Abstract
Slow deep breathing (SDB) has a therapeutic effect on autonomic tone. Our previous studies suggested that coupling of the cardiovascular to the respiratory system mediates plasticity expressed in sympathetic nerve activity. We hypothesized that SDB evokes short-term plasticity of cardiorespiratory coupling (CRC). We analyzed respiratory frequency (fr), heart rate and its variability (HR&HRV), the power spectral density (PSD) of blood pressure (BP) and the ventilatory pattern before, during, and after a 20-min epoch of SDB. During SDB, CRC and the relative PSD of BP at fr increased; mean arterial pressure decreased; but HR varied; increasing (n=3), or decreasing (n=2) or remaining the same (n=5). After SDB, short-term plasticity was not apparent for the group but for individuals differences existed between baseline and recovery periods. We conclude that a repeated practice, like pranayama, may strengthen CRC and evoke short-term plasticity effectively in a subset of individuals.
Keywords: Neural control of heart rate, Neural control of sympathetic nerve activity, Neural control of respiration, Pranayama, Poincaré plots
1. INTRODUCTION
Cardiovascular and respiratory control systems are coupled reciprocally. While each system affects the other, respiration, the slower oscillation, has a stronger influence on the cardiovascular system than arterial blood pressure has on the breathing pattern. Due to the strength of the effect of respiration, the influence of breathing on heart rate (HR) and blood pressure (BP) was recognized early in the development of Western medicine. In his overview of milestones for understanding heart rate variability (HRV), Billman (2011) credited Reverend Stephen Hale for reporting respiration’s influence on HR and BP in 1733 and Walter Cannon for measuring increases in HR and BP during inspiration (Respiratory Sinus Arrhythmia (RSA) and Traube-Hering Waves, respectively) approximately 100 years later. Then just 50 years ago, Hon and Lee (1963a,b) made the translational step from bench to bedside by identifying the clinical relevance of HRV as a biomarker for fetal health. An absence of HRV preceded death.
Although various analytical tools can measure HRV, the standards of measurement, physiological interpretation, and theoretical clinical use of HRV became defined in the 1996 white paper, a joint publication in journals of Cardiology Societies in both North American and Europe. But despite wide spread involvement of leading scientists, the interpretation and usefulness of HRV is not accepted universally. Indeed, these issues have been debated in the literature. For example, “Cardiovascular variability is/is not an index of autonomic control of circulation” was one of the earliest topics considered in Point:Counterpoint published by Journal of Applied Physiology ((Parati et al., 2006) and (Taylor and Studinger, 2006), respectively). This was followed by another Point:Counterpoint entitled “Respiratory sinus arrhythmia is due to a central mechanism vs. respiratory sinus arrhythmia is due to the baroreflex mechanism” ((Eckberg, 2009) and (Karemaker, 2009), respectively).
Even the underlying physiologic purpose for cardio-respiratory coupling (CRC) is not clear. Teleologically, CRC should enhance the efficacy of gas exchange by matching ventilation with perfusion, filling the lungs with well-oxygenated air during inspiration is coupled to increase blood flow to the lungs. Indeed, this concept was verified in a well-designed study performed in canines, where the RSA is dramatic compared to humans (Hayano et al., 1996). However, in a theoretical study using optimal control theory as well as a computational model of gas exchange, RSA minimized the cardiac work while maintaining physiological gas levels (Ben-Tal et al., 2012). While modelling did not support improve gas exchange efficiency, it supported CRC as physiologically significant and not simply an epiphenomenon, the appendix of homeostatic control.
Ironically, a plausible role for CRC acting as pathophysiologic mechanism in hypertension is being established (Braga et al., 2006; Zoccal et al., 2007, 2008, 2009, 2011; Simms et al., 2009a,b; Zoccal and Machado 2010, 2011). In particular, upregulation of chemoreceptor sensitivity and the recruitment of expiratory motor activity have been associated with an additional burst of SNA in expiration, which may lead to neurogenic hypertension (Abdala et al., 2009; Molkov et al., 2010, 2011). While this work was initiated in an animal model of hypertension, in which hypertension was evoked by chronic intermittent hypoxia, enhanced coupling between respiration and sympathetic activity has been extended to other hypertensive rat models (Simms et al., 2009b; Abdala et al., 2012; Paton et al., 2013). Thus, increased activity of the chemoreceptors could trigger sympathetic activity directly and through the network underlying CRC. We have focused on CRC and its role in evoking plasticity.
Western medicine is just one, scientifically as well as empirically based approach to health. Medical practices in the Eastern hemisphere include breathing exercises to enhance health and reduce anxiety. Recently, the National Center for Complementary and Alternative Medicine has suggested slow deep breathing (SDB) exercises as a relaxation technique that could help an individual achieve better health (NIH WEBpage, http://nccam.nih.gov/health/stress/relaxation.htm). In SDB as therapy, relaxation would complement SDB in decreasing heart rate, blood pressure, oxygen consumption and levels of stress hormones. While mechanisms evoked during SDB and other relaxation techniques counteract the stress response, these techniques require that positive effect outlasts the stimulus. Specifically, we speculate that the neural network underlying CRC mediates the neural plasticity evoked by SDB and that the reduction in stress is permissive allowing the plasticity to occur.
In summary, CRC is a biomarker of health and may underlie processes of health and disease. CRC deteriorates in sepsis and increases in highly-trained athletes. Similarly, CRC deteriorates in stress and is enhanced in relaxation and slow-wave sleep. SDB enhances CRC. But in order to act therapeutically, there has to be a lasting effect.
In this paper, we present the effect of a single episode of SDB on HRV assessing HRV in the frequency domain from spectral analysis of arterial blood pressure and in the time domain from Poincaré plots. These data were obtained in healthy human subjects that did not practice yoga. Short-term plasticity is evident after SDB in individuals but not for the group. We discuss these results in the context of our previous reports indicating that plasticity in the cardio-sympathetic control is mediated through mechanisms similar to those that mediate plasticity in the respiratory system.
2. MATERIALS AND METHODS
2.1 Subjects
Healthy young adult male subjects (N=10, mean age 26.7±1.4) were recruited from Rochester, MN and its surrounding area. All experiments and procedures were approved by the Institutional Review Board at the Mayo Clinic and conformed to the Declaration of Helsinki. All subjects signed an approved informed consent form. The data were de-identified to comply with HIPAA rules and regulations for data analysis.
We sought to include subjects that encompassed a wide range of resting breathing frequencies (fr). Although textbooks define a resting fr between 12 and 15 breaths/minute (BrthPM) as normal, fr can range from 6–31 BrthPM (Benchetrit, 2000). A standard pulmonary function test consisting of simple spirometry was administered at the screening day to rule out any pulmonary pathology. Subjects were normotensive (Systolic BP<130 mmHg, Diastolic BP<90 mmHg). The subjects were non-obese (Body Mass Index<30), non-smokers, non-diabetic, and normally active (neither sedentary nor actively training). Subjects were screened rigorously for disease and were ineligible if they had acute or chronic disorders associated with cardiovascular structure or function and if they were being treated with anti-hypertensive or other medications. All subjects reported that they did not practice yoga, meditation and breathing exercises.
2.2 Recording
Arterial pressure was monitored continuously using a 20 gauge, 5 cm catheter, which was inserted aseptically in the brachial artery of the non-dominant arm with ultrasound guidance and local anesthesia (2% lidocaine). The catheter was connected to a pressure transducer and flushed continuously at 3 ml/h with heparinized saline. Heart rate was measured with a standard 3-lead ECG. Respiratory rate and pattern were monitored using a double pneumobelt system (ribcage and abdominal belts) calibrated using a 1L balloon.
2.3 No Respiratory Pacing
Subjects were positioned in a comfortable resting supine position with all instrumentation for the duration of the study. A resting period of measurements of respiration and BP was obtained for 20 min, then subjects were instructed to perform slow breathing for 20 minutes followed by a 20 min recovery period of endogenous rate. The recording was continuous from baseline to slow deep breathing and recovery. In this study, subjects were given minimal instructions on how to breathe slowly and had no visual, auditory or verbal feedback. Subjects were told to breathe at a slower frequency than their normal breathing and at a rate and depth of their choosing based on their comfort level. They were not cued to breathe at a particular pace with any devices or metronome pacing.
2.4 Data Analysis
Data files were converted to a file format compatible with Spike 2 (Fig. 1). Complete 20-min epochs were analyzed; transitions between changes in fr were abrupt. Data were not interpolated; voids in the ABP due to sampling blood at the end of an epoch were not included in the analysis. Breaths were not excluded; sighs were few, and paradoxical rib change contraction with abdominal expansion did not occur. False negatives from not scoring small breaths could have occurred; breaths with a tidal volume less than two standard deviations from the mean were difficult to identify and could have been excluded unintentionally.
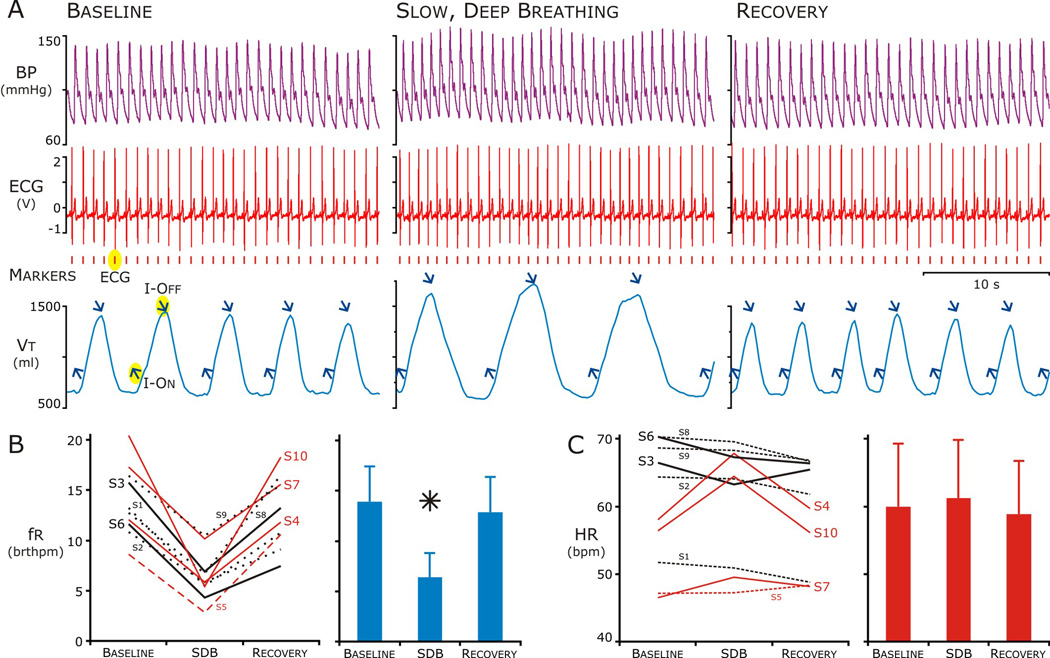
Figure 1. The heart rate (HR) response to slow, deep breathing varied among subjects.
A. Representative tracings at baseline, during, and after Slow, Deep Breathing. Traces during each period from top: blood pressure (purple) ECG (red) and Thoracic-Abdominal volume (blue). Markers; Peak of the R-wave in ECG (red) and onset of inspiration and peak of breath (upward and downward blue arrows respectively).
B. During slow, deep breathing, respiratory frequency (fr) decreased well below ten breaths per minute (20 bthmpm) in all but 2 subjects (S7 and S9, left panel) and this decrease was significant for the group (Right Panel). In the 20-min epoch after SDB (Recovery), fr returned to baseline.
C. Heart Rate (HR) did not change for the group across the epochs (right panel), but HR increased in 3 of 10 subjects (S4, S7, S10) during and in another (S5) after SDB (left panel, red lines).
2.4.1 Heart and Respiratory Rates
Heart Rate (HR) was determined by detecting the peak of the R wave (Fig. 1A red vertical marks) and then the RR Interval was converted to beats per minute (BPM). Similarly, respiratory frequency (fr) was determined from the peak of the sum of the abdominal and thoracic pneumobelts (Fig. 1A, blue arrows slanted down). This was considered to be the end of inspiration; we used a simple threshold crossing paradigm to determine the onset of inspiration (blue arrows slanted up) (Fig. 1A). Respiratory cycle duration was determined as the interval between two downward arrows and that was converted to breaths per minute (BrthPM).
2.4.2 Power Spectral Density
We used the subroutine available on Spike 2, 7.12; to have a frequency resolution of 0.003815 Hz between 0 to 125 Hz, we chose 65536 bins and a Hanning Window for the fast Fourier Transform (FFT). We calculated the relative power of the BP and the ventilatory pattern by summing the power contained at fr and normalizing it to the total power between 0.025 Hz to 0.35 Hz. We performed the FFT on the BP recording.
2.4.3 Arterial Blood Pressure
Mean arterial blood pressure (MAP) is presented as the mean of the calculated mean (= [(2 × diastolic) + systolic] / 3) from the instantaneous pulse pressure for each heart beat in the epoch. We did not examine the dynamics of the change in MAP over the 20-min epoch.
2.4.4 Poincaré Plots
We have developed analytical tools for quantifying Poincaré plots, specifically to analyze the temporal patterns of variability in Poincaré plots (Fishman et al., 2012). We applied temporal Poincaré variability (TPV) to quantify the temporal-dependence of the distribution of points in the Poincaré plot and to detect nonlinear sources underlying the variability. This analysis involved three approaches.
The first approach quantifies how the distribution of the points in Poincaré plots varies as the time delay (τ) increases. The τ is equivalent to the number of RR Intervals separating the plotted values. For instance, when τ=1, next RR Interval (n+1) is plotted against the current RR Interval (n); whereas at τ=5 plots the fifth RR interval (n+5) is plotted against n. Given the tendency for the number of RR Intervals contained in the respiratory cycle to be an integer (Coleman 1920), the distribution of points in a Poincare plot could be expected to shrink at that integer value, this would reflect a non-linear, time-dependent correlation. This approach, we refer to as time-delayed TPV (TPVtd). In Poincaré plots, identical RR Intervals are on the line of identity and the distance of a point from the line of identity reflects the difference between the RR Intervals. TPVtd quantifies the variability of RR Intervals by finding the distance from the line of identity for each point and calculating the standard deviation (SD) of this difference. When τ=1 the difference is between successive RR Intervals and this is referred to as the standard deviation of successive differences (SDSD). In applying TPVtd, we calculate the SDSD for τ=1 to 50 and plot the values of SDSD on the y-axis and τ on the×axis (Note: even at τ>1, we refer to SDSD even though the RR Intervals being compared are no longer consecutive). Usually, at τ=1, the RR Intervals have a high degree of linear correlation, which minimizes the successive differences, so SDSD is small the plotted points have a tendency to form a tight cluster or an ellipse. At greater τ, the linear correlation decreases and the differences between the compared RR Intervals increases and varies more, which TPVtd quantifies. The increase in variability is reflected in the shape and distribution of the plotted points, which form a dispersed cloud more circular than elliptical. So as τ increases from 1, SDSD increases asymptotically to a value, we interpret TPVtd as reflecting the dissipation of linear correlation with stochastic rather than deterministic influences on variability. In unusual circumstances, the Poincaré plots at higher τ form distinct clusters of points indicating that differences in RR Intervals are correlated over longer time delays. In this case as τ increases from 1, TPVtd can increase and decrease repeatedly, oscillating even, then this reflects the emergence of nonlinear dynamics having a deterministic influence on the variability of the compared RR Intervals.
The second approach quantifies how averages of number of consecutive points in Poincaré plots varies and is referred to as TPVa. For TPVa we calculated the difference between the averaged point and the origin for every centroid and the TPVa is the standard deviation of those distances. If the average moves strictly along the line of identity, then TPVa measures long-term variability similar to SD2.
The third approach is simply partitioning the data set. Subdividing the time series data into half or quarters and comparing the dynamics of each to the average entire time series provides insight into the nature of the data set. If the dynamics is uniform across the data set then the variability measurement of the partitions should not be from that of the entire time series and support data set as being stationary. A data set that alternates between regions of high and low variability may produce partitions that have differences.
In summary, TPVtd quantifies short-term variability between intervals whereas TPVA measures longer-term tendencies for clusters to reoccur. The dynamics of this position time series are also used to check for discrete changes in behavior that could signify a nonstationarity in the time series data.
3. RESULTS
All but two of the subjects, which were included in subsequent analysis, were able to breathe in the target range (between 6 and 8 BrthPM) given the instruction to perform slow, deep breathing that felt comfortable without feedback, training and prompting. Nevertheless the HR response to SDB varied among subjects (Fig. 1). During SDB, fr decreased from 13.9 ± 3.5 to 6.4 ± 2.4 BrthPM, which was in the target range. Following SDB, fr returned to baseline. HR did not change for the group across the epochs: 60.0 ± 9.3 BPM, Baseline; 61.3 ± 8.6, SDB; and 58.8 ± 7.9, Recovery). Even the slight increase in HR surprised us and this was due to 3 of 10 subjects (S4, S7, S10), in which HR increased 12.5 ± 5.6 % during SDB. In another individual (S5), HR increased slightly after SDB. Consequently we formed two groups based on whether the HR increased or decreased during or following SDB.
3.1 Power Spectral Density
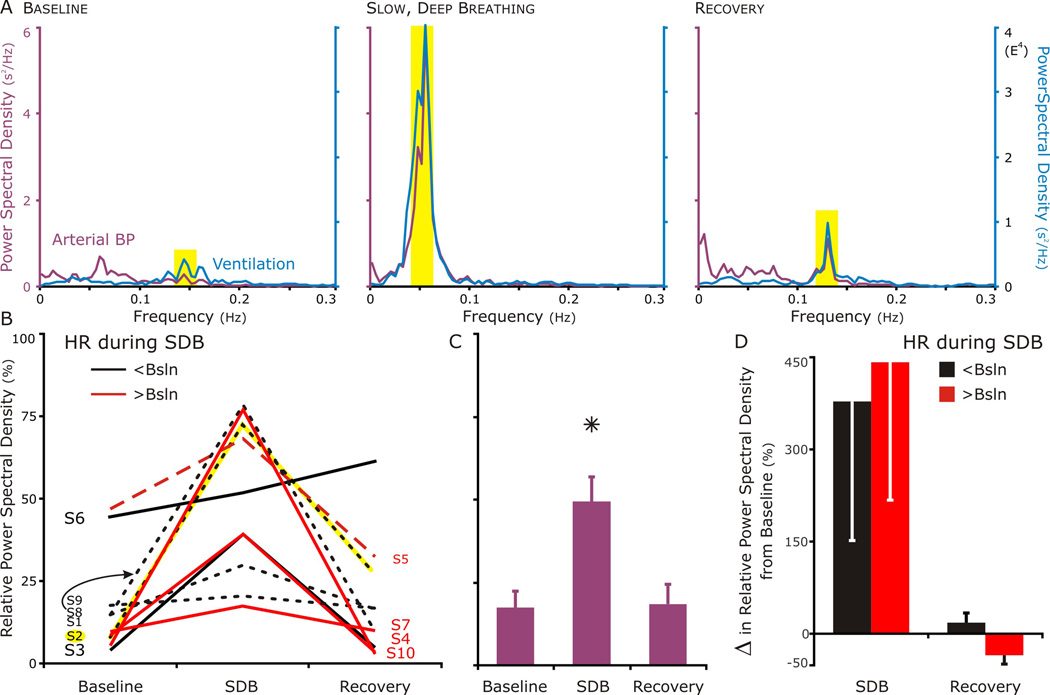
We determined if the high-frequency component of the PSD of the blood pressure increased during SDB. We predicted this effect on HRV for which arterial BP variability was our surrogate measure (Fig. 2). In Fig.2A, the individual (S2) had the response that was completely consistent with our hypothesis. The power spectral density depicted a weak high frequency component at baseline (left panel) which became robust during slow, deep breathing (middle panel), and then decreased but remained greater than that at baseline during recovery (right panel). For the group (Fig. 2B–D), absolute PSD was converted to relative PSD to plot each individual on a bounded y-axis. Even though baseline relative PSD varied (Fig 2B), the relative (and absolute) PSD increased from baseline (Fig. 2C). We then asked if increase in relative PSD from baseline as well as if PSD returning to baseline depended on whether HR decreased or increase during SDB (Fig. 2D). Parsing the individual created small groups with big variability (Fig. 2D). In those subjects that decreased their HR during SDB, their relative PSD trended to be less than those individuals whose HR increased. Surprisingly and despite S2, the relative PSD decreased-HR group returned to baseline and the increased-HR group, if anything, dropped below baseline (Fig. 2D).
Figure 2. Power Spectral Density (PSD) of respiration and blood pressure between 0.005 and 0.30 Hz; at baseline, during, and after slow, deep breathing.
A. A set of PSD graphs representing the result consistent with our hypothesis. The respiratory PSD (blue line) identified the band width for the high-frequency component of cardiovascular PSD (purple line), because it depended on respiratory-modulated vagal nerve activity. The PSD of blood pressure had a small but evident component during relaxed breathing at baseline (left panel); robust, during slow, deep breathing (middle panel); and moderate during recovery (right panel). In this particular subject (S2), in comparing before and after slow deep breathing, the strongest component of the PSD for blood pressure shifted from a low frequency that was independent of respiration to a high frequency that corresponded to respiration.
B. In 9 of 10 subjects, the percent of the total power in the BP PSD that overlapped with the respiratory PSD was highest during SDB (S2, depicted in A highlighted in yellow). The one exception (S6) had a large respiratory-modulated component at baseline, which increased during SDB and continued to increase during recovery.
C. The relative BP-PSD increased for the group during SDB, but returned to baseline in recovery.
D. Separating those subjects that decreased their HR during SDB (black-filled bars) from those that increased it (red-filled bars), revealed a possible effect of SDB on the BP-PSD during recovery may depend on the change in HR during SDB. All 3 of the subjects who increased their HR during SDB had a lower high frequency component in their relative PSD even though they responded well during SDB. The subject that had increase in HR during recovery (S5) also had a lower high frequency component following SDB.
3.2 Arterial Blood Pressure
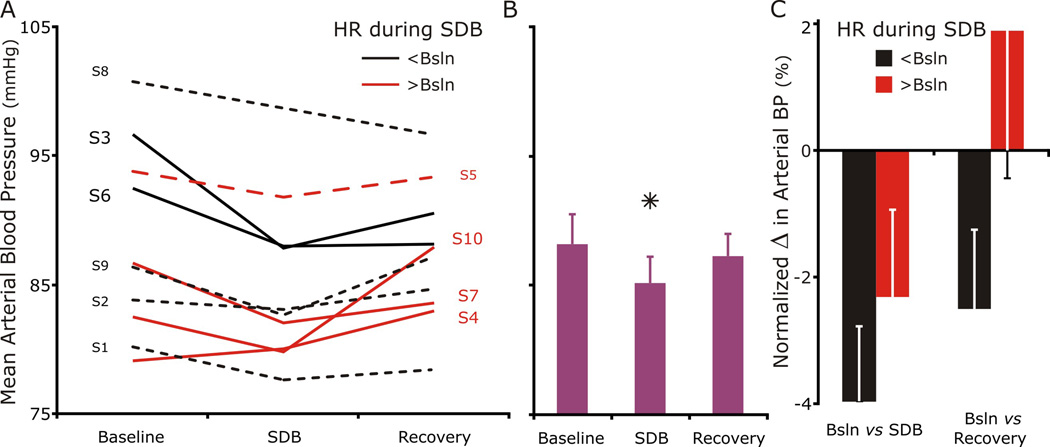
For the group, MAP decreased during SDB but returned to baseline during recovery (Fig. 3). At baseline, the group’s MAP was 88.2 mmHg and ranged between 79 (S4) and 101 (S8) mmHg. During SDB, MAP decreased to 85.2 mmHg which was significant for the group. The percent decrease ranged from 9.1% (S3) to 0.8% (S2) in 9 subjects (MAP decreased ≥ −3% in 6 subjects); MAP increased 1.2% in S4. During recovery, MAP was not significantly different from baseline.
Figure 3. Mean arterial blood pressure decreased during SDB in every subject but S4.
A. Six individuals decreased their BP at least 3 percent (3–9%) during SDB, and three others (S2, S5, and S8) decreased less than 2% (0.8 to 2 %). The individual (S4) whose mean arterial BP increase 1.2% had the lowest baseline BP 79 mmHg).
B. Group mean arterial BP at baseline decreased during SDB and returned to baseline during recovery.
C. Again, separating the individuals in two groups based on their HR during SDB revealed that those that decreased their HR (black-filled bars) had a slightly larger decrease in their percent change in mean ABP from baseline than those that increased their HR (red). Further, blood pressure tended to remain lower in the period following SDB in the groups whose heart rate decreased during SDB.
Separating individuals in two groups based on their change in HR during SDB did not provide insight into HR regulation (decreased HR (black bars) vs. increased (red bars)). If anything; those who decreased their HR during SDB had a potentially a larger decrease in BP than those who increased their HR; but the study was not powered for post hoc analysis.
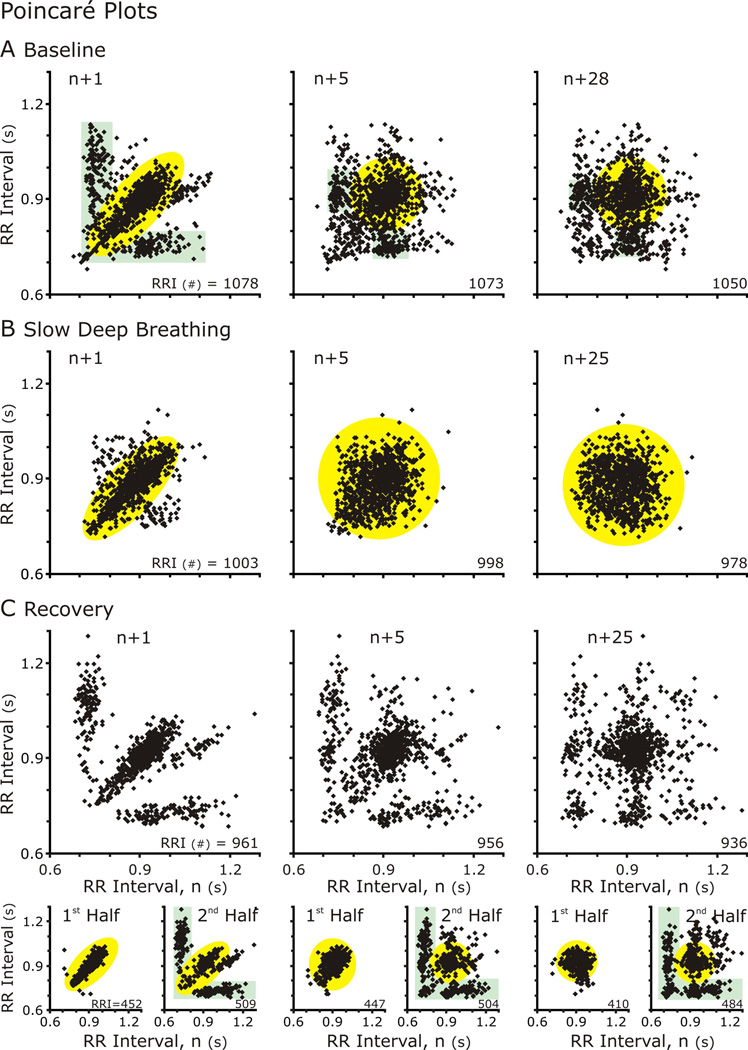
3.3 Poincaré plots
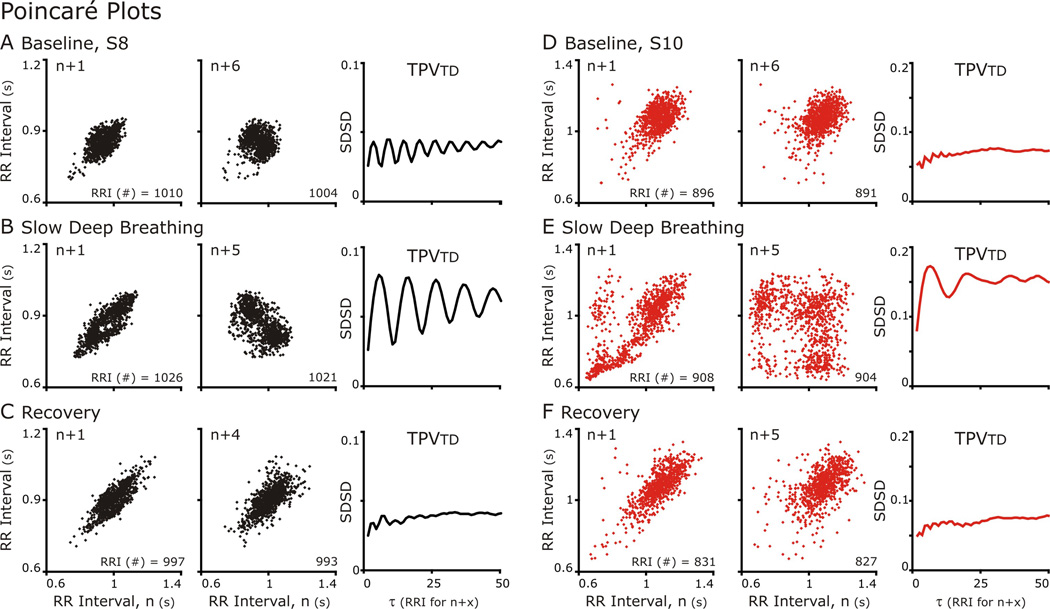
Traditionally in HRV, Poincaré plots show the RR Interval of the next cycle (n+1) plotted against the current cycle (n). In this way, they depict the cycle-to-cycle variability, which often forms an ellipse overlaying the line of identity (Fig. 4). But the delay (τ) defined by the number of cycles separating the cycle compared to the current cycles should not be limited to 1 (as in n+1 versus n) (Fishman et al., 2012). Poincaré plots with (n+(>1) vs n) can reveal the relationship between repetitive events at that occur a multiples of cycles, such as RSA. We generated Poincaré plots with n+τ in which τ varied from 1 to 50 and applied TPVtd, and TPVa to measure variability Poincaré plots (TPV) across multiple time delays (Fig. 4A–E right-hand panels and Fig. 6).
Figure 4. Poincaré Plots of RR Intervals either n+1 (Left Column) or n+5 (Center Column) and TPVtd calculated for 50 Poincare plots plotted against n.
A&D. At baseline for both subjects, n+1 versus n Poincare plots formed an elliptical cloud of points, and n+5 vs n plot had a more circular shape. These shapes are indicative indicative a normal HRV. The n+5 was plotted because the TPVtd plot showed an oscillation in which the nadir of SDSD occurred at 5.
B&C. During SDB. The TPVtd revealed that both subjects had strong RSA with S8 greater than S10. In S8 the n+1 vs n plot had a small void on the line of identity in the center of the ellipse, which is near the mean HR. In the n+5 vs n graph represent the largest SDSD and the distribution forms two clouds. S10 reveals a more complex especially in the n+5 vs n Poincaré plot. C&E. Distribution patterns returned to baseline.
Figure 6. Temporal Poincaré variability (TPV) plots revealed differences in the structure HRV after SDB.
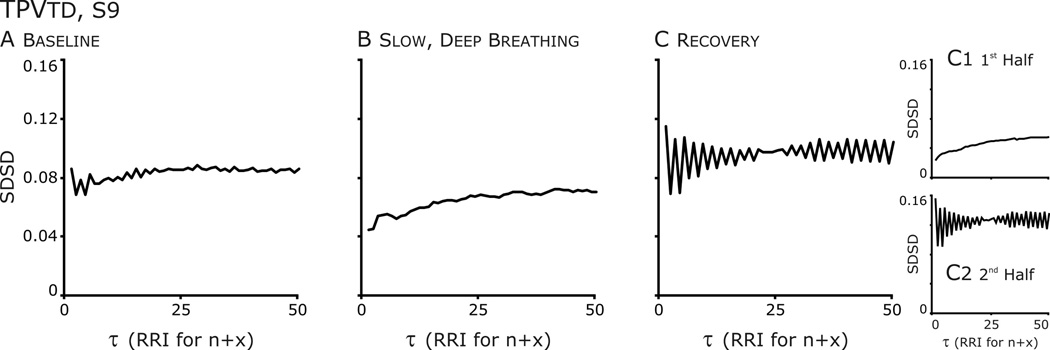
A. Baseline: from τ=1 to 5, an oscillation is present at each increment of 1 RR Interval and there are hints of an oscillation at higher τ.
B. Slow, deep breathing: The oscillation is absent distribution formed an ellipse which became a circular cloud at higher τ.
C. Recovery: The oscillation is accentuated. Further, the oscillation is absent in the Poincaré plot from the 1st half of the data set and SDSD is very low, whereas in the 2nd half SDSD increases and the amplitude of the oscillation is accentuated.
Generally, n versus n+1 Poincaré plots formed an elliptical distribution at baseline, during and after SDB. Further, as n+τ increases, the plots became progressively circular (Fig 4A&D compare n+1 to n+6). This was reflected in TPVtd, which was small at 1 indicating a narrow distribution along the line of identity and increased as a circular distribution formed and the distribution perpendicular to the line of identity widened. CRC affected the distribution of points in Poincaré plots; especially, at τ close to the respiratory cycle length.
A difference between S8 (Fig. 4A–C) and S10 (Fig. 4D–E) was the magnitude of CRC at baseline and during SDB but this difference decreased during recovery. While the values of SDSD were similar at baseline, the lines reflecting TPVtd were different (NOTE: the scales of y-axis of TPVtd are different in A-C from that in Fig. 4D–E). At baseline, TPVtd from S8 oscillated with a period of 6 RR Intervals (Fig 4C) whereas TPVtd from S10 did not. With SDB Rel PSDbp increased for both S8 and S10 (the two greatest increases in Fig 2) and the oscillations in TPVtd had a longer cycle length corresponding to the longer respiratory cycle duration. These patterns were reversed in recovery (Fig. 4C&E). In fact, S8 was the anti-hypothesis: CRC that was enhanced by SDB did not persist in recovery; Rel PSDbp was less in Recovery than baseline and both HR and MAP were less in Recovery than baseline.
Another subject (S9) was similar to S2, in that a persistent effect of SDB on a HRV was observed (Figs. 5–7). But unlike S2, SDB disrupted a pattern of HRV that was not RSA nor related to CRC. At baseline, the n vs.n+1 Poincaré plot of this individual’s RRI had a two overlaying distribution patterns (Fig. 5A). In one pattern the points formed an ellipse whose long axis paralleled the line of identity; the other, ellipses that paralleled the x- and y-axes, which reflected bigeminy, a short followed by a long RRI. These patterns formed clusters at greater τ. Bigeminy was evident in TPVtd in which the SDSD had a sawtooth appearance over initial τ that diminished at longer τ (Fig. 6A). SDB disrupted this pattern and a single distribution around the line of identity was formed (Figs. 5&6). Just from the appearance of the Poincare plots of the complete recovery epoch (Fig 5C), the bigeminy had returned in recovery; TPVtd (Fig. 6C) revealed that bigeminy was accentuated. However partitioning the data in half revealed that the 20-min recovery period was not uniform in that high variability in SD1 occurred at all τ (Fig. 7C). Therefore, we plotted the distributions in the first and second halves of the recovery period (Fig 5, bottom row); the distribution in the first half appeared similar to that of SDB (compare yellow highlighted regions), whereas that of the other half appeared similar to the baseline (compare green highlighted regions). Further, the saw-tooth waveform of TPVtd was accentuated even more in the second half of recovery (Fig. 6C2). Thus, the persistent effect of SDB was the absence of bigeminy for the first 10-min of recovery. The concept that bigeminy was enhanced is revealed by the sharp decrease in SDSD associated with averaging points at τ > 1 (Fig 7A&C orange bent-arrows). The decrease occurred in the second half of recovery (C2 and C3) and least in A3.
Figure 5. TPVtd analysis. Poincaré Plots of RR Intervals either n+1 (Left Column) or n+5 (Center Column) or n+25 (Right Column) plotted against n for subject S9. The complexity of the plots diminished during SDB (middle row) and this effect persisted in the first half of the recovery period.
A. Baseline: rather than a simple ellipse, the distribution of RRI was complex at baseline. With two distinct distributions: 1) an ellipse along the line of identity (highlighted in yellow) and 2) clouds paralleling the x and y axes (highlighted in green).
B. Slow, deep breathing: The distribution formed an ellipse which became a circular cloud at higher τ.
C. Recovery: although the distribution and relationship of RRI returned to baseline during the recovery epoch, it was not immediate. Partitioning 20-min epoch in half revealed that for the first 10 min, the Poincaré plot formed an ellipse and in the second 5-min epoch the two distinct distributions resembling the baseline distribution returned.
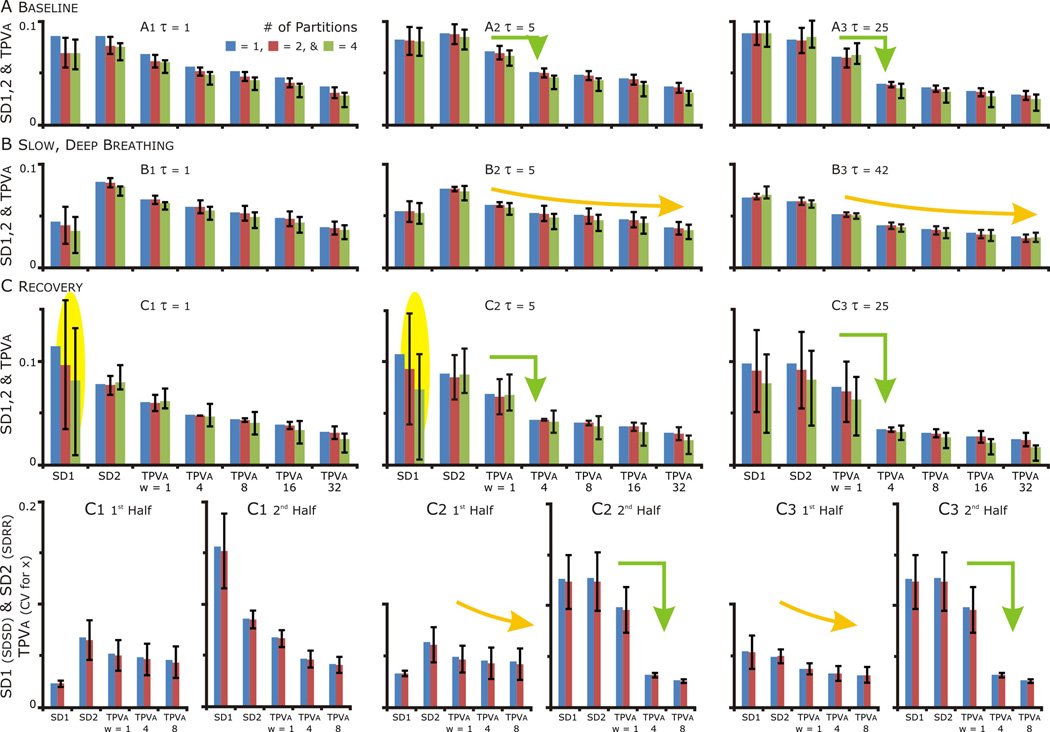
Figure 7. SD1, SD2 and TPVa at τ=1, 5 and ≥25 for the whole and partitioned data set. The variance of SD1 during recovery in the partitioned data set indicative of a nonstationarity in the data. The nonstationarity related to a persistent effect of SDB resolving, and then aggravating bigemney in HR.
A, B & C. Blue bars: the values of SD1, SD2 and TPVa for the whole data set, Red bars: median + 25th and 75th percentile values with the data set partitioned in half. Green bars: values with the data set partitioned in quarters. SD1 = the standard deviation of successive difference (SDSD) and SD2 = the standard deviation of the RR Intervals (SDRR). The Temporal Poincaré Variability for averaged points or TPVa = the coefficient of variation for x, which the distance of the points from the origin. On the x-axis, w = the number of points that were averaged. As w increased, TPVa decreased as averaging reduced the variability caused by beat to beat variability (compare w=1(no averaging) to w>1).
C. In the recovery period, the variability in SD1 indicated a nonstationarity in the data set (yellow oval highlight). The source of this variability was resolved with separate analysis of each half of the recovery data set, the SD1 values for the 1st&2nd halves were an order of magnitude different.
A, B & C. Comparing TPVa: In B: TPVa decreased progressively as the number of points averaged increased (yellow curved arrow) reflecting the the distribution points becoming more circular (as in yellow highlighted regions in Fig. 6). In contrast in A&C: TPVa decreased abruptly with averaging (green arrow) reflecting the clustering of points as τ increased.
3.4 Correlations
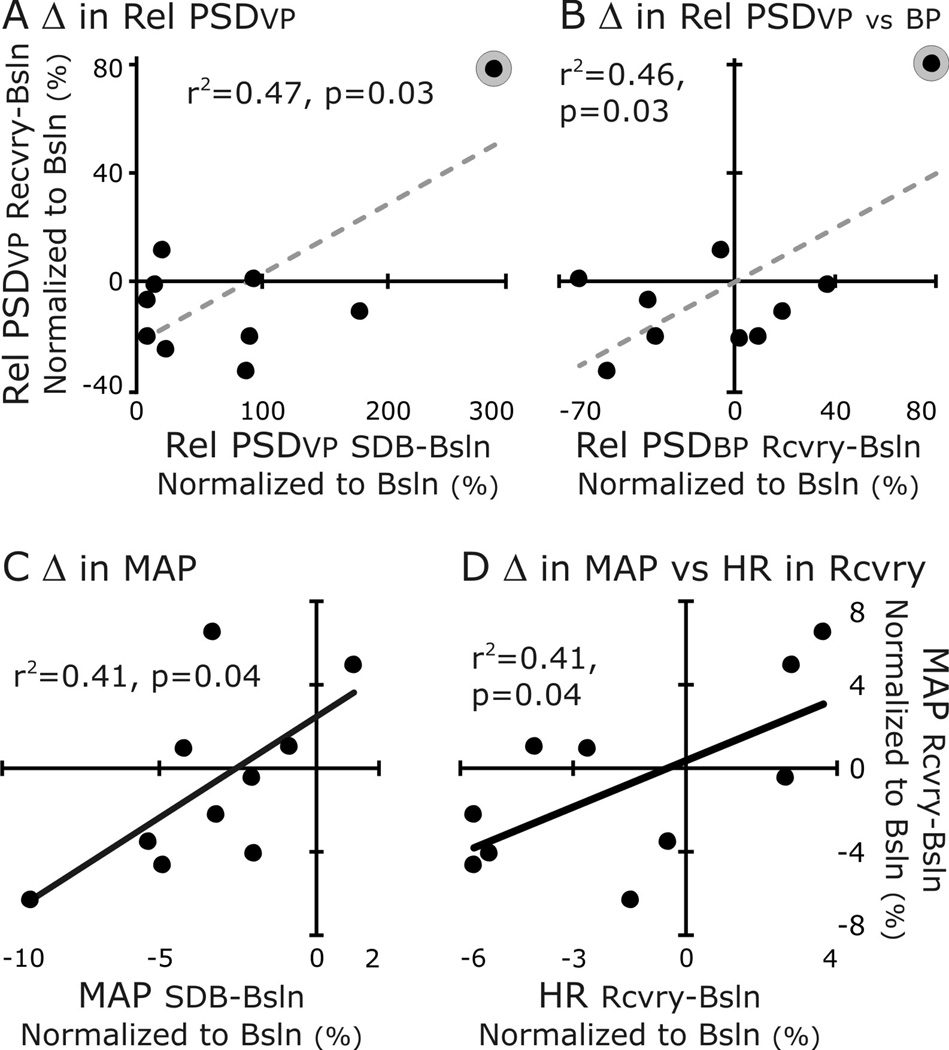
The variability of these data was a predisposing factor lending the data set to regression analysis even with the limitations associated with small data sets. Given this, we tested our hypothesis that changes in cardiovascular control evoked by an acute epoch of SDB persist in Recovery (Fig. 8). We compared whether the changes in relative PSD of the ventilatory pattern (Rel PSDvp) correlated to those of blood pressure (Rel PSDbp) (Fig. 8A&B). The magnitudes of Rel PSDvp and Rel PSDbp at the fr increased during SDB and decreased to above or near or below baseline in the Recovery period. For the ventilatory pattern, the difference in Rel PSDvp between SDB and Baseline was correlated positively to that between Recovery and Baseline (Fig. 8A). For blood pressure, the differences in Rel PSDvp and in Rel PSDbp between Recovery and Baseline were correlated positively (Fig. 8B). Both these correlations depended on a single point (S2, circled in gray and the regression line is dashed to emphasize the potential for a false positive).
Figure 8. Correlations revealed the uniqueness of S2 (A&B) and that a change in mean arterial pressure during recovery varies with its change during SDB (C) and the change in HR (from baseline) during recovery (D).
We correlated changes of MAP between Baseline and SDB to those between Baseline and Recovery (Fig. 8C&D). The normalized difference in the magnitude of MAP during SDB was correlated to that during Recovery. Further, the MAP in Recovery was correlated to the normalized difference in HR between Baseline and Recovery (Fig 8C&D).
4. DISCUSSION
Our study analyzing the ventilatory pattern’s effect on blood pressure and heart rate showed persistent effects of SDB in a subset of humans. Our hypothesis is that in order for SDB to have a beneficial effect of reducing HR or MAP it has to persist after the breathing exercise. With the exception of HR, SDB evoked the expect changes in autonomic tone. The Rel PSDbp increased in all subjects, MAP decreased in nearly all, but HR decreased in a few and even increased in others. During the recovery, even though MAP decreased by more than 2% of baseline in half of the subjects; only one individual had the hypothesized response; that is a persistent increase in CRC evidenced by an increased Rel PSDbp and a 4% drop in HR from baseline with MAP maintained at baseline. The unexpected observation was an individual who had bigeminy; a single 20-min period of SDB dramatically affected his HRV during the recovery period; first alleviating bigeminy and then enhancing it. Clearly, cardiovascular patterning, variability and function can be modulated by SDB. We speculate that the short-term plasticity expressed by one healthy individual reflects a potential for repetitive SDB exercises evoking a long-term plasticity in cardio-vascular control to attenuate hypertension.
Studies by us and others have demonstrated that both short- and long-term plasticity can be evoked in the autonomic nervous system (Dick et al., 2007; Prabhakar et al., 2007&2009; Zoccal and Machado, 2010&2011; Xing and Pilowsky, 2010; Xing et al., 2014). This plasticity is expressed not only as an increase in the magnitude of sympathetic nerve activity (SNA) under baseline conditions, but also the consistency, the strength and the pattern of its coupling to respiration. For instance, in rats exposed to acute (AIH) or chronic intermittent hypoxia (CIH) or acute (ASH) or chronic sustained hypoxia, sympathetic nerve activity (SNA) is recruited but whether this increase in SNA is associated with systemic hypertension depends on numerous factors including the coupling pattern. Increased SNA is not associated with increased MAP after AIH (Dick et al., 2004, 2007) whereas it is, after CIH (Fletcher et al., 1992a 1992b; Fletcher 2001; Braga et al., 2006; Prabhakar et al., 2007; Zoccal et al., 2007, 2008; Simms et al., 2009a; Prabhakar and Kumar 2010; Zoccal and Machado 2010). After AIH, SNA increases in post-inspiration; whereas after CIH, SNA is also recruited in expiration (Zoccal and Machado, 2010). Surprisingly, this coupling pattern is expressed in spontaneously hypertensive but not normotensive Wistar rats (Czyzyk-Krzeska and Trzebski, 1990; Simms et al., 2009). It was in this context of autonomic plasticity apparent in CRC, that we formulated our hypothesis, that a form of short-plasticity would be apparent in CRC as a sustained increase in the component of the PSD relating to respiration.
4.1 Limitations and Considerations
Even though this data set is small it did contain a subject that supported the hypothesis. Respiratory and autonomic control are phylogenetically preserved functions but a broad genetic diversity exists in the performance respiration (Benchetrit, 2000) and the maintenance of BP. So n of 1 is encouraging to an optimist, but this finding indicates that the effect is not robust and must be practiced consistently to be achieved.
The primary reason of performing the spectral analysis was to verify increased CRC during SDB. RelPSDbp should reflect an increase in coupling due to the pronounced effect SDB has on BP. Breathing near 0.1 Hz or 6 BrthPM would not only result in increased venous return but also summation the Mayer and Traube-Hering waves. The following two factors influenced the decision to measure RelPSDbp rather than RelPSDhr: 1) BP decreased significantly for the group, whereas HR varied and 2) the reciprocal property of CRC depends on BP. In other words, cardioventilatory coupling, which is the onset of inspiration occurring at a preferred latency after the last heart beat in expiration is mediated through the baroreceptor activation. Indeed, brainstem respiratory-modulated, especially expiratory-modulated, activity is modulated by arterial pulse pressure (Dick and Morris, 2004 and Dick et al., 2005). However, the role of this feedback loop in inducing plasticity in the CRC is unknown but after chronic sinoaortic baroreceptor denervation the high frequency component of the PSD is absent from BP but remains in H R (Cerutti et al., 1994).
We compare HR and MAP between complete epochs for a cohort of naïve, male subjects. This is the insensitive to differential changes in systolic versus diastolic pressures and to the time course of the induction and resolution of the HR and BP changes and to the difference in males and females in autonomic control. For the first two limitations, we will analyze our data to address the differences in the measures of arterial pressure and in the time course of induction and recovery as well as RelPSDhr. Additional are being planned to address gender difference as well as those who practice pranayama.
Finally, we present a post-hoc analysis of a data set that was collected by Erica Wehrwein. Arterial blood gas samples were gathered as a part the primary study. As expected, PaO2 increased and PaCO2 decreased significantly during SDB, but blood gasses were not different from baseline after the recovery period. The PaCO2 decreased 15%, which could contribute to the decrease MAP during SDB.
4.2 A Respiratory Role for Cardiorespiratory Coupling
Teleologically, CRC acts to match ventilation and perfusion, the influx of air into the lungs with an increased blood flow to the lungs. Indeed, Hayano and Yasuma (1996) have shown in dogs, which have a robust RSA, that compared to evenly spaced RR intervals; imposing a physiologic RSA decreased the ratio of physiological dead space to tidal volume by 10% and the fraction of intrapulmonary shunt by 51%, and increased O2 consumption from the inspired air by 4%; whereas reversing RSA had the opposite effects increasing physiological dead space and decreasing gas exchange (Hayano et al., 1996; Hayano and Yasuma 2003; Yasuma and Hayano 2004). Over a life time of breathing these benefits are substantial and the loss of RSA in the critically-ill could impact the efficiency of gas exchange profoundly.
Even though the data of Hayano and Yasuma (1996) are convincing, a computational model did not support this interpretation (Ben-Tal et al., 2012). Rather than benefitting gas exchange; modelling RSA supported a benefit for the heart (Ben-Tal et al., 2012). In fact, these authors concluded that cardiac work was minimized “most profoundly” during SDB. If data from a single individual can support anything, then our analysis of S2, in which a sustained and enhanced CRC was associated with a decreased HR after SDB supports this interpretation of enhanced CRC.
4.3 Plasticity in the Autonomic Nervous System, a Non-Respiratory Role of Cardiorespiratory Coupling?
Plasticity in the autonomic nervous system is established in pathophysiology rather than physiology. In humans, the intermittent hypoxia that occurs in obstructive sleep apneic patients may elicit the sustained increase in SNA causing hypertension but this occurs in only 40% of OSA patients (Somers et al., 1995). In normal humans, intermittent exposure to hypoxia increases sympathetic tone and upregulates SNA and its response to hypoxia (Xie et al., 2000, 2001; Cutler et al., 2004; Leuenberger et al., 2007; Dempsey et al., 2010). Hypoxia rather than hypercapnia evokes autonomic plasticity; as both hypoxia and hypercapnia can be titrated to drive respiration to the same extent but only hypoxia will result in sustained effects in SNA (Xie et al., 2001; Cutler et al., 2004). Not surprisingly, the plasticity evoked by intermittent hypoxia depends on the carotid bodies (Fletcher et al., 1992), which also express plasticity (Prabhakar et al., 2005, 2012; Prabhakar and Semenza, 2012).
In addition to SNA, CIH may evoke plasticity in RSA (Deng et al., 2006; Sica and Zhao, 2006; Smietanowski et al., 2006; Reynolds et al., 2007; Rey et al., 2008; Iturriaga et al., 2009, 2010). Even though in a piglet model, the high-frequency component of the power spectra of HRV did not change with CIH (Sica and Zhao 2006) in OSA patients their spectra shifts from the HF band (Narkiewicz et al., 1998). Similarly cats exposed to CIH had a higher LF to HF ratio after 4 days of CIH (Rey et al., 2008).
Given the importance of hypoxia and the carotid body in evoking the plasticity in the autonomic nervous system, why do we focus on CRC as a mechanism? Indeed, intermittent hypoxia evokes sympathetic activity that is more robust than respiratory activity (Dick et al., 2007) and even occurs in the absence of a commensurate response in respiratory motor activity (Xing and Pilowsky 2010). In their recent review (Xing et al., 2014), Pilowsky and coworkers examine multiple possibilities of both central and peripheral mechanisms that could contribute to the increase in blood pressure following exposure to hypoxia. While they consider CRC, our focus on CRC developed because of a possible role of the dorsolateral pons (Baekey et al., 2008; Dutschmann and Dick, 2012). Conditioning with either CSH or CIH upregulated SNA but only conditioning with CIH enhanced CRC and caused hypertension (Hsieh et al., 2004, 2008). Even though conditioning with CSH did evoke a sustained in SNA, the activity was poorly coupled respiration and the rats were not hypertensive. Further, we could not induce short-term plasticity in SNA with acute exposures to intermittent hypoxia after CSH. The inability to induce this form of plasticity was associated with the expression of a novel GABAa receptor subtype in the dorsolateral pons. This receptor contains both α6 and δ subunits, which would down-regulate activity (Hsieh et al., 2004, 2008). The dorsolateral pons is essential for sympathetic CRC, for the HR response to baroreceptors and for integrating sensory feedback into the respiratory pattern generator (Baekey et al., 2008). Therefore, we theorize that plasticity expressed as enhanced respiratory modulation of HRV would depend on dorsolateral pontine activity.
4.4 Pranayama - Hindi breathing exercises
Pranayama (in Sanskrit “prana” meaning “breath” and “ayama” meaning “length, extension, restraint”) is viewed as an essential part of yoga practice and various practices us different techniques to manipulate breathing. This includes voluntary control for fast, such as Kapala bhati or slow breathing, such as Bhastrika pranayama, Dirga pranayama (slow, three part diaphragmatic breathing) and Ujjayi breathing. Bhastrika pranayama, which translates to "Bellows Breath", describes forceful, deep breathing with the recruitment of expiratory muscles on exhalation. Breaths are repeated rapidly rather than slowly to form a series of breaths; then following a rest, the series is repeated again. On the Yoga International website (http://yogainternational.com/article/view/bhastrika-pranayama-the-bellows-breath), this practice is not recommended to those who are hypertensive or have heart disease. We theorize that the active expiration and repeated sequences are ideal conditions for evoking expiratory related SNA, reinforcing the underlying hypertensive state.
While the link between voluntary slow diaphragmatic breathing and autonomic function has not been tested rigorously, bursts of SNA are coupled to respiration. During quiet breathing bursts of SNA occur preferentially in expiration and are fewer during inspiration (Eckberg, Nerhed et al., 1985; Seals, Suwarno et al., 1990). During SDB, burst of SNA tend to become even more restricted to the expiratory phase and do not occur at end inspiration (See Fig 1, Limberg et al., 2013). Multiple mechanisms contribute the respiratory rhythmicity of SNA including brainstem coupling of cardio-respiratory networks (Koepchen and Thurau, 1957; Koepchen et al., 1981; Seals et al., 1993; Mandel and Schreihofer, 2006; Eckberg, 2009).
Deep breathing elevates parasympathetic tone but can affect sympathetic tone differentially depending on the type of slow breathing pattern performed (Pal et al., 2004; Jerath et al., 2006; Upadhyay-Dhungel et al., 2008; Pramanik et al., 2009). Thus, subtle differences may provide a basis for the differential effects. For example, the practice of slow Bhastrika pranayama (6 BrthPM without use of the abdomen) reduces systolic and diastolic pressure (Praminik et al., 2009), whereas rapid Bhastrika pranayama (~20 BrthPM, using abdominal muscles for a forceful expiration) may increase SNA.
While MAP returned to baseline values during the Recovery period for most subjects we expect that that multiple mechanisms with different time constants affect autonomic tone even after a single period of slow breathing. SDB promotes relaxation, which itself would enhance CRC. Thus, the mechanisms would not only be the increased vagal afferent nerve activity acting through dl pontine mechanisms but also the reduction of anxiety facilitate parasympathetic and disfacilitate SNA. We also expect that learning is required to optimize the effect of SDB. In a study that reported the effectiveness of SDB on various autonomic functions (for instance, reduced resting HR, MAP was not measured), the subjects were given either a fast or a slow breathing exercise to perform twice a day for 3 months. The breathing sessions were guided and only the group that performed the slow breathing exercise had the reduced HR (Pal et al., 2004). The breathing exercises were initiated, performed and repeated. Dutschmann et al. (2009) have used this approach effectively to teach in situ rat pup preparation to breathe faster (Dutschmann et al., 2009, 2014). We expect that repeated bouts of SDB would be more effective in decreasing HR over a longer period of time than a single bout.
In 10 subjects, 3 increased HR; 5 remained essentially at baseline and only 2 decreased their heart rate. This distribution was unexpected, especially given that 9 of 10 subjects decreased their MAP during SDB. Unexpected increases in HR can occur during breath holding due to reduction in venous return reducing cardiac output eliciting an increase in HR to maintain BP (Craig, 1963). A Valsalva maneuver, which increases intrathoracic pressure reduces venous return even more than breath holding and accentuates the increase in HR (Craig, 1963). Extending this concept to SDB is difficult given the dynamics. SDB should increase venous return, accentuate Traube-Hering waves and decrease MAP. If venous return is great enough to elicit the Bainbridge reflex then HR would increase. Finally, in our study the change in HR from baseline to SDB did not correlate to that in MAP. We would expect this dissociation to change as HR response became more consistent across subjects.
Anxiety and stress disrupt CRC (Bar et al., 2008). The influence of state of mind during the breathing maneuvers may be reflected in the change in MAP this and a recent study (Limberg, Morgan et al., 2013). In a pilot study, subjects reported a feeling of anxiety after being asked to follow visual and auditory cues to control breathing. This feeling was reflected in increased arterial stress hormones (data not shown) but this is the reason we avoided cues and asked subjects to breathe slowly at a level of comfort for them for both rate and depth. In contrast, in the Limberg’s study (2013), subjects were fasted, had already completed a protocol that lasted 1.5h after placement of an electrode in the peritoneal nerve, and adjusted their breathing to cues at the end of inspiration and expiration. In our study 9 of 10 subjects decreased their MAP during SDB; whereas in Limberg’s study, despite no change in average MAP a small subset of subjects had a modest decrease in MAP. Thus, many factors affect the change in autonomic tone during and following SDB, including technique, learning, and state of mind. This underscores the importance of the context in which the effects of pranayama are assessed.
Device-guided breathing has been used to treat hypertension and heart failure with mixed results (Hering et al., 2013; Howorka et al., 2013; Landman et al., 2013; de Barros, da Silva et al., 2014; Harada et al., 2014; van Hateren et al., 2014). The mixed result sparked a debate on what constitutes device-guided breathing (Huang and Subak, 2014; Landman et al., 2014). In performing our study and comparing our results to those of Limberg et al., (2013), we appreciate that what constitutes SDB varies and should be defined clearly to evaluate its use for treatment and to decipher and to determine those factors that to favorable results. Individuals exist for whom SDB evokes favorable effects and for whom SDB would be an effective treatment option. So not only do we need to identify what techniques of the SDB are effective, but we also need to identify those subjects for which this is effective. At this point, the position of the AHA is that SDB is a complementary medicine that shows promise in treating hypertension. Mindfulness and mediation techniques have the best support which is consistent with our suggestion that state of mind is critical in the efficacy of slow breathing. The AHA report noted that Resperate is a FDA-approved device that can be used for slow breathing exercises as supplemental treatment for hypertension (Brook, 2013; Elliott and Brook, 2013).
In summary, the conclusion of Limberg et al (2013) was that the average magnitude rather than the pattern of SNA was a factor in determining MAP. This conclusion focuses on CRC as only a breath-by-breath function in shaping the pattern rather than the magnitude of SNA. On the other, our hypothesis and the point of this manuscript is that CRC functions on a longer time scale and acts to evoke plasticity. Therefore, we suggest evaluating a potential physiologic purpose of CRC primarily on a long-time scale rather than on a breath-by-breath basis or short-time scale. The pathophysiology of CRC reveals dysfunction at a longer time scale in that intermittent hypoxia evokes an increase in the mean SNA. In contrast, Eastern medicine actively engages in breathing exercises and stress relief that may be utilizing CRC to reduce mean SNA. Coupling of cardiovascular activity to respiration may involve a longer time scale that includes dynamics over multiple breaths as well as maintaining vascular elasticity and evoking plasticity in the autonomic nervous system for improving gas exchange and reducing cardiac work by maintaining BP with fewer beats per minute.
HIGHLIGHTS.
Cardio-respiratory coupling (CRC) is the reciprocal interaction of two oscillators.
Increased CRC has been associated with the pathophysiology of hypertension.
Slow deep breathing (SDB) increases CRC but reduces blood pressure (BP).
In subsets of subjects, BP was reduced (>3%) during and after SDB.
A positive effect of SDB and enhanced CRC can persist after SDB.
ACKNOWLEDGEMENTS
This work was supported by NS069220 (TED), R25-HL03152 (JRM), HL 083947 (MJ), T32-DK07352 (EW). The authors acknowledge and thank the funding agencies as well as Professor Michael Joyner for his guidance and mentoring and Professor Stephen Lewis for his critical reading of the manuscript. The authors recognize Christopher Johnson, Nancy Meyer, Shelly Roberts, and Karen Krucker for their assistance in subject recruitment, nursing support, and for their efforts during data collection as well as David Nethery and Cara Campanaro for their assistance in data analysis and statistical testing. Arterial catheters were placed by Timothy Curry and John Eisenach. Finally, we acknowledged and thank Ken Loparo in the Department of Electrical Engineering and Computer Science and the team of engineers and scientists who have worked with us to develop the time-series analytical tools that were applied here.
ABBREVIATIONS
- ABP
Arterial Blood Pressure
- BP
Blood Pressure (In this manuscript, the reader can assume we are referring to ABP.)
- BPM
beats per minute
- BrthPM
breaths per minute
- CRC
Cardiorespiratory Coupling
- ECG
Electro-cardio-graphy
- fr
Respiratory Frequency (brthpm)
- HR
Heart Rate
- HRV
Heart Rate Variability
- PSD
Power Spectral Density
- Rel PSDbp
Relative PSD of Blood Pressure
- Rel PSDvp
Relative PSD of the Ventilatory Pattern
- RSA
Respiratory Sinus Arrhythmia, a property of CRC referring to the influence of, respiration on heart rate.
- RRI
RR Intervals where the time difference in the peaks of two consecutive R waves in the QRS complex is used to measure cardiac cycle duration
- SDB
Slow, Deep Breathing
- SDRR
Standard Deviation of RR Intervals, measured as the distance of the point from the origin in a Poincaré plot
- SDSD
Standard Deviation of Successive Differences between RRIs
- SNA
Sympathetic Nerve Activity
- τ
Time delay, # of RRI from the current RRI
- TPVtd
Temporal Poincaré Variability over multiple time delays
- TPVa
Temporal Poincaré Variability over averages
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abdala AP, McBryde FD, Marina N, Hendy EB, Engelman ZJ, Fudim M, Sobotka PA, Gourine AV, Paton JF. Hypertension is critically dependent on the carotid body input in the spontaneously hypertensive rat. J Physiol. 2012;590:4269–4277. doi: 10.1113/jphysiol.2012.237800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdala AP, Rybak IA, Smith JC, Paton JF. Abdominal expiratory activity in the rat brainstem-spinal cord in situ: patterns, origins and implications for respiratory rhythm generation. J Physiol. 2009;587:3539–3559. doi: 10.1113/jphysiol.2008.167502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baekey DM, Dick TE, Paton JF. Pontomedullary transection attenuates central respiratory modulation of sympathetic discharge, heart rate and the baroreceptor reflex in the in situ rat preparation. Exp Physiol. 2008;93:803–816. doi: 10.1113/expphysiol.2007.041400. [DOI] [PubMed] [Google Scholar]
- Bar KJ, Boettger MK, Schulz S, Neubauer R, Jochum T, Voss A, Yeragani VK. Reduced cardio-respiratory coupling in acute alcohol withdrawal. Drug Alcohol Depend. 2008;98:210–217. doi: 10.1016/j.drugalcdep.2008.05.012. [DOI] [PubMed] [Google Scholar]
- Ben-Tal A, Shamailov SS, Paton JF. Evaluating the physiological significance of respiratory sinus arrhythmia: looking beyond ventilation-perfusion efficiency. J Physiol. 2012;590:1989–2008. doi: 10.1113/jphysiol.2011.222422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benchetrit G. Breathing pattern in humans: diversity and individuality. Respir Physiol. 2000;122:123–129. doi: 10.1016/s0034-5687(00)00154-7. [DOI] [PubMed] [Google Scholar]
- Billman GE. Heart rate variability - a historical perspective. Front Physiol. 2011;2:86. doi: 10.3389/fphys.2011.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga VA, Soriano RN, Machado BH. Sympathoexcitatory response to peripheral chemoreflex activation is enhanced in juvenile rats exposed to chronic intermittent hypoxia. Exp Physiol. 2006;91:1025–1031. doi: 10.1113/expphysiol.2006.034868. [DOI] [PubMed] [Google Scholar]
- Brook RD. Response to evidence for upgrading the ratings for transcendental meditation: response to AHA scientific statement on alternative methods and BP. Hypertension. 2013;62:e43. doi: 10.1161/hypertensionaha.113.02328. [DOI] [PubMed] [Google Scholar]
- Cerutti C, Barrès C, Paultre C. Baroreflex modulation of blood pressure and heart rate variabilities in rats: assessment by spectral analysis. Am J Physiol Heart Circ Physiol. 1994;266:H1993–H2000. doi: 10.1152/ajpheart.1994.266.5.H1993. [DOI] [PubMed] [Google Scholar]
- Coleman WM. On the correlation of the rate of heart beat, breathing, bodily movement and sensory stimuli. J Physiol. 1920;54:213–217. doi: 10.1113/jphysiol.1920.sp001920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AB., Jr Heart Rate Responses to Apneic Underwater Diving and to Breath Holding in Man. J Appl Physiol. 1963;18:854–862. doi: 10.1152/jappl.1963.18.5.854. [DOI] [PubMed] [Google Scholar]
- Cutler MJ, Swift NM, Keller DM, Wasmund WL, Smith ML. Hypoxia-mediated prolonged elevation of sympathetic nerve activity after periods of intermittent hypoxic apnea. J Appl Physiol. 2004;96:754–761. doi: 10.1152/japplphysiol.00506.2003. [DOI] [PubMed] [Google Scholar]
- Czyzyk-Krzeska MF, Trzebski A. Respiratory-related discharge pattern of sympathetic nerve activity in the spontaneously hypertensive rat. J Physiol. 1990;426:355–368. doi: 10.1113/jphysiol.1990.sp018142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Barros S, da Silva GV, de Gusmao JL, de Araujo TG, Mion D., Jr Reduction of sympathetic nervous activity with device-guided breathing. J Clin Hypertens (Greenwich) 2014;16:614–615. doi: 10.1111/jch.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey JA, Veasey SC, Morgan BJ, O'Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90:47–112. doi: 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng ZD, Poon CS, Arzeno NM, Katz ES. Heart rate variability in pediatric obstructive sleep apnea. Conf Proc IEEE Eng Med Biol Soc. 2006;1:3565–3568. doi: 10.1109/IEMBS.2006.260139. [DOI] [PubMed] [Google Scholar]
- Dick TE, Morris KF. Quantitative analysis of cardiovascular modulation in respiratory neural activity. J Physiol. 2004;556:959–970. doi: 10.1113/jphysiol.2003.060418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick TE, Hsieh YH, Morrison S, Coles SK, Prabhakar N. Entrainment pattern between sympathetic and phrenic nerve activities in the Sprague-Dawley rat: hypoxia-evoked sympathetic activity during expiration. Am J Physiol Regul Integr Comp Physiol. 2004;286:R1121–R1128. doi: 10.1152/ajpregu.00485.2003. [DOI] [PubMed] [Google Scholar]
- Dick TE, Shannon R, Lindsey BG, Nuding SC, Segers LS, Baekey DM, Morris KF. Arterial pulse modulated activity is expressed in respiratory neural output. J Appl Physiol. 2005;99:691–698. doi: 10.1152/japplphysiol.01124.2004. [DOI] [PubMed] [Google Scholar]
- Dick TE, Hsieh Y-H, Wang N, Prabhakar N. Acute intermittent hypoxia increases both phrenic and sympathetic nerve activities in the rat. Exp Physiol. 2007;92:87–97. doi: 10.1113/expphysiol.2006.035758. [DOI] [PubMed] [Google Scholar]
- Dutschmann M, Bautista TG, Morschel M, Dick TE. Learning to breathe: habituation of Hering-Breuer inflation reflex emerges with postnatal brainstem maturation. Respir Physiol Neurobiol. 2014;195:44–49. doi: 10.1016/j.resp.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutschmann M, Dick TE. Pontine mechanisms of respiratory control. Compr Physiol. 2012;2:2443–2469. doi: 10.1002/cphy.c100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutschmann M, Morschel M, Rybak IA, Dick TE. Learning to breathe: control of the inspiratory-expiratory phase transition shifts from sensory- to central-dominated during postnatal development in rats. J Physiol. 2009;587:4931–4948. doi: 10.1113/jphysiol.2009.174599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckberg DL. Point:counterpoint: respiratory sinus arrhythmia is due to a central mechanism vs. respiratory sinus arrhythmia is due to the baroreflex mechanism. J Appl Physiol. 2009;106:1740–1742. doi: 10.1152/japplphysiol.91107.2008. discussion 1744. [DOI] [PubMed] [Google Scholar]
- Eckberg DL, Nerhed C, Wallin BG. Respiratory modulation of muscle sympathetic and vagal cardiac outflow in man. J Physiol. 1985;365:181–196. doi: 10.1113/jphysiol.1985.sp015766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott WJ, Brook RD. Response to Call for a re-evaluation of the American Heart Association's standpoint concerning device-guided slow breathing using the RESPeRATE device. Hypertension. 2013;62:e18. doi: 10.1161/hypertensionaha.113.02042. [DOI] [PubMed] [Google Scholar]
- Fishman M, Jacono FJ, Park S, Jamasebi R, Thungtong A, Loparo KA, Dick TE. A method for analyzing temporal patterns of variability of a time series from Poincare plots. J Appl Physiol. 2012;113:297–306. doi: 10.1152/japplphysiol.01377.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher EC. Invited review: Physiological consequences of intermittent hypoxia: systemic blood pressure. J Appl Physiol. 2001;90:1600–1605. doi: 10.1152/jappl.2001.90.4.1600. [DOI] [PubMed] [Google Scholar]
- Fletcher EC, Lesske J, Behm R, Miller CC, 3rd, Stauss H, Unger T. Carotid chemoreceptors, systemic blood pressure, and chronic episodic hypoxia mimicking sleep apnea. J Appl Physiol. 1992;72:1978–1984. doi: 10.1152/jappl.1992.72.5.1978. [DOI] [PubMed] [Google Scholar]
- Fletcher EC, Lesske J, Qian W, Miller CC, 3rd, Unger T. Repetitive, episodic hypoxia causes diurnal elevation of blood pressure in rats. Hypertension. 1992;19:555–561. doi: 10.1161/01.hyp.19.6.555. [DOI] [PubMed] [Google Scholar]
- Harada D, Asanoi H, Takagawa J, Ishise H, Ueno H, Oda Y, Goso Y, Joho S, Inoue H. Slow and Deep Respiration Suppresses Steady-State Sympathetic Nerve Activity in Patients with Chronic Heart Failure: From Modeling to Clinical Application. Am J Physiol Heart Circ Physiol. 2014 doi: 10.1152/ajpheart.00109.2014. (In press). [DOI] [PubMed] [Google Scholar]
- Hayano J, Yasuma F. Hypothesis: respiratory sinus arrhythmia is an intrinsic resting function of cardiopulmonary system. Cardiovasc Res. 2003;58:1–9. doi: 10.1016/s0008-6363(02)00851-9. [DOI] [PubMed] [Google Scholar]
- Hayano J, Yasuma F, Okada A, Mukai S, Fujinami T. Respiratory sinus arrhythmia. A phenomenon improving pulmonary gas exchange and circulatory efficiency. Circulation. 1996;94:842–847. doi: 10.1161/01.cir.94.4.842. [DOI] [PubMed] [Google Scholar]
- Hering D, Kucharska W, Kara T, Somers VK, Parati G, Narkiewicz K. Effects of acute and long-term slow breathing exercise on muscle sympathetic nerve activity in untreated male patients with hypertension. J Hypertens. 2013;31:739–746. doi: 10.1097/HJH.0b013e32835eb2cf. [DOI] [PubMed] [Google Scholar]
- Hon EH, Lee ST. Electronic Evaluation of the Fetal Heart Rate. VIII. Patterns Preceding Fetal Death, Further Observations. Am J Obstet Gynecol. 1963;87:814–826. [PubMed] [Google Scholar]
- Hon EH, Lee ST. The Fetal Electrocardiogram. I. The Electrocardiogram of the Dying Fetus. Am J Obstet Gynecol. 1963;87:804–813. [PubMed] [Google Scholar]
- Howorka K, Pumprla J, Tamm J, Schabmann A, Klomfar S, Kostineak E, Howorka N, Sovova E. Effects of guided breathing on blood pressure and heart rate variability in hypertensive diabetic patients. Auton Neurosci. 2013;179:131–137. doi: 10.1016/j.autneu.2013.08.065. [DOI] [PubMed] [Google Scholar]
- Hsieh YH, Dick TE, Siegel RE. Adaptation to hypobaric hypoxia involves GABA A receptors in the pons. Am J Physiol Regul Integr Comp Physiol. 2008;294:R549–R557. doi: 10.1152/ajpregu.00339.2007. [DOI] [PubMed] [Google Scholar]
- Hsieh YH, Siegel RE, Dick TE. Pontine GABAergic pathways: role and plasticity in the hypoxic ventilatory response. Respir Physiol Neurobiol. 2004;143:141–153. doi: 10.1016/j.resp.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Huang AJ, Subak LL. What constitutes an adequate evaluation of device-guided breathing? JAMA Intern Med. 2014;174:637. doi: 10.1001/jamainternmed.2013.13791. [DOI] [PubMed] [Google Scholar]
- Iturriaga R, Moya EA, Del Rio R. Cardiorespiratory alterations induced by intermittent hypoxia in a rat model of sleep apnea. Adv Exp Med Biol. 2010;669:271–274. doi: 10.1007/978-1-4419-5692-7_55. [DOI] [PubMed] [Google Scholar]
- Iturriaga R, Rey S, Del Rio R, Moya EA, Alcayaga J. Cardioventilatory acclimatization induced by chronic intermittent hypoxia. Adv Exp Med Biol. 2009;648:329–335. doi: 10.1007/978-90-481-2259-2_37. [DOI] [PubMed] [Google Scholar]
- Jerath R, Edry JW, Barnes VA, Jerath V. Physiology of long pranayamic breathing: neural respiratory elements may provide a mechanism that explains how slow deep breathing shifts the autonomic nervous system. Med Hypotheses. 2006;67:566–571. doi: 10.1016/j.mehy.2006.02.042. [DOI] [PubMed] [Google Scholar]
- Karemaker JM. Counterpoint: respiratory sinus arrhythmia is due to the baroreflex mechanism. J Appl Physiol. 2009;106:1742–1743. doi: 10.1152/japplphysiol.91107.2008a. discussion 1744. [DOI] [PubMed] [Google Scholar]
- Koepchen HP, Klussendorf D, Sommer D. Neurophysiological background of central neural cardiovascular-respiratory coordination: basic remarks and experimental approach. J Auton Nerv Syst. 1981;3:335–368. doi: 10.1016/0165-1838(81)90074-6. [DOI] [PubMed] [Google Scholar]
- Koepchen HP, Thurau K. Origin of respiratory arrhythmia. Pflugers Arch. 1957;266:22–23. doi: 10.1007/BF02335148. [DOI] [PubMed] [Google Scholar]
- Landman GW, Drion I, van Hateren KJ, van Dijk PR, Logtenberg SJ, Lambert J, Groenier KH, Bilo HJ, Kleefstra N. Device-guided breathing as treatment for hypertension in type 2 diabetes mellitus: a randomized, double-blind, sham-controlled trial. JAMA Intern Med. 2013;173:1346–1350. doi: 10.1001/jamainternmed.2013.6883. [DOI] [PubMed] [Google Scholar]
- Landman GW, van Hateren KJ, Kleefstra N. What constitutes an adequate evaluation of device-guided breathing?-Reply. JAMA Intern Med. 2014;174:638. doi: 10.1001/jamainternmed.2013.13790. [DOI] [PubMed] [Google Scholar]
- Leuenberger UA, Hogeman CS, Quraishi SA, Linton-Frazier L, Gray KS. Short-term intermittent hypoxia enhances sympathetic responses to continuous hypoxia in humans. J Appl Physiol. 2007;103:835–842. doi: 10.1152/japplphysiol.00036.2007. [DOI] [PubMed] [Google Scholar]
- Limberg JK, Morgan BJ, Schrage WG, Dempsey JA. Respiratory influences on muscle sympathetic nerve activity and vascular conductance in the steady state. Am J Physiol Heart Circ Physiol. 2013;304:H1615–H1623. doi: 10.1152/ajpheart.00112.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel DA, Schreihofer AM. Central respiratory modulation of barosensitive neurones in rat caudal ventrolateral medulla. J Physiol. 2006;572:881–896. doi: 10.1113/jphysiol.2005.103622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molkov YI, Abdala AP, Bacak BJ, Smith JC, Paton JF, Rybak IA. Late-expiratory activity: emergence and interactions with the respiratory CpG. J Neurophysiol. 2010;104:2713–2729. doi: 10.1152/jn.00334.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molkov YI, Zoccal DB, Moraes DJ, Paton JF, Machado BH, Rybak IA. Intermittent hypoxia-induced sensitization of central chemoreceptors contributes to sympathetic nerve activity during late expiration in rats. J Neurophysiol. 2011;105:3080–3091. doi: 10.1152/jn.00070.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narkiewicz K, Montano N, Cogliati C, van de Borne PJ, Dyken ME, Somers VK. Altered cardiovascular variability in obstructive sleep apnea. Circulation. 1998;98:1071–1077. doi: 10.1161/01.cir.98.11.1071. [DOI] [PubMed] [Google Scholar]
- Pal GK, Velkumary S, Madanmohan Effect of short-term practice of breathing exercises on autonomic functions in normal human volunteers. Indian J Med Res. 2004;120:115–121. [PubMed] [Google Scholar]
- Parati G, Mancia G, Di Rienzo M, Castiglioni P. Point: cardiovascular variability is/is not an index of autonomic control of circulation. J Appl Physiol. 2006;101:676–678. doi: 10.1152/japplphysiol.00446.2006. discussion 681–672. [DOI] [PubMed] [Google Scholar]
- Paton JF, Sobotka PA, Fudim M, Engelman ZJ, Hart EC, McBryde FD, Abdala AP, Marina N, Gourine AV, Lobo M, Patel N, Burchell A, Ratcliffe L, Nightingale A. Response to role of the carotid body in obesity-related sympathoactivation. Hypertension. 2013;61:e58. doi: 10.1161/hypertensionaha.113.01301. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR, Dick TE, Nanduri J, Kumar GK. Systemic, cellular and molecular analysis of chemoreflex-mediated sympathoexcitation by chronic intermittent hypoxia. Exp Physiol. 2007;92:39–44. doi: 10.1113/expphysiol.2006.036434. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR, Kumar GK. Mechanisms of sympathetic activation and blood pressure elevation by intermittent hypoxia. Respir Physiol Neurobiol. 2010;174:156–161. doi: 10.1016/j.resp.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar NR, Kumar GK, Peng YJ. Sympatho-adrenal activation by chronic intermittent hypoxia. J Appl Physiol. 2012;113:1304–1310. doi: 10.1152/japplphysiol.00444.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar NR, Peng YJ, Jacono FJ, Kumar GK, Dick TE. Cardiovascular alterations by chronic intermittent hypoxia: importance of carotid body chemoreflexes. Cncl Exper Pharmacol Physiol. 2005;32:447–449. doi: 10.1111/j.1440-1681.2005.04209.x. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR, Semenza GL. Adaptive and maladaptive cardiorespiratory responses to continuous and intermittent hypoxia mediated by hypoxia-inducible factors 1 and 2. Physiol Rev. 2012;92:967–1003. doi: 10.1152/physrev.00030.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pramanik T, Sharma HO, Mishra S, Mishra A, Prajapati R, Singh S. Immediate effect of slow pace bhastrika pranayama on blood pressure and heart rate. J Altern Complement Med. 2009;15:293–295. doi: 10.1089/acm.2008.0440. [DOI] [PubMed] [Google Scholar]
- Rey S, Tarvainen MP, Karjalainen PA, Iturriaga R. Dynamic time-varying analysis of heart rate and blood pressure variability in cats exposed to short-term chronic intermittent hypoxia. Am J Physiol Regul Integr Comp Physiol. 2008;295:R28–R37. doi: 10.1152/ajpregu.00070.2008. [DOI] [PubMed] [Google Scholar]
- Reynolds EB, Seda G, Ware JC, Vinik AI, Risk MR, Fishback NF. Autonomic function in sleep apnea patients: increased heart rate variability except during REM sleep in obese patients. Sleep Breath. 2007;11:53–60. doi: 10.1007/s11325-006-0083-9. [DOI] [PubMed] [Google Scholar]
- Seals DR, Suwarno NO, Dempsey JA. Influence of lung volume on sympathetic nerve discharge in normal humans. Circ Res. 1990;67:130–141. doi: 10.1161/01.res.67.1.130. [DOI] [PubMed] [Google Scholar]
- Seals DR, Suwarno NO, Joyner MJ, Iber C, Copeland JG, Dempsey JA. Respiratory modulation of muscle sympathetic nerve activity in intact and lung denervated humans. Circ Res. 1993;72:440–454. doi: 10.1161/01.res.72.2.440. [DOI] [PubMed] [Google Scholar]
- Sica AL, Zhao N. Heart rate variability in conscious neonatal swine: spectral features and responses to short-term intermittent hypoxia. BMC Physiol. 2006;6:5. doi: 10.1186/1472-6793-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms AE, Paton JF, Allen AM, Pickering AE. Is augmented central respiratory-sympathetic coupling involved in the generation of hypertension? Respir Physiol Neurobiol. 2009a;174:89–97. doi: 10.1016/j.resp.2010.07.010. [DOI] [PubMed] [Google Scholar]
- Simms AE, Paton JF, Pickering AE, Allen AM. Amplified respiratory-sympathetic coupling in the spontaneously hypertensive rat: does it contribute to hypertension? J Physiol. 2009b;587:597–610. doi: 10.1113/jphysiol.2008.165902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smietanowski M, Szelenberger W, Trzebski A. Nonlinear dynamics of the cardiovascular parameters in sleep and sleep apnea. In memory of Alberto Malliani (1935–2006)--a brave heart and beautiful mind. J Physiol Pharmacol. 2006;57(Suppl 11):55–68. [PubMed] [Google Scholar]
- Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–1904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JA, Studinger P. Counterpoint: cardiovascular variability is not an index of autonomic control of the circulation. J Appl Physiol. 2006;101:678–681. doi: 10.1152/japplphysiol.00446.2006. discussion 681. [DOI] [PubMed] [Google Scholar]
- Upadhyay-Dhungel K, Malhotra V, Sarkar D, Prajapati R. Effect of alternate nostril breathing exercise on cardiorespiratory functions. Nepal Med Coll J. 2008;10:25–27. [PubMed] [Google Scholar]
- van Hateren KJ, Landman GW, Logtenberg SJ, Bilo HJ, Kleefstra N. Device-guided breathing exercises for the treatment of hypertension: An overview. World J Cardiol. 2014;6:277–282. doi: 10.4330/wjc.v6.i5.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrwein EA, Wallin G, Charkoudian N, Johnson CP, Drobish JN, Joyner MJ. Immediate effects of Yoga breathing on stress hormones, sympathetic nerve activity, and blood flow [Google Scholar]
- Xie A, Skatrud JB, Crabtree DC, Puleo DS, Goodman BM, Morgan BJ. Neurocirculatory consequences of intermittent asphyxia in humans. J Appl Physiol. 2000;89:1333–1339. doi: 10.1152/jappl.2000.89.4.1333. [DOI] [PubMed] [Google Scholar]
- Xie A, Skatrud JB, Puleo DS, Morgan BJ. Exposure to hypoxia produces long-lasting sympathetic activation in humans. J Appl Physiol. 2001;91:1555–1562. doi: 10.1152/jappl.2001.91.4.1555. [DOI] [PubMed] [Google Scholar]
- Xing T, Pilowsky PM. Acute intermittent hypoxia in rat in vivo elicits a robust increase in tonic sympathetic nerve activity that is independent of respiratory drive. J Physiol. 2010;588:3075–3088. doi: 10.1113/jphysiol.2010.190454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing T, Pilowsky PM, Fong AY. Mechanism of sympathetic activation and blood pressure elevation in humans and animals following acute intermittent hypoxia. Prog Brain Res. 2014;209:131–146. doi: 10.1016/B978-0-444-63274-6.00007-2. [DOI] [PubMed] [Google Scholar]
- Yasuma F, Hayano J. Respiratory sinus arrhythmia: why does the heartbeat synchronize with respiratory rhythm? Chest. 2004;125:683–690. doi: 10.1378/chest.125.2.683. [DOI] [PubMed] [Google Scholar]
- Zoccal DB, Bonagamba LG, Oliveira FR, Antunes-Rodrigues J, Machado BH. Increased sympathetic activity in rats submitted to chronic intermittent hypoxia. Exp Physiol. 2007;92:79–85. doi: 10.1113/expphysiol.2006.035501. [DOI] [PubMed] [Google Scholar]
- Zoccal DB, Bonagamba LG, Paton JF, Machado BH. Sympathetic-mediated hypertension of awake juvenile rats submitted to chronic intermittent hypoxia is not linked to baroreflex dysfunction. Exp Physiol. 2009;94:972–983. doi: 10.1113/expphysiol.2009.048306. [DOI] [PubMed] [Google Scholar]
- Zoccal DB, Huidobro-Toro JP, Machado BH. Chronic intermittent hypoxia augments sympatho-excitatory response to ATP but not to L-glutamate in the RVLM of rats. Auton Neurosci. 2011;165:156–162. doi: 10.1016/j.autneu.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Zoccal DB, Machado BH. Coupling between respiratory and sympathetic activities as a novel mechanism underpinning neurogenic hypertension. Curr Hypertens Rep. 2011;13:229–236. doi: 10.1007/s11906-011-0198-7. [DOI] [PubMed] [Google Scholar]
- Zoccal DB, Machado BH. Sympathetic overactivity coupled with active expiration in rats submitted to chronic intermittent hypoxia. Respir Physiol Neurobiol. 2010;174:98–101. doi: 10.1016/j.resp.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Zoccal DB, Paton JF, Machado BH. Do changes in the coupling between respiratory and sympathetic activities contribute to neurogenic hypertension? Clin Exp Pharmacol Physiol. 2009;36:1188–1196. doi: 10.1111/j.1440-1681.2009.05202.x. [DOI] [PubMed] [Google Scholar]
- Zoccal DB, Simms AE, Bonagamba LG, Braga VA, Pickering AE, Paton JF, Machado BH. Increased sympathetic outflow in juvenile rats submitted to chronic intermittent hypoxia correlates with enhanced expiratory activity. J Physiol. 2008;586:3253–3265. doi: 10.1113/jphysiol.2008.154187. [DOI] [PMC free article] [PubMed] [Google Scholar]