Abstract
There is ongoing debate concerning the functions of resting-state brain activity. Prior work demonstrates that memory encoding enhances subsequent resting-state functional connectivity within task-relevant networks and that these changes predict better recognition. Here, we used functional connectivity MRI (fcMRI) to examine whether task-induced changes in resting-state connectivity correlate with performance improvement after sleep. In two separate sessions, resting-state scans were acquired before and after participants performed a motor task. In one session participants trained on the motor sequence task (MST), a well-established probe of sleep-dependent memory consolidation, and were tested the next day, after a night of sleep. In the other session they performed a motor control task (MCT) that minimized learning. In an accompanying behavioral control study, participants trained on the MST and were tested after either a night of sleep or an equivalent interval of daytime wake. Both the fcMRI and the sleep control groups showed significant improvement of MST performance, while the wake control group did not. In the fcMRI group, increased connectivity in bilateral motor cortex following MST training correlated with this next-day improvement. This increased connectivity did not appear to reflect initial learning since it did not correlate with learning during training and was not greater after MST training than MCT performance. Instead, we hypothesize that this increased connectivity processed the new memories for sleep-dependent consolidation. Our findings demonstrate that physiological processes immediately after learning correlate with sleep-dependent performance improvement and suggest that the wakeful resting brain prepares memories of recent experiences for later consolidation during sleep.
Keywords: sleep, motor learning, memory consolidation, resting state, functional connectivity MRI, procedural memory
1.0 Introduction
When not actively engaged in an external task, the human brain sustains a high level of spontaneous activity that is synchronized within distinct networks (Smith et al., 2009). Recent studies support a role for this wakeful resting state activity in memory processing. Resting state activity in motor networks is modulated after motor learning, but not after movement per se (Albert et al., 2009; Vahdat et al., 2011). Moreover, both increased resting state functional connectivity in task-relevant networks (Stevens et al., 2010; Tambini et al., 2010) and a ‘replay’ of stimulus-specific neural activity (Deuker et al., 2013) during wakeful rest periods that follow memory encoding predict better recognition (i.e., fewer items forgotten). These findings suggest that resting state activity in task-relevant neural networks after learning contributes to the retention of declarative memories. In the present study we examined whether the modulation of resting state activity by procedural motor learning correlates with subsequent performance enhancement that is known to depend on sleep.
Following active encoding, memory consolidation proceeds off-line, during both wake and sleep, without requiring conscious intent, effort or awareness (Stickgold and Walker, 2007). Not all memories last, however, some rapidly fade. For a memory to be retained for subsequent sleep-dependent consolidation it must be selected for retention and further processing during the intervening wake period (Stickgold and Walker, 2013). We hypothesized that this selection for subsequent sleep-dependent consolidation would be reflected in the modulation of activity in task-relevant brain networks immediately after learning as measured by functional connectivity MRI (fcMRI). To test this, we used a procedural motor learning task and examined the relations between changes in motor network connectivity following learning and improved performance after a night of sleep.
We acquired resting state scans of healthy young participants before and after performing a finger-tapping task. In one scanning session, participants were trained on the finger-tapping motor sequence task (MST, Karni et al., 1998; Walker et al., 2002) a simple motor procedural learning task that is known to undergo sleep-dependent consolidation (Albouy et al., 2013; Kuriyama et al., 2004; Nishida and Walker, 2007; Walker et al., 2003a; Walker et al., 2002; Walker et al., 2003b). Significant improvement of MST performance occurs after sleep but not after an equivalent period of wake and correlates both with the amount of stage 2 NREM sleep (Walker et al., 2002) and the number and density of sleep spindles (Albouy et al., 2013; Barakat et al.; Nishida and Walker, 2007). (In a separate behavioral control study, we confirmed that MST improvement depended on sleep rather than the mere passage of time.) During a control scanning session, participants performed a paced motor control task (MCT) that involves approximately the same number of finger movements as the MST but is externally paced and employs a simpler sequence to minimize learning. We first identified differential changes in resting state activity within the motor network due to learning during MST training vs. movement during MCT performance. To test our primary hypothesis, we examined whether changes in motor network functional connectivity following MST training correlated with sleep-dependent improvement in performance measured the next day.
2.0 Methods
2.1 Participants
Fifteen young healthy participants enrolled in the fcMRI study and 12 (age 25±2 years, 4 males) successfully completed both scanning sessions and were included in the analyses. Participants endorsed strong right-hand preference (laterality score ≥70) on the modified Edinburgh Handedness Inventory (Oldfield, 1971; White and Ashton, 1976). The 20 participants (age 22±4 years, 7 males) in the behavioral control study provided complete data and were included in the analyses. All participants gave written informed consent and the study was approved by the Partners Human Research Committee.
2.2 Procedures
2.2.1 fcMRI study overview
Participants completed two scan sessions in a counter-balanced order one week apart (Fig. 1). Each session began at 3pm and included two rest scans, one before and one after performing a finger-tapping task with their left-hand while being scanned. During one session, participants trained on the MST and 24 hours later, they were tested on the MST in a mock scanner, which replicated the noise and conditions of the original scan. A 24-hour interval was used to avoid possible circadian effects on task performance. During the other session, participants performed the motor control task (MCT), which involved the same finger movements but minimal learning.
Figure 1.
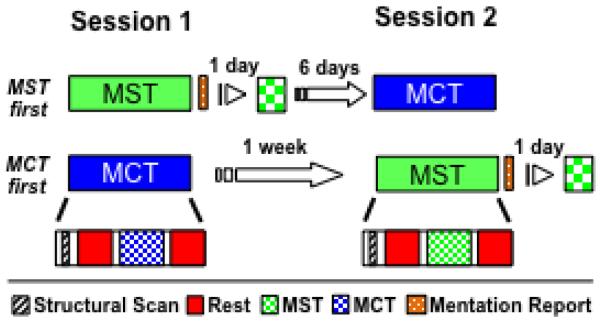
Experimental Protocol for the fcMRI study.
2.2.2 Behavioral control study overview
Participants were pseudorandomly assigned to either the Sleep condition (n=11) or the Wake condition (n=9). Participants trained on the MST and were tested 12 hours later. Sleep participants were trained at 9pm and tested at 9am the following morning. Wake participants were trained at 9am and tested at 9pm after a day of wakefulness. The MST sequence and instructions were identical to the fMRI session, but instead of using a hand mold to respond as they did in fMRI (see below), participants pressed four numerically labeled keys on a standard computer keyboard with the fingers of their left hand. In addition, because participants could look at the labels on the keys, there was no preparatory teaching of the mapping between fingers and keys, nor was there any viewing of the red and green screens in advance of beginning the MST (as described below).
2.2.3 Finger Tapping Motor Sequence Task (MST)
The MST involves pressing four keys with the fingers of the left hand, repeating a five digit sequence (e.g., 4-1-3-2-4) "as quickly and accurately as possible" for 30s (Walker et al., 2003b). During both the training and test sessions, participants performed twelve 30s tapping trials each of which was followed by a 30s break. During tapping trials, the computer screen was green with the numeric sequence displayed at the top, and dots appearing from left to right beneath the sequence with each keystroke. During the breaks, the display was red, and instead of showing the sequence, numbers (displayed as words) counted down the seconds until the next trial. Three seconds before the display turned green again, the words were replaced by flashing dots, which alerted the participant to get ready.
The primary outcome measure was the number of correct sequences per 30s tapping trial, which reflects the speed and accuracy of performance. Any unfinished sequence at the end of a trial was added to the total, as a fraction of a correct sequence. Next-day improvement was calculated as the percent increase in correct sequences from the last three training trials to the first three test trials, and learning during training was calculated as the percent increase in correct sequences from the first training trial to the average of the last three training trials (Walker et al., 2002).
2.2.4 Motor Control Task (MCT)
The MCT used the same displays as the MST and like the MST it involved pressing four keys with the fingers of the left hand, with twelve 30s tapping trials alternating with 30s breaks. It differed from the MST in that during tapping trials, participants repeatedly typed the simple four-digit sequence 1-2-3-4 at a paced rate of 3.3 finger taps per second. As in the MST, the sequence appeared at the top of the screen, but now dots appeared beneath it automatically, indicating the pace of one tap per dot. This pace approximated the total number of finger taps expected in the MST session based on prior work in young healthy participants (Walker et al., 2002).
2.2.5 Preparation and instructions for scanning
To introduce the task design prior to the fMRI sessions, participants viewed three alternations of the green and red displays shown during the motor tasks, but did no actual tapping and were not told which hand they would use or what sequence they would perform. The displays were identical to those seen during the task except that X’s appeared in place of the sequence and dots appeared beneath the X’s at a paced rate of one every 3.3s. Participants were told that during task performance the X’s would be replaced by a sequence that they would tap repeatedly and that they would rest during the red display.
Once in the scanner, participants placed their left hand in a custom-built plastic hand mold that rested on their thigh. The mold had a key for each finger except the thumb, corresponding to the digits one through four. Before each rest scan, participants were asked to keep their eyes open and to remain still and awake for the duration of the 6 minute scan. After the first rest scan and immediately prior to the task, participants were told the mapping of each finger to a digit – pinky for one, ring finger for two, middle finger for three, index finger for four – and practiced until their performance demonstrated that they had learned the mapping. They were then provided with instructions for either the MST (type the sequence as quickly and accurately as possible and repeat until the screen turns red) or MCT (type one digit of the sequence every time a dot appears on the screen and repeat until the screen turns red).
2.2.6 Mentation Questionnaire
Immediately after the second rest scan of the MST session only, participants were asked to remember what they were thinking about during that scan. Once out of the scanner they were given a mentation questionnaire that included a pie chart. Participants divided the pie chart into a maximum of five slices to indicate the proportion of time during the post-MST rest scan they spent thinking about: (i) the past, (ii) the future, (iii) the finger tapping task, (iv) other things and (v) nothing.
2.2.7 MRI image acquisition
Images were acquired with a 3.0 T Siemens Trio whole body high-speed imaging device equipped for echo planar imaging (Siemens Medical Systems, Erlangen, Germany) and a 12-channel headcoil. We first acquired a high-resolution structural scan followed by three functional scans: rest, task, rest (Figure 1). The structural scan took 6’03” and was acquired in the sagittal plane using a 3D rf-spoiled magnetization prepared rapid gradient echo (MP-RAGE) sequence (TR/TE/Flip = 2530ms/1.64ms/7°; FOV=256mm, 176 1mm isotropic slices). Functional images were collected using gradient echo T2* weighted sequences and contiguous slices parallel to the intercommissural plane. Rest scans took 6’12” each (TR/TE/Flip = 3000ms/30ms/85°; FOV=216mm; 47 3mm isotropic slices, acquired interleaved). The task performance scan took 12’42” (TR/TE/Flip = 3000ms/30ms/77°; FOV=190mm; 47 3mm isotropic slices, acquired interleaved) and included prospective acquisition correction (PACE) for head motion (Thesen et al., 2000).
2.2.8 MRI data analysis
Analyses were conducted using AFNI (Cox, 1996). The first four images of each functional run were discarded and the remaining images were slice-time corrected and corrected for residual motion. Functional images were aligned to the MPRAGE for each participant, transformed to Montreal Neurological Institute (MNI) space, spatially smoothed (6mm FWHM), and bandpass filtered between 0.008Hz and 0.10 Hz.
2.2.8.1 Analysis of activation during task performance
To identify brain regions showing activation during the finger tapping tasks we performed a group level t-test on the regression results of each particpant’s spatially normalized data with condition (tapping vs. break epochs) as the regressor of interest and the six directions of residual head motion from AFNI as nuisance regressors. These analyses was corrected for multiple comparisons using a False Discovery Rate (FDR) (Genovese et al., 2002) corrected threshold that set the overall probability to p<.01 (Table 1 lists clusters showing significant activation during the finger tapping tasks).
Table 1.
Clusters showing significant task-related activation in the averaged MST and MCT data. List of maxima locations, Brodmann Areas (BA), cluster sizes, Montreal Neurological Institute (MNI) coordinates, peak T-score and peak FDR probability values (q-values) for clusters with ten or more voxels.
| Location of maxima | Cluster Size (mm3) |
MNI Coordinates | Peak T-score | FDR q- value |
||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| L Cerebellum* | 7479 | −21 | −54 | −18 | 11.0 | 0.002 |
| R Precentral Gyrus (BA 4)** | 6804 | 57 | −15 | 39 | 11.3 | 0.002 |
| R Thalamus | 5589 | 18 | −18 | 15 | 13.0 | 0.002 |
| R Cerebellum* | 3456 | 30 | −51 | −30 | 9.8 | 0.002 |
| L Angular Gyrus (BA 39) | 1890 | −53 | −69 | 36 | −10.0 | 0.002 |
| L Thalamus | 1242 | −12 | −18 | 12 | 9.5 | 0.003 |
| L Insula (BA 13) | 1215 | −36 | 18 | 6 | 10.1 | 0.002 |
| L Cerebellum | 1107 | −21 | −57 | −50 | 8.6 | 0.003 |
| L Putamen* | 702 | −27 | 3 | 9 | 10.5 | 0.002 |
| L Inferior Parietal Lobule (BA 40) | 540 | −53 | −33 | 30 | 8.1 | 0.003 |
| L Inferior Frontal Gyrus (BA 45) | 513 | −53 | 18 | 6 | 9.9 | 0.002 |
| R Inferior Frontal Gyrus (BA 44) | 405 | 53 | 12 | 6 | 7.7 | 0.004 |
| L Superior Frontal Gyrus (BA 10) | 378 | −27 | 68 | 9 | −7.4 | 0.005 |
| L Caudate | 351 | −21 | 0 | 27 | 7.5 | 0.005 |
| R Superior Frontal Gyrus (BA 6)* | 351 | 9 | 0 | 54 | 7.6 | 0.004 |
| R Precentral Gyrus (BA 6) | 351 | 33 | −15 | 71 | 7.7 | 0.004 |
| R Inferior Parietal Lobule (BA 40) |
324 | 62 | −30 | 36 | 8.1 | 0.003 |
| R Cerebellum | 297 | 21 | −60 | −50 | 7.1 | 0.005 |
| R Cerebellum | 270 | 15 | −66 | −24 | 7.5 | 0.005 |
Maxima used as the centers of seeds used to define resting state motor networks for control analyses.
Maximum used as the center of the seed used to define the resting state motor network region of interest in the primary analyses (see Table 2).
2.2.8.2 Functional connectivity methods
Artifact Detection Tools (ART; www.nitrc.org/projects/artifact_detect) were used to exclude time points in the functional scans that had a global signal more than three standard deviations from the mean across all time points or greater than1mm of absolute movement (Whitfield-Gabrieli and Nieto-Castanon, 2012). Anatomic component-based noise correction (aCompCor; Behzadi et al., 2007; Chai et al., 2012) was used to correct for spurious correlations in the data. After regressing out five principal components extracted from a combined CSF/white matter mask and residual head motion from each functional run, functional connectivity maps were created for the seed region (see below). This involved computing the Pearson correlations of the average signal across voxels in the seed with the signal at every other voxel in the brain and transforming the correlation coefficients to z-scores.
2.2.8.3 Definition of the motor network region of interest
To identify the resting state motor network region of interest, we chose the voxel in the primary motor cortex (M1) of the right hemisphere (contralateral to the hand that performed the tasks) with the maximum t-statistic in the comparison of the 30 s tapping vs. break epochs (x, y, z: 57, −15, 39, BA 4) in the averaged MST and MCT activation data to be the center of a 6mm radius spherical seed region (activation analysis described in section 2.2.81). (See Biswal et al. (1995) for an early example of using sensorimotor fMRI activation during hand movements as seeds to identify functionally connected motor regions during a resting state scan). The motor network was defined as voxels that showed positive connectivity with this right M1 seed in the averaged pre-task rest scans from both the MST and MCT sessions using a one-sample t-test of the z-scores and an FDR-corrected threshold that set the overall probability to p<.01 (Fig. 2, top; Table 2 lists significant clusters).
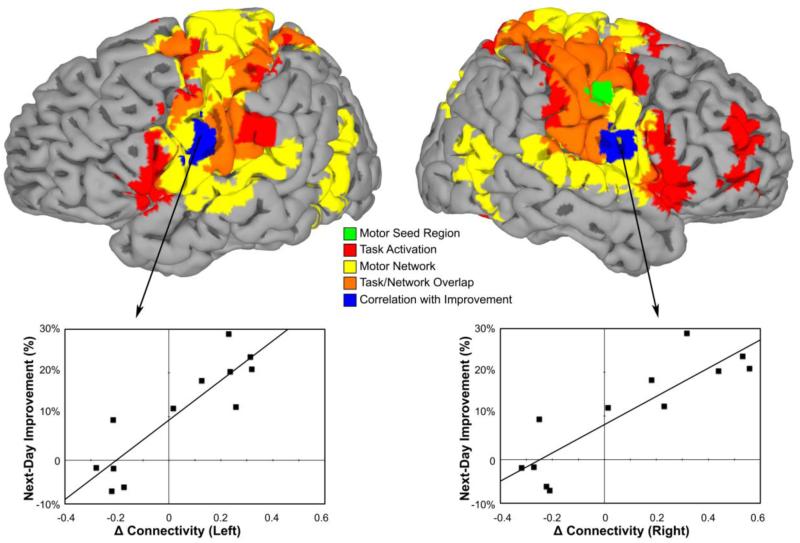
Figure 2.
Summary of results. Top row: Statistical maps of the resting state motor network (yellow), task-related activation (red), overlap between the motor network and task-related activation (orange), the seed region (green), and regions in which enhanced post-MST training connectivity predicted next-day improvement (blue) displayed on the Montreal Neurological Institute N27 brain surface. Bottom row: Scatter plots of next-day MST improvement by connectivity change at the maximum voxel of the right and left motor cortex regions in which enhanced post-MST training connectivity predicted next-day improvement (blue) for the n=12 fcMRI participants.
Table 2.
Clusters comprising the motor network region of interest. List of maxima locations, Brodmann Areas (BA), cluster sizes, Montreal Neurological Institute (MNI) coordinates, T-scores and FDR probability values (q-values) of the peak voxel for all clusters comprising ten or more voxels. Sub-peaks of the largest cluster, which spanned both hemispheres, are indented and italicized.
| Location of maxima | Cluster Size (mm3) |
MNI Coordinates | Peak T-score | FDR q- value |
||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| R Precentral Gyrus (BA 4) | 207900 | 57 | −12 | 42 | 20.2 | 0.00001 |
| L Postcentral Gyrus (BA 2) | −51 | −24 | 45 | 12.0 | 0.00005 | |
| L Precentral Gyrus (BA 4) | −24 | −27 | 69 | 10.0 | 0.0001 | |
| R Cingulate Gyrus (BA 24) | 6 | −3 | 45 | 9.0 | 0.0002 | |
| L Cingulate Gyrus (BA 24) | −12 | −15 | 45 | 11.0 | 0.00008 | |
| L Middle Temporal Gyrus (BA 39) | 6885 | −39 | −75 | 21 | 10.6 | 0.0001 |
| R Middle Temporal Gyrus (BA 39) | 6777 | 48 | −66 | 9 | 10.4 | 0.0001 |
| R Cuneus (BA 19) | 2673 | 9 | −84 | 36 | 8.3 | 0.0003 |
| L Fusiform Gyrus (BA 37) | 1458 | −42 | −42 | −21 | 5.8 | 0.002 |
| R Fusiform Gyrus (BA 37) | 783 | 45 | −42 | −18 | 6.0 | 0.001 |
| R Thalamus | 675 | 12 | −30 | 6 | 7.5 | 0.0005 |
2.2.8.4 Analyses of resting state motor network connectivity
Functional connectivity (z-score) maps were created for each of the four rest scans (pre-MST, post-MST, pre-MCT, post-MCT) and for the scan acquired during MST training, using the motor seed region. A repeated measures ANOVA with factors for task (MCT vs. MST) and time (pre- vs. post-task) and their interaction was used to identify regions that showed greater changes in resting motor connectivity after performing the MST compared to the MCT (task by time interaction). To test the primary hypothesis that increased connectivity in the motor network following MST performance correlates with next-day improvement (defined in section 2.2.3), we calculated the difference between the post- and pre-MST rest scan z-scores at each voxel for each participant and correlated it with next-day MST improvement using Pearson correlations.
2.2.8.5 Control analyses
We conducted a series of control analyses to evaluate other possible correlates of next-day improvement. We correlated next-day improvement with motor network connectivity in the pre-MST rest scan, the post-MST rest scan and in the scan acquired during MST training with and without modeling and removing task-evoked activity and during the break periods alone. In addition, we examined whether learning during training affected resting state functional connectivity by correlating it with motor network connectivity changes in the post-pre MST rest scans and with connectivity in the post-MST rest scan alone.
To determine whether functional connectivity with the seed during the MST scan correlated with next-day improvement, we performed the same fcMRI analysis on the MST scan as for the rest scans, as we have done in prior studies examining connectivity during task performance (Agam et al., 2011; Agam et al., 2010; Tu et al., 2010). In this analysis the correlations derive from a combination of task-evoked and spontaneous activity. To examine functional connectivity during the training scan with the effects of task modeled and removed, we also performed the fcMRI analysis on the residual error time-series from the regression of task-related activation (Rogers and Gore, 2008). To the extent that the task model accurately corresponds to task-evoked activation, the residuals reflect spontaneous activity. We also examined functional connectivity during the concatenated break epochs. For this analysis we excluded the first 3 time points (9 s) of each break and included the first time point (3 s) of the following typing epoch to account for the hemodynamic delay. The resulting z-score maps from these analyses were correlated with next-day improvement.
We also correlated next-day improvement with activation during MST training. The statistical activation map was correlated with next-day improvement on a voxel-wise basis.
Our primary fcMRI analyses used a right M1 seed. This choice was based on electroencephalography (EEG) and magnetoencephalography (MEG) evidence that contralateral M1 is involved in sleep-dependent consolidation of the MST (Nishida and Walker, 2007; Tamaki et al., 2013) and that contralateral M1 showed the strongest cortical activation during the finger tapping tasks. Using the same procedures as in M1, we determined whether next-day improvement correlated with changes in motor networks identified by seeds in other regions that were activated during finger tapping performance and contribute to motor learning. We defined resting state networks using seeds in the left and right cerebellum, left putamen and right supplementary motor area. Local maxima of task activation in these regions formed the center of these 6 mm seeds (Table 1 provides the coordinates). Each seed was used to define a resting state network based on its positive connectivity in the averaged pre-task rest scans from both the MST and MCT sessions using a one-sample t-test of the z-scores and an FDR-corrected threshold that set the overall probability to p<.01. Within each network, we calculated the difference between the post- and pre-MST rest scan z-scores at each voxel` for each participant and correlated it with next-day MST improvement.
Finally, we computed correlations of next-day improvement with the proportion of time spent thinking about the task (based on the mentation questionnaire) during the post-MST rest scan and with performance improvement during training (defined in section 2.2.3).
2.2.8.6 Correction for multiple comparisons
Except when otherwise noted (i.e., task-related activation and definition of the motor network region-of-interest), analyses were corrected for multiple comparisons using a cluster threshold based on 1,000,000 Monte Carlo simulations of synthesized white Gaussian noise with the smoothing and resampling parameters of the functional analyses and an uncorrected threshold of p<.01 (Nichols, 2012). This determined the likelihood that a cluster of a certain size would be found by chance. A cluster threshold of 22 voxels set this probability to ≤.05 within the motor network and a threshold of 38 voxels set the probability to ≤.05 in the entire brain. Although we restricted our hypothesis space to the motor network and base the reported cluster-sizes and CWP values on this region-of-interest, all of the clusters reported also met the more stringent threshold for the entire brain. (One cluster is reported in results as having only 31 voxels, but although its maximum is in the motor network, it extended beyond the motor network for a total cluster size of 42 voxels).
3.0 Results
3.1 Finger-tapping Performance
During MST training, all groups showed significant improvement, indicative of learning (fcMRI: 25±6% sem; t(11)=4.58, p<.001; Sleep group: 42±11%; t(10)=3.81, p=.003; Wake group: 35±8% sem; t(8)=4.26, p=.004) and did not differ significantly in this regard (F(2,28)=1.08, p=.35). Both the fcMRI participants and the Sleep group of the behavioral control study showed significant next-day improvement in MST performance (fcMRI:11±4% sem, t(11)=3.01, p=.01; Sleep: 17±3%; t(10)=5.64, p =.0002), which did not differ significantly between groups (t(21)=1.33; p=.20). In contrast, the Wake group of the behavioral control study showed no significant MST improvement (4±6%, t(8)=.61, p=.56) and significantly less than the Sleep group (t(18)=2.10, p=.05). This replicates previous findings that off-line improvement of MST performance requires sleep. For the fcMRI participants, the number of correctly typed keystrokes did not differ significantly between MST training (81±6) and MCT performance (88±3, t(12)=1.40; p=.18), and the amount of next-day MST improvement did not differ significantly based on the order of the MST and MCT sessions (t(10)=.17; p=.87).
3.2 MST vs. MCT effects on resting state connectivity
To assess the effects of learning vs. finger tapping per se on resting state connectivity within the motor network, we used ANOVA to examine the differential effects of MST training vs. MCT performance by comparing the pre- and post-task rest scans (interaction of task by time). This interaction revealed a single significant region within the motor network (right post-central gyrus, Montreal Neurological Institute (MNI) x, y, z coordinates: 3, −42, 72, Brodmann Area (BA) 5, cluster size: 31 voxels, cluster-wise probability (CWP)=0.007). This region showed increased connectivity with the seed after the MST compared with the MCT, which may reflect the greater learning demands of the MST. Increased connectivity in this region, however, did not correlate with next-day MST improvement.
3.3 Changes in resting state motor network connectivity in relation to next-day improvement
We calculated the difference between the post- and pre-MST rest scan z-scores at each voxel for each participant and correlated it with next-day MST improvement. Increased connectivity of the seed with bilateral motor cortex (left: −51, −9, 30, BA 4, 58 voxels, CWP=0.00003; right: 63, −3, 18, BA 6; 38 voxels, CWP=0.002) in the post-MST (relative to pre-MST) rest scan correlated with next-day improvement (Fig. 2, top). These regions were distinct from the seed region. Moreover, the connectivity of these regions with the seed was not significantly increased during the post-training rest scan at a group level. Instead, increased connectivity was seen only in participants showing next-day improvement. Post-hoc examination of the scatter plots (Fig. 2, bottom) reveals an almost perfect split: all but one of the 12 participants either showed both next-day improvement and increased connectivity bilaterally, or showed both next-day deterioration and reduced connectivity bilaterally (chi-square test with simulated p-value based on 105 replicates: χ2=8.4, p=.01). Among the eight participants showing next-day improvement, the one with the least improvement was the only one who did not show increased connectivity in bilateral motor cortex. This raises the possibility that increased connectivity in these regions during post-training rest was necessary to realize sleep-dependent improvement.
3.4 Control analyses
A series of control analyses evaluated other possible correlates of next-day improvement. First, only the change in motor network connectivity correlated with next-day improvement; connectivity in neither the pre- nor post-MST rest scan alone significantly correlated with improvement (i.e., there were no significant clusters) suggesting that generally higher levels of connectivity do not enhance sleep-dependent motor memory consolidation.
Second, motor network connectivity during the scan acquired during MST training did not correlate with next-day improvement. This was true regardless of whether motor network connectivity during the training scan was measured in the break periods alone or in the entire scan either before or after modeling and removing the effect of task-related activation. Moreover, changes in motor network connectivity in the MST training scan relative to the pre-training rest scan did not significantly correlate with next-day improvement. Finally, MST-related activation (as opposed to connectivity) also showed no significant correlation with next-day improvement. Thus, motor network connectivity during training, changes in connectivity during training, and task-related activation during MST training were not significantly related to next-day improvement.
We examined whether next-day improvement correlated with changes in motor networks defined by seeds in other regions (left and right cerebellum, left putamen and right supplementary motor area) that were activated by finger tapping and contribute to motor learning and consolidation (e.g., Tzvi et al., 2014). There were no regions in which a change in connectivity with these seeds correlated with next-day improvement.
Immediately after completing the MST scan session, participants indicated the proportion of time spent thinking about the task during the post-training rest scan. This measure bore no relation to next-day task improvement (r=−.03, p=.92).
Unexpectedly, learning during training was inversely related to next-day improvement in fcMRI participants (r=−.66, p=.02), although not in the Sleep control group (r=−.09, p=.80), or in prior MST studies (Walker et al., 2003b). Learning during training, however, was not significantly correlated either with changes in motor network connectivity from the pre- to post-MST rest scan or with connectivity in the post-MST rest scan alone.
4.0 Discussion
During a period of wakeful rest immediately after training on a finger tapping motor sequence task (MST) that involves procedural learning, enhanced connectivity between a seed in the hand region of M1 and distinct bilateral motor cortex regions correlated with improved task performance measured the following day. Only participants who showed increased connectivity in these motor cortex regions showed next-day improvement, raising the possibility that increased connectivity was necessary for improvement. Importantly, while the increase in motor network connectivity following training significantly correlated with next-day improvement, connectivity present before, during or after training did not. These observations suggest that the increased connectivity that predicted next-day improvement was induced by the MST training. While prior studies have demonstrated that enhanced resting state connectivity after declarative memory encoding predicts better recognition when measured shortly thereafter (Stevens et al., 2010; Tambini et al., 2010), this is the first demonstration that enhanced connectivity during wakeful rest correlates with actual gains in task performance over the level achieved at the end of training. Moreover, as these gains in performance were measured the following day and depend on sleep, our findings demonstrate that physiological processes immediately after learning are associated with sleep-dependent improvements in performance. We hypothesize that these changes in connectivity within the motor network following learning prepare motor memories for subsequent consolidation during sleep.
A series of control analyses evaluated other possible correlates of next-day improvement. (As a caveat, we note that the lack of significant relations in these control analyses may reflect lack of power due to the limited sample size of the present study.) First, motor network connectivity in neither the pre- nor post-MST rest scan alone significantly correlated with next-day improvement, suggesting that generally increased levels of connectivity do not enhance sleep-dependent motor memory consolidation. Second, motor network connectivity during MST training, changes in connectivity during MST training compared with the pre-training rest scan, and task-related activation did not significantly correlate with next-day improvement. This suggests that within the motor network it is the change in connectivity during post-training rest that is associated with next-day improvement rather than a continuation of connectivity present during training. Third, next-day improvement did not correlate with the amount of time spent thinking about the task during post-training rest, suggesting that changes in connectivity that correlate with next-day improvement do not depend on conscious thought. Finally, the correlations we observed are unlikely to reflect initial learning of the task, since the amount of learning during training was not related either to changes in resting state motor network connectivity (i.e., post vs. pre) or to connectivity in the post-training rest scan alone. In addition, the regions in which increased connectivity correlated with next-day improvement were not differentially affected by MST training compared with performing a non-learning motor control task (MCT).
The location of the motor network regions in which enhanced connectivity correlates with next-day improvement may provide clues to the function of this connectivity during post-training rest. While their maxima fall in right premotor cortex and left M1, both clusters span premotor cortex and M1, with the left cluster extending onto the post-central gyrus. These regions are considerably ventral to the seed region in the M1 hand area that was activated during the task. They instead include face regions of the motor homunculus. Similar motor and premotor face regions are bilaterally activated in fMRI studies during both speech production (Brown et al., 2005; Ghosh et al., 2008) and covert speech (Callan et al., 2006; Shergill et al., 2002). Participants report covertly vocalizing the digit sequence during MST training, presumably to guide their sequential finger movements. (That these regions were not significantly activated during MST training may reflect continued covert vocalization of the sequence during the break epochs, which served as the baseline condition.) In the rest scan after MST training, increased coordination between these bilateral ‘covert vocalization’ regions and the right M1 seed region involved in the actual finger movements may reflect a simultaneous reactivation of the verbal declarative and motor procedural memories. This simultaneous reactivation is unlikely to be conscious or to reflect covert vocalization since participants reportedly spent most of their time during the post-training rest scan thinking about other things or nothing, and the percentage of time spent thinking about the task (14±21%) did not correlate with next-day improvement.
Indirect evidence for contralateral (right) M1 involvement in consolidation of the MST during sleep comes from both an electroencephalography (EEG) study and an anatomically-constrained magnetoencephalography (MEG) source localization study. Specifically, increased sleep spindles in the C4 (right) relative to the C3 (left) electrode, which lie approximately over motor cortex, predicts MST improvement after sleep (Nishida and Walker, 2007). In addition, in the sleep that follows MST training relative to a no-task condition, there is increased sigma activity, which corresponds to the frequency of sleep spindles, in contralateral M1 (Tamaki et al., 2013). These studies, in conjunction with the present findings, raise the question of whether motor regions important in sleep-dependent consolidation also contribute to processing motor memories during the interval of wake between learning and sleep.
How coordinated activity within a motor network during rest would lead to greater sleep-dependent consolidation is unclear. The selection of memories for retention is thought to occur during or shortly after encoding and may depend on the expectation of future relevance. Post-training assignment of salience to recently learned material in response, for example, to informing participants of a test or a performance-based reward to be given the next day, leads to enhanced sleep-dependent consolidation (Rauchs et al., 2011; van Dongen et al., 2012; Wilhelm et al., 2011). In the present study, participants knew that they would return the next-day to be tested on the MST. The increased connectivity between the hand region and regions hypothetically involved in the declarative memory of the sequence in the present study might reflect the action of mechanisms by which the salience of these memories leads to their selection for subsequent sleep-dependent consolidation (Stickgold and Walker, 2013). While the actual mechanisms underlying the assignment of salience and selection are unknown, animal studies propose that memories are selected and sustained for system level consolidation in neocortical networks by ‘synaptic tagging’ in the immediate aftermath of learning (Cassini et al., 2013; Frey and Morris, 1997; Redondo and Morris, 2011).
Limitations of our study design prevent us from making stronger claims. First, the MST differed from the MCT not only with regard to learning, but also in that the typing was not externally paced, it involved a more complex sequence, and participants were scheduled to return for testing the following day. These differences, rather than the greater learning requirements, may account for differences in post-task processing. It also would have been informative to measure performance immediately after scanning as was done in prior work (Stevens et al., 2010; Tambini et al., 2010). Had we done so, we might have also identified regions in which post-MST training changes in resting state connectivity correlated with immediate as opposed to next-day performance. In addition, participants slept at home following MST training, and we did not measure MST performance prior to sleep nor did we record sleep with polysomnography. Such measures would have allowed us to determine whether variations in the retention of the MST across the day could be predicted by resting state connectivity, and to examine whether next-day improvement correlated with specific characteristics of post-training sleep (e.g., the density of sleep spindles). Finally, given the limited sample size of our study and concerns about capitalizing on chance by using multiple tests, we restricted our hypotheses to the motor network defined using an M1 seed. This choice was based on prior findings that resting state activity in task-relevant networks is modified by learning (Albert et al., 2009; Vahdat et al., 2011) and correlates with subsequent recognition memory (Deuker et al., 2013; Stevens et al., 2010; Tambini et al., 2010). Thus we cannot exclude the possibility that connectivity within other networks or between the motor network and other regions are also correlated with next-day improvement. These limitations, however, do not detract from our main finding that task-induced modulation of resting state motor network connectivity immediately following learning correlates with sleep-dependent enhancement of task performance. This is consistent with the hypothesis that the resting brain prepares memories of recent experiences for later consolidation during sleep.
Acknowledgments
The authors are grateful to Susan Whitfield-Gabrieli, Randy Buckner, and Frank Guenther for consultation. Support provided by the National Alliance for Research on Schizophrenia and Depression (DSM); Milton Fund, Harvard University (ER, DSM); National Institute for Mental Health, F32 MH088081 (YA) R01 MH48832 (RS); MGH-ECOR Fund for Medical Discovery (YA); National Institute of Neurological Disorders and Stroke, 5T32NS51151-5 (MDG); Biogen IDEC, Clinical Fellowship in Multiple Sclerosis (MDG); National Center for Research Resources, P41RR14075. The authors declare no competing financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agam Y, Hamalainen MS, Lee AK, Dyckman KA, Friedman JS, Isom M, Makris N, Manoach DS. Multimodal neuroimaging dissociates hemodynamic and electrophysiological correlates of error processing. Proc Natl Acad Sci U S A. 2011;108:17556–17561. doi: 10.1073/pnas.1103475108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agam Y, Joseph RM, Barton JJS, Manoach DS. Reduced cognitive control of response inhibition by the anterior cingulate in autism spectrum disorders. Neuroimage. 2010;52:336–347. doi: 10.1016/j.neuroimage.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert NB, Robertson EM, Miall RC. The resting human brain and motor learning. Curr Biol. 2009;19:1023–1027. doi: 10.1016/j.cub.2009.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albouy G, Fogel S, Pottiez H, Nguyen VA, Ray L, Lungu O, Carrier J, Robertson E, Doyon J. Daytime sleep enhances consolidation of the spatial but not motoric representation of motor sequence memory. PLoS ONE. 2013;8:e52805. doi: 10.1371/journal.pone.0052805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barakat M, Doyon J, Debas K, Vandewalle G, Morin A, Poirier G, Martin N, Lafortune M, Karni A, Ungerleider LG, Benali H, Carrier J. Fast and slow spindle involvement in the consolidation of a new motor sequence. Behav Brain Res. 2011;217:117–121. doi: 10.1016/j.bbr.2010.10.019. [DOI] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37:90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Brown S, Ingham RJ, Ingham JC, Laird AR, Fox PT. Stuttered and fluent speech production: an ALE meta-analysis of functional neuroimaging studies. Hum Brain Mapp. 2005;25:105–117. doi: 10.1002/hbm.20140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callan DE, Tsytsarev V, Hanakawa T, Callan AM, Katsuhara M, Fukuyama H, Turner R. Song and speech: brain regions involved with perception and covert production. Neuroimage. 2006;31:1327–1342. doi: 10.1016/j.neuroimage.2006.01.036. [DOI] [PubMed] [Google Scholar]
- Cassini LF, Sierra RO, Haubrich J, Crestani AP, Santana F, de Oliveira Alvares L, Quillfeldt JA. Memory reconsolidation allows the consolidation of a concomitant weak learning through a synaptic tagging and capture mechanism. Hippocampus. 2013. [DOI] [PubMed]
- Chai XJ, Castanon AN, Ongur D, Whitfield-Gabrieli S. Anticorrelations in resting state networks without global signal regression. Neuroimage. 2012;59:1420–1428. doi: 10.1016/j.neuroimage.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Deuker L, Olligs J, Fell J, Kranz TA, Mormann F, Montag C, Reuter M, Elger CE, Axmacher N. Memory consolidation by replay of stimulus-specific neural activity. J Neurosci. 2013;33:19373–19383. doi: 10.1523/JNEUROSCI.0414-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey U, Morris RG. Synaptic tagging and long-term potentiation. Nature. 1997;385:533–536. doi: 10.1038/385533a0. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Ghosh SS, Tourville JA, Guenther FH. A neuroimaging study of premotor lateralization and cerebellar involvement in the production of phonemes and syllables. J Speech Lang Hear Res. 2008;51:1183–1202. doi: 10.1044/1092-4388(2008/07-0119). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karni A, Meyer G, Rey-Hipolito C, Jezzard P, Adams MM, Turner R, Ungerleider LG. The acquisition of skilled motor performance: fast and slow experience-driven changes in primary motor cortex. Proc Natl Acad Sci U S A. 1998;95:861–868. doi: 10.1073/pnas.95.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama K, Stickgold R, Walker MP. Sleep-dependent learning and motor-skill complexity. Learn Mem. 2004;11:705–713. doi: 10.1101/lm.76304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols TE. Multiple testing corrections, nonparametric methods, and random field theory. Neuroimage. 2012;62:811–815. doi: 10.1016/j.neuroimage.2012.04.014. [DOI] [PubMed] [Google Scholar]
- Nishida M, Walker MP. Daytime naps, motor memory consolidation and regionally specific sleep spindles. PLoS ONE. 2007;2:e341. doi: 10.1371/journal.pone.0000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Rauchs G, Feyers D, Landeau B, Bastin C, Luxen A, Maquet P, Collette F. Sleep contributes to the strengthening of some memories over others, depending on hippocampal activity at learning. J Neurosci. 2011;31:2563–2568. doi: 10.1523/JNEUROSCI.3972-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redondo RL, Morris RG. Making memories last: the synaptic tagging and capture hypothesis. Nat Rev Neurosci. 2011;12:17–30. doi: 10.1038/nrn2963. [DOI] [PubMed] [Google Scholar]
- Rogers BP, Gore JC. Empirical comparison of sources of variation for FMRI connectivity analysis. PLoS ONE. 2008;3:e3708. doi: 10.1371/journal.pone.0003708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shergill SS, Brammer MJ, Fukuda R, Bullmore E, Amaro E, Jr., Murray RM, McGuire PK. Modulation of activity in temporal cortex during generation of inner speech. Hum Brain Mapp. 2002;16:219–227. doi: 10.1002/hbm.10046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF. Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens WD, Buckner RL, Schacter DL. Correlated low-frequency BOLD fluctuations in the resting human brain are modulated by recent experience in category-preferential visual regions. Cereb Cortex. 2010;20:1997–2006. doi: 10.1093/cercor/bhp270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickgold R, Walker MP. Sleep-dependent memory consolidation and reconsolidation. Sleep Med. 2007;8:331–343. doi: 10.1016/j.sleep.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickgold R, Walker MP. Sleep-dependent memory triage: evolving generalization through selective processing. Nat Neurosci. 2013;16:139–145. doi: 10.1038/nn.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki M, Huang TR, Yotsumoto Y, Hamalainen M, Lin FH, Nanez JE, Sr., Watanabe T, Sasaki Y. Enhanced spontaneous oscillations in the supplementary motor area are associated with sleep-dependent offline learning of finger-tapping motor-sequence task. J Neurosci. 2013;33:13894–13902. doi: 10.1523/JNEUROSCI.1198-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambini A, Ketz N, Davachi L. Enhanced brain correlations during rest are related to memory for recent experiences. Neuron. 2010;65:280–290. doi: 10.1016/j.neuron.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thesen S, Heid O, Mueller E, Schad LR. Prospective acquisition correction for head motion with image-based tracking for real-time fMRI. Magn Reson Med. 2000;44:457–465. doi: 10.1002/1522-2594(200009)44:3<457::aid-mrm17>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Tu P, Buckner RL, Zollei L, Dyckman KA, Manoach DS. Reduced functional connectivity in a right-hemisphere network for volitional ocular motor control in schizophrenia. Brain. 2010;133:625–637. doi: 10.1093/brain/awp317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzvi E, Munte TF, Kramer UM. Delineating the cortico-striatal-cerebellar network in implicit motor sequence learning. Neuroimage. 2014;94:222–230. doi: 10.1016/j.neuroimage.2014.03.004. [DOI] [PubMed] [Google Scholar]
- Vahdat S, Darainy M, Milner TE, Ostry DJ. Functionally specific changes in resting-state sensorimotor networks after motor learning. J Neurosci. 2011;31:16907–16915. doi: 10.1523/JNEUROSCI.2737-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dongen EV, Thielen JW, Takashima A, Barth M, Fernandez G. Sleep supports selective retention of associative memories based on relevance for future utilization. PLoS ONE. 2012;7:e43426. doi: 10.1371/journal.pone.0043426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MP, Brakefield T, Hobson JA, Stickgold R. Dissociable stages of human memory consolidation and reconsolidation. Nature. 2003a;425:616–620. doi: 10.1038/nature01930. [DOI] [PubMed] [Google Scholar]
- Walker MP, Brakefield T, Morgan A, Hobson JA, Stickgold R. Practice with sleep makes perfect: sleep-dependent motor skill learning. Neuron. 2002;35:205–211. doi: 10.1016/s0896-6273(02)00746-8. [DOI] [PubMed] [Google Scholar]
- Walker MP, Brakefield T, Seidman J, Morgan A, Hobson JA, Stickgold R. Sleep and the time course of motor skill learning. Learn Mem. 2003b;10:275–284. doi: 10.1101/lm.58503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White K, Ashton R. Handedness assessment inventory. Neuropsychologia. 1976;14:261–264. doi: 10.1016/0028-3932(76)90058-0. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Wilhelm I, Diekelmann S, Molzow I, Ayoub A, Molle M, Born J. Sleep selectively enhances memory expected to be of future relevance. J Neurosci. 2011;31:1563–1569. doi: 10.1523/JNEUROSCI.3575-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]