Abstract
Aedes aegypti males transfer sperm and seminal fluid proteins (Sfps), primarily produced by male accessory glands (AGs), to females during mating. When collectively injected or transplanted into females, AG tissues and/or seminal fluid homogenates have profound effects on Aedes female physiology and behavior. To identify targets and design new strategies for vector control, it is important to understand the biology of the AGs. Thus, we examined characteristics of AG secretion and development in Ae. aegypti, using the AG-specific seminal fluid protein, AAEL010824, as a marker. We showed that AAEL010824 is first detectable by 12h post-eclosion, and increases in amount over the first 3 days of adult life. We then showed that the amount of AAEL0010824 in the AG decreases after mating, with each successive mating depleting it further; by 5 successive matings with no time for recovery, its levels are very low. AAEL010824 levels in a depleted male are replenished by 48hr post-mating. In addition to examining the level of AAEL010824 protein, we also characterized the expression of its gene. We did this by making a transgenic mosquito line that carries an Enhanced Green Fluorescence Protein (EGFP) fused to the AAEL0010824 promoter that we defined here. We showed that AAEL010824 is expressed in the anterior cells of the accessory glands, and that its RNA levels also respond to mating. In addition to further characterizing AAEL010824 expression, our results with the EGFP fusion provide a promoter for driving AG expression. By providing this information on the biology of an important male reproductive tissue and the production of one of its seminal proteins, our results lay the foundation for future work aimed at identifying novel targets for mosquito population control.
Keywords: Aedes aegypti, mating, seminal fluid, dengue vector, accessory gland, transgenic mosquitoes
1. Introduction
The mosquito, Aedes aegypti, is an important vector of viruses that cause dengue and Chikungunya infections (Gubler, 2002). Dengue affects an estimated 390 million people worldwide each year, and the majority of the world’s population is now at risk for infection (Bhatt et al., 2013). With no vaccine available and no treatment for dengue or Chikungunya, control of these diseases is focused on limiting the spread of, or transmission by, their mosquito vector (Vazquez-Prokopec, 2011). However, vector control has been difficult due to numerous factors, including expanding urbanization and the lack of effective mosquito control tools (Morrison et al., 2008). Accordingly, it is essential to develop novel tools for vector control that will ultimately reduce the burden of vector-borne infections.
Reproduction of the mosquito vector is a key process in the spread of mosquito-borne diseases, and thus a worthwhile target to consider for disease control. In numerous insect species, including Ae. aegypti, Sfps are transferred along with sperm from males to females during copulation. Sfp receipt in the mated female effects numerous physiological and behavioral changes (reviewed in Avila et al., 2011; Gillott, 2003). These changes include stimulating oviposition (Judson, 1967), increasing fertility (Adlakha and Pillai, 1975), changing female host seeking and feeding behavior (Lee and Klowden, 1999), and increasing female mating refractoriness (Fuchs et al., 1968; Helinski and Harrington, 2011).
In insects, Sfps are typically produced in the male accessory glands (AGs), yet the structure and secretory activity of these glands have received little attention. Most AGs consist of a secretory epithelium surrounded by a muscle sheath. Secretory epithelia of the AG, like most insect epithelia, are monolayers, in which each secretory cell produces, stores and exports secreted products (Happ, 1984; Gligorov et al., 2013). Ae. aegypti AGs are divided into anterior and posterior zones that differ in cell type, density and in the nature of the secretory material that they contain (Dapples, 1974). Transplantation of cells from the anterior compartment of the AGs into unmated females inhibits multiple inseminations and stimulates oviposition in females (Ramalingam, 1976), whereas the cells of the posterior region synthesize and secrete a mucus that serves to bind the secretory granules produced in the anterior region (Ramalingam, 1983).
Development of the male Ae. aegypti AGs begins during the larval stage, but is not complete until 24 hrs post adult eclosion, when the lumens of the ejaculatory duct and AGs fill with secreted products (Clements, 1999). Several potential secretion mechanisms have been suggested to operate in the AGs of various insects. These mechanisms include merocrine (secretion via exocytosis), holocrine (secretion products are released by rupture of the cell membrane) and apocrine secretion (where the apical portion of the cell is released along with secretion products). Merocrine and perhaps holocrine secretion have been suggested for Drosophila AGs (Chen, 1984; Perotti, 1971) and the flour moth, Anagasta (Riemann and Thorson, 1976). Merocrine secretion has also been described for the AGs of the butterfly Calpodes (Lai-Fook, 1982) and the darkling beetle Tenebrio, and apocrine secretion has been reported in the AGs of the Colorado potato beetle (Leptinotarsa decemlineata) (reviewed in Happ, 1984).
In Ae. aegypti, a variety of secretory mechanisms have been suggested for the AGs. Jones (1970) observed numerous male AG-derived cellular organelles and fragments, including mitochondria and membranes, inside the female bursa, leading him to suggest a holocrine secretion. Subsequent electron microscopy studies by Dapples (1974) suggested apocrine secretion by both anterior and posterior AG cells. Further studies attributed apocrine secretion to the AG anterior cells, and holocrine secretion in the AG posterior cells in both Ae. aegypti and Ae. triseriatus males (Ramalingam, 1983; Ramalingam, 1978; Sirot et al., 2011). Thus, there remains debate about the secretory mechanism used by Ae. aegypti AG cells.
Another debate concerns the effect of mating on synthesis of male AG secreted proteins. Similarly to the majority of male insects, female Aedes are primarily monogamous and male Aedes are polygynous (Clements, 1999); during each copulation, a proportion of the AGs’ secreted material is transferred at ejaculation and, subsequently, must be replenished. Dapples (1974) and Foster (1975) reported that males’ depleted AGs regenerated and refilled over few days. However, Ramalingam (1983; 1976) and Hausermann and Nijhout (1975) argued that recovery of secretory capacity did not occur after depletion.
Elucidating male AGs function—the role of individual cell-types and their patterns of secretion—is a first step in dissecting the overall contribution of this organ to mosquito reproductive biology and might potentially identify targets for the purposes of reproductive control. Therefore, we conducted a comprehensive investigation of the male AGs. Previously, we identified 93 seminal proteins from Ae. aegypti (Sirot et al., 2011). We used one of these, the AG-specific protein AAEL010824 as a marker for the present studies. We characterized the expression of AAEL010824, examining both its transcript and protein levels during development, as well as before and after mating. We determined how quickly males that mated in rapid succession became depleted of AAEL010824, and how much time was required until the protein was replenished in these males. Finally, we used the upstream region of the AAEL010824 gene to drive the reporter Enhanced Green Fluorescence Protein (EGFP) in transgenic mosquitoes. We showed that this regulatory region drives expression in anterior cells of AGs, specifically. We then examine protein transfer from the anterior cells of the AGs.
2. METHODS
2.1 Mosquitoes
Ae. aegypti (Thai strain) were originally collected in Bangkok, Thailand (15°7193′N, 101°752′E) in 2011, and supplemented with field material in 2012. This colony was held in an environmental chamber at 25.9 ± 0.6 °C with 71.9 ± 9.5% relative humidity (RH), with a photoperiod of 10-hour light:10-hour dark with a 2 h simulated dusk and dawn period. Mosquitoes were reared to obtain uniform medium body size adults. Larvae were fed on Cichlid gold pellets (Hikari, Himeji, Japan) using four pellets per tray of 200 larvae. Adults had constant access to 10% sucrose. Individual pupae were transferred to vials to ensure virginity and sorted by sex upon adult eclosion. Two hundred individuals were transferred into 12L plastic mating cages by sex and held until experiments commenced.
2.2 Mosquito matings
Matings were conducted as described previously (Helinski and Harrington, 2011). Five-day-old medium body size Ae. aegypti males and females were used in our experiments. One virgin male was released into a 5 L observation cage containing approximately 8 virgin females. Male and female couples were observed carefully and copulating pairs were removed using a mouth aspirator after a minimum mating duration of 10 sec. For our multiple mating experiments, males were mated in succession to a different numbers of females (from 1 to 5) by transferring them to subsequent cages with virgin females following the process described above. Males from each mating frequency group (those males mated to 1,2,3,4 or 5) plus virgin males were collected each day for three days. These males were frozen and stored at −80°C for Western blot analysis or RNA extraction.
2.3 Rapid Amplification of cDNA Ends (RACE)
RACE was employed to determine the 5′ and 3′ UTR sequences and to validate the open reading frame (ORF) of AAEL010824. Total RNA from whole males was isolated using TRIzol solution (Invitrogen, Carlsbad, California), followed by chloroform/isopropanol extraction and ethanol precipitation according to standard protocols. Prior to cDNA synthesis, RNA was treated with RNase-free DNAse (Clontech, Madison, Wisconsin). RACE was performed using the GeneRacer system (Invitrogen. Carlsbad CA, USA) with SuperScript III (Invitrogen. Carlsbad CA, USA), following the manufacturer’s instructions. Oligo dT primer (5′ GCT GTC AAC GAT ACG CTA CGT AAC GGC ATG ACA TGT (T)24 3′) was used to reverse transcribe the 3′ end of the mRNA. Primer 5′cDNAsynthGSP: 5′ TCG GAG CCC CTT ATG TAG ACG TA 3′ and Random (N6) primers were used to reverse transcribe the 5′ end of the mRNA. The samples containing the cDNA for analysis of the 3′ and 5′ ends were diluted 10 fold, and 1μl was used as PCR template. Primer GeneRacer 3′ (5′ GCT GTC AAC GAT ACG CTA CGT AAC G 3′) and 3′ RACE-GSPF1 (5′ TGG CGA CAT GTG GGT CAT TAC CAG AA 3′) were used for 3′ ends PCR. Primer GeneRacer 5′ (5′ CGACTG GAG CAC GAG GAC ACT GA 3′) and 5′ RACE-GPSR3 (5′ ACG CGA CTT TCG CAC GGA CA 3′) were used for 5′ ends PCR. PCR products of 3′ and 5′ ends were cloned into pCR4-TOPO vector (Invitrogen, Carlsbad CA, USA) and transformed into TOP10 Escherichia coli competent cells (Invitrogen, Carlsbad CA, USA). Plasmids were sequenced by the Cornell University Life Science Core Facility. Sequences were analyzed to identify the 3′ and 5′ UTR of the AAEL010824 mRNA.
2.4 Gene analysis
Gene analyses were carried out using the Genious software package (Pro 5.6.5, Biomatters, Auckland, New Zealand). DNA sequence alignments to the Ae. aegypti genome were performed with BLAST (https://www.vectorbase.org/blast), while multiple sequence alignment and phylogenetic analysis were done using ClustalW (http://www.ebi.ac.uk/Tools/msa/clustalw2/). SignalP 4.1. (http://www.cbs.dtu.dk/services/SignalP/) was used to predict the putative signal peptide. Sequences from predicted Ae. albopictus proteins were obtained from Boes et al. (2014). Sequences from predicted Ae. aegypti proteins were obtained from Sirot et al. (2011) and Vectorbase.org.
2.5 AAEL010824 promoter- EGFP construct
In order to study AAEL010824 expression and to characterize secretion by the AG cells, we created a construct using the promoter of AAEL010824 to drive expression of EGFP. The NCBI Map Viewer (http://www.ncbi.nlm.nih.gov/mapview), Promoter 2.0 Prediction Server (CBS, Technical University of Denmark DTU, http://www.cbs.dtu.dk/services/Promoter/) and our 5′ RACE data were used to estimate a predicted regulatory region of the AAEL010824 gene. We BLASTed the sequence against the Ae. aegypti genome in Vectorbase (www.vectorbase.org) and amplified the sequence extending from the transcription start site determined in the 5′ RACE to approximately ~5 kb upstream using iProof High Fidelity DNA Polymerase (Biorad, Hercules, CA, USA) following the manufacturer’s protocol. Primers used to amplify this region were 10824(5K)-FseI-F: TAA TAG GCC GGC CCT GGG CTC GTT AAT CTC GAA and 10824(5K)-FseI-R: TAA TAG GCC GGC CGA ATA ACG GAT GTC ACA AGA ACT GGC TG. The final PCR product was cloned into pCR-XLTOPO (Invitrogen Carlsbad, CA, USA) and transferred to pBAC[3xP3-EGFP-DsRedaf] using the FseI sites of the final pBAC[3xP3-EGFP-DsRedaf] plasmid (Fig. 3A). To generate pBAC[3xP3-EGFP-DsRedaf], an EGFP-SV40 fragment was amplified from pBac[3xP3-EGFPafm] plasmid and cloned into the FseI-AscI sites of pBAC[3xP3-DsRedaf]. The EGFP fragment amplified does not include a secretion signal. The final construct was sent to the Insect Transformation Facility at the University of Maryland for injection into Ae. aegypti strain to create a transgenic line. The line was maintained by continuously crossing EGFP -positive females (verified by PCR) with EGFP-positive males.
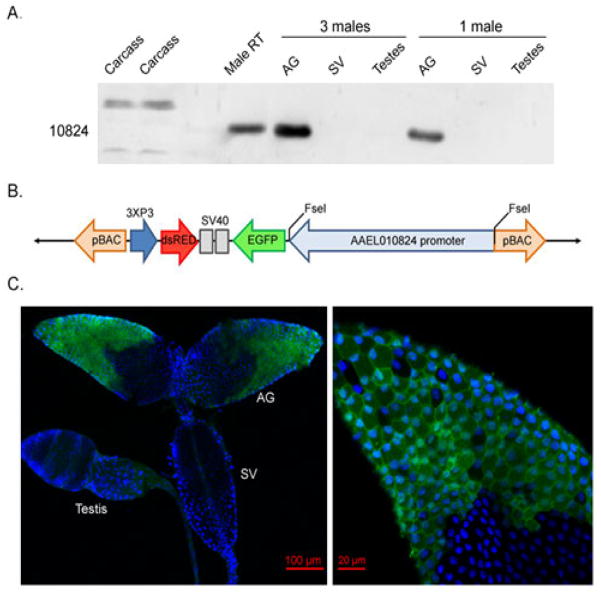
Fig. 3.
Localization of AAEL010824 within the AGs. (A) AAEL010824 distribution in the male reproductive tract. Tissues tested included the carcass, AG, SV (seminal vesicle) and testes. (B) Overview of the final promoter construct: pBac[3xP3- AAEL010824EGFP-DSRedaf] (Not to scale). (C) Expression of EGFP under the control of the AAEL010824 promoter is specific to the anterior cells of the AGs. Nuclei were stained blue using DAPI.
2.6 RNA isolation and Quantitative PCR analysis
To quantify AAEL010824 transcript levels at all developmental stages, and before and after mating, total RNA from whole males was isolated using TRIzol solution (Invitrogen, Carlsbad, California), followed by chloroform/isopropanol extraction and ethanol precipitation according to standard protocols. Prior to cDNA synthesis, RNA was treated with RNase-free DNAse (Clontech, Madison, Wisconsin). Reverse transcription was conducted using 1ug of total RNA following the manufacturer’s instructions (Clontech, Mountain View, CA). Relative transcript levels of AAEL010824 were measured using quantitative PCR conducted with a CFV96 Real-Time System (Bio-Rad, USA). Two AAEL010824 gene specific primers 5′ GAT ATT GGG TCT TGT GTT AAG TGC3′ and 5′ AGT TGC CGG TCG CTG TTC G3′ were used to amplify an 82 bp amplicon. Both S17 and Actin (Cook et al., 2006) were used as controls. Primers 5′ ACG ACA GCA GCG AAA CTT GAA TCA3′ and 5′ ATC TTA TTG CGC AGG GGC TTC G3′ were employed to amplify 78 nucleotides of the S17 gene. Primers 5′ ATC GTA CGA ACT TCC CGA TG3′ and 5′ GAA CGA TGG CTG GAA GAG AG3′ amplified 81 nucleotides of the Actin gene. Amplifications were carried out in a total volume of 15uL containing 7.5uL of iQ SYBR Green Supermix (Biorad, USA), 1uL cDNA and 0.5uL of each primer. Cycle differences between AAEL010824 and either S17 or actin genes (ΔCT) were compared to generate the relative expression of AAEL010824 at different time points and life stages.
2.7 Western blotting
Western blotting was used to examine levels of AAEL010824 and EGFP proteins. Western blotting was carried out as in Ravi Ram and Wolfner (2009). Briefly, male reproductive tracts were dissected in 1X PBS under a dissecting microscope. Tissue was placed in 10μl of 1X PBS in an Eppendorf tube, and ground briefly with a pestle. After adding 10μl of 2X SDS sample buffer, samples were boiled for 5 min and centrifuged. The supernatant was loaded into a SDS 5–15% gradient polyacrylamide gel. After electrophoresis, the proteins were transferred onto Immobilon PVDF membrane (Millipore, Billerica, MA). Membranes were blocked in 5% non-fat dry milk blocking solution for 1h, followed by probing using rabbit anti-AAEL010824 at a concentration of 1:1000, mouse anti-alpha-Tubulin (Sigma T5168, clone B-5-1-2) at 1:10,000 or rabbit anti-GFP (Life Technologies, Grand Island, NY) at 1:1000, overnight at 4°C. Incubation with secondary goat anti-rabbit and goat anti-mouse was performed at 1:2000 (Jackson ImmunoResearch, West Grove, PA) for 2 h at room temperature. Blots were treated with Pierce ECL 2 Substrate (Thermo Scientific Pierce, Logan, UT) and imaged using a Typhoon 8600 imager (GE Healthcare, Piscataway, NJ). Rabbit anti-AAEL010824 was raised against the synthetic peptide 173YVDDEKHDQRNAYN186 and affinity-purified (Genscript, NJ, USA). Band intensity was measured using Image studio lite software (Li-COR corporate, Lincoln, Nebraska, USA). AAEL010824 signals normalized to tubulin loading control were compared to generate the relative AAEL010824 protein content at different mating groups during different time points.
2.8 Immunofluorescence and microscopy
We used confocal microscopy to determine the expression pattern driven by the regulatory region of AAEL010824. Specifically, we examined EGFP localization in transgenic mosquitoes that expressed the fluorescent protein under the control of the AAEL010824 upstream regulatory region. Accessory glands were dissected in 1X PBS and fixed with 4% paraformaldehyde (Sigma, MO, USA) at room temperature for 30 min. Fixed tissue was washed, permeabilized in 0.2% Triton X-100 (Sigma, MO, USA) for 10 min, blocked for 30 min with bovine serum albumin (Sigma, MO, USA) and incubated with anti-EGFP (1:1000) at 4°C overnight. Slides were washed and then incubated with secondary goat-anti-rabbit-Alexa 488 (Jackson Immunoresearch, PA, USA) for 1 h at room temperature (1:200). Accessory glands were then incubated with DAPI in PBS at 1ug/mL for 20 min at room temperature to stain DNA. Prolong gold antifade reagent (Invitrogen, Carlsbad, California) was used as mounting medium. For confocal microscopy, sequential excitation was performed at 488 nm (for fluorescein) and 405 nm (for DAPI) with a Zeiss 710 confocal microscope. The images were processed using Zen software (Zen 2011).
3. Results and Discussion
3.1 Characterization of the AAEL010824 gene
Our previous work demonstrated that AAEL010824 is transferred to females during mating (Sirot et al., 2011). To examine the structure of the RNA encoding this protein, we used Rapid Amplification of cDNA ends (RACE). The AAEL010824 transcript contains a 22bp 5′-untranslated region (UTR) and a 624b coding sequence that is followed by either of two 3′-UTR variants. The shorter 3′-UTR variant is 48b in length, while the other variant is 726b. AAEL010824 is predicted to encode a protein of 207 amino acids. A paralog of AAEL010824, AaegSfp8, was previously described in Ae. aegypti (Sirot et al., 2011). AAEL010824 and AaegSfp8 are located on the same contig, within 34 kb of each other. These two sequences have 70% identity (E value = 2e−74) due primarily to variability in the N-terminal region of the predicted proteins. Potential signal peptide cleavage sites were detected in both amino acid sequences, indicating that both proteins might be secreted (Fig. 1A). Given the high similarity between these two proteins, we suggest that the genes could be the result of a tandem duplication of ancestral gene, followed by divergence. Such genesis of seminal protein-encoding genes has been documented previously in numerous cases in Drosophila (e.g. Clark et al., 2007; Wagstaff and Begun, 2007; Findlay et al., 2008; Sirot et al., 2014)
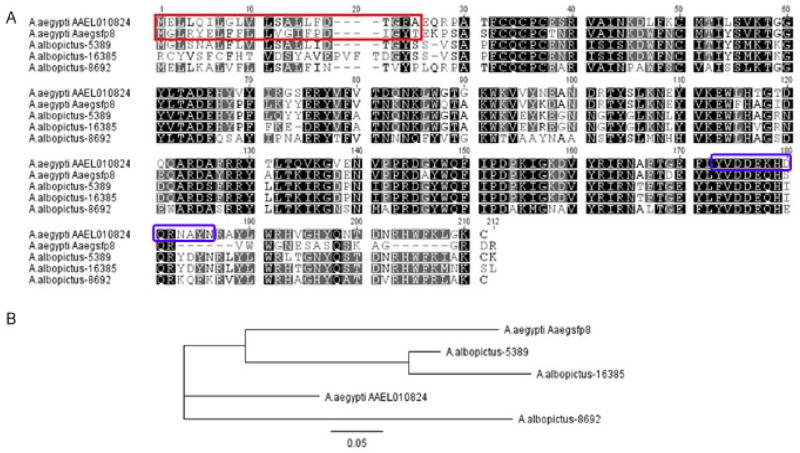
Fig. 1.
Characterization of AAEL010824. (A) Multiple amino acid sequence alignment of AAEL010824 and AaegSfp8 from Ae. aegypti and 5389, 16385 and 8692 from Ae. albopictus. Identical amino acid residues in all sequences are shaded in black. Similar amino acids found in at least three of the sequences are shaded in grey. Predicted signal peptides in AAEL010824 and AaegSfp8 from Ae. aegypti and 5389 from Ae. albopictus are boxed in red. The peptide used for anti-AAEL010824 antibody production is boxed in blue (B) Phylogenetic analysis derived by neighbor-joining analysis using Ae. aegypti AAEL010824 and Aaegsfp8 and Ae. albopictus 5389, 16385 and 8692 sequences. The bar represents a sequence divergence of 5%.
A BLAST database search revealed that the amino acid sequences of AAEL010824 or AaegSfp8 are not similar to known proteins in other insect species, including proteins in Anopheles and Culex mosquitoes. However, there are three transferred Ae. albopictus Sfps that have 68% identity with AAEL010824 over the entire length of the protein (Boes et al., 2014). This finding is interesting as Leahy (1965) showed that implantation of Ae. albopictus male AGs into unmated Ae. aegypti females increased oviposition. In addition, Tripet et al. (2011) showed that injection of Ae. albopictus Sfp homogenates into unmated Ae. aegypti females induced female mating refractory behavior, suggesting that Ae. albopictus males have proteins that can trigger some post-mating responses in the Ae. aegypti females. To investigate the phylogenetic relationship of these proteins, we constructed a phylogenetic tree based on the amino acid sequences of the three Ae. albopictus genes, AaegSfp8 and AAEL010824. Phylogenetic analysis using the Neighbor Joining method (Saitou and Nei, 1987) indicated that these sequences may share a common ancestor, where AaegSfp8 and Ae. albopictus 16385 and 5389 are positioned in the same sub-clade that is located within a main clade along with AAEL010824 and Ae. albopictus 8692 (Fig 1B). These phylogenetic data provide support for a gene duplication event in which all the family members are proteins transferred from male to female during copulation; the divergence of these two groups could reflect differences in their post-copulatory functions after transfer.
3.2 AAEL010824 expression in pupae and adult males
Aedes mosquitoes’ AGs begin development during the larval stage. They mature rapidly during the late pre-adult stages, reaching full secretory activity in young adults (Clements, 1999). Previous studies characterized the physical changes of Ae. aegypti AGs that follow accumulation of secretory material (Dapples, 1974; Foster, 1975). In those studies, investigators noted an increase in AGs diameter within 1h after eclosion (interpreted as an indicator of seminal fluid accumulation in the AGs increasing their volume). This increase ceased after approximately 2 days. However, a direct relationship between AGs diameter, seminal fluid protein content and AGs activity was never established in those studies.
To characterize the expression of genes in the male AGs, we first examined transcript levels of AAEL010824 across development, using quantitative PCR. Low mRNA levels were detected in the pupal stage. AAEL010824 mRNA levels increased after eclosion for at least 3 days (Fig. 2A). We then examined AAEL010824 protein levels, which we expected might be more directly related to AGs diameter. Using a purified AAEL010824 antibody raised against a synthetic peptide, we did not detect AAEL010824 in western blots of proteins from pupae or unmated male adults at 3h post-eclosion (Fig. 2B). However, AAEL010824 was detected in unmated male samples from 12h post-eclosion to 3 days post-eclosion, with increasing levels seen for up to 2–3 days post-eclosion. These data support the hypothesis (Ramalingam, 1983) that increased AGs diameter during male adult maturation reflects accumulation of secreted Sfps. AAEL010824 was not detected in 3 day-old unmated females. Consistent with our phylogenetic data (above), we detected a signal with anti-AAEL010824 in protein samples from four Ae. albopictus AGs, suggesting the presence of an AAEL010824 ortholog in this species.
Fig. 2.
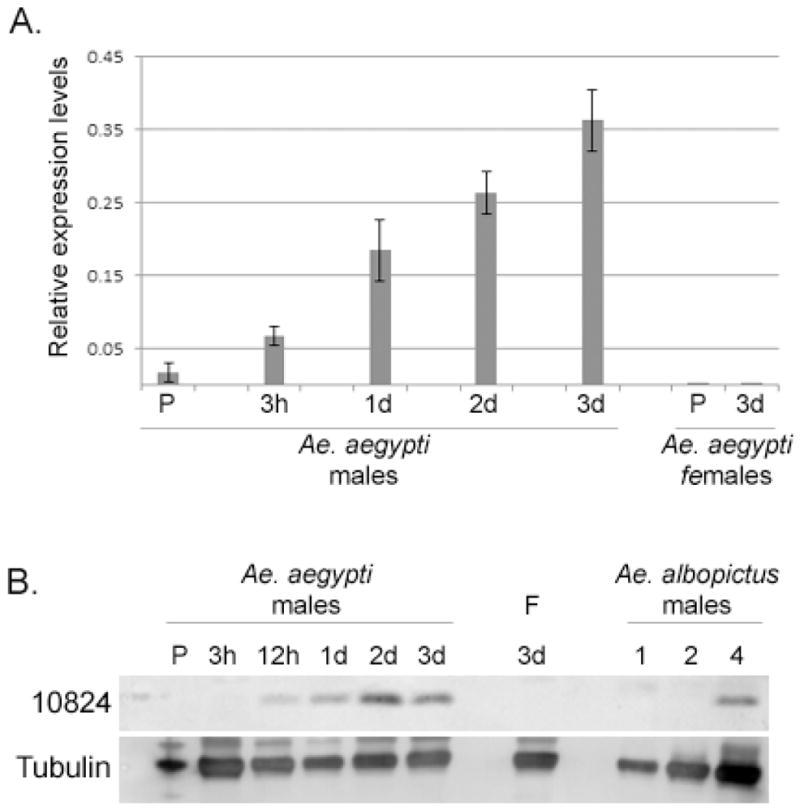
AAEL010824 expression. (A) Expression patterns of AAEL010824 gene using quantitative PCR. Each sample was obtained from 3, late pupae (P), 3h, 1d, 2d or 3d old males and 3d old female. Each sample represents three different biological replicates. Relative expression values were calculated by normalizing the expression with RPS17. (B) Protein levels of AAEL010824 of an entire pupae or single reproductive tracts from virgin adults (males as indicated; F = females) were determined by Western blot. In addition, samples from 1, 2 and 4 reproductive tracts of Ae. albopictus males were tested.
3.3 Expression driven by the AAEL010824 regulatory region is in the anterior cells of male AGs
AAEL010824 was previously described as a protein found in the male Ae. aegypti seminal fluid, that was encoded by a male-specific mRNA (Sirot et al., 2011; Sirot et al., 2008). To confirm its AGs origin, we used anti-AAEL010824 to probe Western blots of proteins from specific tissue(s) of the male reproductive tract. AAEL010824 was detected in the AGs and not in the testes or the seminal vesicle (Fig 3A).
Male Ae. aegypti AGs are composed of two distinct types of secretory cells, forming the anterior and posterior regions of the glands (Dapples, 1974). To determine if AAEL010824 is expressed in the anterior cells of the AGs, the posterior cells, or both, we generated a transgenic line in which the expression of the fluorescent reporter, Enhanced Green Fluorescent Protein (EGFP), is driven by the AAEL010824 regulatory region. Sequences comprising 5kb of DNA upstream of the ATG that initiates translation of AAEL010824 and contain the apparent transcriptional start site, as identified by 5′ RACE, were cloned into pBAC[3xP3-EGFP-DsRedaf] (Fig 3B). Confocal microscopy of AGs from transgenic males containing this construct revealed differential distribution of the EGFP signal: only cells in the anterior portion of the AGs showed a fluorescent signal (Fig 3C); the signal was seen in the cytoplasm of those cells.
Expression of EGFP under the control of the AAEL010824 regulatory region in the anterior region of the AGs is interesting, as secretions from these cells have been associated with inhibition of multiple inseminations, stimulation of oviposition (Ramalingam, 1983) and changes in host seeking behavior (Naccarati et al., 2012) in females. The AAEL010824 regulatory region drives expression only in a portion of AG cells suggesting that the two cell-types might differ in the proteins they secrete or that they might have an essential, non-secretory role in reproduction. There is evidence that the two major cell-types, main and secondary cells, that constitute the D. melanogaster AGs have unique functions, with the main cells products, such as ovulin and sex peptide, playing an important role in female post-mating responses (Kalb et al., 1993) and with the secondary cells (those at the distal end of the gland) potentially responsible for post-translational modification of some secreted AG proteins (Gligorov et al., 2013). To fully understand the role of the anterior and posterior cells of the Ae. aegypti AGs, it will be important to further identify and characterize the products of these cells to determine their individual roles in reproduction.
3.4 Depletion of AAEL010824 in males and transfer to females
When the AGs mature, their lumens become filled with proteins secreted from the anterior and posterior cells. Since Aedes males are polygynous, at each copulation, some of AGs luminal content is transferred to females during ejaculation. After transfer, secretions must then be replenished by the secretory cells of the AGs. In Drosophila, main cells of the AGs increase gene expression after mating, presumably to replenish secreted proteins transferred at mating. However, gene expression in the secondary cells of the AGs remains stable, regardless of male age or sexual activity (Bertram et al., 1992; DiBenedetto et al., 1990). We used AAEL010824 as a marker to determine: 1) how levels of the gene change after mating, 2) how many sequential matings are required to deplete AAEL010824, and 3) how long it takes for the AGs to replenish their complement of AAEL010824.
To answer the first question, we used qPCR to measure AAEL010824 mRNA levels from virgin males and males mated successively to 1, 2 or 3 females at 0, 24 and 48h after copulation. Levels of AAEL010824 mRNA in virgin males remained stable. However, immediately after copulation, AAEL010824 mRNA levels of mated males (mated 1, 2 or 3 times) decreased around 2-fold relative to the levels in virgins. AAEL010824 mRNA levels of mated males then generally increased with time, reaching similar levels to those of virgin males at 48 hours post-mating, indicating high levels of mating-induced gene expression (Fig 4A). For reasons that are unclear, males that are mated twice have higher levels of AAEL010824 mRNA after 24 and 48h. However, this may be an effect of natural variation in transcript levels or possibly result from the sensitivity of qPCR in detecting differences in sample preparation.
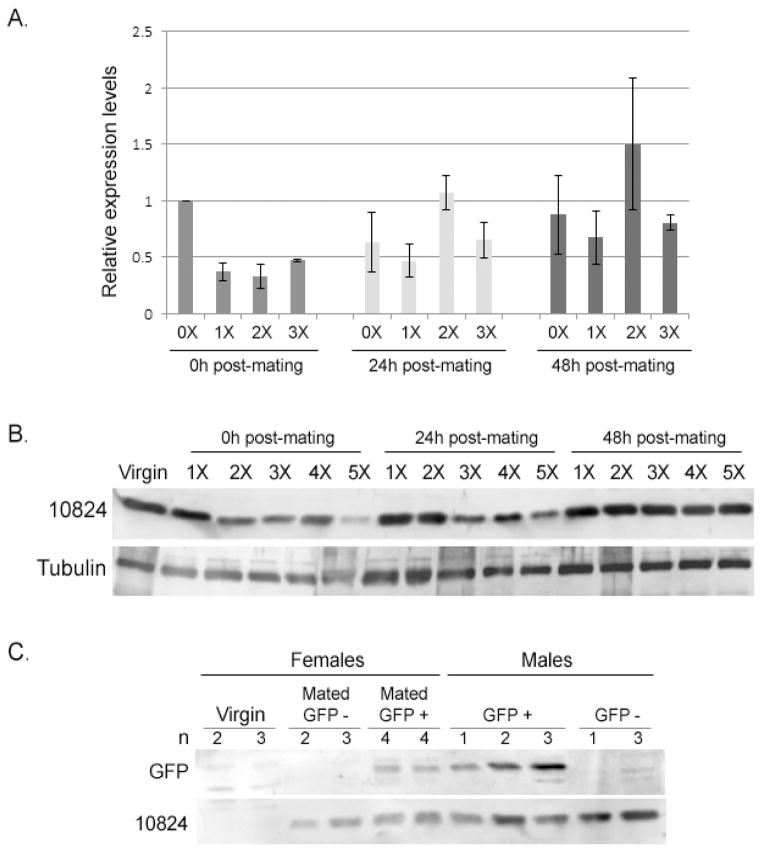
Fig. 4.
Transfer, depletion and replenishment of AAEL010824. (A) mRNA levels of AAEL010824 using quantitative PCR. Samples were obtained from virgin males and males mated to 1, 2 and 3 females successively (1X, 2X, 3X). Samples were collected at 0, 24 and 48h after mating. Each sample represents four different biological replicates. Relative expression values were calculated by normalizing the expression with RPS17. Columns followed by the same letter are not significantly different from each other within age group (Univariate analysis: F=7.06, df =3, P = 0.001). Values across time classes are significantly different (ANOVA, df=14, F=4.6, P=0.03; LSD separation of means, 0hrs is different from 24hrs (P=0.02), 0 hrs is different from 48 hrs (P=0.03), no significant difference between 24 and 48 hrs (P=0.91)) (B) Protein levels of AAEL010824 were analyzed by Western blotting. Protein samples were extracted from single reproductive tracts of virgin males and males mated to 1,2,3,4 and 5 females. Samples were collected 0, 24 and 48h post-mating. The figure shows a representative blot from one of three replicates of this experiment. Anti-tubulin was use as loading control. (C) EGFP transfer from male to females was analyzed by Western blotting. Proteins were extracted from reproductive tracts from males expressing EGFP under the AAEL010824 promoter (GFP+), wild-type males (GFP−), from virgin females and females mated to EGFP males and wild-type males. Anti-AAEL010824 was used as control for Sfp transfer.
We used Western blotting and Image Studi Lite software to examine AAEL010824 levels after 1, 2, 3, 4 or 5 successive matings. AGs of males were depleted of AAEL010824 by approximately 44% after 2 matings and 72% after mating with 5 females within a 5h period. This result is consistent with previous studies that showed that after 4–5 consecutive matings, males were depleted of factors that affected fecundity and longevity of subsequent female mates (Helinski and Harrington, 2011). We next examined the replenishment of AAEL010824 in 5x-mated males that were allowed to recover (in the absence of females) for 0h, 24h and 48h. We observed that virgin-levels of AAEL010824 in AGs were replenished by 48h of recovery (Fig. 4B). These findings—consistent with previous work showing AG size in sequentially mated mosquitoes was reduced by half following multiple matings, returning to their normal size after a rest period of 3 days (Dapples, 1974; Foster, 1975)—further refine our knowledge of the reproductive ability of Aedes males and potentially give insight into determining the number of males required, and the timing of their release, in genetic mosquito control programs.
We next tested for transfer of EGFP to females during copulation. Previous studies of the anterior AG cells suggested that they used apocrine secretion mechanisms, in which the cytoplasm pinches off and falls freely into the lumen of the gland (Dapples, 1974; Ramalingam, 1983). Since the EGFP in our construct lacks a predicted signal sequence, we used our AAEL010824 promoter-EGFP line to test whether this mechanism of secretion might be operating in the anterior cells. Virgin females were mated with AAEL010824 promoter-EGFP males. The females’ reproductive tracts were dissected immediately post mating, and probed for EGFP on Western blots, using anti-GFP. As a control, anti-AAEL010824 was used to detect transfer of protein from male to female (Fig 4C). We detected EGFP in reproductive tract extracts from the mated females (Fig. 4C). Our observation of EGFP in the females’ reproductive tracts is consistent with apocrine secretion from the anterior cells. However, we cannot rule out that the EGFP derived from its release by dying or lysing cells, or that whole cells (containing EGFP) were transferred to the females, in a mechanism recently reported for Drosophila AG secondary cells (Leiblich et al., 2012).
4. Conclusions
Previous studies indicated that male AGs play an important role in the reproductive success of mosquitoes (reviewed in Sirot et al., 2011). AG secretions, including Sfps, are transferred to females during mating. Sfp receipt by the female results in significant physiological and behavioral changes which include a decrease in mating probability (Fuchs et al., 1968; Helinski and Harrington, 2011) and an increase in blood feeding behavior (Lee and Klowden, 1999). Although the nature and sequences of these proteins have been investigated in other species, the structure and regulation of the proteins in the male mosquito AGs have received little attention to date. Our results provide new information on the function of these important reproductive organs in male mosquitoes.
Here, we characterized the Ae. aegypti male AGs, using AAEL010824, or EGFP fused to its gene-regulatory region, as a marker. We previously identified this Sfp as a male-derived protein that is transferred to females during mating (Sirot et al., 2011). We show here that AAEL010824 is found exclusively in the AGs of the male reproductive tract. Our AAEL010824 expression data shows that this AGs protein expressed after eclosion, with its levels increasing for at least 3d. In addition, we showed that a male’s complement of AAEL010824 is largely depleted after mating with 5 consecutive females in succession, and that 48h of recovery suffice for it to be fully replenished. If AAEL010824 is representative of AG proteins, then these results demonstrate the limits of depletion and replenishment of AG secretions in males and will ultimately guide functional studies of AG proteins with RNAi. Finally, we generated a transgenic line in which EGFP is driven by the AAEL010824 regulatory region. Using this line, we showed that the AAEL010824 regulatory region drives expression exclusively in the anterior cells of the AGs. Surprisingly, we observed transfer of EGFP (which lacks a signal sequence) from male to females during copulation, suggesting an apocrine mechanism for secretion. However, other processes can be involved in protein transfer during mating. Because of the tissue-specificity of our transgenic line, we suggest that the AAEL010824 regulatory region can be used to express other proteins into the male AGs. Our results on the function and secretion pattern of the male AGs in mosquitoes, coupled with our identification of an AG-specific regulatory region, will enable future studies of mosquito mating biology and thus will significantly advance the study of reproductive targets for vector control.
Highlights.
Ae. aegypti seminal protein AAEL010824 accumulates in virgin males post-eclosion.
Males’ AAEL010824 levels drop post-mating, and are depleted by 5 successive matings.
48-hr of recovery restores mating-depleted levels of AAEL010824 to virgin levels.
5kb upstream of AAEL010824 drives transgenic EGFP in anterior accessory glands.
Acknowledgments
We thank Sylvie Pitcher, Amber Krauchunas, Norene Buehner, and members of the Harrington and Wolfner lab for comments and technical support. We thank Susan Rottschaefer and Brian Lazzaro for use of the CFV96 Real-Time System, and Robert Harrell from the Insect Transformation Facility at the University of Maryland. This study was supported by NIH/NIAID grant R01AI095491 (to LCH and MFW) and NYC 2011-12-219 (to LCH and MFW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adlakha V, Pillai MK. Involvement of male accessory gland substance in the fertility of mosquitoes. Journal of insect physiology. 1975;21:1453–1455. doi: 10.1016/0022-1910(75)90207-3. [DOI] [PubMed] [Google Scholar]
- Avila FW, Sirot LK, LaFlamme BA, Rubinstein CD, Wolfner MF. Insect seminal fluid proteins: identification and function. Annual review of entomology. 2011;56:21–40. doi: 10.1146/annurev-ento-120709-144823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram MJ, Akerkar GA, Ard RL, Gonzalez C, Wolfner MF. Cell type-specific gene expression in the Drosophila melanogaster male accessory gland. Mechanisms of development. 1992;38:33–40. doi: 10.1016/0925-4773(92)90036-j. [DOI] [PubMed] [Google Scholar]
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GR, Simmons CP, Scott TW, Farrar JJ, Hay SI. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boes KE, Ribeiro JM, Wong A, Harrington LC, Wolfner MF, Sirot LK. Identification and Characterization of Seminal Fluid Proteins in the Asian Tiger Mosquito, Aedes albopictus. PLoS neglected tropical diseases. 2014;8:e2946. doi: 10.1371/journal.pntd.0002946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PS. The Functional Morphology and Biochemistry of Insect Male Accessory Glands and their Secretions. Annual review of entomology. 1984;29:233–255. [Google Scholar]
- Clark NL, Findlay GD, Yi X, MacCoss MJ, Swanson WJ. Duplication and selection on abalone sperm lysin in an allopatric population. Molecular biology and evolution. 2007;24:2081–2090. doi: 10.1093/molbev/msm137. [DOI] [PubMed] [Google Scholar]
- Clements AN. The Biology of Mosquitoes: Sensory reception and behaviour. Chapman & Hall; Wallingford, United Kingdom: 1999. [Google Scholar]
- Cook PE, Hugo LE, Iturbe-Ormaetxe I, Williams CR, Chenoweth SF, Ritchie SA, Ryan PA, Kay BH, Blows MW, O’Neill SL. The use of transcriptional profiles to predict adult mosquito age under field conditions. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:18060–18065. doi: 10.1073/pnas.0604875103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dapples CC, Foester WA, Lea AO. Ultrastructure of the accessory gland of the male mosquito, AEDES AEGYPTI (L.) (Diptera: Culicidae) int J insect morphol & embryol. 1974:279–291. [Google Scholar]
- DiBenedetto AJ, Harada HA, Wolfner MF. Structure, cell-specific expression, and mating-induced regulation of a Drosophila melanogaster male accessory gland gene. Developmental biology. 1990;139:134–148. doi: 10.1016/0012-1606(90)90284-p. [DOI] [PubMed] [Google Scholar]
- Findlay GD, Yi X, Maccoss MJ, Swanson WJ. Proteomics reveals novel Drosophila seminal fluid proteins transferred at mating. PLoS biology. 2008;6:e178. doi: 10.1371/journal.pbio.0060178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster WAL, AO Renewable fecundity of male Aedes aegypti following replenishment of seminal vesicles and accessory glands. Journal of insect physiology. 1975;21:1085–1090. doi: 10.1016/0022-1910(75)90120-1. [DOI] [PubMed] [Google Scholar]
- Fuchs MS, Craig GB, Jr, Hiss EA. The biochemical basis of female monogamy in mosquitoes. I. Extraction of the active principle from Aedes aegypti. Life sciences. 1968;7:835–839. doi: 10.1016/0024-3205(68)90114-8. [DOI] [PubMed] [Google Scholar]
- Gillott C. Male accessory gland secretions: modulators of female reproductive physiology and behavior. Annual review of entomology. 2003;48:163–184. doi: 10.1146/annurev.ento.48.091801.112657. [DOI] [PubMed] [Google Scholar]
- Gligorov D, Sitnik JL, Maeda RK, Wolfner MF, Karch F. A novel function for the Hox gene Abd-B in the male accessory gland regulates the long-term female post-mating response in Drosophila. PLoS genetics. 2013;9:e1003395. doi: 10.1371/journal.pgen.1003395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler DJ. The global emergence/resurgence of arboviral diseases as public health problems. Archives of medical research. 2002;33:330–342. doi: 10.1016/s0188-4409(02)00378-8. [DOI] [PubMed] [Google Scholar]
- Happ GM. Structure and development of male accesory glands in insect, Insect Ultrastructure. Plenum press; New York, NY: 1984. [Google Scholar]
- Hausermann W, Nijhout HF. Permanent loss of male fecundity following sperm depletion in Aedes aegypti (L.) Journal of medical entomology. 1975;11:707–715. doi: 10.1093/jmedent/11.6.707. [DOI] [PubMed] [Google Scholar]
- Helinski ME, Harrington LC. Male mating history and body size influence female fecundity and longevity of the dengue vector Aedes aegypti. Journal of medical entomology. 2011;48:202–211. doi: 10.1603/me10071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JCSHG. The fine structure of the seminal bursa of Aedes aegypti. Mosq news. 1970;30:270–271. [Google Scholar]
- Judson CL. Feeding and oviposition behavior in the mosquito Aedes aegypti (L.). I. Preliminary studies of physiological control mechanisms. Biol Bull. 1967;133:369–378. doi: 10.2307/1539832. [DOI] [PubMed] [Google Scholar]
- Kalb JM, DiBenedetto AJ, Wolfner MF. Probing the function of Drosophila melanogaster accessory glands by directed cell ablation. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:8093–8097. doi: 10.1073/pnas.90.17.8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai-Fook J. Structure of the accessory glands and duplex of the internal male reproductive system of Calpodes ethlius (Hesperiidae, Lepidoptera) Canadian Journal of Zoology. 1982;60:1202–1215. [Google Scholar]
- Leahy MGCJG. Accessory gland substance as a stimulant for oviposition in Aedes aegypti and Ae. albopictus. Mosq news. 1965;25:448–452. [Google Scholar]
- Lee JJ, Klowden MJ. A male accessory gland protein that modulates female mosquito (Diptera: Culicidae) host-seeking behavior. Journal of the American Mosquito Control Association. 1999;15:4–7. [PubMed] [Google Scholar]
- Leiblich A, Marsden L, Gandy C, Corrigan L, Jenkins R, Hamdy F, Wilson C. Bone morphogenetic protein- and mating-dependent secretory cell growth and migration in the Drosophila accessory gland. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:19292–19297. doi: 10.1073/pnas.1214517109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison AC, Zielinski-Gutierrez E, Scott TW, Rosenberg R. Defining challenges and proposing solutions for control of the virus vector Aedes aegypti. PLoS medicine. 2008;5:e68. doi: 10.1371/journal.pmed.0050068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naccarati C, Audsley N, Keen JN, Kim JH, Howell GJ, Kim YJ, Isaac RE. The host-seeking inhibitory peptide, Aea-HP-1, is made in the male accessory gland and transferred to the female during copulation. Peptides. 2012;34:150–157. doi: 10.1016/j.peptides.2011.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perotti ME. Microtubules as components of Drosophila male paragonia secretion: An electron microscope study with enzymatic test. J Submicro Cytol. 1971;3:27. [Google Scholar]
- Ramalingam S. Secretion in the male accesory glands of Aedes aegypti (L.) (Diptera: Culicidae) Int J Insect Morphol & Embryol. 1983:87–96. [Google Scholar]
- Ramalingam SC, GB Functions of the male accessory gland secretions of Aedes mosquitoes (Diptera:Culicidae): Transplantation studies. Can Entomol. 1976;108:955–960. [Google Scholar]
- Ramalingam SCJ, GB Fine structure of the male accessory glands in Aedes triseriatus. Journal of insect physiology. 1978;24:251–259. [Google Scholar]
- Ravi Ram K, Wolfner MF. A network of interactions among seminal proteins underlies the long-term postmating response in Drosophila. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:15384–15389. doi: 10.1073/pnas.0902923106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemann JG, Thorson BJ. Ultrastructure of the ductus ejaculatoris duplex of the mediterranean flour moth, Anagasta Kühniella (Zeller) (Lepidoptera : Pyralidae) International Journal of Insect Morphology and Embryology. 1976;5:227–240. [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Molecular biology and evolution. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Sirot LK, Findlay GD, Sitnik JL, Frasheri D, Avila FW, Wolfner MF. Molecular characterization and evolution of a gene family encoding both female- and male-specific reproductive proteins in Drosophila. Molecular biology and evolution. 2014;31:1554–1567. doi: 10.1093/molbev/msu114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirot LK, Hardstone MC, Helinski ME, Ribeiro JM, Kimura M, Deewatthanawong P, Wolfner MF, Harrington LC. Towards a semen proteome of the dengue vector mosquito: protein identification and potential functions. PLoS neglected tropical diseases. 2011;5:e989. doi: 10.1371/journal.pntd.0000989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirot LK, Poulson RL, McKenna MC, Girnary H, Wolfner MF, Harrington LC. Identity and transfer of male reproductive gland proteins of the dengue vector mosquito, Aedes aegypti: potential tools for control of female feeding and reproduction. Insect biochemistry and molecular biology. 2008;38:176–189. doi: 10.1016/j.ibmb.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripet F, Lounibos LP, Robbins D, Moran J, Nishimura N, Blosser EM. Competitive reduction by satyrization? Evidence for interspecific mating in nature and asymmetric reproductive competition between invasive mosquito vectors. The American journal of tropical medicine and hygiene. 2011;85:265–270. doi: 10.4269/ajtmh.2011.10-0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Prokopec GM. Dengue control: the challenge ahead. Future microbiology. 2011;6:251–253. doi: 10.2217/fmb.11.10. [DOI] [PubMed] [Google Scholar]
- Wagstaff BJ, Begun DJ. Adaptive evolution of recently duplicated accessory gland protein genes in desert Drosophila. Genetics. 2007;177:1023–1030. doi: 10.1534/genetics.107.077503. [DOI] [PMC free article] [PubMed] [Google Scholar]