Abstract
A positive association between cardiorespiratory fitness (CRF) and white matter integrity has been consistently reported in older adults. However, it is unknown whether this association exists in adults over 80 with a range of chronic disease conditions and low physical activity participation, which can influence both CRF and brain health. This study examined whether higher CRF was associated with greater microstructural integrity of gray and white matter in areas related to memory and information processing in adults over 80 and examined moderating effects of chronic diseases and physical activity. CRF was measured as time to walk 400m as quickly as possible with concurrent 3Telsa diffusion tensor imaging in 164 participants (57.1%female, 40.3%black). Fractional anisotropy (FA) was computed for cingulum, uncinate and superior longitudinal fasciculi. Mean diffusivity (MD) was computed for dorsolateral prefrontal cortex, hippocampus, parahippocampus, and entorhinal cortex. Moderating effects were tested using hierarchical regression models. Higher CRF was associated with higher FA in cingulum and lower MD in hippocampus and entorhinal cortex (β, sex-adjusted p: −0.182, 0.019; 0.165, 0.035; and 0.220, 0.006, respectively). Hypertension attenuated the association with MD in entorhinal cortex. Moderating effects of chronic diseases and physical activity in walking and climbing stairs on these associations were not significant. The association of higher CRF with greater microstructural integrity in selected subcortical areas appears robust, even among very old adults with a range of chronic diseases. Intervention studies should investigate whether increasing CRF can preserve memory and information processing by improving microstructure and potential effects of hypertension management.
Keywords: Cardiorespiratory Fitness, Diffusion Tensor Imaging, Microstructural Integrity, Very Old Adults, Neuroepidemiology
1. Introduction
Cumulative evidence suggests higher cardiorespiratory fitness (CFR) is associated with improved cognitive function in older adults [for meta-analysis, see (Colcombe and Kramer, 2003); for review, see (Kramer et al., 2005; Kramer et al., 2006)]. However, neuroimaging evidence of the mechanisms underlying the neuroprotective effect of CRF is sparse, especially at the microstructural level. Most neuroimaging studies have focused on macrostructure of the brain and have found that higher CRF is associated with fewer white matter (WM) lesions (Sen et al., 2012) and greater brain volume, mostly localized in prefrontal cortex and medial temporal lobe (MTL) among cognitively healthy adults in their mid-sixties (Alosco et al., 2013; Colcombe et al., 2003; Colcombe et al., 2006; Erickson et al., 2009; Erickson et al., 2011; McAuley et al., 2011; Weinstein et al., 2012). These regions support executive control function and memory (Park et al., 2001) and are highly susceptible to changes in blood oxygenation levels (Bladin et al., 1993).
However, few studies have examined the association between CRF and microstructural integrity. Diffusion tensor imaging (DTI) provides accurate measures of brain parenchyma microstructure and can also indicate early stages of cognitive impairment (Sasson et al., 2013). Disruption of WM integrity is frequently observed as decreased fractional anisotropy (FA) (Alexander et al., 2007), while increased mean diffusivity (MD) suggests the loss of microstructural integrity in gray matter (GM) (Whitwell et al., 2010). Among the few studies focusing on parenchymal microstructure, DTI was applied to examine WM only in relatively young and well-functioning older adults (Johnson et al., 2012; Marks et al., 2007; Marks et al., 2011; Tseng et al., 2013) and in patients with multiple sclerosis (Voss et al., 2010). Overall, results indicated a positive association between CRF and WM integrity in corpus callosum, cingulum, and uncinate fasciculus. One small intervention study reported increases in fitness from walking were associated with greater WM integrity in prefrontal, parietal, and temporal regions in well-functioning older adults (Voss et al., 2012). However, the microstructural integrity of GM in relation to CRF has not been examined. Examining the spatial distribution of the microstructure in both WM and GM can further the understanding of the neuroprotective effects of CRF.
It is also unclear whether CRF would have a neuroprotective effect in very old adults who are living with chronic disease conditions and low physical activity participation. This is important, because chronic disease and physical activity could affect both CRF and brain health. For example, chronic diseases may lower CRF levels directly or indirectly through limited physical activity participation. Previous investigations have not examined the contribution of chronic disease or physical activity to the neuroprotective effects of higher CRF. It is important to quantify the association between CRF and structural integrity of the brain in the context of chronic diseases and physical activity participation, in order to identify the optimal CRF level for preserving brain health in older age.
One challenge in examining the relationship between CRF and brain health in very old age is the limited availability of safe and accurate measures of CRF. Most prior studies focusing on well-functioning adults in their mid-sixties to seventies applied the graded maximal exercise test (Colcombe et al., 2003; Erickson et al., 2009; Johnson et al., 2012; Marks et al., 2011) which can pose safety risks for adults over 80 years of age (Hollenberg et al., 1998; Wilson et al., 1986). By contrast, the self-paced 400-meter long-distance corridor walk test has high safety in measuring CRF and it has been previously validated against the graded maximal exercise test (Simonsick et al., 2006). The 400-m walk time is shown to be a valid indicator of CRF in adults aged 60 and older, which is highly correlated with peak VO2 and explains 75.5% of the variance of peak VO2 (Simonsick et al., 2006).
This study examined the cross-sectional association between CRF, measured as time to complete the 400-m walk as quickly as possible (Simonsick et al., 2006), and neuroimaging markers of microstructural integrity of WM and GM in a cohort of adults over 80 years of age, while accounting for chronic diseases and physical activity. It was hypothesized that higher CRF would be associated with greater brain integrity in prefrontal cortex and MTL because of their localizations within watershed areas and their known associations with memory (Erickson et al., 2011; McAuley et al., 2011) and processing speed (Spirduso, 1980). It was also hypothesized that these associations would be moderated by higher burden of prevalent diseases and lower physical activity levels.
2. Results
Demographic characteristics of the 164 participants who completed the 400-m walk test and had DTI data in 2006–2008 (51.8% female, 40.3% black as shown in Table 1) were similar to those who completed the 400-m walk test in the parent cohort at study entry (48.8% female, 37.7% black) (Newman et al., 2006). Compared to those who received a brain Magnetic Resonance Imaging (MRI) but did not complete the 400-m walk test (n = 62), these 164 completers were more likely to be men (p = 0.016) and had higher digit symbol substitution test (DSST) score (p = 0.019), faster gait speed (p < 0.001), and lower body mass index (BMI) (p = 0.016). The 164 completers also tended to have lower prevalence of hypertension and diabetes than those who did not complete the 400-m walk test (n = 62), although the group differences were not statistically significant (p = 0.058 and 0.060, respectively).
Table 1.
Characteristics of the analytic sample by sex (N = 164)
| Completed (n = 164) | Men (n = 79, 48.2%) | Women (n = 85, 51.8%) | p-value | |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 82.9 ± 2.6** | 82.8 ± 2.8 | 82.9 ± 2.5 | 0.772 |
| Female | 85 (51.8) | - | - | - |
| Black race | 63 (38.4)** | 22 (27.8) | 41 (48.2) | 0.007 |
| Cardiorespiratory fitness | ||||
| 400m time, seconds | 344.4 ± 63.7 | 329.3 ± 61.8 | 358.4±62.6 | 0.003 |
| Physical Activity | ||||
| Walking and climbing stairs, kcal/kg/week | 6.2 ± 10.5* | 6.0 ± 10.7 | 6.4 ± 10.3 | 0.810 |
| Chronic disease conditions1 | ||||
| Cardiovascular disease | 42 (25.6) | 23 (29.1) | 19 (22.4) | 0.322 |
| Hypertension | 102 (62.2)* | 41 (51.9) | 62 (72.9) | 0.005 |
| Myocardial infarction | 20 (12.2) | 13 (16.5) | 7 (8.2) | 0.108 |
| Stroke | 12 (7.3) | 1 (1.3) | 11 (12.9) | 0.004 |
| Diabetes | 37 (22.6) | 23 (29.1) | 14 (16.5) | 0.053 |
| Cognitive function | ||||
| DSST score (range 0–90) | 38.5 ± 13.2** | 38.2 ± 12.7 | 38.8 ± 13.8 | 0.768 |
| 3MSE score (range 0–100) | 93.9 ± 5.8* | 94.4 ± 5.4 | 93.4 ± 6.2 | 0.313 |
| Depressive symptoms | ||||
| CES-D score (range 0–60) | 6.2 ± 5.4* | 5.9 ± 4.7 | 6.4 ± 6.0 | 0.572 |
| Physical function | ||||
| Gait speed, meters/second | 1.01 ± 0.23** | 0.98 ± 0.18 | 0.94 ± 0.17 | 0.104 |
| Other characteristics related to CRF | ||||
| Postsecondary Education | 89 (54.6) | 49 (62.8) | 40 (47.1) | 0.092 |
| Current or former smokers | 75 (45.7) | 51 (64.5) | 24 (28.2) | < 0.001 |
| Alcohol consumption ever ≥ 5drinks/day | 9 (5.8) | 9 (11.4) | 0 (0) | 0.001 |
| Body mass index, kg/m2 | 26.8 ± 4.1** | 27.3 ± 3.8 | 26.4 ± 4.3 | 0.192 |
| Systolic blood pressure, mm Hg | 133.6 ± 19.0 | 132.5 ± 18.4 | 134.7 ± 19.6 | 0.483 |
| FEV1/FVC | 70.8 ± 9.3 | 69.1 ± 9.0 | 72.4 ± 9.3 | 0.026 |
Abbreviations: DSST = digit symbol substitution test. 3MSE = modified mini-mental state examination. CES-D = Center for Epidemiologic Studies Depression Scale. FEV1/FVC = forced expiratory volume in first second/forced vital capacity, unitless. Note: Values are mean ± SD or N (%) as noted.
Disease prevalence at the time of MRI and LDCW measurements.
sex-adjusted correlations with 400-m time p < 0.001,
p < 0.05.
Partial correlation coefficient ranged from 0.18 to 0.32 and from −0.55 to −0.23. P-values for sex differences were computed from independent t-test or χ2 test as appropriate.
All 164 participants included in this study had 3MSE greater than 80, indicating free of cognitive impairment or dementia. The associations between 400-m time and health characteristics were similar to those previously reported in the large cohort (Newman et al., 2003). Specifically, faster 400-m time was associated with younger age, white race, male sex, higher physical activity level in walking and climbing stairs, lower prevalence of hypertension, higher DSST and modified mini-mental status examination (3MSE) scores, lower scores on Center for Epidemiologic Studies Depression Scale (CES-D), faster gait speed, and lower BMI (sex-adjusted p < 0.05 for all) (Table 1). Compared to women, men had faster 400-m time, lower prevalence of hypertension and stroke, were more likely to be current or former smokers, reported more alcohol consumption, and had lower pulmonary function as measured by the ratio of forced expiratory volume in the first second and forced viral capacity (FEV1/FVC) (Table 1).
After adjustment for sex, faster 400-m time was significantly associated with higher FA in the cingulum, but not with FA in uncinate or superior longitudinal fasciculi (Table 2). Faster 400-m time was also associated with lower MD in MTL, but not with MD in dorsolateral prefrontal cortex (Table 2). Among the sub-regions of MTL, faster 400-m time was associated with MD in the hippocampus and the entorhinal cortex, but not in parahippocampus (Table 2). Among tracts and regions examined, the sex-adjusted association with FA in the entorhinal cortex remained significant after Bonferroni correction (p < 0.007, Table 2).
Table 2.
FA in tracts of interest and MD in regions of interest and sex-adjusted correlations with 400m time (n = 164)
| Regions and tracts of interest | Mean ± SD | Partial correlations, r | p-value |
|---|---|---|---|
| Memory-related network | |||
| FA in the uncinate fasciculus | 0.3284 ± 0.0274 | 0.05 | 0.531 |
| FA in the cingulum | 0.3973 ± 0.0229 | −0.18 | 0.019 |
| MD in the medial temporal lobe | 0.00157 ± 0.00021 | 0.18 | 0.022 |
| in the hippocampus | 0.00165 ± 0.00024 | 0.17 | 0.035 |
| in the parahippocampus | 0.00154 ± 0.00020 | 0.15 | 0.062 |
| in the entorhinal cortex | 0.00144 ± 0.00025 | 0.22 | 0.006^ |
| Executive control-related network | |||
| FA in the superior longitudinal fasciculus | 0.3546 ± 0.0200 | −0.08 | 0.321 |
| MD in the dorsolateral prefrontal cortex | 0.00108 ± 0.00011 | −0.06 | 0.466 |
Note: FA = fractional anisotropy (higher = greater integrity); MD = mean diffusivity (lower = greater integrity); 400m time, faster = higher cardiorespiratory fitness.
p < 0.007 (Bonferroni adjusted).
The associations between faster 400-m time and higher FA and lower MD were significant in sex-adjusted linear regression models (Table 3). Each standard deviation difference in 400-m time (standard deviation = 63.7 seconds) was associated with a difference of 0.004 (= 0.182 × 0.0229) in FA in the cingulum, corresponding to a 1% of FA in the cingulum of this sample. Similarly, each standard deviation difference in 400-m time was associated with a difference of .00004 (= 0.165 × 0.00024) in MD in the hippocampus, and with a difference of 0.00006 (= 0.220 × 0.00025) in MD in the entorhinal cortex, corresponding to 2.4% of MD in the hippocampus and 4.0% of MD in the entorhinal cortex, respectively.
Table 3.
Sex-adjusted multivariate regression models of 400-m time predicting FA in the cingulum and MD in the hippocampus and the entorhinal cortex, standardized units (n = 164)
| Covariates | FA in cingulum | MD in hippocampus | MD in entorhinal cortex |
|---|---|---|---|
|
| |||
| β (95% CI), p
|
|||
| sex | −0.182 (−0.334, −0.031), 0.019 | 0.165 (0.012, 0.317), 0.035 | 0.220 (0.064, 0.376), 0.006 |
| sex + age | −0.145 (−0.303, 0.012), 0.070* | 0.068 (−0.083, 0.219), 0.376* | 0.169 (0.008, 0.329), 0.040* |
| sex + physical activity | −0.168 (−0.324, −0.011), 0.036 | 0.163 (0.005, 0.321), 0.043 | 0.213 (0.052, 0.374), 0.010 |
| sex + cardiovascular disease | −0.184 (−0.335, −0.032), 0.018 | 0.163 (0.010, 0.315), 0.036 | 0.222 (0.066, 0.378), 0.006 |
| sex + hypertension | −0.168 (−0.323, −0.013), 0.034 | 0.153 (−0.003, 0.310), 0.055 | 0.196 (0.037, 0.355), 0.016* |
| sex + myocardial infarction | −0.182 (−0.334, −0.030), 0.019 | 0.165 (0.012, 0.318), 0.035 | 0.220 (0.064, 0.376), 0.006 |
| sex + stroke | −0.178 (−0.328, −0.028), 0.020 | 0.164 (0.011, 0.317), 0.036 | 0.217 (0.062, 0.373), 0.006 |
| sex + diabetes | −0.180 (−0.334, −0.027), 0.021 | 0.166 (0.012, 0.321), 0.035 | 0.216 (0.058, 0.373), 0.008 |
Note: FA = fractional anisotropy; MD = mean diffusivity. Covariates included age, physical activity, and chronic disease conditions which were known to be associated with the 400m walk performance.
Changes in regression coefficients compared to Model 1 > 10%.
After adjustment for sex, for each standard deviation decrease in 400-m time (63.7 seconds), there was an increase of 0.004 (= 0.182 × 0.0229) in FA in the cingulum, corresponding to 1% of the average FA in the cingulum of this sample. For each standard deviation decrease in 400-m time, there was a decrease of 0.00004 (= 0.165 × 0.00024) in MD in the hippocampus (2.4% of the average MD in the hippocampus of this sample) and a decrease of 0.00006 (= 0.220 × 0.00025) in MD in the entorhinal cortex (4.0% of the average MD in the entorhinal cortex of this sample).
The associations between faster 400-m time with higher FA in the cingulum and with lower MD in the hippocampus and the entorhinal cortex were attenuated after adjustment for age, but the association with MD in the entorhinal cortex remained significant (Table 3). Changes in regression coefficient of 400-m time were 20.0%, 58.8% and 23.2%, respectively (Table 3). The association of faster 400-m time with lower MD in the entorhinal cortex was attenuated after further adjustment for hypertension (Δβ = 10.9%). The interaction between 400-m time and hypertension on the association with MD in the entorhinal cortex was not significant (p > 0.05) and trends were similar for those with hypertension and without. Adjustment for cardiovascular disease, stroke, myocardial infarction, diabetes, or self-reported physical activity in walking and climbing stairs, did not substantially attenuate the associations with FA in the cingulum or with MD in the hippocampus or the entorhinal cortex (Table 3).
In additional sensitivity analyses, the estimated peak VO2 was obtained based on parameters from the 400-m walk test using a previously published formula (Simonsick et al., 2006). The association of estimated peak VO2 with FA in the cingulum and MD in the entorhinal cortex remained similar (standardized β, p: 0.241, 0.002; −0.185, 0.018, respectively). The association with MD in the hippocampus was not significant (standardized β, p: −0.089, 0.257).
3. Discussion
In adults over 80 years of age, higher CRF was associated with greater microstructural integrity in the cingulum and the entorhinal cortex. Although the presence of chronic diseases and low physical activity participation were common in this sample, the observed associations remained robust after accounting for these measures. Using the long-distance corridor walk allowed us to test the association between CRF and brain health in a sample of very old adults. This sample had a mean age of 83 and only 14.9% walked at a pace of at least 1.34 meters/second which was the typical starting pace for treadmill protocols (Newman et al., 2006). Thus, the majority of these participants would not have been able to perform a treadmill test to quantify their CRF levels.
The application of DTI extended previous work by examining both WM and GM in specific regions and tracts (Johnson et al., 2012; Marks et al., 2007; Marks et al., 2011). Associations appeared to be strong for MD in the entorhinal cortex. For every minute difference in 400-m time, there was a 4% difference in MD in the entorhinal cortex. This difference may appear small in an absolute term, but it is relatively large in considering that MD in GM increases by approximately 2% per year in older age (Abe et al., 2008; Benedetti et al., 2006). One recent study with a small sample found physically fit older adults had lower MD in the cingulum and the hippocampus than sedentary older adults in their early seventies (Tseng et al., 2013). Since the entorhinal cortex plays an important role in memory (Park et al., 2001), these associations may explain the beneficial effects of CRF on memory (McAuley et al., 2011). Because of our cross-sectional design and the lack of extensive memory assessment, our study could not test this hypothesis. Future intervention studies in very old adults with a wide range of fitness levels are needed to test the hypothesis that CRF improves memory because it has an effect on entorhinal microstructure.
The observed associations were localized in MTL, which was known to be vulnerable to low perfusion mainly because of the watershed vascularization (Bladin et al., 1993). CRF may preserve structural integrity in these regions by improving oxygen transport and utilization in cerebral vascular system and increase oxidative capacity in the brain (Ainslie et al., 2008; Etnier and Landers, 1995). The finding that hypertension attenuated the association with structural integrity in the entorhinal cortex was consistent with the pathway linking higher CRF and greater microstructural integrity reported in a prior study (Gow et al., 2012). However, we did not observe a significant interaction effect of hypertension on the association between fitness integrity in the entorhinal cortex. Whether changes in blood pressure underlie the relationship between CRF and brain microstructural integrity needs further investigation.
Because lower 400-m walk performance was associated with higher prevalence and severity of chronic disease conditions, such as cardiovascular disease, stroke, and diabetes (Enright et al., 2003; Newman et al., 2003; Newman et al., 2006), we hypothesized that these conditions would moderate the associations between CRF and neuroimaging markers of interest. However, adjustment for cardiovascular disease, myocardial infarction, stroke, and diabetes, did not substantially attenuate the association between CRF and neuroimaging outcomes. It is possible that adults who survive to very old ages free from clinically overt disabilities, even though have been exposed to chronic diseases, are exceptionally robust and have developed resilience to such chronic conditions. Other factors, such as genetics, environmental conditions, and motivation, contribute to fitness and brain health but are not directly accounted for in the analyses (Bouchard and Rankinen, 2001). As motivation is one important determinant of physical activity, it is to some extent controlled for in the analysis by adjusting for the self-reported physical activity level.
We also hypothesized that self-reported physical activity would moderate the association between CRF and microstructural integrity. We have recently reported greater microstructural integrity in this cohort is associated with greater amounts of physical activity assessed by a comprehensive self-report questionnaire ten years prior (at study entry of the original cohort) (Tian et al., 2014). However, contrary to our expectations, neither physical activity by the comprehensive self-report questionnaire at study entry nor self-reported physical activity in walking and climbing stairs at the time of MRI attenuated the association of CRF with microstructural integrity in the cingulum and the entorhinal cortex. Although CRF and physical activity are strongly interrelated, fitness and physical activity can be differentially determined by an individual’s health profile, such as age, sex, genotypes, clinical and subclinical diseases, and social and environmental factors (Eaton et al., 1995; Tager et al., 1998). These findings suggest that fitness and physical activity may have independent contributions to brain health. The fact that the adjustment for physical activity did not attenuate the association between fitness and microstructural integrity was also likely due to the type of physical activity measure. Specifically, physical activity was quantified based on self-reported information on a very limited range of activities, including only walking and climbing stairs. Thus, it did not capture the whole spectrum of free-living activities, especially for those activities with light-to-low intensity. In addition, compared to objective measures of physical activity, self-report measurement may be less accurate and reliable for very old adults with declining memory and low activity participation (Fruin and Rankin, 2004). Using the self-report measurement focusing on limited activity domains may underestimate the association between physical activity and brain health. Thus, the contribution of physical activity to the association between CRF and brain structure may not have been accurately quantified. Future studies with objective measures of physical activity, are needed to clarify the role of physical activity in the neuroprotective effects of CRF.
Although the observed associations were attenuated after adjustment for age, the interactions between 400-m time and age were not significant. Because the age range of this analytic sample was narrow (79–90 years old), the effect of age on these associations may be due to the collinearity between 400-m time and age, rather than a true age-related moderation. It is possible that the attenuated associations after adjustment for age are due to the accelerated decline in WM integrity in very old age (Kochunov et al., 2012). Others also reported that the association between CRF and microstructural integrity was attenuated after adjustment for age and sex in older adults (Marks et al., 2011).
It is worth noting that results for the cingulum and the entorhinal cortex did not substantially change when the estimated peak VO2 was used in the model instead of 400-m time. These findings are consistent with prior work showing that 400-m time provides a valid estimate of peak VO2 and of CRF in very old adults (Simonsick et al., 2006).
Because of the cross-sectional design, this study cannot assess a causal relationship between CRF and brain structural integrity and reverse causality cannot be ruled out. For example, those with greater brain integrity may be more life-style conscious and thus more likely to engage in CRF-promoting activities. A second limitation is that the long-distance corridor walk test cannot estimate CRF levels for those ineligible to participate and those who stopped. Lastly, our study participants are likely to be healthier than the general population due to their ability to complete the long distance corridor test and the eligibility for brain MRI. In sum, while our findings may be applicable only to very old adults, they are not likely to be driven by illness or frailty status.
4. Methods
4.1 Study population
Participants were recruited from the Health, Aging and Body Composition study cohort, an ongoing longitudinal study that began in March 1997 in Pittsburgh, PA and Memphis, TN to assess the relationship between changes in body composition and health outcomes in 3,075 community-dwelling older adults (52% women, 42% Black) aged 70 to 79 (Simonsick et al., 2001). Among the 1,527 participants seen at the Pittsburgh site at study entry (1997–1998), 819 were alive and seen in the clinic or at home in 2006–2008. Of these 819, 325 participants had a brain MRI. Of these 325, 226 had DTI and data on the 400-meter walk test. Twenty-three of the 226 (10.2%) were excluded from the test due to medical contraindications, including electrocardiogram abnormalities, mobility-related exclusions or recent chest pain, shortness of breath, or fainting, 39 (17.3%) were unable to complete the test, and 164 (72.5%) completed the test. These 164 participants with DTI and 400-meter time were included in this study. The study protocol was approved by the University of Pittsburgh and all participants provided informed consent.
4.2 MRI image acquisition
MRI scans were obtained at the MR Research Center of the University of Pittsburgh with a 12-channel head coil on 3Tesla Siemens TIM TRIO scanners equipped for echo-planer imaging using the protocol previously described (Rosano et al., 2012). Four series of MRI images were acquired on the MR scanner. Magnetization-prepared rapid gradient echo (MPRAGE) T1-weighted images were acquired in the axial plane: TR = 2300 ms; TE = 3.43 ms; TI = 900 ms; Flip angle = 9°; slice thickness = 1 mm; FOV = 256 mm × 224 mm; voxel size = 1 mm × 1 mm; matrix size = 256 × 224; and number of slices = 176. Fluid-attenuated inversion recovery (FLAIR) images were acquired in the axial plane: TR = 9160 ms; TE = 89 ms; TI = 2500 ms; FA = 150°; FOV = 256 mm × 212 mm; slice thickness = 3 mm; matrix size = 256 × 240; number of slices = 48 slices; and voxel size = 1 mm × 1 mm. Diffusion Tensor Images (DTI) were acquired using single-short spin-echo sequence with 12 directions. The acquisition parameters were as follows: TR = 5300 ms; TE = 88 ms; TI = 2500 ms; Flip angle = 90°; FOV = 256 mm × 256 mm; voxel size = 2 mm × 2 mm; two diffusion values of b = 0 and 1000 s/mm2; four repeats; four B0 images; slice thickness = 3 mm; and GRAPPA = 2. There were no pathological findings from MR images for this study as verified by a neuroradiologist.
4.3 Image processing and analysis
MPRAGE T1-weighted images were acquired to obtain volumes of GM and WM, respectively. Volumes of GM, WM, and cerebrospinal fluid were calculated by segmenting the skull-stripped T1-weighted image in native anatomical space using the FAST-FMRIB Automated Segmentation Tool (Zhang et al., 2001). T2-weighted FLAIR images were acquired to obtain the WM hyperintensities (WMH) volume. The WMH quantification was done using a fuzzy connected algorithm (Wu et al., 2006). The diffusion-weighted images were pre-processed using the FMRIB’s Diffusion Toolbox (Smith et al., 2004) to remove unwanted distortions which were eddy current distortions leading to geometric distortions of the image and the tensor were computed (Basser et al., 1994). Four repeats were concatenated and processed with FMRIB Software Library to generate FA and MD maps. The FA map was registered to the FMRIB58_FA template (Smith et al., 2004) using the FMRIB’s non-linear image registration tool (FNIRT; Andersson et al. 2007). The transformation was also applied to the MD map. Using the segmentation of GM, WM, and WMH, the FA and MD maps were restricted to normal-appearing WM and GM (Rosano et al., 2012). Using Automated Labeling Pathway (Aizenstein et al., 2005; Wu et al., 2006), neuroimaging markers in regions and tracts were identified based on the Automated Anatomical Labeling Atlas (Tzourio-Mazoyer et al., 2002) and the Johns Hopkins University White Matter Atlas (Wakana et al., 2004), respectively.
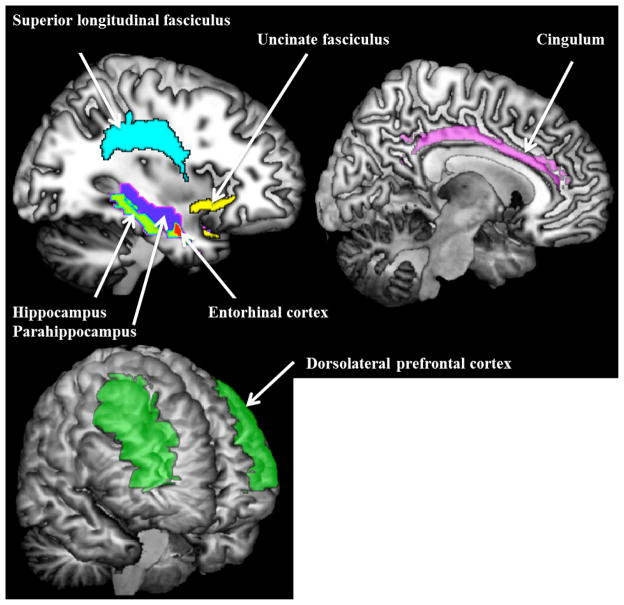
In this study, neuroimaging outcomes were FA from normal-appearing WM and MD from normal-appearing GM. Regions and tracts of interest were selected a priori (Figure 1), including MTL (hippocampus, parahippocampus, entorhinal cortex), dorsolateral prefrontal cortex, uncinate and superior longitudinal fasciculi, and cingulum. MD in left and right hemispheres was computed as the average weighted by GM volume (i.e. MD in bilateral region was weighted by the percentage of voxels in that region from each hemisphere). FA in tracts of interest from left and right hemispheres was computed as the average weighted by WM volume.
Figure 1.
Regions (dorsolateral prefrontal cortex, hippocampus, parahippocampus, entorhinal cortex) and tracts (superior longitudinal fasciculus, uncinate fasciculus, cingulum) of interest
4.4 Cardiorespiratory fitness
Cardiorespiratory fitness was measured as time to complete the 400-m walk test as quickly as possible at a pace that participants could maintain (Newman et al., 2003). The test consists of a 2-minute walk followed immediately by a 400-m walk with the instruction to “walk as quickly as possible”. Time to complete the 400-m walk is shown as a valid estimate of peak VO2 among older adults (Simonsick et al., 2006) and it is here referred to as “400-m time”. 400-m time was computed in seconds and reported in standardized units. In additional sensitivity analyses, peak VO2 was also estimated based on heart rate at the end of the 400-m walk, stride length at a usual pace during the first 20 meters of the 2-min walk, and 400-m time using a previously published formula (Simonsick et al., 2006).
4.5 Other measures of interest
Chronic diseases, including prevalent cardiovascular disease (CVD), hypertension, myocardial infarction, stroke, and diabetes, were all obtained concurrently with the 400-m walk test and brain MRI in 2006–2008 using prevalent disease algorithms according to self-reported diagnoses by physicians and record of medication use. Prevalent CVD was defined by self-reported prevalent coronary heart disease or cerebrovascular disease. Prevalent diabetes and hypertension were defined by self-report and confirmed by medication use.
Cognitive function was measured by DSST and 3MSE and depressive symptoms were assessed using CES-D. Physical function was measured as gait speed using the GaitMat II™ system (EQ Inc., Chalfont, Pennsylvania). Participants were asked to walk at their usual pace on the 4-m-long GaitMat II™ walkway. For those who did not have the GaitMat measure, gait speed was computed in meters/second while walking at a usual pace over 3, 4 or 6 meters (Inzitari et al., 2007; Klepin et al., 2010). Gait speed is a valid and reliable assessment of physical function among older adults in both clinical settings and in aging research (Guralnik et al., 2000; Studenski et al., 2003). Slower gait speed is strongly associated with severity of health conditions and a higher mortality risk (Montero-Odasso et al., 2005; Studenski et al., 2011).
In addition to age, sex, and race obtained at study entry, other characteristics related to CRF were measured at the time of the MRI: education, BMI (kg/m2), smoking status, alcohol consumption, and systolic blood pressure (mm Hg). Systolic blood pressure was obtained as the average from two measurements. Pulmonary function was assessed by FEV1/FVC. Physical activity was measured as walking and climbing stairs in the past week by self-report at the time of the MRI (Taylor et al., 1978). Physical activity was computed as a continuous variable in kcal/kg/week.
4.6 Statistical analyses
The associations of 400-m time with population characteristics were adjusted for sex using partial correlation analyses, due to the sex difference in CRF. Since this was an exploratory analysis, significant associations were reported with p value less than 0.05. Associations between 400-m time and neuroimaging outcomes that were significant at p < 0.05 were first adjusted for sex and further adjusted for age, physical activity, and chronic diseases including cardiovascular disease, hypertension, myocardial infarction, stroke, and diabetes using multiple linear regression models.
Moderating effects of chronic diseases or physical activity on the strength of the associations between 400-m time and neuroimaging outcomes were tested using hierarchical multiple regression analyses: (1) the predictor (400-m time) and the moderator (if continuous) were set to be at the mean, (2) the interaction term between scaled 400-m time and the moderator was created, and (3) hierarchical multiple regression models were conducted by first entering 400-m time and the moderator and then adding the interaction term. A significant model change after adding the interaction term and also the significance of the interaction term at p < 0.05 indicated a statistically significant moderating effect.
In additional sensitivity analyses, the associations between the estimated peak VO2 and neuroimaging outcomes were tested using linear regression models. Stride length, which was closely correlated with sex (r = 0.369, p < 0.001), was already included in the formula to estimate peak VO2. Thus, models were not adjusted for sex to avoid collinearity.
We examined the association between fitness and microstructure in very old adults.
Higher fitness was associated with greater microstructural integrity.
Associations were localized in the cingulum and the medial temporal lobe.
Associations were independent of physical activity and chronic diseases.
Acknowledgments
This research was supported by National Institute on Aging (NIA) Contracts N01-AG-6-2101, N01-AG-6-2103, N01-AG-6-2106, K23-AG028966-01; NIA grants R01-AG028050, R01-AG029232, P30-AG024827, and NINR grant R01-NR012459. This research was supported in part by the Intramural Research Program of the NIH, National Institute on Aging.
Footnotes
Conflict of Interest
The authors declare no conflict of interest related to this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersson JL, Jenkinson M, Smith S. FMRIB Analysis Group of the University of Oxford. 2007. Non-linear registration, aka Spatial normalisation FMRIB technical report TR07JA2. [Google Scholar]
- Abe O, Yamasue H, Aoki S, Suga M, Yamada H, Kasai K, Masutani Y, Kato N, Kato N, Ohtomo K. Aging in the CNS: comparison of gray/white matter volume and diffusion tensor data. Neurobiol Aging. 2008;29:102–16. doi: 10.1016/j.neurobiolaging.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Ainslie PN, Cotter JD, George KP, Lucas S, Murrell C, Shave R, Thomas KN, Williams MJ, Atkinson G. Elevation in cerebral blood flow velocity with aerobic fitness throughout healthy human ageing. J Physiol. 2008;586:4005–10. doi: 10.1113/jphysiol.2008.158279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizenstein HJ, Butters MA, Figurski JL, Stenger VA, Reynolds CF, 3rd, Carter CS. Prefrontal and striatal activation during sequence learning in geriatric depression. Biol Psychiatry. 2005;58:290–6. doi: 10.1016/j.biopsych.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4:316–29. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alosco ML, Brickman AM, Spitznagel MB, Griffith EY, Narkhede A, Raz N, Cohen R, Sweet LH, Colbert LH, Josephson R, Hughes J, Rosneck J, Gunstad J. Poorer physical fitness is associated with reduced structural brain integrity in heart failure. J Neurol Sci. 2013;328:51–7. doi: 10.1016/j.jns.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B. 1994;103:247–54. doi: 10.1006/jmrb.1994.1037. [DOI] [PubMed] [Google Scholar]
- Benedetti B, Charil A, Rovaris M, Judica E, Valsasina P, Sormani MP, Filippi M. Influence of aging on brain gray and white matter changes assessed by conventional, MT, and DT MRI. Neurology. 2006;66:535–9. doi: 10.1212/01.wnl.0000198510.73363.c6. [DOI] [PubMed] [Google Scholar]
- Bladin CF, Chambers BR, Donnan GA. Confusing stroke terminology: watershed or borderzone infarction? Stroke. 1993;24:477–8. doi: 10.1161/01.str.24.3.477. [DOI] [PubMed] [Google Scholar]
- Bouchard C, Rankinen T. Individual differences in response to regular physical activity. Med Sci Sports Exerc. 2001;33:S446–51. doi: 10.1097/00005768-200106001-00013. discussion S452–3. [DOI] [PubMed] [Google Scholar]
- Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14:125–30. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Raz N, Webb AG, Cohen NJ, McAuley E, Kramer AF. Aerobic fitness reduces brain tissue loss in aging humans. J Gerontol A Biol Sci Med Sci. 2003;58:176–80. doi: 10.1093/gerona/58.2.m176. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, Elavsky S, Marquez DX, Hu L, Kramer AF. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61:1166–70. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- Eaton CB, Lapane KL, Garber CE, Assaf AR, Lasater TM, Carleton RA. Physical activity, physical fitness, and coronary heart disease risk factors. Med Sci Sports Exerc. 1995;27:340–6. [PubMed] [Google Scholar]
- Enright PL, McBurnie MA, Bittner V, Tracy RP, McNamara R, Arnold A, Newman AB. The 6-min walk test: a quick measure of functional status in elderly adults. Chest. 2003;123:387–98. doi: 10.1378/chest.123.2.387. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Prakash RS, Voss MW, Chaddock L, Hu L, Morris KS, White SM, Wojcicki TR, McAuley E, Kramer AF. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. 2009;19:1030–9. doi: 10.1002/hipo.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108:3017–22. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etnier JL, Landers DM. Brain function and exercise. Current perspectives. Sports Med. 1995;19:81–5. doi: 10.2165/00007256-199519020-00001. [DOI] [PubMed] [Google Scholar]
- Fruin ML, Rankin JW. Validity of a multi-sensor armband in estimating rest and exercise energy expenditure. Med Sci Sports Exerc. 2004;36:1063–9. doi: 10.1249/01.mss.0000128144.91337.38. [DOI] [PubMed] [Google Scholar]
- Gow AJ, Bastin ME, Munoz Maniega S, Valdes Hernandez MC, Morris Z, Murray C, Royle NA, Starr JM, Deary IJ, Wardlaw JM. Neuroprotective lifestyles and the aging brain: Activity, atrophy, and white matter integrity. Neurology. 2012;79:1802–1808. doi: 10.1212/WNL.0b013e3182703fd2. [DOI] [PubMed] [Google Scholar]
- Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, Studenski S, Berkman LF, Wallace RB. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–31. doi: 10.1093/gerona/55.4.m221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenberg M, Ngo LH, Turner D, Tager IB. Treadmill exercise testing in an epidemiologic study of elderly subjects. J Gerontol A Biol Sci Med Sci. 1998;53:B259–67. doi: 10.1093/gerona/53a.4.b259. [DOI] [PubMed] [Google Scholar]
- Inzitari M, Newman AB, Yaffe K, Boudreau R, de Rekeneire N, Shorr R, Harris TB, Rosano C. Gait speed predicts decline in attention and psychomotor speed in older adults: the health aging and body composition study. Neuroepidemiology. 2007;29:156–62. doi: 10.1159/000111577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson NF, Kim C, Clasey JL, Bailey A, Gold BT. Cardiorespiratory fitness is positively correlated with cerebral white matter integrity in healthy seniors. Neuroimage. 2012;59:1514–23. doi: 10.1016/j.neuroimage.2011.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klepin HD, Geiger AM, Tooze JA, Newman AB, Colbert LH, Bauer DC, Satterfield S, Pavon J, Kritchevsky SB. Physical performance and subsequent disability and survival in older adults with malignancy: results from the health, aging and body composition study. J Am Geriatr Soc. 2010;58:76–82. doi: 10.1111/j.1532-5415.2009.02620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, Williamson DE, Lancaster J, Fox P, Cornell J, Blangero J, Glahn DC. Fractional anisotropy of water diffusion in cerebral white matter across the lifespan. Neurobiol Aging. 2012;33:9–20. doi: 10.1016/j.neurobiolaging.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer AF, Colcombe SJ, McAuley E, Scalf PE, Erickson KI. Fitness, aging and neurocognitive function. Neurobiol Aging. 2005;26(Suppl 1):124–7. doi: 10.1016/j.neurobiolaging.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Erickson KI, Colcombe SJ. Exercise, cognition, and the aging brain. J Appl Physiol. 2006;101:1237–42. doi: 10.1152/japplphysiol.00500.2006. [DOI] [PubMed] [Google Scholar]
- Marks BL, Madden DJ, Bucur B, Provenzale JM, White LE, Cabeza R, Huettel SA. Role of aerobic fitness and aging on cerebral white matter integrity. Ann N Y Acad Sci. 2007;1097:171–4. doi: 10.1196/annals.1379.022. [DOI] [PubMed] [Google Scholar]
- Marks BL, Katz LM, Styner M, Smith JK. Aerobic fitness and obesity: relationship to cerebral white matter integrity in the brain of active and sedentary older adults. Br J Sports Med. 2011;45:1208–15. doi: 10.1136/bjsm.2009.068114. [DOI] [PubMed] [Google Scholar]
- McAuley E, Szabo AN, Mailey EL, Erickson KI, Voss M, White SM, Wojcicki TR, Gothe N, Olson EA, Mullen SP, Kramer AF. Non-Exercise Estimated Cardiorespiratory Fitness: Associations with Brain Structure, Cognition, and Memory Complaints in Older Adults. Ment Health Phys Act. 2011;4:5–11. doi: 10.1016/j.mhpa.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero-Odasso M, Schapira M, Soriano ER, Varela M, Kaplan R, Camera LA, Mayorga LM. Gait velocity as a single predictor of adverse events in healthy seniors aged 75 years and older. J Gerontol A Biol Sci Med Sci. 2005;60:1304–9. doi: 10.1093/gerona/60.10.1304. [DOI] [PubMed] [Google Scholar]
- Newman AB, Haggerty CL, Kritchevsky SB, Nevitt MC, Simonsick EM. Walking performance and cardiovascular response: associations with age and morbidity--the Health, Aging and Body Composition Study. J Gerontol A Biol Sci Med Sci. 2003;58:715–20. doi: 10.1093/gerona/58.8.m715. [DOI] [PubMed] [Google Scholar]
- Newman AB, Simonsick EM, Naydeck BL, Boudreau RM, Kritchevsky SB, Nevitt MC, Pahor M, Satterfield S, Brach JS, Studenski SA, Harris TB. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295:2018–26. doi: 10.1001/jama.295.17.2018. [DOI] [PubMed] [Google Scholar]
- Park DC, Polk TA, Mikels JA, Taylor SF, Marshuetz C. Cerebral aging: integration of brain and behavioral models of cognitive function. Dialogues Clin Neurosci. 2001;3:151–65. doi: 10.31887/DCNS.2001.3.3/dcpark. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosano C, Aizenstein HJ, Newman AB, Venkatraman V, Harris T, Ding J, Satterfield S, Yaffe K, Health ABCS. Neuroimaging differences between older adults with maintained versus declining cognition over a 10-year period. Neuroimage. 2012;62:307–13. doi: 10.1016/j.neuroimage.2012.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasson E, Doniger GM, Pasternak O, Tarrasch R, Assaf Y. White matter correlates of cognitive domains in normal aging with diffusion tensor imaging. Front Neurosci. 2013;7:32. doi: 10.3389/fnins.2013.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen A, Gider P, Cavalieri M, Freudenberger P, Farzi A, Schallert M, Reichmann F, Watzinger N, Zweiker R, Schmidt R, Schmidt H. Association of cardiorespiratory fitness and morphological brain changes in the elderly: results of the Austrian Stroke Prevention Study. Neurodegener Dis. 2012;10:135–7. doi: 10.1159/000334760. [DOI] [PubMed] [Google Scholar]
- Simonsick EM, Newman AB, Nevitt MC, Kritchevsky SB, Ferrucci L, Guralnik JM, Harris T. Measuring higher level physical function in well-functioning older adults: expanding familiar approaches in the Health ABC study. J Gerontol A Biol Sci Med Sci. 2001;56:M644–9. doi: 10.1093/gerona/56.10.m644. [DOI] [PubMed] [Google Scholar]
- Simonsick EM, Fan E, Fleg JL. Estimating cardiorespiratory fitness in well-functioning older adults: treadmill validation of the long distance corridor walk. J Am Geriatr Soc. 2006;54:127–32. doi: 10.1111/j.1532-5415.2005.00530.x. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Spirduso WW. Physical fitness, aging, and psychomotor speed: a review. J Gerontol. 1980;35:850–65. doi: 10.1093/geronj/35.6.850. [DOI] [PubMed] [Google Scholar]
- Studenski S, Perera S, Wallace D, Chandler JM, Duncan PW, Rooney E, Fox M, Guralnik JM. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51:314–22. doi: 10.1046/j.1532-5415.2003.51104.x. [DOI] [PubMed] [Google Scholar]
- Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, Brach J, Chandler J, Cawthon P, Connor EB, Nevitt M, Visser M, Kritchevsky S, Badinelli S, Harris T, Newman AB, Cauley J, Ferrucci L, Guralnik J. Gait speed and survival in older adults. JAMA. 2011;305:50–8. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tager IB, Hollenberg M, Satariano WA. Association between self-reported leisure-time physical activity and measures of cardiorespiratory fitness in an elderly population. Am J Epidemiol. 1998;147:921–31. doi: 10.1093/oxfordjournals.aje.a009382. [DOI] [PubMed] [Google Scholar]
- Taylor HL, Jacobs DR, Jr, Schucker B, Knudsen J, Leon AS, Debacker G. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis. 1978;31:741–55. doi: 10.1016/0021-9681(78)90058-9. [DOI] [PubMed] [Google Scholar]
- Tian Q, Erickson KI, Simonsick EM, Aizenstein HJ, Glynn NW, Boudreau RM, Newman AB, Kritchevsky SB, Yaffe K, Harris TB, Rosano C. Physical Activity Predicts Microstructural Integrity in Memory-Related Networks in Very Old Adults. J Gerontol A Biol Sci Med Sci. 2014 doi: 10.1093/gerona/glt287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng BY, Gundapuneedi T, Khan MA, Diaz-Arrastia R, Levine BD, Lu H, Huang H, Zhang R. White matter integrity in physically fit older adults. Neuroimage. 2013;82:510–6. doi: 10.1016/j.neuroimage.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Voss MW, Prakash RS, Erickson KI, Basak C, Chaddock L, Kim JS, Alves H, Heo S, Szabo AN, White SM, Wojcicki TR, Mailey EL, Gothe N, Olson EA, McAuley E, Kramer AF. Plasticity of brain networks in a randomized intervention trial of exercise training in older adults. Front Aging Neurosci. 2010:2. doi: 10.3389/fnagi.2010.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss MW, Heo S, Prakash RS, Erickson KI, Alves H, Chaddock L, Szabo AN, Mailey EL, Wojcicki TR, White SM, Gothe N, McAuley E, Sutton BP, Kramer AF. The influence of aerobic fitness on cerebral white matter integrity and cognitive function in older adults: Results of a one-year exercise intervention. Hum Brain Mapp. 2012 doi: 10.1002/hbm.22119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- Weinstein AM, Voss MW, Prakash RS, Chaddock L, Szabo A, White SM, Wojcicki TR, Mailey E, McAuley E, Kramer AF, Erickson KI. The association between aerobic fitness and executive function is mediated by prefrontal cortex volume. Brain Behav Immun. 2012;26:811–9. doi: 10.1016/j.bbi.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Avula R, Senjem ML, Kantarci K, Weigand SD, Samikoglu A, Edmonson HA, Vemuri P, Knopman DS, Boeve BF, Petersen RC, Josephs KA, Jack CR., Jr Gray and white matter water diffusion in the syndromic variants of frontotemporal dementia. Neurology. 2010;74:1279–87. doi: 10.1212/WNL.0b013e3181d9edde. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson PW, Paffenbarger RS, Jr, Morris JN, Havlik RJ. Assessment methods for physical activity and physical fitness in population studies: report of a NHLBI workshop. Am Heart J. 1986;111:1177–92. doi: 10.1016/0002-8703(86)90022-0. [DOI] [PubMed] [Google Scholar]
- Wu M, Rosano C, Butters M, Whyte E, Nable M, Crooks R, Meltzer CC, Reynolds CF, 3rd, Aizenstein HJ. A fully automated method for quantifying and localizing white matter hyperintensities on MR images. Psychiatry Res. 2006;148:133–42. doi: 10.1016/j.pscychresns.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]