Abstract
Yellow fever (YF) causes an acute hemorrhagic fever disease in tropical Africa and Latin America. To develop a novel experimental YF vaccine, we applied iDNA infectious clone technology. The iDNA represents plasmid that encodes the full-length RNA genome of 17D vaccine downstream from a cytomegalovirus (CMV) promoter. The vaccine was designed to transcribe the full-length viral RNA and to launch 17D vaccine virus in vitro and in vivo. Transfection with 10ng of iDNA plasmid was sufficient to start replication of vaccine virus in vitro. Safety of the parental 17D and iDNA-derived 17D viruses was confirmed in AG129 mice deficient in receptors for IFN-α/β/γ. Finally, direct vaccination of BALB/c mice with a single 20µg dose of iDNA plasmid resulted in seroconversion and elicitation of virus-specific neutralizing antibodies in animals. We conclude that iDNA immunization approach combines characteristics of DNA and attenuated vaccines and represents a promising vaccination strategy for YF.
Keywords: Yellow fever virus, 17D vaccine, DNA vaccine, live attenuated vaccine, flavivirus, YFV
1. Introduction
Yellow fever (YF) virus (YFV) is a major public health problem in Africa and South America, with a history of causing epidemics worldwide including the U.S. (Ishikawa et al., 2014; Paessler and Walker, 2013; Weaver, 2013). The World Health Organization estimates 200 000 cases of YF and 30 000 deaths worldwide annually. Geographic distribution of YF endemicity currently covers mostly tropical areas of Africa and South America (Beck et al., 2013; Jentes et al., 2011). Phylogenetic and epidemiological analysis supports a model of YFV emergence that is dependent upon both the presence of competent mosquito vectors and nonhuman primate reservoir hosts (Grard et al., 2010; Quaresma et al., 2013). The dynamic ecological and climatic changes associated with international travel, trade, and urbanization increase the potential of YF spread to previously non-endemic regions of the world (Weaver, 2013).
The enveloped, 50 nm YFV particle contains a single-stranded, positive-sense RNA genome of approximately 10.8 kb, including a 5′ cap, as well as 5′ and 3′ terminal untranslated regions, without 3′ polyA tail (Heinz and Stiasny, 2012; Ishikawa et al., 2014; Paessler and Walker, 2013). Following isolation of YF virus in 1927, the YF 17D vaccine was developed in 1937 by 176 passages of Asibi strain in chicken embryo tissue to remove its neurotropic characteristics (Ishikawa et al., 2014; Staples and Monath, 2008). Currently, substrains 17D-204, 17D-213, and 17DD are used for vaccine manufacturing. Although genetically distinct, all three substrains are immunogenic and efficacious, with amino acid substitutions likely responsible for attenuation (dos Santos et al., 1995; Jennings et al., 1993; Lang et al., 1999). A single dose of vaccine confers life-long protective immunity; therefore, a booster dose is not needed in most cases (Gotuzzo et al., 2013; Patel and Simons, 2013).
The available safety data support continued use of YF 17D vaccine for preventive vaccination campaigns in endemic areas (Breugelmans et al., 2013; Cottin et al., 2013; Garske et al., 2014). The vaccine has also been used as a backbone to develop other flavivirus vaccines (Bredenbeek et al., 2006; Guy et al., 2011; Guy et al., 2010; Ishikawa et al., 2014; Stoyanov et al., 2010). Active case-analyses in several countries found only rare 17D vaccine-associated adverse effects including YF vaccine-associated neurologic disease (YEL-AND) and viscerotropic disease (YEL-AVD), as well as allergic reactions (Breugelmans et al., 2013; Ishikawa et al., 2014; Rafferty et al., 2013). Some adverse events appear to be associated with host factors rather than with the vaccine (Barban et al., 2007). Because the vaccine is still manufactured using chicken embryos, egg allergy is a contraindication for vaccination (Rutkowski et al., 2013). Thus, alternative strategies are still needed to improve YF vaccination. Attempts to prepare new vaccines were hampered due to genetic instability of the YF cDNA clones in E. coli (Bredenbeek et al., 2003; Rice et al., 1989). In order to develop a novel experimental vaccine and a reverse genetics system for YF, we applied recently described “immunization DNA” (iDNA®) platform, which is based on a molecular clone technology and combines advantages of DNA immunization and efficacy of live attenuated vaccines (Tretyakova et al., 2014; Tretyakova et al., 2013). We showed that iDNA can successfully launch the YF 17D vaccine virus in vitro. Moreover, injection of iDNA plasmid into BALB/c mice resulted in virus-specific immune responses in vivo, suggesting the potential use of iDNA technology as novel YF vaccine.
2. Materials and Methods
2.1. Cell lines and viruses
African green monkey Vero cell line was obtained from the American Type Culture Collection (ATCC, Manassas, VA) and maintained in a humidified incubator at 37°C and 5% CO2 in αMEM medium supplemented with 10% fetal bovine serum (FBS) and gentamicin sulfate (10 µg/ml) (Life Technologies, Carlsbad, CA). The YF live attenuated vaccine strain 17D (NR-116) was obtained from the NIH Biodefense and Emerging Infections Research Resources Repository, NIAID, NIH. The sequence X03700 is available from Genbank. The 17D vaccine virus was propagated in Vero cells in 75 cm2 flask to generate passage 1 (P1) virus. At 9 days post infection, the 17D P1 virus was harvested, clarified by centrifugation at 3000×g for 10 min, and frozen at −80°C.
2.2. Plasmids and preparation of iDNA
Plasmid pACNR-FLYF\17D encoding the full-length YF 17D cDNA downstream from the bacteriophage SP6 promoter was a kind gift from Dr. Peter Bredenbeek (LUMC, Leiden, Netherlands) and was described elsewhere (Bredenbeek et al., 2003). The CMV major immediate-early promoter was inserted into the pACNR- FLYF\17D plasmid upstream from the full-length 17D cDNA. This resulted in the pYF17D-5 iDNA plasmid that encoded the 17D full-length genomic RNA under transcriptional control of the CMV promoter (Fig. 1). Additional iDNA plasmid pYF17D-16 was prepared by inserting synthetic intron between 17D nucleotides (nt) 9152 and 9153 within the pYF17D-5 plasmid. Intron sequence was derived from mouse immunoglobulin H chain V-region precursor gene (Genbank accession M12880) and inserted into 17D cDNA to inactivate cryptic E. coli promoters and eliminate a potentially toxic polypeptide that may reduce genetic stability of the YF cDNA. The E. coli promoters have been predicted by using BPROM software (SoftBerry, Mount Kisco, NY). The resulting iDNAs were isolated from E.coli strains DH5α or Stbl3 as indicated.
Fig. 1.
Preparation of the YF 17D iDNA plasmids encoding the full-length 17D RNA downstream from the CMV promoter. (a) Schematic depiction of two iDNA plasmids, pYF17D-5 and pYF17D-16. Indicated are CMV promoter (shaded arrow), positions of the 5’ and 3’ ends of the full-length 17D cDNA, as well as intron within pYF17D-16 (shaded box). The 17D nucleotides are indicated according to 17D genome, Genbank X03700 (b) Plasmid yields in E. coli DH5α and Stbl3 cells. Miniprep DNA isolations were performed from each E. coli strain as indicated. The DNA yields were compared by gel densitometry analysis. (c) Infectious center assay (ICA) of Vero cells transfected with 200 ng of pYF17D-5 and pYF17D-16 iDNA. Transfected cells were covered with 1% agarose overlay and incubated for 4 days to form plaques, which were visualized using neutral red. (d) Indirect immunofluorescence assay (IFA) of Vero cells transfected with 200 ng of pYF17D-5 and pYF17D-16 iDNA. After transfection, aliquots of transfected Vero cells were seeded in 8-well chamber slides, fixed at 72 h in cold acetone and processed by IFA using YF-specific mouse polyclonal antiserum and FITCI-conjugated goat antibody to mouse IgG (H+L). Propidium iodide (PI) counterstain was used to visualize cell nuclei. Expression of 17D antigens after transfection of iDNA plasmid is indicated by green fluorescent Vero cells.
2.3. Transfections and assays in vitro
Vero cells were transfected by electroporation with indicated plasmid iDNA at concentrations ranging from 10 ng to 1 µg. Transfection was carried out essentially as described previously (Messer et al., 2012; Tretyakova et al., 2013). As 17D virus controls, Vero cells were infected with 103 PFU of 17D vaccine virus. Production of virus and expression of 17D antigens in the iDNA-transfected cells were determined by the infectious center assay (ICA), indirect immunofluorescence assay (IFA) and western blot. For ICA, iDNA-transfected Vero cells were diluted 10-fold in complete αMEM containing 10% FBS, allowed to adhere for 4 h in in 6-well plates, and covered with 1% agarose overlay. Plates were incubated at 37°C in 5% CO2 for 4 days to form plaques, which were visualized using neutral red. For IFA, iDNA-transfected or 17D-infected Vero cells were seeded in 8-well chamber slides in complete αMEM. At 48 h posttransfection, cells were fixed with cold acetone, and IFA was done using YF-specific mouse antiserum followed by secondary fluorescein-labeled antibody to mouse IgG (H+L). For western blot, Vero cells transfected with iDNA or infected with 17D virus were harvested on day 9, solubilized in the protein sample buffer, and proteins were separated by 4–12% SDS-PAGE. Finally, the virus presence in the growth medium was confirmed by plaque assay. For virus growth curves, samples were taken at indicated time intervals. Average and standard deviation values were determined. Each experiment was conducted at least two times to ensure reproducibility.
2.4. Infection of AG129 mice with YF 17D and pYF17D-16 viruses
The AG129KO mice (129/Sv/Ev background), female, 4–5 weeks old, deficient for IFN-α/β/γ receptors were purchased from B&K Universal Ltd (Grimston, Aldbrough Hill, UK). Mice were randomly placed into two groups (n=30) and inoculated with a single 100 µl intraperitoneal (i.p.) injection (~105 PFU/mouse) of either parental YF 17D vaccine or with iDNA-derived pYF17D-16 virus. Between days 0 and 12 post inoculation (p.i.) 3 mice from each group were sacrificed every 3 days. Beginning at day 12 p.i., mice were euthanized upon exhibiting signs of morbidity. Tissue samples were collected and stored at liquid nitrogen before RNA purification. Quantitative RT/PCR with the 8280f primer, CCACTCATGAAATGTACTACGTGTCTG; 8354r primer, GGAGGCGGGATGTTTGGT, and fluorogenic 8308pr probe, AGCCCGCAGCAATGTCACATTTACTGT, was performed as previously described (Thibodeaux et al., 2012) and viral genome copies were calculated and expressed as viral genome equivalents per 100 mg of tissues. At day 0, 6, and 15 two additional mice from each group were euthanized, samples of liver, brain, kidney, and spleen were removed, fixed in 10% formalin, then samples were prepared for paraffin-embedding, sectioning and hematoxylin and eosin (H&E) staining.
2.5. Immunizations and serology
The iDNA plasmid was isolated from E.coli and formulated in phosphate-buffered saline (PBS) to a final concentration of 0.4 mg/ml. Four-week-old female BALB/c mice were anesthetized with isoflurane and vaccinated intramuscularly (i.m.) with a dose of 50 µl of pYF17D-16 iDNA vaccine in the medial thighs (Noble Life Sciences, Gaithersburg, MD). After injection of iDNA, animals were electroporated as described elsewhere (Tretyakova et al., 2013). As a control, the YF 17D virus (104 PFU) was injected subcutaneously (s.c.). After vaccinations, animals were observed daily for clinical signs of infection. Sera were collected on days 2 and 4 for viremia test, and at day 21 for antibody response determination. For viremia detection, sera were pooled and incubated with Vero cells for 3 days followed by observation for cytopathic effects (CPE) and plaque assay. To determine antibody responses, plaque reduction neutralization test (PRNT), western blot, and IFA were performed. For PRNT, an equal volume (0.1 ml) of virus suspension containing 500 PFU/ml and serial twofold dilutions of heat-inactivated serum were incubated 1 h at 37°C, and the serum-virus mixture was plated onto Vero cell monolayers in 12-well plates. An agarose overlay of in αMEM was added and plates were incubated at 37°C for 3 days prior to neutral red staining and plaque count determination. The endpoint PRNT50 or PRNT80 were expressed as the highest dilution of serum that reduces plaques by 50% or 80%, respectively, as compared to control serum collected before vaccination. For IFA, Vero cells were initially infected with 102 PFU/well of 17D virus in chamber slides for 24 h in complete αMEM. Then, infected Vero cells were fixed with acetone and used as immobilized antigen to detect YF-specific antibodies in the sera from experimental mice.
3. Results
3.1. Design and preparation of YF 17D iDNA
The YF 17D iDNA plasmids were prepared by inserting CMV major immediate-early promoter upstream from the full-length YF 17D cDNA. For this purpose we used plasmid pACNR- FLYF\17D that contained the full-length cDNA of YF 17D virus downstream from the SP6 RNA polymerase promoter (Bredenbeek et al., 2003). The SP6 promoter within the pACNR- FLYF\17D plasmid was replaced with the CMV promoter to generate the YF 17D iDNA plasmid pYF17D-5 containing the full-length cDNA of YF strain 17D genomic RNA downstream from the CMV promoter (Fig. 1a). Because the authentic 5’ terminus of RNA is important for flavivirus replication (Khromykh et al., 2001), the distance between the CMV promoter and the 5’ of 17D RNA was optimized to ensure transcription of the functional YF 5’ terminus of the 17D genomic RNA. The resulting pYF17D-5 was confirmed by sequencing and contained the full-length YF 17D cDNA. Consistent with previous observations (Bredenbeek et al., 2003; Rice et al., 1989), pYF17D-5 showed low plasmid production yields in E. coli strain DH5α as well as in strain Stbl3 (Fig. 1b). It has been hypothesized that YF cDNA contains cryptic bacterial promoters that drive synthesis of toxic proteins thus affecting genetic stability and DNA yields in E. coli (Bredenbeek et al., 2003). In an attempt to improve genetic stability and increase plasmid production, we prepared iDNA plasmid pYF17D-16 (Fig. 1a), which was essentially identical to the pYF17D-5, except it contained an 82nt-long intron inserted at nucleotide 9152 of the full-length YF 17D cDNA. The site for intron insertion was chosen by predicting bacterial promoters within 17D sequence using BPROM software. Putative bacterial promoters have been identified at nt 8617 and 9012. The intron was inserted at nt 9152 and contained five stop codons to prevent translation of downstream putative polypeptides in E. coli. The plasmids pYF17D-16 and pYF17D-5 were isolated from E. coli DH5α or Stbl3 cells. The yield pYF17D-16 was equivalent or higher than that of the pYF17D-5. For example, in one experiment, the yield of pYF17D-16 from DH5α cells was approximately 2 times higher than that of pYF17D-5, as determined by gel densitometry (Fig. 1b).
3.2 Launch of YF vaccine virus replication from iDNA in vitro
The YF 17D pYF17D-5 and pYF17D-16 iDNA plasmids were isolated from E.coli Stbl3, resulting in a sterile, endotoxin-free DNA with 95% supercoiled fraction and an A260/A280 ratio of ~1.9. In order to launch replication of live 17D vaccine virus in vitro, iDNA plasmids were transfected into Vero cells by electroporation. The transfected Vero cells were analyzed for expression of 17D antigens by ICA, IFA and western blot. For ICA, a suspension of electroporated Vero cells was seeded into 6-well plates and overlaid with 1% agarose. At 96 h, plaques were detected, indicating replication of virus from the infectious centers, IC (Fig. 1c). Specific infectivity of pYF17D-5 and pYF17D-16 was calculated at 1.0×103 IC/µg and 1.3×103 IC/µg, respectively. Expression of YF 17D antigens in transfected cells was confirmed at 72 h posttransfection by IFA using mouse anti-YF polyclonal antiserum (Fig. 1d). As expected for a flavivirus, expression of 17D antigen was found in the cytoplasm of transfected cells.
Expression of 17D antigens in iDNA-transfected cells was further examined by SDS-PAGE and western blot. As a control, mock-electroporated Vero cells were infected with 103 PFU of 17D virus. The antigen bands detected in iDNA-transfected Vero cells were similar to those in the cells infected with 17D virus control (Fig. 2a, lanes 1–3). SDS-PAGE and western blot confirmed the presence of 17D antigens that were consistent with molecular weight of E, NS1, and prM proteins. As expected, no bands were detected in the Vero uninfected control cells (Fig 2a, lane 4). The growth medium from iDNA-transfected Vero cells was examined for the presence of replicating virus by plaque assay (Fig. 2b). Plaques were detected in the supernatant samples of Vero cells transfected with 200 ng of iDNA plasmids or infected with 103 PFU of 17D virus suggesting that both pYF17D-5 and pYF17D-16 iDNA plasmids have launched replication of live vaccine viruses and that insertion of 82nt-long intron did not affect the ability of pYF17D-16 to initiate replication of live virus. Plaques from iDNA-transfected cells appeared to be more uniform in size as compared to the 17D virus. Growth curves of viruses from the transfected/infected cells are shown on Fig. 2c. In the culture media from iDNA-transfected cells, the titers reached 106 PFU/ml on day 5 posttransfection, similar to the control 17D-infected cells. We observed approximately 24 h delay for 17D control virus, which can be explained by low amount (103 PFU) of virus used for infection.
Fig. 2.
Expression of YF 17D antigens and secretion of viruses in Vero cells transfected with 200 ng of iDNA or infected with 103 PFU of 17D virus, by western blot and plaque assay. (a) Western blot of Vero cells transfected with indicated iDNA plasmid or infected with 17D virus. Cells were harvested at day 9 posttransfection or postinfection and solubilized in SDS-PAGE sample buffer with no 2-Mercaptoethanol. Lane 1, pYF17D-5 transfected cells; lane 2, pYF17D-16 transfected cells; lane 3, 17D-infected cells; lane 4, untreated control Vero cells; lane 5, SeeBlue Plus2 protein standard. (b) Plaque assay of growth medium from Vero cells transfected with indicated iDNA or infected with 17D virus. Growth medium samples were taken at day 8. Plaque assay was performed on Vero cell monolayers for 4 days and plaques were visualized with neutral red. (c) Growth curves of 17D viruses in iDNA-transfected (solid lines) and 17D virus-infected (dashed lines) Vero cells. Vero cells were transfected by electroporation with 200 ng of indicated iDNA plasmids (Fig. 1a) or infected with 103 PFU of 17D virus. Designations of the viruses and plasmids are shown. Plaque assay was done in duplicates. Each data point represents average of two measurements. Standard deviations are not shown to improve clarity of the graph.
In the next experiment, we determined the minimal dose of iDNA sufficient to launch live 17D virus in vitro. Vero cells were transfected with pYF17D-16 iDNA plasmid with escalating doses ranging from 10 ng to 1 µg. As a control, Vero cells were mock-electroporated using PBS. As expected, no replicating virus was detected in the mock-electroporated Vero cells. Transfection of only 10 ng of iDNA resulted in the replication of 17D virus, with virus titers reaching approximately 106 PFU/ml, similar to transfections with higher quantities of DNA (Fig. 3). Since 17D is a replicating virus, dependence of virus titer on the iDNA dose was minimal. However, iDNA dose dependence was detectable as a delayed onset of replication when 10 ng or 100 ng of iDNA is used. These results suggest that the minimal dose of iDNA to launch 17D virus in Vero cells is below 10 ng (Fig. 3), which corroborates our previous findings with iDNA plasmids encoding VEEV or CHIKV alphaviruses (Tretyakova et al., 2014; Tretyakova et al., 2013).
Fig. 3.
Transfections of Vero cells with escalating amounts of iDNA to start replication of YF 17D virus. Vero cells were transfected by electroporation with indicated amounts of pYF17D-16 iDNA or mock-transfected using PBS. Aliquots of medium from transfected cells were taken every 24 h and virus titer in the medium samples was determined by plaque assay. Plaque assay was done in duplicates. Standard deviations are not shown to improve clarity of the graph. The result was reproduced three times with various quantities of iDNA.
3.3. Replication of iDNA-derived YF17D in tissues of AG129 mice
To confirm safety of the iDNA-derived virus, we used AG129 mice. It was previously determined that virulence of wild-type YFV in mice is IFN-α/β-dependent (Lee and Lobigs, 2008; Meier et al., 2009). Based on this observation, s.c. infection of IFN-α/β deficient mice is currently considered to be a biologically relevant model of wild-type YFV infection (Meier et al., 2009). Mice deficient for receptors for both types of IFN, α/β and γ (AG129) are also susceptible to YF 17D in a dose-dependent manner (Thibodeaux et al., 2012). YF 17D virus does not persist in AG129 mice. Peripheral challenge with YF 17D results in 100% lethality at dose-dependent manner ranging between 104 – 106 PFU. The YF 17D infection of AG129 mice results in high viral load in brain and liver tissues providing a suitable model of human YF in BSL2 containment. We have used this model to compare replication kinetics in brain tissues (neurotropism) and in liver (viscerotropism) of the parental YF 17D virus and the 17D virus derived from pYF17D-16 iDNA-transfected Vero cells. Two groups of AG129 mice were inoculated with viruses (~105 PFU/mouse) and 3 mice from each group were sacrificed every 3 days to collect tissues to extract RNA and measure viral load.
As seen in Fig. 4a, both viruses replicated similarly in brain tissues of infected mice. The viral RNA gradually accumulated starting with day 3 and peaked on days 12–15 after infection. In agreement with the previous study (Thibodeaux et al., 2012), infected animals at this time point showed clinical signs of neurotropic disease (paresis, high limb paralysis) and met euthanasia criteria shorty after this time point. Replication kinetics of YF 17D and pYF17D-16 iDNA-derived virus in the liver tissues differed from those in the brain. Both viruses replicated very rapidly at early stage of the infection. At day 3, 17D was detected in liver and replicated efficiently peaking at day 6 with viral burden comparable with those in the brain on day 12–15. While replication kinetic of both viruses was similar, replication of YF17D-16 virus in liver was less active in comparison with the parental YF 17D (Fig. 4b).
Fig. 4.
Replication of YF 17D viruses in AG129 mice. AG129KO mice (129/Sv/Ev background) were inoculated with parental biological YF 17D virus or with YF 17D virus recovered from YF17D-16 iDNA. Neurotropic, viscerotropic properties and induction of IFN-γ were assessed. Briefly, three mice from each group were sacrificed at indicated time points and neurotropic and viscerotropic features as well as induction of IFN-γ were measured as described in Materials and Methods. Panels (a) and (b), replication kinetics and viral loads in brain and liver tissue, respectively. Panels (c) and (d), induction of IFN-γ mRNA in brain and liver tissue, respectively.
Early events after YF17D vaccination are critical for induction of robust adaptive immune responses and correlate with early production of IFN-γ (Neves et al., 2009; Neves et al., 2013). We measured IFN-γ mRNA expression in the spleen and liver tissues of AG129 mice infected with parental 17D and the 17D virus derived from pYF17D-16 iDNA. Both vaccine viruses rapidly induced strong expression of IFN-γ RNA in spleen, with no significant differences either in the scale or timing of induction (Fig. 4c). As expected, IFN-γ mRNA induction was moderate in liver tissues (less cells are susceptible for induction); however, the response in mice vaccinated with YF17D-16 virus peaked ~72h earlier in comparison with animals injected with parental 17D. The magnitude of IFN-γ mRNA induction in both groups of mice was similar (Fig. 4d). Histological findings for both groups of infected mice by H&E-stained sections prepared on day 6 and 15 after infection did not reveal distinct differences, with only a few foci with mild mononuclear inflammation were observed in liver sections (data not shown).
3.4. Immunogenicity of YF 17D-16 iDNA vaccine in BALB/c mice
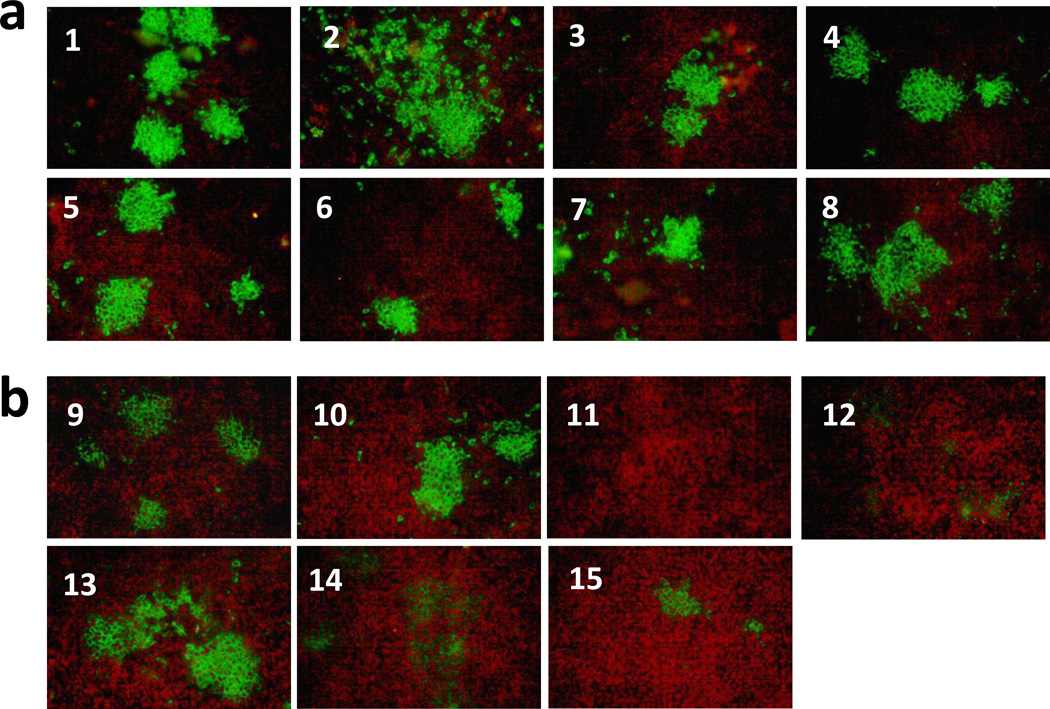
To determine if pYF17D-16 iDNA plasmid is immunogenic in vivo, BALB/c mice were vaccinated by injection-electroporation with a single dose of pYF17D-16 iDNA. As a control, mice were vaccinated by injection with YF parental 17D live virus vaccine. Briefly, mice were vaccinated with either a single i.m. injection of 20 µg of iDNA followed by electroporation, or injected s.c. with 104 PFU of YF 17D vaccine virus. After immunizations, all mice remained healthy with no detectable pathology at the site of injection or adverse effects due to vaccinations. No viremia was detected on days 2 and 4 in both iDNA- and virus-vaccinated groups either by direct plaque assay, or after incubating pooled sera with Vero cells for 10 days followed by CPE analysis and plaque assays. This result indicates no significant presence of replicating virus on days 2 and 4 after iDNA injection. In order to detect antibodies by IFA, Vero cells were infected with 100 PFU of 17D virus in chamber slides, fixed, and then probed with immunized mouse sera at 1:10 dilution (Fig. 5). On day 21, all experimental iDNA-vaccinated mice, as well as 6 out of 7 virus-control animals have seroconverted, as shown by IFA (Fig. 5). Seroconvertion was confirmed by PRNT (Table 1). Neutralizing antibodies were detected in the serum of iDNA vaccinated animals and were equivalent with, or exceeded, the neutralizing antibody titers in the virus-vaccinated control animals (Table 1).
Fig. 5.
Detection of serum antibody in sera of iDNA-vaccinated or 17D virus-vaccinated BALB/c mice, by IFA. (a) BALB/c mice 1–8 were injected i.m. with 20 µg of pYF17D-16 iDNA that encoded the full-length 17D genomic RNA as a cDNA copy downstream from the CMV promoter (Fig. 1a). After iDNA injection, mice were electroporated in order to launch live attenuated 17D vaccine in vivo and to elicit 17D-specific antibody response. (b) Mice 9–15 were vaccinated i.m. by injection with 104 PFU of 17D virus. Sera were taken at day 21 post-vaccination and probed at 1:10 dilution with acetone-fixed monolayers of Vero cell that were previously incubated with 100 PFU of 17D virus. After incubation with mouse antisera, monolayers were incubated with FITC-conjugated goat antimouse IgG antibody to visualize foci of YF 17D-expressing cells. The YF 17D-specific mouse antisera showed green fluorescent cell foci indicating binding to 17D-infected Vero cells.
Table 1.
Virus neutralizing antibody of pYF17D-16 iDNA and parental 17D virus in mice.
| YF Vaccine a |
No. of animals |
Seroconverted /total (%) By WB and IFA b, |
Virus Neutralization Titer c, PRNT80 (No. of Mice) |
Virus Neutralization Titer c, PRNT50 (No. of Mice) |
|---|---|---|---|---|
| pYF17D-16 | n=8 | 8/8 (100%) | <10 (n=4) | 10 (n=2) |
| iDNA, i.m. | 10 (n=2) | 20 (n=2) | ||
| 20 (n=1) | 40 (n=2) | |||
| 40 (n=1) | 80 (n=1) | |||
| 640 (n=1) | ||||
| YF 17D | n=7 | 6/7 (86%) | <10 (n=7) | <10 (n=2) |
| Virus, s.c. | 10 (n=2) | |||
| 20 (n=1) | ||||
| 40 (n=2) | ||||
BALB/c mice were vaccinated either by injection-electroporation i.m. with 20 µg of pYF17D-16 iDNA (Fig. 1a) or by injection s.c. of 104 PFU of YF 17D live attenuated vaccine virus.
WB, western blot; IFA, immunofluorescence assay.
PRNT80, PRNT50, plaque reduction neutralization test demonstrating 80% and 50% reduction in plaque numbers, respectively, after incubation with serum antibody from vaccinated BALB/c mice.
4. Discussion
Pathogenesis of YF is not fully understood, and there is no approved specific antiviral therapy for YF (Julander, 2013; Woodson et al., 2013). The 17D vaccine has been used in the YF control programs in endemic areas as well as in travelers, and the vaccine elicits long-term immunity (Gotuzzo et al., 2013; Ishikawa et al., 2014; Patel and Simons, 2013). Rare adverse effects including YEL-AND and YEL-AVD have been reported (Breugelmans et al., 2013; Ishikawa et al., 2014). The development of improved YF vaccine would be beneficial (Levine, 2011; Monath, 2005). A potentially safer Vero cell-derived inactivated vaccine was evaluated in phase I clinical trial (Monath et al., 2011). Candidate experimental vaccines also include NS1 protein vaccine (Schlesinger et al., 1986), as well as the modified vaccinia Ankara and the D4R-defective vaccinia viruses expressing 17D prME protein (Schafer et al., 2011). In this study, we configured recently developed iDNA® approach (Tretyakova et al., 2014; Tretyakova et al., 2013) to prepare a novel experimental vaccine for YFV. We showed that the YF 17D iDNA plasmids launched live vaccine virus in vitro and elicited immune response in vivo. In AG129 mice, a biologically relevant model of YFV infection, both parental 17D and iDNA-derived 17D viruses were found safe with a tendency to be less hepatotropic for iDNA-derived virus.
The iDNA approach resembles the traditional “infectious clone” technology (Bredenbeek et al., 2003; Rice et al., 1989) but does not involve in vitro RNA transcription, thus allowing the use of iDNA plasmid for direct vaccination in vivo. Essentially, iDNA combines advantages of DNA and live attenuated vaccines when used in vivo (Pushko, 2014; Tretyakova et al., 2014; Tretyakova et al., 2013). Recently, we reported that two alphavirus iDNA vaccines, one based on the TC-83 strain of Venezuelan equine encephalitis (VEEV), and another based on the 181/25 strain of chikungunya virus (CHIKV), initiated replication of live attenuated vaccines in vitro and elicited protective immune responses in mice (Tretyakova et al., 2014; Tretyakova et al., 2013). In addition to extending the use of DNA immunization, iDNA technology potentially can provide improvement to the live attenuated 17D vaccine. Although YF 17D vaccine showed remarkable genetic stability (Beck et al., 2014; Mantel et al., 2011), it cannot be excluded that reversion mutations are present at a low frequency within the manufactured vaccine. In such a case, direct vaccination with iDNA in vivo would reduce probability of reversion mutations. Analysis of population structure of the attenuated YF17D-204 and wild-type Asibi virus revealed that the attenuated YFV population was homogeneous with a restricted pattern of quasispecies variability and very limited evidence of the existence of the Asibi consensus sequence, while Asibi virus expressed diverse quasispecies (Beck et al., 2014). Lack of YF 17D quasispecies was confirmed by previously reported evidence of the high fidelity of YF 17D RNA polymerase (Pugachev et al., 2004).
Because iDNA represents a novel approach, more research is needed. Although genetic stability of YF 17D virus has been demonstrated (Beck et al., 2014; Pugachev et al., 2004), serial passages of iDNA-derived vaccine virus in animals would be useful to directly confirm stability and safety of this vaccine in vivo. Furthermore, additional studies including efficacy can be done in other animal models such as nonhuman primates (NHP) (Monath et al., 1981), hamsters (Li et al., 2008) and A129 mice deficient in the IFN-α/β receptor (Meier et al., 2009). NHP remain the best model of human disease (Monath et al., 1981; Tesh et al., 2001). The absence of appropriate small animal model has been the major obstacle for YF vaccine studies. Although immunocompromised animals are not considered by FDA as appropriate challenge models for vaccine studies, recent A129 and AG129 mouse models lacking receptors for IFN-α/β or IFN-α/β/γ, respectively, can mimic some viscerotropic and neurotropic characteristics of YF Asibi virus and can be used for proof-of-concept efficacy studies. These experiments are currently being planned. In conclusion, the iDNA vaccine approach offers certain advantages over both the traditional DNA vaccines and the live attenuated vaccines. As any DNA vaccine, the iDNA can be formulated to be stable at ambient temperatures (van der Heijden et al., 2013). Potentially, the iDNA clone can serve as a reverse genetic system for preparation of improved 17D vaccines or chimeric 17D-vectored vaccines, such as Lassa vaccine (Bredenbeek et al., 2006) or live dengue vaccine (Guy et al., 2011; Guy et al., 2010). Finally, we have shown that the 17D iDNA vaccine induces immune response in vivo after a single vaccination, which is a necessity for prophylactic vaccine in the developing nations (Levine, 2011). Therefore, we conclude that the iDNA approach may represent a feasible strategy for YF immunization.
Highlights.
-
-
The iDNA® platform combines advantages of DNA and live attenuated vaccines
-
-
Yellow fever (YF) 17D vaccine was launched from iDNA plasmid in vitro and in vivo
-
-
Safety of iDNA-generated 17D virus was confirmed in AG129 mice
-
-
BALB/c mice seroconverted after a single-dose vaccination with iDNA
-
-
YF virus-neutralizing response was elicited in iDNA-vaccinated mice
Acknowledgements
We thank Peter Bredenbeek for pACNR-FLYF\17D plasmid and Elena Klyushnenkova for help with immunizations. The YF 17D vaccine was obtained through the NIH Biodefense and Emerging Infections Research Resources Repository, NIAID, NIH: Yellow Fever Virus, 17D, NR-116. Research reported in this publication was supported in part by the NIH National Institute of Allergy and Infectious Diseases grants R43AI088923 (PP) and R01AI052367 (ISL). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare that they have no competing financial interests.
References
- Barban V, Girerd Y, Aguirre M, Gulia S, Petiard F, Riou P, Barrere B, Lang J. High stability of yellow fever 17D-204 vaccine: a 12-year restrospective analysis of large-scale production. Vaccine. 2007;25:2941–2950. doi: 10.1016/j.vaccine.2006.06.082. [DOI] [PubMed] [Google Scholar]
- Beck A, Guzman H, Li L, Ellis B, Tesh RB, Barrett AD. Phylogeographic reconstruction of African yellow fever virus isolates indicates recent simultaneous dispersal into east and west Africa. PLoS Negl Trop Dis. 2013;7:e1910. doi: 10.1371/journal.pntd.0001910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A, Tesh RB, Wood TG, Widen SG, Ryman KD, Barrett AD. Comparison of the live attenuated yellow fever vaccine 17D-204 strain to its virulent parental strain Asibi by deep sequencing. J. Infect. Dis. 2014;209:334–344. doi: 10.1093/infdis/jit546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredenbeek PJ, Kooi EA, Lindenbach B, Huijkman N, Rice CM, Spaan WJ. A stable full-length yellow fever virus cDNA clone and the role of conserved RNA elements in flavivirus replication. J. Gen. Virol. 2003;84:1261–1268. doi: 10.1099/vir.0.18860-0. [DOI] [PubMed] [Google Scholar]
- Bredenbeek PJ, Molenkamp R, Spaan WJ, Deubel V, Marianneau P, Salvato MS, Moshkoff D, Zapata J, Tikhonov I, Patterson J, Carrion R, Ticer A, Brasky K, Lukashevich IS. A recombinant Yellow Fever 17D vaccine expressing Lassa virus glycoproteins. Virology. 2006;345:299–304. doi: 10.1016/j.virol.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breugelmans JG, Lewis RF, Agbenu E, Veit O, Jackson D, Domingo C, Bothe M, Perea W, Niedrig M, Gessner BD, Yactayo S, group YA. Adverse events following yellow fever preventive vaccination campaigns in eight African countries from 2007 to 2010. Vaccine. 2013;31:1819–1829. doi: 10.1016/j.vaccine.2013.01.054. [DOI] [PubMed] [Google Scholar]
- Cottin P, Niedrig M, Domingo C. Safety profile of the yellow fever vaccine Stamaril(R): a 17-year review. Expert Rev Vaccines. 2013;12:1351–1368. doi: 10.1586/14760584.2013.836320. [DOI] [PubMed] [Google Scholar]
- dos Santos CN, Post PR, Carvalho R, Ferreira II, Rice CM, Galler R. Complete nucleotide sequence of yellow fever virus vaccine strains 17DD and 17D-213. Virus Res. 1995;35:35–41. doi: 10.1016/0168-1702(94)00076-o. [DOI] [PubMed] [Google Scholar]
- Garske T, Van Kerkhove MD, Yactayo S, Ronveaux O, Lewis RF, Staples JE, Perea W, Ferguson NM Yellow Fever Expert, C. Yellow Fever in Africa: estimating the burden of disease and impact of mass vaccination from outbreak and serological data. PLoS Med. 2014;11:e1001638. doi: 10.1371/journal.pmed.1001638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotuzzo E, Yactayo S, Cordova E. Efficacy and duration of immunity after yellow fever vaccination: systematic review on the need for a booster every 10 years. Am. J. Trop. Med. Hyg. 2013;89:434–444. doi: 10.4269/ajtmh.13-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grard G, Moureau G, Charrel RN, Holmes EC, Gould EA, de Lamballerie X. Genomics and evolution of Aedes-borne flaviviruses. J. Gen. Virol. 2010;91:87–94. doi: 10.1099/vir.0.014506-0. [DOI] [PubMed] [Google Scholar]
- Guy B, Barrere B, Malinowski C, Saville M, Teyssou R, Lang J. From research to phase III: preclinical, industrial and clinical development of the Sanofi Pasteur tetravalent dengue vaccine. Vaccine. 2011;29:7229–7241. doi: 10.1016/j.vaccine.2011.06.094. [DOI] [PubMed] [Google Scholar]
- Guy B, Guirakhoo F, Barban V, Higgs S, Monath TP, Lang J. Preclinical and clinical development of YFV 17D-based chimeric vaccines against dengue, West Nile and Japanese encephalitis viruses. Vaccine. 2010;28:632–649. doi: 10.1016/j.vaccine.2009.09.098. [DOI] [PubMed] [Google Scholar]
- Heinz FX, Stiasny K. Flaviviruses and their antigenic structure. J. Clin. Virol. 2012;55:289–295. doi: 10.1016/j.jcv.2012.08.024. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Yamanaka A, Konishi E. A review of successful flavivirus vaccines and the problems with those flaviviruses for which vaccines are not yet available. Vaccine. 2014;32:1326–1337. doi: 10.1016/j.vaccine.2014.01.040. [DOI] [PubMed] [Google Scholar]
- Jennings AD, Whitby JE, Minor PD, Barrett AD. Comparison of the nucleotide and deduced amino acid sequences of the structural protein genes of the yellow fever 17DD vaccine strain from Senegal with those of other yellow fever vaccine viruses. Vaccine. 1993;11:679–681. doi: 10.1016/0264-410x(93)90317-q. [DOI] [PubMed] [Google Scholar]
- Jentes ES, Poumerol G, Gershman MD, Hill DR, Lemarchand J, Lewis RF, Staples JE, Tomori O, Wilder-Smith A, Monath TP Informal, W.H.O.W.G.o.G.R.f.Y.F. The revised global yellow fever risk map and recommendations for vaccination, 2010: consensus of the Informal WHO Working Group on Geographic Risk for Yellow Fever. Lancet Infect. Dis. 2011;11:622–632. doi: 10.1016/S1473-3099(11)70147-5. [DOI] [PubMed] [Google Scholar]
- Julander JG. Experimental therapies for yellow fever. Antiviral Res. 2013;97:169–179. doi: 10.1016/j.antiviral.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khromykh AA, Meka H, Guyatt KJ, Westaway EG. Essential role of cyclization sequences in flavivirus RNA replication. J. Virol. 2001;75:6719–6728. doi: 10.1128/JVI.75.14.6719-6728.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang J, Zuckerman J, Clarke P, Barrett P, Kirkpatrick C, Blondeau C. Comparison of the immunogenicity and safety of two 17D yellow fever vaccines. Am. J. Trop. Med. Hyg. 1999;60:1045–1050. doi: 10.4269/ajtmh.1999.60.1045. [DOI] [PubMed] [Google Scholar]
- Lee E, Lobigs M. E protein domain III determinants of yellow fever virus 17D vaccine strain enhance binding to glycosaminoglycans, impede virus spread, and attenuate virulence. J. Virol. 2008;82:6024–6033. doi: 10.1128/JVI.02509-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine MM. "IDEAL" vaccines for resource poor settings. Vaccine. 2011;29(Suppl 4):D116–D125. doi: 10.1016/j.vaccine.2011.11.090. [DOI] [PubMed] [Google Scholar]
- Li G, Duan T, Wu X, Tesh RB, Soong L, Xiao SY. Yellow fever virus infection in Syrian golden hamsters: relationship between cytokine expression and pathologic changes. Int J Clin Exp Pathol. 2008;1:169–179. [PMC free article] [PubMed] [Google Scholar]
- Mantel N, Girerd Y, Geny C, Bernard I, Pontvianne J, Lang J, Barban V. Genetic stability of a dengue vaccine based on chimeric yellow fever/dengue viruses. Vaccine. 2011;29:6629–6635. doi: 10.1016/j.vaccine.2011.06.101. [DOI] [PubMed] [Google Scholar]
- Meier KC, Gardner CL, Khoretonenko MV, Klimstra WB, Ryman KD. A mouse model for studying viscerotropic disease caused by yellow fever virus infection. PLoS Pathog. 2009;5:e1000614. doi: 10.1371/journal.ppat.1000614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messer WB, Yount B, Hacker KE, Donaldson EF, Huynh JP, de Silva AM, Baric RS. Development and characterization of a reverse genetic system for studying dengue virus serotype 3 strain variation and neutralization. PLoS neglected tropical diseases. 2012;6:e1486. doi: 10.1371/journal.pntd.0001486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monath TP. Yellow fever vaccine. Expert Rev Vaccines. 2005;4:553–574. doi: 10.1586/14760584.4.4.553. [DOI] [PubMed] [Google Scholar]
- Monath TP, Brinker KR, Chandler FW, Kemp GE, Cropp CB. Pathophysiologic correlations in a rhesus monkey model of yellow fever with special observations on the acute necrosis of B cell areas of lymphoid tissues. Am. J. Trop. Med. Hyg. 1981;30:431–443. doi: 10.4269/ajtmh.1981.30.431. [DOI] [PubMed] [Google Scholar]
- Monath TP, Fowler E, Johnson CT, Balser J, Morin MJ, Sisti M, Trent DW. An inactivated cell-culture vaccine against yellow fever. N Engl J Med. 2011;364:1326–1333. doi: 10.1056/NEJMoa1009303. [DOI] [PubMed] [Google Scholar]
- Neves PC, Matos DC, Marcovistz R, Galler R. TLR expression and NK cell activation after human yellow fever vaccination. Vaccine. 2009;27:5543–5549. doi: 10.1016/j.vaccine.2009.07.028. [DOI] [PubMed] [Google Scholar]
- Neves PC, Santos JR, Tubarao LN, Bonaldo MC, Galler R. Early IFN-gamma production after YF 17D vaccine virus immunization in mice and its association with adaptive immune responses. PLoS One. 2013;8:e81953. doi: 10.1371/journal.pone.0081953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paessler S, Walker DH. Pathogenesis of the viral hemorrhagic fevers. Annu Rev Pathol. 2013;8:411–440. doi: 10.1146/annurev-pathol-020712-164041. [DOI] [PubMed] [Google Scholar]
- Patel D, Simons H. Yellow fever vaccination: is one dose always enough? Travel Med Infect Dis. 2013;11:266–273. doi: 10.1016/j.tmaid.2013.08.007. [DOI] [PubMed] [Google Scholar]
- Pugachev KV, Guirakhoo F, Ocran SW, Mitchell F, Parsons M, Penal C, Girakhoo S, Pougatcheva SO, Arroyo J, Trent DW, Monath TP. High fidelity of yellow fever virus RNA polymerase. J. Virol. 2004;78:1032–1038. doi: 10.1128/JVI.78.2.1032-1038.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pushko P, Lukashevich I. Patent 8,691,563. USA: Medigen, Inc.; IDNA vaccines and methods for using the same U.S. 2014
- Quaresma JA, Pagliari C, Medeiros DB, Duarte MI, Vasconcelos PF. Immunity and immune response, pathology and pathologic changes: progress and challenges in the immunopathology of yellow fever. Rev. Med. Virol. 2013;23:305–318. doi: 10.1002/rmv.1752. [DOI] [PubMed] [Google Scholar]
- Rafferty E, Duclos P, Yactayo S, Schuster M. Risk of yellow fever vaccine-associated viscerotropic disease among the elderly: a systematic review. Vaccine. 2013;31:5798–5805. doi: 10.1016/j.vaccine.2013.09.030. [DOI] [PubMed] [Google Scholar]
- Rice CM, Grakoui A, Galler R, Chambers TJ. Transcription of infectious yellow fever RNA from full-length cDNA templates produced by in vitro ligation. New Biol. 1989;1:285–296. [PubMed] [Google Scholar]
- Rutkowski K, Ewan PW, Nasser SM. Administration of yellow fever vaccine in patients with egg allergy. Int. Arch. Allergy Immunol. 2013;161:274–278. doi: 10.1159/000346350. [DOI] [PubMed] [Google Scholar]
- Schafer B, Holzer GW, Joachimsthaler A, Coulibaly S, Schwendinger M, Crowe BA, Kreil TR, Barrett PN, Falkner FG. Pre-clinical efficacy and safety of experimental vaccines based on non-replicating vaccinia vectors against yellow fever. PLoS One. 2011;6:e24505. doi: 10.1371/journal.pone.0024505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger JJ, Brandriss MW, Cropp CB, Monath TP. Protection against yellow fever in monkeys by immunization with yellow fever virus nonstructural protein NS1. J. Virol. 1986;60:1153–1155. doi: 10.1128/jvi.60.3.1153-1155.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staples JE, Monath TP. Yellow fever: 100 years of discovery. Jama. 2008;300:960–962. doi: 10.1001/jama.300.8.960. [DOI] [PubMed] [Google Scholar]
- Stoyanov CT, Boscardin SB, Deroubaix S, Barba-Spaeth G, Franco D, Nussenzweig RS, Nussenzweig M, Rice CM. Immunogenicity and protective efficacy of a recombinant yellow fever vaccine against the murine malarial parasite Plasmodium yoelii. Vaccine. 2010;28:4644–4652. doi: 10.1016/j.vaccine.2010.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesh RB, Guzman H, da Rosa AP, Vasconcelos PF, Dias LB, Bunnell JE, Zhang H, Xiao SY. Experimental yellow fever virus infection in the Golden Hamster (Mesocricetus auratus). I. Virologic, biochemical, and immunologic studies. J. Infect. Dis. 2001;183:1431–1436. doi: 10.1086/320199. [DOI] [PubMed] [Google Scholar]
- Thibodeaux BA, Garbino NC, Liss NM, Piper J, Blair CD, Roehrig JT. A small animal peripheral challenge model of yellow fever using interferon-receptor deficient mice and the 17D-204 vaccine strain. Vaccine. 2012;30:3180–3187. doi: 10.1016/j.vaccine.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretyakova I, Hearn J, Wang E, Weaver S, Pushko P. DNA Vaccine Initiates Replication of Live Attenuated Chikungunya Virus In Vitro and Elicits Protective Immune Response in Mice. J. Infect. Dis. 2014 doi: 10.1093/infdis/jiu114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretyakova I, Lukashevich IS, Glass P, Wang E, Weaver S, Pushko P. Novel vaccine against Venezuelan equine encephalitis combines advantages of DNA immunization and a live attenuated vaccine. Vaccine. 2013;31:1019–1025. doi: 10.1016/j.vaccine.2012.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heijden I, Beijnen JH, Nuijen B. Long term stability of lyophilized plasmid DNA pDERMATT. Int. J. Pharm. 2013;453:648–650. doi: 10.1016/j.ijpharm.2013.06.010. [DOI] [PubMed] [Google Scholar]
- Weaver SC. Urbanization and geographic expansion of zoonotic arboviral diseases: mechanisms and potential strategies for prevention. Trends Microbiol. 2013;21:360–363. doi: 10.1016/j.tim.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodson SE, Freiberg AN, Holbrook MR. Coagulation factors, fibrinogen and plasminogen activator inhibitor-1, are differentially regulated by yellow fever virus infection of hepatocytes. Virus Res. 2013;175:155–159. doi: 10.1016/j.virusres.2013.04.013. [DOI] [PubMed] [Google Scholar]