Abstract
Silk-elastinlike protein polymers (SELPs) have been fabricated as matrices for controlled delivery of bioactive agents. In this application the need for an environmentally responsive, degradable polymer has risen to improve treatment outcomes. To satisfy this need, we have designed, synthesized, and expressed SELPs with matrix metalloproteinase (MMP) degradable sequences inserted in distinct regions of the polymer backbone. Upon characterization of the physiochemical properties of newly synthesized analogues, it was determined that conditioning of the polymers was necessary for normalization of batch properties, and to generate a more robust polymer network suitable for delivery. In this report we have examined the use of shear stress to condition synthesized material prior to application as a controlled release matrix. The application of high shear to SELPs results in significant changes in physiochemical properties as assayed by swelling ratio, soluble fraction release, rate of gel formation, stiffness of hydrogels, and nanoparticle release from matrices. These observed changes in material characteristics may be caused by the removal of semi-stable secondary and tertiary structure from single polymer strands leading to a more robust hydrogel with greater intermolecular interaction.
Keywords: Recombinant polymers, Silk-elastinlike polymers, Shear, Drug delivery, Matrix metalloproteinase
1. Introduction
Silk-elastinlike protein polymers (SELPs) are block copolymers consisting of repeating amino acid sequences modeled from silkworm silk fibroin (GAGAGS) and mammalian elastin (GVGVP) synthesized using recombinant DNA technology.[1] SELPs possess the unique property of transitioning from an aqueous solution into a physical network triggered by increase in temperature and dictated by the specific sequence and ratio of silk to elastin blocks. SELPs are liquid at room temperature to facilitate mixing with bioactive agents. When raised to body temperature they form insoluble hydrogels suitable for controlled release. The use of recombinant DNA technology in synthesis allows for a high degree of control over the sequence and length of the polymers formed, as well as the amino acid sequence-level precision at which modifications can be made to existing polymers.[2-7] SELPs have undergone extensive characterization of physicochemical and release properties.[8-14] In the context of intratumoral gene delivery, it has been demonstrated that one particular analog of SELPs, namely SELP815K (Figure 1), holds promise for localized release of adenoviruses in the treatment of head and neck solid tumors.[15, 16]
Figure 1.
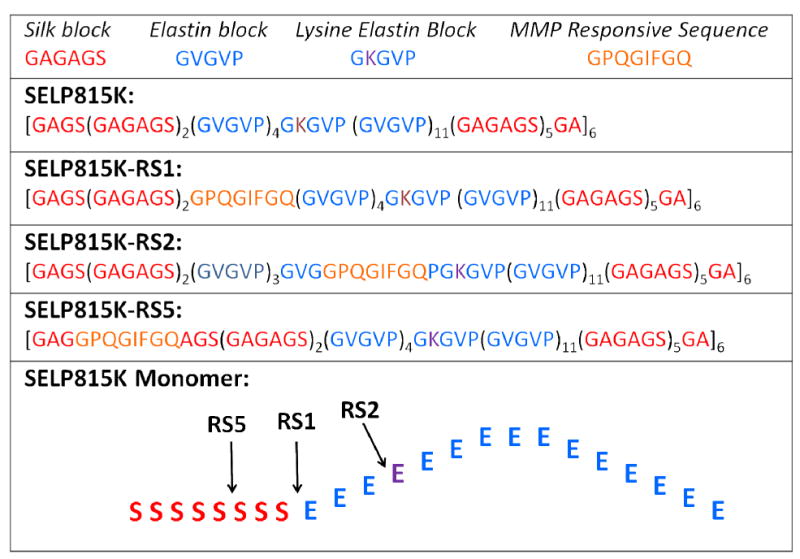
Single letter amino acid structures of SELP815K, SELP815K-RS1, SELP815K-RS2, and SELP815K-RS5 with graphical representation of matrix metalloproteinase responsive sequence insertion sites into the SELP815K monomer.
While SELPs including SELP815K have shown potential for use as controlled release matrices, their observed in vivo durability as biomaterials in murine models leads to progressive encapsulation over 12 weeks.[17] In order to promote more rapid degradation, a SELP analog with sequences degradable by matrix metalloproteinases (MMPs) was synthesized.[18] It was demonstrated that these hydrogels degrade in the presence of MMPs, a family of naturally occurring proteases that function to breakdown extracellular matrix proteins that are over expressed in a variety of solid tumors.[19] The influence that the location of MMP responsive sequences in the SELP backbone may have on physicochemical properties of the resulting hydrogels and release characteristics from these matrices is however unknown. In this work several analogues of SELPS were biosynthesized by inserting the MMP responsive sequences into two structurally distinct regions of each monomer repeat, namely the silk block and the elastin block (Figure 1). Following synthesis and initial experiments on the physiochemical properties of new analogues, it was determined that mechanical conditioning of the synthesized material could be used for normalization of properties and to obtain a more robust polymer network.
Shear stress has long been postulated to contribute to protein denaturation and has been shown to be utilized in living systems to affect conformational change in circulating proteins.[20, 21] Experimentally, conformational change can be induced mechanically via high fluid flow, particularly in molecules such as DNA whose conformation depends on weaker non-covalent bonds.[22] Applying mechanical shear stress to stable globular proteins, however, has shown to be less effective than expected in completely breaking secondary and tertiary conformational bonds due to the combination of weak interactions together with covalent bonds.[23] Proteins with weaker, less defined single strand tertiary structure such as SELPs may be more susceptible to the denaturing effects of shear stress. This will render linearized polymer strands that can form a higher density of intermolecular interactions. Here we report the biosynthesis and characterization of new MMP responsive SELPs and the influence of high shear rate on the physiochemical properties of these polymers in the context of controlled release.
2. Materials and Methods
2.1 Materials
SELP815K[9] and SELP815K-RS2 with MMP responsive sequence in the middle of elastin blocks [18] were synthesized and characterized as previously described (Figure 1). SELP815K-RS1 with MMP responsive site located in the junction between silk and elastin blocks, and SELP815K-RS5 (RS5) with MMP responsive sequence in the middle of silk blocks (Figure 1) were synthesized and characterized using the methods previously described for SELP815K-RS2. All restriction enzymes, T4 ligase, and phosphatases were purchased from New England Biolabs (Ipswich, MA) or Fermentas (Vilnius, LT). C5 Emulsilex was purchased from Avestin (Ottawa, ON) and modified using high pressure valves and fittings from Autoclave Engineers (Erie, PA). Fluorescently labeled polystyrene beads were acquired from Invitrogen (Carlsbad, CA). Materials for Lowry assay were purchased from Thermo Fisher Scientific (Waltham, MA).
2.2 Methods
2.2.1 SELP815K-RS5 Synthesis
SELP815K-RS1 and SELP815K-RS5 were synthesized and characterized using methods previously described for SELP815K-RS2.[18] Briefly DNA Oligomers encoding the MMP responsive amino acid sequence GPQGIFGQ were inserted into the SELP815K monomer in a construction plasmid using restriction endonucleases to linearize plasmid, and T4 DNA ligase to recircularize with responsive sequence inserted. MMP responsive monomer population was then expanded and digested from the construction plasmid using BanI. The population of MMP-responsive monomers was multimerized by random concatemerization into a high efficiency expression plasmid using T4 DNA ligase and screened by size using XcmI, NcoI double digest run on a DNA agarose gel. Expression was confirmed using shake flask culture and SDS-PAGE protein gel. Protein was then expressed at 10L fermentation scale and purified from 1Kg wet cell pellet using several precipitation, ion exchange chromatography, and ultra filtration steps. Product was characterized via SDS-PAGE protein gel, matrix-assisted laser desorption/ionization time of flight (MALDI-ToF, University of California at Los Angeles proteomics core, Los Angeles, CA), and vapor phase hydrolysis amino acid analysis (the University of Nebraska Medical Center protein structure core facility, Omaha, NE).
2.2.3 Shearing
Protein polymers were lyophilized and stored at -80° C following synthesis and purification. Lyophilized stocks were reconstituted completely in cold 0.7× PBS and kept on ice. Avestin C5 Emulsiflex homogenizer was washed thoroughly using depyrogenated deionized water and PBS. Homogenizer was then submerged in an ice bath to maintain low temperature during shear processing. Reconstituted protein polymer solution was pipetted aseptically into sample vessel and pressurized to 80 psi using nitrogen. The homogenizing valve was opened to allow a flow rate of approximately 5 mL/min and sample fluid was pumped through a needle valve at 17,000 psi. Material was then collected using a sterile luer connection. Sample fluid was aliquoted and snap frozen in liquid nitrogen. Frozen sheared polymers were stored at -80° C.
2.2.4 Minimum Gel Forming Concentration
Minimum gel forming concentration was determined by qualitative observation of mechanical stability following extrusion from the barrel of a 1 mL tuberculin syringe with the tip removed. Frozen polymer samples were thawed in a room temperature water bath and diluted in PBS to concentrations with a 1% step from 0% wt/wt polymer to 15% wt/wt polymer. Resulting solutions were drawn into 1mL slip tip syringes and incubated overnight at 37°C. Syringe tips were then removed with an autoclaved razor blade and contents were extruded and sectioned into 50uL gel discs. Minimum gel forming concentration was defined as the concentration at which no lower concentration would hold a stable disc shape following sectioning.
2.2.5 Swelling Ratio
Swelling ratio was assayed on gel discs formed by extrusion from 1mL syringes in the same method as minimum gel forming concentration. Gel cylinders were extruded from syringe barrels and sectioned into 50uL discs using an autoclaved razor blade. Subsequently, discs were incubated in 500uL release media composed of 1× PBS + 0.2mM sodium azide for 7 days to remove soluble fraction. Following incubation, gels were removed from release media, blotted dry with a lint free wipe, and weighed. Gels were then frozen and lyophilized for 3 days to remove water content and weighed again. Swelling ratio was calculated by dividing wet weight by dry weight.
2.2.6 Soluble Fraction
Soluble fraction was assayed using chromogenic modified Lowry assay and UV-vis spectrophotometer with standard curve consisting of serial dilution of SELP and MMP-responsive SELP protein to determine amount of protein in release media collected in swelling ratio experiment. Soluble fraction was calculated by dividing soluble protein by soluble protein plus dry weight of respective hydrogel.
2.2.7 Nanoparticle Release
Model nanoparticle release was assayed using 50uL gel discs loaded with 110nm fluorescently labeled polystyrene spheres prepared in the same method as detailed above. Gel discs were incubated for 28 days in 500uL release media composed of 1× PBS + 0.2mM azide. Release media was collected, gels were washed, and media replaced on days 1, 3, 5, 9, 15, 21, 28 to simulated infinite sink conditions. Bead release was assayed by fluorescence quantification of FITC label after excitation at 260nm with emission at 515nm. Fluorescence photo bleaching and degradation were accounted for with internal polystyrene bead control group.
2.2.8 Rheological Analysis
Rheological analysis was performed using an AR 550 stress-controlled rheometer from TA Instruments (New Castle, DE) in a cone-and-plate configuration using a steel 20 mm diameter, 4 degree cone. Oscillatory time sweeps were performed on each sample consisting of an equilibration time sweep at 23°C, angular frequency of 6.283 rad/s, and 1.0 % strain for 1 min followed by a 5 hr sweep at 37°C, angular frequency of 6.283 rad/s, and 0.1% strain. Briefly, individual polymer samples previously prepared to the correct concentration by dilution with 1× PBS and flash frozen were thawed just prior to assay in cool water and immediately transferred to the Peltier plate pre-heated to 23°C at a volume of 150μL. The equilibration step ended with a temperature ramp to 37°C in 30-60 seconds before start of the 5hr run. The time sweep resulted in traces for G′ and G″, the storage and loss moduli respectively. The G′ plateau represents dynamic gel strength, and was extracted for each sample at the 4hr time point. Due to the crystalline nature of the gelation which in some cases caused cone detachment from the sample, several runs were unable to be completed to 5hrs; therefore 4hr point was chosen for consistency. Experiments were conducted in triplicate.
2.3 Analysis
2.3.3 Statistical analysis
Students T-test was used to compute statistical significance between two groups. One way ANOVA with Bonferroni post-test was used to compute statistical analysis of groups of 3 or more. A value of p ≤ 0.05 was considered statistically significant and p ≤ 0.01 was considered highly statistically significant. All error bars displayed represent standard deviation of mean.
3. Results
3.1. Synthesis of SELPs
SELP815K and SELP815K-RS2 were synthesized and purified as previously described[9, 18] and found to be identical to previous confirmed sequence batches by plasmid DNA sequencing, SDS-PAGE, mass spectrometry, and amino acid analysis. SELP815K-RS1 and SELP815K-RS5 were synthesized using similar strategy to SELP815K-RS2 and confirmed to be correct size via plasmid DNA sequencing, gel electrophoresis (not shown), and mass spectroscopy (Figure 2). The predicted size of SELP815K-RS1 is 69,443 DA, SELP815K-RS5 is 70,211 Da, and SELP815K is 64,733. Mass spectroscopy found the sizes to be 69,298 Da, 69,142 Da, and 63,766 respectively. Amino acid analysis confirmed the presence of additional residues of the low occurrence amino acids isoleucine, phenylalanine, and glutamine in SELP815K-RS5 and SELP815K-RS1 as compared to the confirmed sequence of SELP815K corresponding to the addition of a single MMP responsive sequence GPQGIFGQ within each monomer (Figure 2).
Figure 2.
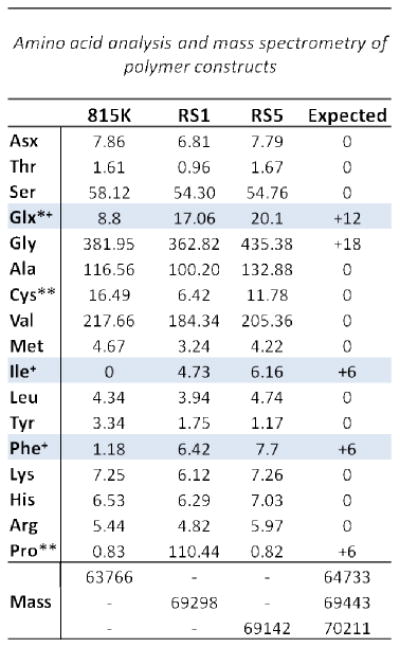
Characterization of SELP815K-RS5: (Lower panel) matrix-assisted laser desorption/ionization time of flight (MALDI-ToF) mass spectrometry of SELP815K-RS5 and SELP815K-RS1 with previously confirmed sequence SELP815K internal control. (Upper panel) HCl vapor hydrolysis amino acid analysis of SELP815K-RS5 and SELP815K-RS1 with previously confirmed sequence SELP815K internal control. +Amino acid additions characteristic of responsive sequence insertion. *Glutamine converted to glutamate (glx) by hydrolysis. **Quantification of these amino acids error prone by this method. 815K: SELP815K, RS1: SELP815K-RS1, RS5: SELP815K-RS5. N=3. For structure of polymers see Figure 1.
3.2. Minimum Gel Forming Concentration
Minimum gel forming concentration (MGFC) was determined as an initial indication of the change in mechanical properties exhibited by each polymer before and after shearing. Uniformly and repeatedly, shearing resulted in a lower MGFC. Native SELP815K showed the least change, decreasing its MGFC from 3% to 2% following shearing. SELP815K-RS5 showed the greatest change with shearing as the MGFC decreased over three fold from 13% to 4% while shearing of SELP815K-RS2 and SELP815K-RS1 resulted in decreases from 4% to 3% and 4% to 2% respectively (Table 1).
Table 1.
Minimum gel forming concentrations of SELP815K, SELP815K-RS1, SELP815K-RS2, and SELP815K-RS5 samples solvated in cold PBS in unsheared form or sheared at 17,000 psi. Polymers were allowed to gel for 24 hrs at 37 °C prior to extrusion from the barrel of 1 mL tuberculin syringes for qualitative analysis of ability to hold form after sectioning. Polymers were assayed at 1% wt/wt concentration increments. Concentrations shown are % w/w. For structure of polymers see Figure 1.
| Minimum Gel Forming Concentration | ||
|---|---|---|
|
| ||
| No Shear | Shear 17k psi | |
| SELP815K | 3% | 2% |
| SELP815K-RS1 | 4% | 3% |
| SELP815K-RS2 | 4% | 2% |
| SELP815K-RS5 | 13% | 4% |
3.3. Swelling ratio
The swelling ratio of each polymer was also assayed following the shearing. The change in swelling ratio was affected not only by the presence or absence of shear, but also the shear rate (Figure 3A). This can be observed in SELP815K, where lower shear pressure of 4,000 psi resulted in properties intermediate to unsheared and the maximum shearing pressure of 17,000 psi. Similar trends were observed for polymers SELP815K-RS1 and SELP815K-RS2 as SELP815K where higher weight percent hydrogels resulted in a lower swelling ratio, and the greatest difference between sheared and unsheared manifested at the lowest weight percent tested (Figure 3B and Figure 3C). Testing between sheared and unsheared SELP815K-RS5 showed the least variability across concentrations from 14% wt/wt to 18% wt/wt (Figure 3D). Comparing across polymer types showed no significant difference between SELP815K, SELP815K-RS1, and SELP815K-RS2 at 10.7% wt/wt and 8% wt/wt, but SELP815K-RS5 showed a significant increase in swelling ratio compared to the other two polymers at gel forming concentrations (Figure 3E). The lowest concentration tested of SELP815K, SELP815K-RS1, and SELP815K-RS2 of 4% wt/wt was not tested for SELP815K-RS5 due to its higher MGFC. In all polymers shearing decreased the measured swelling ratio.
Figure 3.
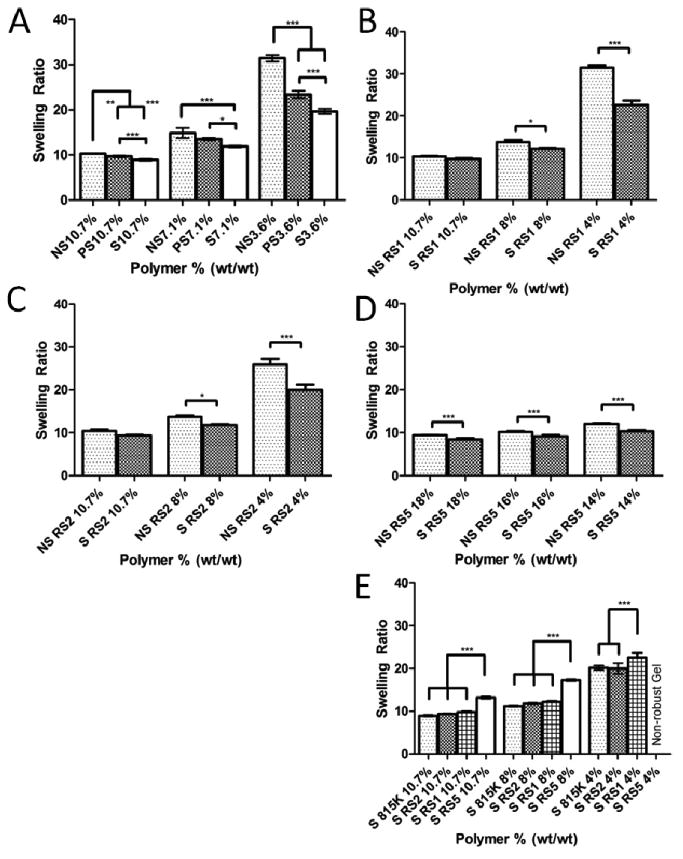
Swelling ratio of (A) SELP815K, (B) SELP815K-RS1, (C) SELP815K-RS2, and (D)SELP815K-RS5 calculated by (wet weight/dry weight) (E) Comparison of swelling ratio of low concentration SELP815K-RS5 with SELP815K, SELP815K-RS1, and SELP815K-RS2. NS: Not Sheared, PS: Partial Shear (4000psi), FS/S: (Full) Shear (17000psi). All percentages expressed as % wt/wt. N=4-5. For structure of polymers see Figure 1. *-p ≤ 0.05, **-p ≤ 0.01, ***-p ≤ 0.001
3.4. Soluble Fraction
The soluble fraction of SELP815K, SELP815K-RS1, and SELP815K-RS2 showed similar trends, and statistical significance was achieved between sheared and unsheared forms of the polymers at all concentrations tested (Figure 4). It was further observed that SELP815K-RS5 soluble fraction did not differ as highly between sheared and unsheared at the concentrations that enabled this comparison (Figure 4B). Additionally SELP815K-RS5 showed the highest soluble fraction release of any polymer tested at 8% wt/wt or higher. SELP815K-RS5 showed greater than 25% soluble fraction release at 10.7% wt/wt and 8% wt/wt while SELP815K, SELP815K-RS1,and SELP815K-RS2 showed less than 13% soluble fraction release at 10.7% wt/wt and 8% wt/wt. SELP815K-RS1 showed the highest soluble fraction at 4% wt/wt in both sheared and unsheared forms.
Figure 4.
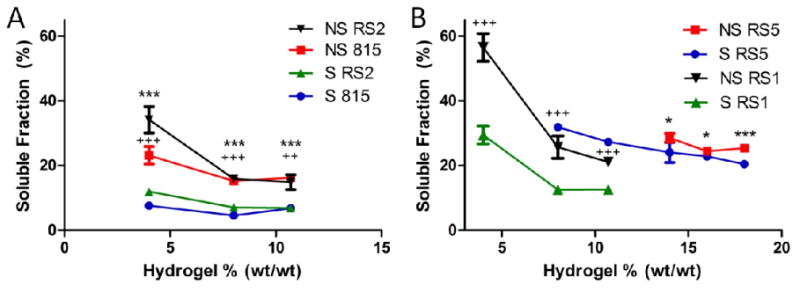
Soluble fraction of (A) SELP815K, SELP815K-RS2, (B) SELP815K-RS1, and SELP815K-RS5 assayed using chromogenic modified Lowry assay for protein content in release media of hydrogels after 7 days. Soluble fractions computed by (weight soluble protein / (weight soluble protein + hydrogel dry weight at day 7)). S: Sheared, NS: Not Sheared, 815: SELP815K, RS1: SELP815K-RS1, RS2: SELP815K-RS2, RS5: SELP815K-RS5 (sequences in Figure 1). N=4-5. (A)***-p ≤ 0.001 for SELP815K points, +++-p ≤ 0.001 for SELP815K-RS2 points, ++-p ≤ 0.01 for SELP815K-RS2 points, (B) *-p ≤ 0.05 for SELP815K-RS5, ***-p ≤ 0.001 for SELP815K-RS5, +++-p ≤ 0.001 for SELP815K-RS1
3.5. Rheology
Rheological properties were compared after shearing for the polymers using a TA Instruments cone-and-plate rheometer. The storage modulus G′ quantifying gel stiffness was measured over 4 hours at 37°C for each polymer with and without shear (Figure 5). Sheared samples showed more rapid gel formation and higher modulus plateau for all polymers. Sheared samples also showed closer adherence to ideal stiffness curves with rapid initial increase in modulus without disruption followed by plateau and curing. The stiffness at 4 hours obtained for each polymer is compared in Figure 5E. The largest difference was observed in SELP815K-RS5, which was statistically significant when sheared and unsheared polymers at identical concentrations were compared. Sheared SELP815K-RS5 showed an over 2-fold increase in stiffness at 14 % wt/wt. Statistically significant increases in stiffness were also recorded for SELP815K-RS1 at 4% wt/wt and 10.7% wt/wt. Increases in stiffness were also observed in SELP815K and SELP815K-RS2 although these changes were not found to be statistically significant.
Figure 5.
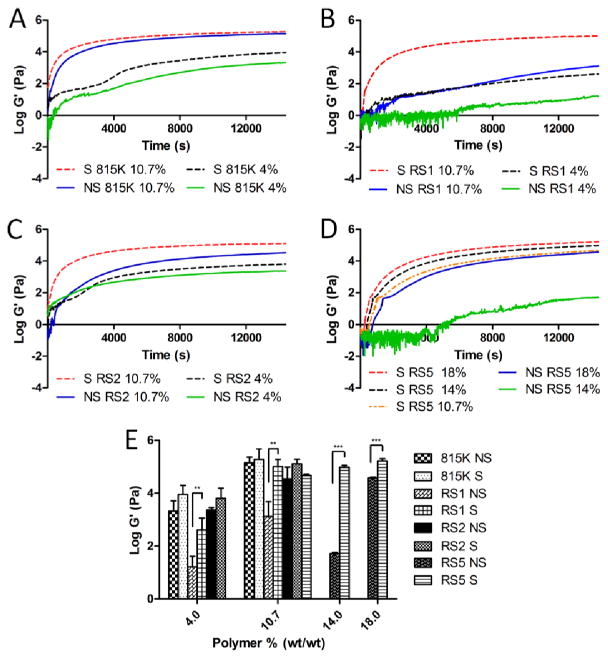
Rheological analysis performed on sheared and unsheared (A) SELP815K at 4% and 10.7% wt/wt polymer respectively, (B) SELP815K-RS1 at 4% and 10.7% wt/wt, (C) SELP815K-RS2 at 4% and 10.7% wt/wt, and (D) SELP815K-RS5 at 18%, 14%, and 10.7% wt/wt polymer respectively. (E) Final gel stiffness at the end of 4 hour run at 37° C for each polymer and concentration. S: Sheared, NS: Not Sheared, 815: SELP815K, RS1: SELP815K-RS1, RS2: SELP815K-RS2, RS5: SELP815K-RS5 (sequences in Figure 1). N=3. *-p ≤ 0.05, **-p ≤ 0.01, ***-p ≤ 0.001
3.6. Nanoparticle Release
Release of 110nm polystyrene beads showed the least change as a function of shear, although several trends were observed across polymer types and concentrations (Figure 6). Unsheared hydrogels released more over 28 days than their sheared counterparts except in a single instance in which the two differed by less than one standard deviation. On average, SELP815K-RS5 released the highest amount over 28 days followed by SELP815K-RS2. SELP815K released the least on average although the differences in the three polymers were not statistically significant. SELP815K-RS1 showed the broadest range of release between sheared and unsheared polymers which resulted in statistical significance. SELP815K, SELP815K-RS1, and SELP815K-RS2 exhibited release inversely correlated with concentration, as expected from previous results with other SELPs.[10] SELP815K-RS5 deviated from the expected trend with low weight percentages releasing slower than high weight percentages. While this result was not statistically significant, similar results were obtained when the experiment was replicated.
Figure 6.
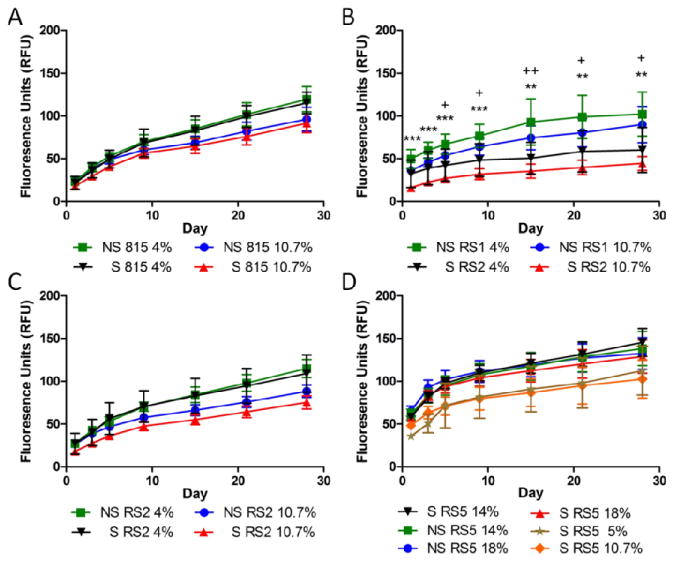
Release of 110nm fluorescently labeled polystyrene beads from (A) SELP815K, (B) SELP815K-RS1, (C) SELP815K-RS2, and (D) SELP815K-RS5 over 28 days from 50uL gels into 500uL of release media consisting of PBS + 0.2mM sodium azide. Release media was removed and gels were washed at each time point to simulate infinite sink conditions. Graphs represent running total of fluorescence released assayed by UV-Vis corrected for photo bleaching and degradation. S: Sheared, NS: Not Sheared, 815: SELP815K, RS1: SELP815K-RS1, RS2: SELP815K-RS2, RS5: SELP815K-RS5 (sequences in Figure 1). N=4. **-p ≤ 0.01, ***-p ≤ 0.001 for 10.7% wt/wt, and +-p ≤ 0.05, ++-p ≤ 0.01 for 4% wt/wt
4. Discussion
The physiochemical properties of protein based polymers can be manipulated by changing the amino acid sequence of the polymer backbone. Due to the exquisite control of recombinant DNA technology one is able to incorporate changes into polymer sequences precisely with near monodisperse precision. By measuring classical hydrogel properties such as swelling ratio and soluble fraction release along with the mechanical properties as exhibited by the MGFC and rheology, we were able to examine the structure - function relationship of each insertion into the polymer sequence. As these polymers are to be used as controlled release depots, it was also important to assay the change in temporal release caused by responsive sequence insertion. We predicted responsive sequence insertion into the silk domain of SELP815K would result in greater structure disruption than insertion into the elastin domain or the junction between silk and elastin units due to the nature of the silk domains to form structurally important beta-sheets. Unexpected was the extent to which shear processing was able to rescue the mechanical stability lost by sequence disruption in SELP815K-RS5.
Minimum gel forming concentration (MGFC) as an initial indicator of mechanical properties behaved as expected with respect to shear and polymer. We assumed the application of shear stress to polymer solutions would disrupt secondary and tertiary interactions from the polymers leading to less intramolecular interactions, and as a result allow for more intermolecular bond formation upon gel formation creating a robust polymer network. Data showed this hypothesis to be true as the application of shear lowered the MGFC across the board and mirrored the predicted mechanical structure disruption. SELP815K-RS5 with MMP-responsive sequences located in the main structural component of the polymer, the silk, would have the highest MGFC while the native SELP815K with no structural disruption to the silk or elastin units would have the lowest MGFC (Table 1).
As with MGFC, the swelling ratios of tested polymers were uniformly affected by shear processing. Increased shear rate resulted in a marked decrease in swelling ratio (Figure 3). This was likely the result of greater intermolecular interaction due to the disruption of weak non-covalent intramolecular bonds by shear stress. Interestingly, the effect on swelling ratio is shear rate dependent with 4,000psi shear pressure having less effect on the polymer systems than 17,000psi indicating that the intramolecular interactions are of varying strength requiring variable shear rate to disrupt these structures from the polymers (Figure 3A). Comparing between polymers, it is observed that SELP815K, SELP815K-RS1 and SELP815K-RS2 with the MMP-responsive sequence in the elastin blocks and in the junction between silk and elastin blocks do not differ significantly at higher concentrations. SELP815K-RS1 did show a statistical difference at the lowest concentration tested 4% wt/wt. However, SELP815K-RS5 shows significant difference from other polymers with a much higher swelling ratio at the same concentration likely due to the disruption of crystal structure normally formed by silk blocks by the insertion of the MMP-responsive sequence (Figure 3E).
The soluble fractions released from hydrogels formed from each polymer were similarly affected by shearing. Across all polymers, hydrogels formed from unsheared polymers had significantly higher soluble fraction release compared to hydrogels formed from sheared polymers at the same concentration. Additionally lower weight percent hydrogels had higher soluble fraction release compared to higher weight percent gels (Figure 4). This was expected as the likely higher percent of intramolecular interactions prior to shear allow for less intermolecular forces to hold polymer strands within the hydrogel network. It is also apparent that structural disruption of the polymer network plays a significant role in soluble fraction release as sheared SELP815K-RS5 showed significantly higher soluble fraction release than sheared SELP815K, SELP815K-RS1, or SELP815K-RS2 at the same concentrations. Additionally, despite high weight percent sheared formulations, SELP815K-RS5 was unable to retain more than 80% of initial polymer strands while SELP815K and SELP815K-RS2 were able to retain over 93%. SELP815K-RS1 was intermediate to these cases retaining approximately 87% of polymer strands at 10.7% wt/wt. This result may indicate responsive sequence insertion adjacent to the silk block can cause some long range structural disruption.
Gelation behavior of polymers tested using rheology gave further data supporting the hypothesis that shearing promotes more intermolecular interaction between polymer strands for a more robust gel network. This is illustrated in the increase in mechanical stiffness seen in all polymers after shearing (Figure 5). Furthermore, similar to observed MGFCs of each polymer, the polymer with the most disrupted structure, SELP815K-RS5, achieved the greatest increase in stiffness after shearing suggesting restoration of some of the crystallization potential of the silk blocks lost by the introduction of the responsive sequence (Figure 5E). As with soluble fraction release, an intermediate but statistically significant increase in rigidity was also observed in SELP815K-RS1 indicating increased structural disruption by the location of the responsive sequence as compared to SELP815K and SELP815K-RS2.
Release from SELP gels was expected to follow trends observed previously in other SELP polymers such as SELP415K and SELP47K with lower weight percent gels releasing particle load faster as a result of less crosslinking density and, by extension, a larger pore size.[10] Further, any parameter which increases crosslinking density and robustness of the network such as shearing should yield lower release rates. These trends were seen in SELP815K, SELP815K-RS1, and SELP815K-RS2. However, the reverse trend was observed in SELP815K-RS5 with lower weight percent gels releasing particle load slower in the sheared group (Figure 6). While interesting, this result was not statistically significant and may be a result of study artifacts. In all release studies, ideal low burst release and linear character of release thereafter was observed. It is important to note that less than 1% of the particle load was released by all gels at day 28. This shows the release kinetics of this gel system will be ideal for delivery of drug containing nanoparticles based on degradation enzyme levels present in the injection site.
5. Conclusion
Two new SELP analogs with MMP responsive sequences placed in silk units and the junction between silk and elastin units were synthesized. The application of shear stress to this and other SELP analogs with and without MMP responsive sequences resulted in quantifiable changes in physiochemical properties. These included influencing the minimum gel forming concentration, soluble fraction, swelling ratio, and mechanical stiffness. Further, shearing enables the use of polymer compositions with disruption in structurally important subunits by significantly lowering the concentration of polymer needed to form hydrogels. These findings have significant implications in the design of recombinant silk-elastinlike polymers for controlled delivery of bioactive agents where responsiveness to matrix-metalloproteinases is desired.
Acknowledgments
Financial Support was provided by the NIH grant (R01-CA107621), Utah Science Technology and Research (USTAR) Initiative, University of Utah Intramural Research Instrumentation Grant, PhRMA Foundation predoctoral fellowship (RP) and an NIH pre-doctoral fellowship (1F30CA176922) (AP). Manuscript review and advice by Joshua Gustafson is highly appreciated.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cappello J, Crissman J, Dorman M, Mikolajczak M, Textor G, Marquet M, Ferrari F. Genetic engineering of structural protein polymers. Biotechnology Progress. 1990;6(3):198–202. doi: 10.1021/bp00003a006. [DOI] [PubMed] [Google Scholar]
- 2.Langer R, Tirrell DA. Designing materials for biology and medicine. Nature. 2004;428(6982):487–492. doi: 10.1038/nature02388. [DOI] [PubMed] [Google Scholar]
- 3.Frandsen JL, Ghandehari H. Recombinant protein-based polymers for advanced drug delivery. Chemical Society Reviews. 2012;41(7):2696–2706. doi: 10.1039/c2cs15303c. [DOI] [PubMed] [Google Scholar]
- 4.MacEwan SR, Chilkoti A. Elastin-like polypeptides: biomedical applications of tunable biopolymers. Biopolymers. 2010;94(1):60–77. doi: 10.1002/bip.21327. [DOI] [PubMed] [Google Scholar]
- 5.Qi Y, Chilkoti A. Growing polymers from peptides and proteins: a biomedical perspective. Polymer Chemistry. 2014;5(2):266–276. [Google Scholar]
- 6.Ngo JT, Tirrell DA. Noncanonical amino acids in the interrogation of cellular protein synthesis. Accounts of Chemical Research. 2011;44(9):677–685. doi: 10.1021/ar200144y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson JA, Lu YY, Van Deventer JA, Tirrell DA. Residue-specific incorporation of non-canonical amino acids into proteins: recent developments and applications. Current Opinion in Chemical Biology. 2010;14(6):774–780. doi: 10.1016/j.cbpa.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cresce AW, Dandu R, Burger A, Cappello J, Ghandehari H. Characterization and realtime imaging of gene expression of adenovirus embedded silk-elastinlike protein polymer hydrogels. Molecular Pharmaceutics. 2008;5(5):891–7. doi: 10.1021/mp800054w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dandu R, Cresce AV, Briber R, Dowell P, Cappello J, Ghandehari H. Silk-elastinlike protein polymer hydrogels: Influence of monomer sequence on physicochemical properties. Polymer. 2009;50(2):366–374. [Google Scholar]
- 10.Dandu R, Ghandehari H, Cappello J. Characterization of structurally related adenovirus-laden silk-elastinlike hydrogels. Journal of Bioactive and Compatible Polymers. 2008;25:441–454. [Google Scholar]
- 11.Dandu R, Ghandehari H. Delivery of bioactive agents from recombinant polymers. Progress in Polymer Science. 2007;32(8-9):1008–1030. [Google Scholar]
- 12.Dinerman AA, Cappello J, El-Sayed M, Hoag SW, Ghandehari H. Influence of solute charge and hydrophobicity on partitioning and diffusion in a genetically engineered silk-elastin-like protein polymer hydrogel. Macromolecular Bioscience. 2010;10(10):1235–47. doi: 10.1002/mabi.201000061. [DOI] [PubMed] [Google Scholar]
- 13.Dinerman AA, Cappello J, Ghandehari H, Hoag SW. Swelling behavior of a genetically engineered silk-elastinlike protein polymer hydrogel. Biomaterials. 2002;23(21):4203–10. doi: 10.1016/s0142-9612(02)00164-3. [DOI] [PubMed] [Google Scholar]
- 14.Dinerman AA, Cappello J, Ghandehari H, Hoag SW. Solute diffusion in genetically engineered silk–elastinlike protein polymer hydrogels. Journal of Controlled Release. 2002;82(2-3):277–287. doi: 10.1016/s0168-3659(02)00134-7. [DOI] [PubMed] [Google Scholar]
- 15.Gustafson J, Greish K, Frandsen J, Cappello J, Ghandehari H. Silk-elastinlike recombinant polymers for gene therapy of head and neck cancer: from molecular definition to controlled gene expression. Journal of Controlled Release. 2009;140(3):256–61. doi: 10.1016/j.jconrel.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gustafson JA, Ghandehari H. Silk-elastinlike protein polymers for matrix-mediated cancer gene therapy. Advanced Drug Delivery Reviews. 2010;62(15):1509–23. doi: 10.1016/j.addr.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 17.Price R, Gustafson J, Greish K, Cappello J, McGill L, Ghandehari H. Comparison of silk-elastinlike protein polymer hydrogel and poloxamer in matrix-mediated gene delivery. International Journal of Pharmaceutics. 2012;427(1):97–104. doi: 10.1016/j.ijpharm.2011.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gustafson JA, Price RA, Frandsen J, Henak CR, Cappello J, Ghandehari H. Synthesis and characterization of a matrix-metalloproteinase responsive silk–elastinlike protein polymer. Biomacromolecules. 2013;14(3):618–625. doi: 10.1021/bm3013692. [DOI] [PubMed] [Google Scholar]
- 19.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nature Reviews Cancer. 2002;2(3):161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 20.Schneider S, Nuschele S, Wixforth A, Gorzelanny C, Alexander-Katz A, Netz R, Schneider M. Shear-induced unfolding triggers adhesion of von Willebrand factor fibers. Proceedings of the National Academy of Sciences. 2007;104(19):7899–7903. doi: 10.1073/pnas.0608422104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaspe J, Hagen SJ. Do protein molecules unfold in a simple shear flow? Biophysical Journal. 2006;91(9):3415–3424. doi: 10.1529/biophysj.106.089367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Szymczak P, Cieplak M. Proteins in a shear flow. The Journal of Chemical Physics. 2007;127(15):155106. doi: 10.1063/1.2795725. [DOI] [PubMed] [Google Scholar]
- 23.Virkar P, Narendranathan T, Hoare M, Dunnill P. Studies of the effect of shear on globular proteins: Extension to high shear fields and to pumps. Biotechnology and Bioengineering. 1981;23(2):425–429. [Google Scholar]


