selective OTR and V1aR antagonists (OTA and V1aA, respectively) block OT-induced flank marking. There was a significant main effect of drug treatment on the number of flank marks produced (F(3, 22)=49.0, p< 0.0001; Fig.3). Hamsters injected with OT and any of the three concentrations of V1aA flank marked significantly less than those injected with OT alone (P<0.0001 for all comparisons). In fact, all three concentrations of the V1aR antagonist almost completely blocked OT-induced flank marking. In contrast, the same concentrations of the OTA did not significantly alter the amount of OT-induced flank marking (Fig.3). There were no significant differences in the number of flank marks among hamsters injected with OT and any of the three concentrations of OTA and those injected with OT alone (F(3, 22)=0.674, p=0.577). Hamsters in drug control groups injected with only V1aA (90μM) or OTA (90μM) did not differ from saline control animals in flank marking (flank marks: 90μM OTA: 0.0±0.0, n=7; 90μM V1aA: 0.9±1.3, n=6; saline: 0.1±0.2, n=7; F(2,17)=0.760, p=0.483).
Figure 3.
Effects of OTR and V1aR antagonists on OT-induced flank marking. All three concentrations of the V1aR antagonist completely blocked OT induced flank marking. The same concentrations of the OTR antagonist did not significantly affect flank marking. Note: * indicates a significant difference compared to the OT group. A: antagonist.
3.4 Effects of Alpha-MSH on Odor-stimulated Flank Marking
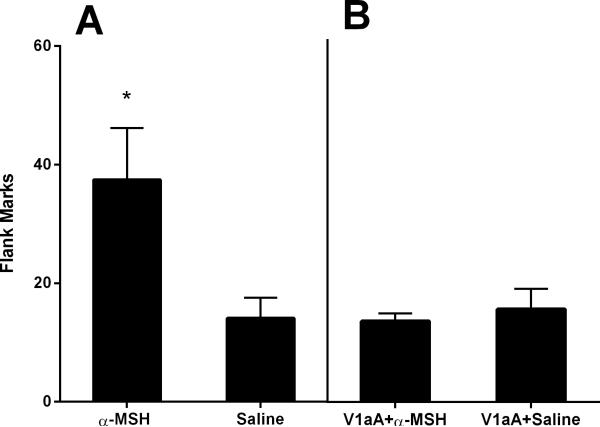
This experiment examined whether α-MSH, a peptide that stimulates endogenous OT release in rats, would enhance odor-induced flank marking. Hamsters injected with α-MSH displayed significantly more odor-induced flank marks than did hamsters injected with saline (independent samples t-test: t(16)=2.338, p<0.05; Fig.4A). This enhancement in flank marking by α-MSH was blocked by an injection of V1aR antagonist prior to testing. After administration of the V1aA, no differences in the number of flank marks were observed between groups of hamsters injected with α-MSH or saline (paired t-test: t(4)=0.611, p=0.574; Fig.4B).
Figure 4.
Effects of alpha-MSH on odor-stimulated flank marking and involvement of V1aR. (A) Hamsters injected with α-MSH flank marked more than those injected with saline. (B) V1aA blocked this α-MSH-induced enhancement in flank marking. Note: * indicates a significant difference compared to the saline group.
4. Discussion
The results of the present study support the hypothesis that OT can induce flank marking by activating V1aRs and not OTRs. The data reveal that selective V1aR but not OTR agonists mimic the effects of OT in inducing flank marking, and that selective V1aR but not OTR antagonists block OT-induced flank marking. In addition, we demonstrated that α-MSH, a peptide that stimulates endogenous release of OT (Sabatier et al., 2003), enhances odor stimulated flank marking and that this enhancement by α-MSH can be blocked by applying a V1aR antagonist prior to testing. Together, these findings suggest that the endogenous release of OT might contribute to the regulation of flank marking as well as other social behavior by acting on V1aRs and that the V1aR should be considered to be an OTR as well as an AVP receptor.
Comparison of OT and AVP induced flank marking reveals that OT stimulates flank marking at concentrations between 9 and 90 μM, while AVP induces flank marking at concentrations between 0.09 and 0.9 μM, suggesting that OT is approximately 100-fold less potent in activating V1aRs than is AVP. Because the levels of OT and AVP released within the hamster brain are not known, it is not clear whether OT released endogenously might contribute to the regulation of the expression of flank marking. In an attempt to investigate whether endogenously released OT might contribute to the induction of flank marking normally stimulated by exposure to flank gland secretions of conspecifics (i.e., odor-stimulated flank marking), we injected α-MSH, which has been shown in rats to induce endogenous OT, but not AVP release, from hypothalamic neurons (Sabatier et al., 2003). Although α-MSH was not sufficient to induce flank marking in the absence of conspecific odors, α-MSH did significantly enhance odor-stimulated flank marking, and this enhancement was blocked by a V1aR antagonist. The inability of α-MSH to induce flank marking in the absence of conspecific odors might be the result of an insufficient amount of endogenously released OT or the release of OT in a brain site outside the optimal region for induction of flank marking. It is also possible that α-MSH induced “priming” in the OT neurons so that more OT was released when the hamsters were subsequently exposed to conspecific odors. (Stoop, 2012). Even though α-MSH, by itself, did not induce flank marking, it did significantly enhance its expression when it was stimulated by relevant environmental cues.
In the present study, the effects of OTR/V1aR agonists and antagonists were investigated following their administration by ICV injection. Previous studies of the effects of AVP on flank marking examined its role in specific brain regions including the medial preoptic–anterior hypothalamus (MPOA-AH), periaqueductal gray, and lateral septum (Albers and Cooper, 1995; Albers et al., 1986; Hennessey et al., 1992; Irvin et al., 1990). The effects of AVP injections within these regions on flank marking are similar to the effects of ICV administration. In both cases, AVP-stimulated flank marking has an ED50 (the dose that elicits a half-maximal behavioral response) of 0.9μM, and the effect lasts for 5-10 minutes (Albers et al., 1986; Caldwell and Albers, 2003; Ferris et al., 2013; Ferris et al., 1988). Although several studies have examined the ability of OT to induce flank marking following its injection into the MPOA-AH, complete dose-response relationships are not available in most cases (Albers et al., 1986; Harmon et al., 2002). In general, the effects of different concentrations of OT into the MPOA-AH in inducing flank marking are consistent with the effects of OT given ICV in the present study (cf. Ferris et al., 1984).
Recent evidence from other studies also supports the hypothesis that OT can act on V1aRs. Central administration of OT appears to produce analgesic effects (Gao and Yu, 2004; Ge et al., 2002) by activating V1aRs (Schorscher-Petcu et al., 2010). OT does not induce analgesia in V1aR knock-out mice and OT-induced analgesia in wild-type mice can be blocked by pretreatment of V1aR but not OTR antagonists (Schorscher-Petcu et al., 2010). Another study also suggests that OT can produce behavioral effects by acting on V1aRs; OT rescues autistic-like deficits of OTR null mice in sociability, aggression, as well as cognitive flexibility by acting on V1aRs (Sala et al., 2011).
In summary, the present study demonstrated that OT induces flank marking by activating V1aRs. Our results raise the important possibility that endogenously released OT might influence other social behaviors by acting on V1aRs. These studies emphasize the importance of pharmacological profiling when studying the behavioral responses to OT and AVP.
Highlights.
Oxytocin, like AVP, induces flank marking in a dose-dependent manner in hamsters.
Selective V1aR agonists but not selective OTR agonists induce flank marking.
V1aR antagonists but not OTR antagonists inhibit oxytocin-induced flank marking.
α-MSH, a potent OT releaser, increases odor induced flank marking.
V1a receptor should be considered to be an OT receptor as well as an AVP receptor.
Abbreviations
- OT
oxytocin
- AVP
arginine vasopressin
- i.c.v
intracerebroventricle
- α-MSH
alpha-melanocyte-stimulating hormone
- OTR
oxytocin receptor
- V1aR
AVP receptor V1a subtype
- OTA
oxytocin receptor antagonist
- V1aA
V1a receptor antagonist
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors:
Song designed and participated in conducting all experiments, analyzed the data, and also wrote the first draft of this manuscript. McCann and McNeill helped design and participated in conducting the experiments, and contributed to the editing of the manuscript. Larkin participated in conducting the experiments, scored the videos, and contributed to the editing of the manuscript. Drs. Albers and Huhman supervised all the experiments, data analyses, and also contributed significantly in the preparation and editing of the manuscript.
References
- Albers HE. The regulation of social recognition, social communication and aggression: vasopressin in the social behavior neural network. Horm Behav. 2012;61:283–292. doi: 10.1016/j.yhbeh.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Albers HE, Cooper TT. Effects of testosterone on the behavioral response to arginine vasopressin microinjected into the central gray and septum. Peptides. 1995;16:269–273. doi: 10.1016/0196-9781(94)00188-x. [DOI] [PubMed] [Google Scholar]
- Albers HE, Pollock J, Simmons WH, Ferris CF. A V1-like receptor mediates vasopressin-induced flank marking behavior in hamster hypothalamus. J Neurosci. 1986;6:2085–2089. doi: 10.1523/JNEUROSCI.06-07-02085.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamshad M, Albers HE. Neural circuitry controlling vasopressin-stimulated scent marking in Syrian hamsters (Mesocricetus auratus). J Comp Neurol. 1996;369:252–263. doi: 10.1002/(SICI)1096-9861(19960527)369:2<252::AID-CNE6>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Caldwell HK, Albers HE. Short-photoperiod exposure reduces vasopressin (V1a) receptor binding but not arginine-vasopressin-induced flank marking in male Syrian hamsters. J Neuroendocrinol. 2003;15:971–977. doi: 10.1046/j.1365-2826.2003.01086.x. [DOI] [PubMed] [Google Scholar]
- Caldwell HK, Young WS., 3rd . Oxytocin and Vasopressin: Genetics and Behavioral Impolications. In: Lim R, editor. Neuroactive Proteins and Peptides. Springer; New York: 2006. pp. 573–607. [Google Scholar]
- Ferris CF, Albers HE, Wesolowski SM, Goldman BD, Luman SE. Vasopressin injected into the hypothalamus triggers a stereotypic behavior in golden hamsters. Science. 1984;224:521–523. doi: 10.1126/science.6538700. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Melloni RH, Albers HE. In: Role of vasopressin in flank marking and aggression Oxytocin, Vasopressin and Related Peptides in the Regulation of Behavior Edited by Elena Choleris. Pfaff Donald W., editor. Martin Kavaliers; 2013. pp. 213–231. [Google Scholar]
- Ferris CF, Singer EA, Meenan DM, Elliott Albers H. Inhibition of vasopressin-stimulated flank marking behavior by V1-receptor antagonists. European journal of pharmacology. 1988;154:153–159. doi: 10.1016/0014-2999(88)90092-1. [DOI] [PubMed] [Google Scholar]
- Gao L, Yu LC. Involvement of opioid receptors in the oxytocin-induced antinociception in the central nervous system of rats. Regul Pept. 2004;120:53–58. doi: 10.1016/j.regpep.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Ge Y, Lundeberg T, Yu LC. Blockade effect of mu and kappa opioid antagonists on the anti-nociception induced by intra-periaqueductal grey injection of oxytocin in rats. Brain Res. 2002;927:204–207. doi: 10.1016/s0006-8993(01)03346-7. [DOI] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. The Oxytocin Receptor System: Structure, Function, and Regulation. Physiological reviews. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Gutzler SJ, Karom M, Erwin WD, Albers HE. Seasonal regulation of social communication by photoperiod and testosterone: effects of arginine-vasopressin, serotonin and galanin in the medial preoptic area-anterior hypothalamus. Behav Brain Res. 2011;216:214–219. doi: 10.1016/j.bbr.2010.07.042. [DOI] [PubMed] [Google Scholar]
- Harmon AC, Huhman KL, Moore TO, Albers HE. Oxytocin inhibits aggression in female Syrian hamsters. J Neuroendocrinol. 2002;14:963–969. doi: 10.1046/j.1365-2826.2002.00863.x. [DOI] [PubMed] [Google Scholar]
- Hennessey AC, Whitman DC, Albers HE. Microinjection of arginine-vasopressin into the periaqueductal gray stimulates flank marking in Syrian hamsters (Mesocricetus auratus). Brain Res. 1992;569:136–140. doi: 10.1016/0006-8993(92)90379-n. [DOI] [PubMed] [Google Scholar]
- Irvin RW, Szot P, Dorsa DM, Potegal M, Ferris CF. Vasopressin in the septal area of the golden hamster controls scent marking and grooming. Physiol Behav. 1990;48:693–699. doi: 10.1016/0031-9384(90)90213-n. [DOI] [PubMed] [Google Scholar]
- Johnston RE, Brenner D. Species-specificity of scent marking in hamsters. Behavioral and Neural Biology. 1982;35:46–55. [Google Scholar]
- Johnston RE, Lee NA. Persistence of the odor deposited by two functionally distinct scent marking behaviors of golden hamsters. Behavioral Biology. 1976;16:199–210. doi: 10.1016/s0091-6773(76)91310-9. [DOI] [PubMed] [Google Scholar]
- Lee H-J, Macbeth AH, Pagani J, Young WS. Oxytocin: The Great Facilitator of Life. Prog Neurobiol. 2009 doi: 10.1016/j.pneurobio.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light A, Du Vigneaud V. On the nature of oxytocin and vasopressin from human pituitary. Proceedings of the Society for Experimental Biology and Medicine. Society for Experimental Biology and Medicine (New York, N.Y.) 1958;98:692–696. doi: 10.3181/00379727-98-24154. [DOI] [PubMed] [Google Scholar]
- Manning M, Misicka A, Olma A, Bankowski K, Stoev S, Chini B, Durroux T, Mouillac B, Corbani M, Guillon G. Oxytocin and vasopressin agonists and antagonists as research tools and potential therapeutics. J Neuroendocrinol. 2012;24:609–628. doi: 10.1111/j.1365-2826.2012.02303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann ID, Landgraf R. Balance of brain oxytocin and vasopressin: implications for anxiety, depression, and social behaviors. Trends Neurosci. 2012;35:649–659. doi: 10.1016/j.tins.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Sabatier N, Caquineau C, Dayanithi G, Bull P, Douglas AJ, Guan XM, Jiang M, Van der Ploeg L, Leng G. Alpha-melanocyte-stimulating hormone stimulates oxytocin release from the dendrites of hypothalamic neurons while inhibiting oxytocin release from their terminals in the neurohypophysis. J Neurosci. 2003;23:10351–10358. doi: 10.1523/JNEUROSCI.23-32-10351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala M, Braida D, Lentini D, Busnelli M, Bulgheroni E, Capurro V, Finardi A, Donzelli A, Pattini L, Rubino T, Parolaro D, Nishimori K, Parenti M, Chini B. Pharmacologic Rescue of Impaired Cognitive Flexibility, Social Deficits, Increased Aggression, and Seizure Susceptibility in Oxytocin Receptor Null Mice: A Neurobehavioral Model of Autism. Biol Psychiatry. 2011;69:875–882. doi: 10.1016/j.biopsych.2010.12.022. [DOI] [PubMed] [Google Scholar]
- Schorscher-Petcu A, Sotocinal S, Ciura S, Dupre A, Ritchie J, Sorge RE, Crawley JN, Hu SB, Nishimori K, Young LJ, Tribollet E, Quirion R, Mogil JS. Oxytocin-induced analgesia and scratching are mediated by the vasopressin-1A receptor in the mouse. J Neurosci. 2010;30:8274–8284. doi: 10.1523/JNEUROSCI.1594-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoop R. Neuromodulation by oxytocin and vasopressin. Neuron. 2012;76:142–159. doi: 10.1016/j.neuron.2012.09.025. [DOI] [PubMed] [Google Scholar]
- Whitman DC, Albers HE. Role of oxytocin in the hypothalamic regulation of sexual receptivity in hamsters. Brain Research. 1995;680:73–79. doi: 10.1016/0006-8993(95)00233-g. [DOI] [PubMed] [Google Scholar]