Abstract
Objective
To quantify the impact of objectively-recorded hot flashes on objective sleep in perimenopausal women.
Design
Cross-sectional study. Participants underwent 1–5 laboratory-based polysomnographic recordings for a total of 63 nights, including sternal skin conductance measures, from which 222 hot flashes were identified according to established criteria. Data were analyzed with hierarchical mixed-effect models and Spearman correlations.
Setting
Sleep laboratory.
Patients
34 perimenopausal women (Age±SD:50.4±2.7y).
Intervention
None.
Main Outcome Measures
Perceived and polysomnographic sleep measures (sleep quality, amount of wake after sleep onset and number of awakenings). Subjective (frequency and bother) and objective (frequency and amount of hot flash-associated wake time) hot flash measures.
Results
Women had an average of 3.5 (95%CI:2.8–4.2, range=1– 9) objective hot flashes per night. 69.4% of hot flashes were associated with an awakening. Hot flash-associated wake time per night was, on average, 16.6 min (95%CI:10.8–22.4), which accounted for 27.2% (SD 27.1) of total wakefulness per night. Hot flash-associated wake, but not frequency, was negatively associated with sleep efficiency and positively associated with wake after sleep onset. Also, self-reported wakefulness correlated with hot flash-associated wake, suggesting that women’s estimates of wakefulness are influenced by the amount of time spent awake in association with hot flashes during the night. More perceived and bothersome hot flashes correlated with more perceived wakefulness and awakenings and more objective hot flash-associated wake time and hot flash frequency.
Conclusions
The presence of physiological hot flashes accounts for a significant proportion of total objective wakefulness during the night in perimenopausal women.
Keywords: hot flash, sleep, menopause, skin conductance, polysomnography
Introduction
Hot flashes are depicted as a sensation of heat, sweating, flashing, anxiety, and chills lasting 1 to 5 min (1) and are reported by up to 84% of women in natural menopause (2). A hot flash is a heat-dissipation response, consisting of peripheral vasodilation, and sweating beginning in the upper part of the body (3). Sleep disturbance in menopause has often been attributed to the occurrence of hot flashes (4, 5) and both hot flashes and sleep disturbance are primary reasons for women seeking medical care during perimenopause and postmenopause (4).
Several studies have found that self-reported hot flashes are associated with perceived poor sleep (6–14). Studies that have investigated the association between subjective hot flashes and objective measures of sleep have, however, produced variable results, with some showing that hot flashes are associated with a poorer sleep (6, 15, 16), and others showing no relationship between hot flashes and objective measures of sleep quality (12, 13, 17, 18).
Similarly, studies that have assessed the relationship between objectively-recorded nocturnal hot flashes, based on skin conductance or skin temperature measures, with objective sleep parameters have produced mixed results, with studies showing that objective hot flashes are associated with a poorer objective sleep profile (16, 19–23), or no association between objective hot flash and sleep measures (15, 24), or a relationship between objective hot flashes and PSG-defined awakenings only in the first half of the night (25). The nature of the relationship between hot flashes and sleep, therefore, depends on whether hot flashes and sleep are subjectively or objectively assessed (15), and it remains unclear whether physiological hot flashes are a significant cause of objective sleep disturbance in peri- and post-menopausal women. Indeed, the question of whether hot flashes disturb sleep has recently been a source of discussion in the literature (26–28). Recently, Joffe and colleagues (29) took an experimental approach and showed that night time subjective and objective hot flashes correlated with subjective and objective sleep disturbance in young women treated with a Gonadotropin-releasing hormone agonist that simulated menopause, providing indirect evidence that menopausal hot flashes likely interrupt PSG-defined sleep.
Few studies have directly quantified the impact of hot flashes on sleep architecture. Two early studies reported a strong association between waking episodes and hot flashes in postmenopausal women (19, 20), an association also reported in breast cancer survivors with insomnia (22). However, Freedman and Roehrs (24) reported that of awakenings occurring within two minutes of a hot flash, the majority (55%) occurred before the onset of a hot flash, leading them to conclude that hot flashes do not disturb sleep. In a subsequent study, these authors reported that hot flashes disturb sleep only in the first half of the night; awakenings were more likely to occur after than before a hot flash in the first but not the second half of the night (25). Recently, Savard et al. (23) reported that time to reach the peak in skin conductance (but not hot flash frequency) correlated with more wake after sleep onset, less sleep efficiency and higher number of awakenings in breast cancer patients.
Overall, these approaches have provided some insight into the relationship between physiological hot flashes and disrupted sleep and suggest that factors other than hot flash frequency may be important when examining the impact of hot flashes on sleep. The main aim of the current study was to quantify the impact of objectively-recorded hot flashes on objective sleep in perimenopausal women. We adopted a novel approach by calculating the amount of hot flash-associated wakefulness during the night in perimenopausal women. We also explored the relationships between objective and subjective measures of hot flashes and sleep. We hypothesized that hot flash-associated wake would make a significant contribution to PSG-defined wakefulness after sleep, validating women’s reports that hot flashes disturb sleep.
Material and methods
Participants
Perimenopausal women were recruited from the San Francisco Bay area community over a four year period. Women included here were participating in an ongoing study about sleep quality during the menopausal transition. The study was reviewed and approved by SRI International’s Institutional Review Board and all participants provided written informed consent. Screening procedures for the study are fully described elsewhere (8). All women were perimenopausal based on self-report menstrual cycle length, i.e. they were either in the menopausal transition or early post-menopausal according to the Stages of Reproductive Aging Workshop criteria (30). Additional inclusion criteria were body mass index (BMI) of 32 kg.m−2 or lower, intact uterus and at least one ovary. Exclusions were use of hormone therapy or hormonal contraception for the previous 3 months, hypertension or arrhythmias, chronic medical conditions such as Diabetes Fibromyalgia, history of breast cancer, current antidepressant use, or current depressive disorder, assessed from clinical interview. Women were only included in this analysis if they experienced at least one objectively-recorded night-time hot flash during a PSG recording. They contributed between one and five overnight recordings to the analysis. Thirty-four women (Age, mean±SD: 50.4±2.7 years; BMI, mean±SD: 24.3±3.6 kg.m−2; 23 white) met inclusion criteria and were included in the analysis. A blood sample was drawn from the majority of women (n = 28) and plasma was analyzed for follicle stimulating hormone (FSH) and estradiol using standard immunoassay kits. The intraassay and interassay coefficients of variations were 6.7% and 7.6%, respectively, for the sensitive estradiol assay (Beckman Coulter Inc., Fullerton, CA, USA), and 2.6% and 5.5%, respectively, for the FSH assay (Siemens Healthcare Diagnostics, Los Angeles, CA, USA).
Procedure
Participants maintained their usual bedtimes while in the laboratory (SRI International, Menlo Park, CA, US) and slept in sound-attenuated and temperature controlled rooms.
Polysomnographic recordings
EEG (C3-A2, C4-A1, O1-A2, and O2-A1), electrooculogram (EOG; E1-A2, E2-A1), and submental electromyogram (EMG) were recorded using Compumedics amplifiers and Profusion software (Compumedics, Abbotsford, Victoria, Australia). EEG signals were sampled at 256 Hz, high-pass-filtered at 0.3 Hz, and low-pass-filtered at 30 Hz. Consecutive 30-s epochs in each record were scored (Wake, N1, N2, N3, and rapid-eye-movement [REM] sleep) by two experienced scorers following American Academy of Sleep Medicine rules (AASM) (31). Time bed (TIB, min), sleep efficiency (SE[obj], %; calculated as the percentage ratio of time spent asleep over TIB), wake after sleep onset (WASO[obj], min), time spent in REM (min) and non-REM (NREM, min) sleep, and number of awakenings (Awakenings[obj]) were calculated according to AASM rules (31).
Objective hot flash recordings
Objective hot flashes were assessed using measures of sternal skin conductance with a BioDerm Skin Conductance Meter (model 2701; UFI, Morro Bay, CA). Two 1.5 cm in diameter Ag/AgCl electrodes filled with 0.05 M potassium chloride Velvachol/glycol gel (32) were placed on either side of the sternum (about 4 cm apart). A 0.5-V constant voltage circuit (33) was maintained between them. The Bioderm was connected via an optically isolated DC input to the Compumedics amplifiers and the skin conductance signal was sampled at 16 Hz and co-registered online as a polysomnographic channel. Hot flashes were manually evaluated by two scorers (F.C.B. and M.d.Z.), blinded to PSG scoring, for fluctuations meeting accepted standard criteria for physiological hot flashes, i.e. an increase of 2 micro Siemens (µmho) within 30-s(34), which marked the onset of a hot flash. A 20-min refractory period following an identified hot flash was applied during which no additional hot flashes were scored (35) (in the current dataset two hot flashes were not considered in the analyses because they occurred within this lockout period). The total number of hot flashes was calculated (HF-number[obj]).
Quantification of the overall impact of hot flashes on sleep
After sleep scoring and hot flash detection had been independently completed, the association between epochs of wake and the onset of hot flashes was assessed using the following rules:
The period around the onset of a hot flash, ranging from 1 min before to 2 min after the start of a rise in skin conductance was evaluated for PSG epochs of wakefulness;
When an awakening started within the range of hot flash onset, 30-s epochs of wake were summed together until the first epoch of N2 or N3 sleep was scored, marking the return to stable sleep, independently from the return of skin conductance to a baseline level post-hot flash (e.g. a woman could wake up at the onset of a hot flash but fall asleep again before skin conductance returned to baseline; similarly, a woman could return to sleep only several minutes after the hot flash had ended, i.e. after the skin conductance returned to baseline);
When an awakening started more than 1 min before or more than 2 min after the start of a rise in skin conductance, it was not included in the calculation.
The amount of wake associated with each hot flash identified during the night was summed together to provide a measure reflecting the total number of minutes spent awake in association with hot flashes on each recording night, which we defined as hot flash-associated wake time (HF-associated wake[obj], min). An example of the calculation of HF-associated wake is provided in Figure 1. The criteria chosen to quantify the impact of hot flashes on sleep are based on the literature and on our experience in PSG recordings and characterization of hot flashes and are discussed fully in the Discussion. Even applying less conservative criteria (extending the windows up to 5 min before and 5 min after the onset of a hot flash), less than 5% of the total sample would be categorized as associated with an awakening.
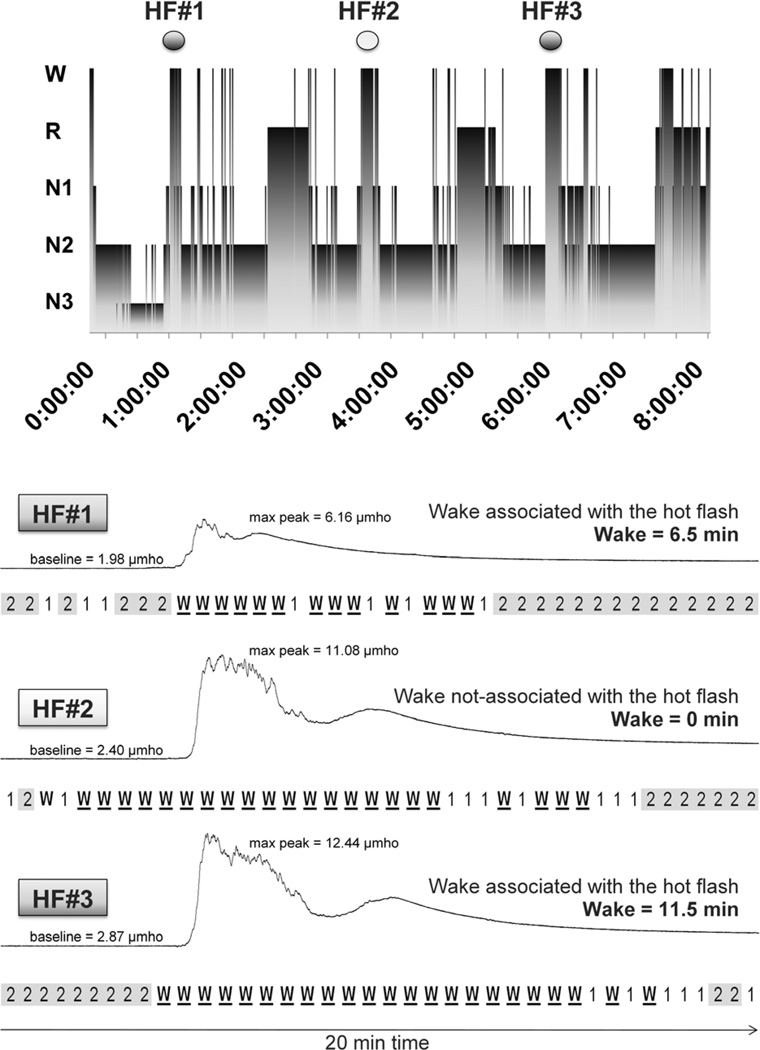
Figure 1.
Calculation of the amount of wake associated with the presence of hot flashes. In the top panel, the stages of sleep (wake, REM, N1, N2 and N3) are represented as a function of time (hypnogram), as obtained from the polysomnographic (PSG) recording from one participant. Above the hypnogram, three physiological hot flashes (defined as an increase in skin conductance >2 µmho in 30 s) are marked. Below the hypnogram, the skin conductance signal for the three hot flashes is plotted with a time resolution of 20 min (from 5 min before to 15 min after the onset of the hot flashes); the PSG sleep stages for each of the forty 30-s epochs within the 20-min window is displayed under each hot flash. Hot flashes HF#1 and HF#3 were included in the calculation of hot flash impact (HF-impact[obj], min) as the sum of the minutes of PSG wake time associated with each hot flash (6.5 min [HF#1] + 11.5 min [HF#3]). For this night, the total amount of wake associated with hot flashes (HF-impact[obj]) was 18 min. The hot flash impact index (wake time associated with hot flashes as a percentage of total wakefulness after sleep onset) was calculated as 32.17 %. HF#2 was not included in the calculation of HF-impact[obj] since the wake period started more than 1 min before the start of a rise in skin conductance, making the association between the hot flash and the awakening uncertain.
We also converted HF-associated wake[obj] into a percentage of minutes spent awake in association with hot flashes over the total wake after sleep onset to provide a global index (%).
Self-report sleep and hot flash characterization
Upon awakening in the morning, women completed sleep and hot flash questionnaires. They reported the number of perceived awakenings (Awakenings[sub]), wake after sleep onset (WASO[sub], min), and sleep quality on a 100 mm visual analogue scale, ranging from 0, “very bad”, to 100, “very good” (SQ[sub]), as used elsewhere (36).
They also were asked to report the number of nocturnal hot flashes experienced (HF-number[sub]) and how bothered they were by hot flashes/night sweats during the night (HF-bother[sub]), as used elsewhere (37).
Statistical analyses
A total of 222 objectively recorded hot flashes were identified over 63 PSG nights in 34 women having at least one physiological hot flash per night. We used hierarchical mixed-effect models to describe and quantify each of the subjective (sleep: SQ[sub], WASO[sub], Awakenings[sub]; hot flashes: HF number[sub], HF bother[sub]) and objective (sleep: SE[obj], WASO[obj], Awakenings[obj]; hot flashes: HF number[obj], HF-associated wake[obj]) variables, accounting for the amount of woman-to-woman and night-to-night variance. In the regressions, for the purposes of calculating standard errors, all independent variables are considered to be fixed except for a random effect for participant and a random effect for each individual measurement of the dependent variable. Estimated variance and 95% confidence intervals (CI) of random-effects parameters are provided. In addition, we calculated Spearman's rank correlation coefficients between all subjective and objective sleep and hot flash measures.
P values < 0.05 were considered significant. All analyses were performed using Stata/SE version 12.1 for Windows.
Results
Estradiol and FSH concentrations
Women had estradiol (34.7 ± 30.3 pg.ml−1, 95%CI [23.9, 41.2], n = 28) and FSH (48.9 ± 43.5 IU.l−1, 95%CI [34.4, 59.2], n = 28) levels consistent with being perimenopausal.
Quantification of subjective and objective hot flash and sleep measures
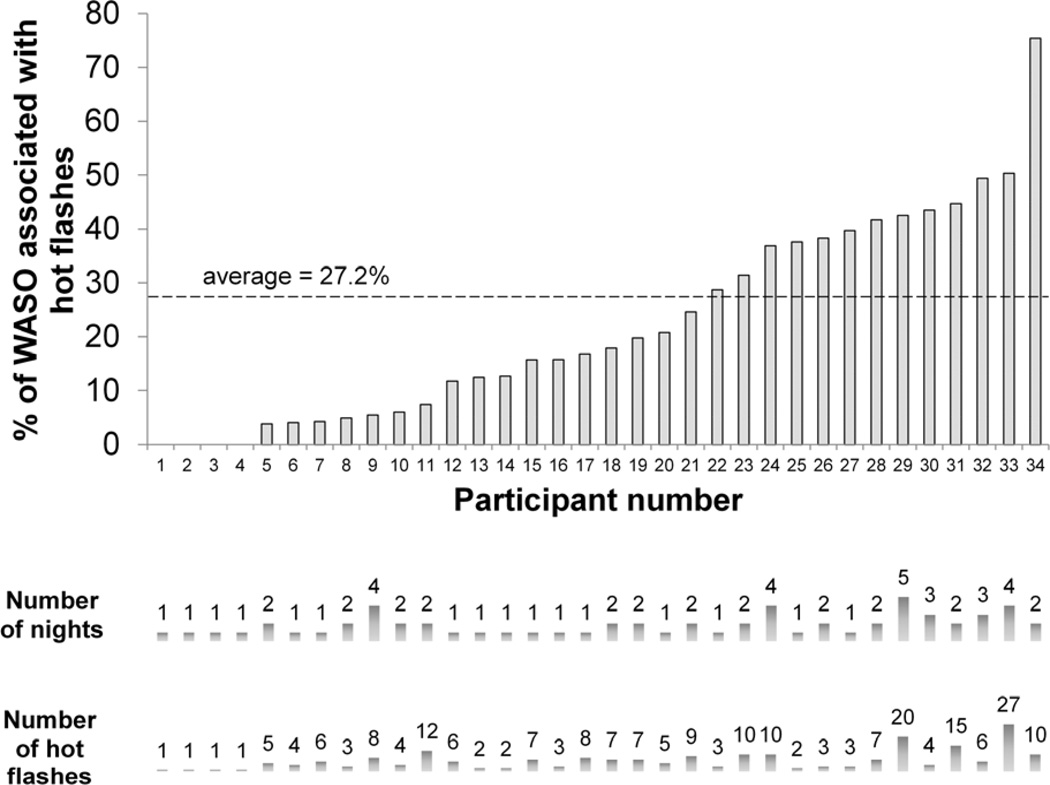
HF-associated wake accounted for, on average, 27.2% of WASO on a given night, with a wide range between nights of 0 to 89% (Figure 2).
Figure 2.
Amount of wake associated with the presence of hot flashes. Percentage of wake after sleep onset (WASO) associated with hot flashes for each woman, showing the contribution of number of nights and hot flashes to each percentage value.
An awakening was triggered during hot flash onset in 69.4% of the 222 hot flashes identified. 19.8% of hot flashes occurred without disturbing sleep in our defined interval and 10.8% occurred when the participant had already been awake for more than one minute. 3.1% of hot flashes occurred at the beginning of the night before sleep onset. The number of hot flashes associated with or without an awakening, or that occurred when participants were already awake are shown in Supplemental figure 1 as a function of time after lights-out. The number of hot flashes peaked in hour 5. The majority of hot flashes occurred in N2 and N3 sleep; 3.6% hot flashes were identified during REM sleep.
Objective and subjective sleep and hot flash variables are shown in Table 1. Women tended to subjectively underestimate objective measures of WASO, number of awakenings and number of hot flashes.
Table 1.
Mean, standard error and 95% confidence interval for objective and subjective sleep and hot flash variables recorded from 64 nights in 34 women having at least one objectively-recorded hot flash per night.
| Variable | Mean | Standard Error |
95% CI | ||
|---|---|---|---|---|---|
| Objective PSG sleep | TIB[obj] (min) | 451.3 | 8.7 | 434.3 | 468.3 |
| SE[obj] (%) | 84.1 | 1.0 | 82.1 | 86.1 | |
| WASO[obj] (min) | 60.1 | 4.5 | 51.3 | 68.8 | |
| Number of awakenings[obj] | 26.6 | 1.8 | 23.1 | 30.1 | |
| Time spent in REM (min) | 82.0 | 3.3 | 75.6 | 88.5 | |
| Time spent in NREM (min) | 293.3 | 5.5 | 282.5 | 304.0 | |
| Self-report Sleep | SQ[sub] (mm) | 43.9 | 4.6 | 34.9 | 52.9 |
| WASO[sub] (min) | 45.7 | 7.4 | 31.1 | 60.3 | |
| Number of awakenings[sub] | 3.6 | 0.4 | 2.9 | 4.3 | |
| Objective hot flashes | HF-number[obj] | 3.5 | 0.4 | 2.8 | 4.2 |
| HF-associated wake[obj] (min) | 16.6 | 3.0 | 10.8 | 22.4 | |
| Self-report hot flashes | HF-number[sub] | 1.5 | 0.3 | 0.9 | 2.1 |
| HF-bother[sub] (mm) | 29.8 | 5.1 | 19.7 | 39.9 | |
HF-associated wake (time awake associated with hot flashes in min); HF-bother (rated on a 100 mm visual analog scale, not at all bothered - very bothered); PSG (polysomnography); REM (rapid-eye-movement sleep); NREM (non-rapid-eye-movement sleep); SE (sleep efficiency, %); SQ (sleep quality; 0–100 mm visual analogic scale, very bad - very good); TIB (time in bed, min); WASO (wake after sleep onset, min).
Association between subjective and objective hot flashes and sleep measures
A correlation matrix of Spearman's rank correlations between objective and subjective sleep and hot flash measures is shown in Table 2. Hot flash frequency (HF-number[obj]) and wake time associated with hot flashes (HF-associated wake[obj]) were correlated. HF-associated wake[obj], but not HF-number[obj], was negatively associated with the objective quality of sleep (SE[obj] (%)) and positively associated with the amount of wake after sleep onset (WASO[obj]). HF-number[obj] correlated with objective and subjective number of awakenings. Perceived wake time (WASO[sub]) was associated with HF-associated wake[obj] and amount of wake after sleep onset (WASO[obj]). In addition, more perceived (HF-number[sub]) and more bothersome (HF-bother[sub]) hot flashes were related to more perceived wakefulness (WASO[sub]) and awakenings (Number of awakenings[sub]), and to more objective hot flash-associated wake time (HF-associated wake[obj]) and frequency (HF-number[obj]). Neither HF-associated wake[obj] nor frequency of hot flashes (HF-number[obj]) was related to REM or NREM sleep duration (P’s > 0.1).
Table 2.
Spearman's rank correlation coefficients between subjective and objective assessments of sleep and hot flashes in 34 perimenopausal women. Significant correlations are highlighted in the matrix.
| Spearman's rank correlation coefficients |
PSG sleep |
Self-report sleep |
Objective hot flashes |
Self-report hot flashes |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TIB[obj] | SE[obj] | WASO[obj] | Awakenings[obj] | SQ[sub] | WASO[sub] | Awakenings[sub] | HF-number[obj] | HF-associated wake[obj] |
HF-number[sub] | HF-bother[sub] | ||
| PSG sleep | TIB[obj] | 1.00 | ||||||||||
| SE[obj] | −0.31* | 1.00 | ||||||||||
| WASO[obj] | 0.49*** | −0.93*** | 1.00 | |||||||||
| Awakenings[obj] | 0.56*** | −0.18 | 0.36** | 1.00 | ||||||||
|
Self-report sleep |
SQ[sub] | 0.30* | 0.15 | −0.06 | 0.25 | 1.00 | ||||||
| WASO[sub] | 0.22 | −0.42*** | 0.44*** | −0.16 | −0.42** | 1.00 | ||||||
| Awakenings[sub] | 0.32* | −0.10 | 0.21 | 0.42*** | −0.14 | 0.54*** | 1.00 | |||||
|
Objective hot flashes |
HF-number[obj] | 0.29* | −0.16 | 0.22 | 0.27* | −0.01 | 0.29* | 0.44*** | 1.00 | |||
| HF-associated wake[obj] | 0.07 | −0.27* | 0.32* | 0.08 | −0.18 | 0.33* | 0.21 | 0.53*** | 1.00 | |||
|
Self-report hot flashes |
HF-number[sub] | 0.23 | 0.07 | 0.01 | 0.28* | −0.08 | 0.34* | 0.52*** | 0.42*** | 0.46*** | 1.00 | |
| HF-bother[sub] | −0.00 | 0.01 | 0.02 | 0.11 | −0.31* | 0.40** | 0.38** | 0.42*** | 0.43*** | 0.82*** | 1.00 | |
p < 0.05,
p < 0.01,
p < 0.001.
HF-associated wake (time awake associated with hot flashes in min); HF-bother (rated on a 100 mm visual analog scale, not at all bothered - very bothered); PSG (polysomnography); SE (sleep efficiency, %); SQ (sleep quality; 0–100 mm visual analogic scale, very bad - very good); TIB (time in bed, min); WASO (wake after sleep onset, min).
Discussion
We found that hot-flash associated wake time accounted for, on average, 27.2% of PSG-defined wakefulness during the night in peri-menopausal women. As expected, a greater number of objective hot flashes correlated with a greater amount of hot flash-associated wakefulness. This index provides a measure of the magnitude of the effect of hot flashes on sleep taking into account that not all hot flashes are associated with awakenings and that hot flashes are associated with a variable amount of wake time. We also show that hot flash-associated wake time is more highly correlated with poor PSG-defined sleep efficiency than is objective hot flash frequency. Our findings show that hot flashes are a significant contributor to objective sleep disturbance in perimenopausal women with nocturnal hot flashes, validating long-standing evidence from self-reports that hot flashes disrupt sleep.
The calculation of the amount of wake time associated with hot flashes offered a quantifiable index reflecting the impact of hot flashes on sleep. Objective hot flash frequency was not associated with PSG-defined poor sleep quality, apart from number of awakenings, possibly because not all hot flashes disturb sleep; 19.8% of hot flashes in our sample occurred without interfering with sleep. Similarly, 34% of physiological hot flashes were not associated with an awakening in a hormonal model of menopause in young women (29). Conversely, not all wakefulness during the night is attributed to hot flashes, as evident in our study and elsewhere (19). The fact that not all hot flashes are associated with disturbed sleep and that not all wakefulness is associated with hot flashes may explain, in part, why studies have not consistently found evidence of objective sleep disturbances in comparisons of menopausal women with and without objectively-measured hot flashes (38). Also, the presence of a primary sleep disorder but not objective hot flash frequency predicted objective sleep efficiency in a large sample of midlife women (39). Our findings suggest that a measure of wakefulness associated with hot flashes could be a better indicator of hot flash impact on objective sleep than is hot flash frequency. While our results show a strong association between objective measures of hot flashes and PSG-defined measures of wake, there was no association between objective hot flash measures and amounts of NREM or REM sleep, implying that hot flashes interfere mostly with sleep continuity, as shown by others (29). Hot flashes have also been associated with more subtle disturbances during sleep such as increased beta EEG activity (16) and decreased vagal activity that is evident even when hot flashes are not associated with an arousal (40).
The windows of analysis of the association between hot flashes and PSG measures have varied between studies and have not always been clearly defined. We chose a 3-min window to mark hot flash onset based on several findings in the literature. While the rise in skin conductance is the recognized presentation of a hot flash, physiological changes are evident at least one minute earlier. Our previous study showed changes in physiological variables (e.g. heart rate) occurring within two minutes before the rise in skin conductance (40), with changes most evident closer to the onset of the rise in skin conductance. Similarly, Thurston et al. (41) found an abrupt decrease in high frequency power of heart rate variability starting in the minute before the rise in skin conductance. Thus, we considered an awakening occurring within one minute before the start of a rise in skin conductance to be associated with a hot flash. We considered the first 2-min following the start of a rise in skin conductance as part of hot flash onset because this interval encompasses the rise in skin conductance towards a peak level, before it plateaus, for the majority of hot flashes (23). Given the clear association between awakenings and hot flash onset for the majority of hot flashes, our results support the theory that a hot flash and awakening are driven by a common central process, such as sympathetic activation (25); however, it is still possible that sweating triggered by a hot flash contributes to or extends the interval of waking. There is a circadian variation in the incidence of hot flashes, peaking in the early evening (42). Within the night period, similar to Freedman and colleagues (42), we found a peak in the number of hot flashes in the second part of the night. Interestingly, Freedman and Roehrs (25) found that only hot flashes in the first half of the night were more likely to precede an awakening or arousal. We found no apparent variation in the number of hot flashes associated with or without an awakening across the night (Supplemental figure 1). Possibly, the different findings relate to how hot flashes were classified as associated or not with awakenings and how the windows of analysis were defined. We confirmed Freedman and Roehrs (25) findings that most hot flashes occurred during NREM sleep (25).
As part of our analysis, we explored the univariate correlations between subjective and objective measures of hot flashes and sleep. The subjective number of hot flashes correlated with the number of objectively quantified hot flashes, although they were under-reported, as to be expected given that events are recalled the following morning and may therefore not be accurate (11). Hot flash bother correlated with the number of physiological hot flashes experienced, suggesting that the more physiological hot flashes a woman has during the night, the more bothered she is by them, consistent with previous findings (43). Hot flash bother also correlated with hot flash-associated wake time. Additional factors, including affect, sleep problems, symptom sensitivity, general health, and race are also associated with night-time hot flash bother (43), which we did not consider in our analysis.
Self-reported hot flashes correlated with subjective sleep measures, with more bothersome and frequent hot flashes being related to a poorer sleep quality, more perceived WASO and awakenings, confirming previous findings (6–14). More hot flash-associated wake time correlated with more perceived WASO in the morning, suggesting that a woman’s estimate of her WASO is strongly influenced by the amount of time spent awake during the night in association with hot flashes. To confirm this, we calculated the objective time spent awake after sleep onset not associated with a hot flash as WASO[obj] minus HF-associated wake[obj] and we found no relation between this measure and perceived WASO. Thus, women may be more aware of time spent awake in association with hot flashes than other wake periods during the night. Our results should be considered within the context of the study limitations. Our study included a non-clinical, community sample of women in the menopause transition or within the first year post-menopause who experienced between one and nine physiological hot flashes per night. The magnitude of impact of hot flashes on sleep in treatment-seeking women may be even greater than what we found. Also, we were conservative in our calculation of hot flash-associated wake time in that we considered a hot flash to be associated with wake only when an awakening began within 1 minute before and 2 minutes after hot flash onset. The more advanced an awakening is relative to the rise in skin conductance, the lower the certainty that it is related with the hot flash. Also, we only counted hot flashes if they met standard criteria of an increase in skin conductance of at least 2 µmho within 30-s (34). Others have suggested that using a more liberal criterion for detecting hot flashes may be more clinically relevant (23), although this remains to be confirmed. However, in our dataset only 5 “potential hot flashes” were discharged due to the failure of meeting the 2 µmho increase in skin conductance. Future studies are needed to determine if hot flash-associated wake time varies between groups of women. Some women may have more difficulty falling back to sleep after being woken by a hot flash than others, leading to more prolonged bouts of wakefulness; women who are anxious or stressed have difficulty returning to sleep following nocturnal awakenings, regardless the cause (44). Further studies could also consider more subtle measures of sleep alterations such as EEG spectral indices, already found to be related to the frequency of hot flashes (23).
Conclusions
We have shown that hot flashes – while not the only cause of sleep disturbance - account for 27.2% of wakefulness during the night and therefore contribute significantly to disturbed PSG-defined sleep in perimenopausal women. The calculation of hot flash-associated wake time could be clinically useful in the assessment and management of sleep disturbances in women with nocturnal hot flashes.
Supplementary Material
Acknowledgments
We would like to thank Justin Greco, Rebecca Carr, David Sugarbaker, David Dresser, Stephanie Claudatos, Sarah Inkelis and Ben Mayer for their effort in the data collection process. Blood sample analysis was supported by NICHD (SCCPRR) Grant U54-HD28934, “University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core”.
Financial disclosure. This study was supported by National Institutes of Health, Bethesda, MD, USA; Grant HL103688 to FCB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest. I.M.C. reports grants (or pending grants) with Apnicure Inc.
Contributor Information
Massimiliano de Zambotti, Email: massimiliano.dezambotti@sri.com.
Ian M. Colrain, Email: ian.colrain@sri.com.
Harold S. Javitz, Email: harold.javitz@sri.com.
Fiona C. Baker, Email: fiona.baker@sri.com.
References
- 1.Kronenberg F. Hot flashes: epidemiology and physiology. Ann N Y Acad Sci. 1990;592:52–86. doi: 10.1111/j.1749-6632.1990.tb30316.x. [DOI] [PubMed] [Google Scholar]
- 2.Melby MK, Anderson D, Sievert LL, Obermeyer CM. Methods used in cross-cultural comparisons of vasomotor symptoms and their determinants. Maturitas. 2011;70:110–119. doi: 10.1016/j.maturitas.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 3.Freedman R. Physiology of hot flashes. Am J Hum Biol. 2001;13:453–464. doi: 10.1002/ajhb.1077. [DOI] [PubMed] [Google Scholar]
- 4.Moe K. Hot flashes and sleep in women. Sleep Med Rev. 2004;8:487–497. doi: 10.1016/j.smrv.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Ameratunga D, Goldin J, Hickey M. Sleep disturbance in menopause. Intern Med J. 2012;42:742–747. doi: 10.1111/j.1445-5994.2012.02723.x. [DOI] [PubMed] [Google Scholar]
- 6.Ensrud K, Stone K, Blackwell T, Sawaya G, Tagliaferri M, Diem S, et al. Frequency and severity of hot flashes and sleep disturbance in postmenopausal women with hot flashes. Menopause. 2009;16:286–292. doi: 10.1097/gme.0b013e31818c0485. [DOI] [PubMed] [Google Scholar]
- 7.Otte JL, Carpenter JS, Russell KM, Bigatti S, Champion VL. Prevalence, Severity, and Correlates of Sleep-Wake Disturbances in Long-Term Breast Cancer Survivors. J Pain Symptom Manage. 2010;39:535–547. doi: 10.1016/j.jpainsymman.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sassoon S, de Zambotti M, Colrain I, Baker F. Association between personality traits and DSM-IV diagnosis of insomnia in peri-and postmenopausal women. Menopause. 2014 doi: 10.1097/GME.0000000000000192. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kravitz H, Ganz P, Bromberger J, Powell L, Sutton-Tyrrell K, Meyer P. Sleep difficulty in women at midlife: a community survey of sleep and the menopausal transition. Menopause. 2003;10:19–28. doi: 10.1097/00042192-200310010-00005. [DOI] [PubMed] [Google Scholar]
- 10.Ohayon M. Severe hot flashes are associated with chronic insomnia. Arch Intern Med. 2006;166:1262–1268. doi: 10.1001/archinte.166.12.1262. [DOI] [PubMed] [Google Scholar]
- 11.Thurston R, Blumenthal J, Babyak M, Sherwood A. Association between hot flashes, sleep complaints, and psychological functioning among healthy menopausal women. Int J Behav Med. 2006;13:163–172. doi: 10.1207/s15327558ijbm1302_8. [DOI] [PubMed] [Google Scholar]
- 12.Polo-Kantola P, Erkkola R, Irjala K, Helenius H, Pullinen S, Polo O. Climacteric symptoms and sleep quality. Obstet Gynecol. 1999;94:219–224. doi: 10.1016/s0029-7844(99)00284-7. [DOI] [PubMed] [Google Scholar]
- 13.Young T, Rabago D, Zgierska A, Austin D, Laurel F. Objective and subjective sleep quality in premenopausal, perimenopausal, and postmenopausal women in the Wisconsin Sleep Cohort Study. Sleep. 2003;26:667–672. doi: 10.1093/sleep/26.6.667. [DOI] [PubMed] [Google Scholar]
- 14.Thurston RC, Joffe H. Vasomotor symptoms and menopause: findings from the Study of Women’s Health across the Nation. Obstet Gynecol Clin North Am. 2011;38:489–501. doi: 10.1016/j.ogc.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thurston R, Santoro N, Matthews K. Are vasomotor symptoms associated with sleep characteristics among symptomatic midlife women? Comparisons of self-report and objective measures. Menopause (New York, NY) 2012;19:742–748. doi: 10.1097/gme.0b013e3182422973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell I, Bromberger J, Buysse D, Hall M, Hardin K, Kravitz H, et al. Evaluation of the association of menopausal status with delta and beta EEG activity during sleep. Sleep. 2011;34:1561–1568. doi: 10.5665/sleep.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaver J, Giblin E, Lentz M, Lee K. Sleep patterns and stability in perimenopausal women. Sleep. 1988;11:556–561. doi: 10.1093/sleep/11.6.556. [DOI] [PubMed] [Google Scholar]
- 18.Sharkey K, Bearpark H, Acebo C, Millman R, Cavallo A, Carskadon M. Effects of menopausal status on sleep in midlife women. Behav Sleep Med. 2003;1:69–80. doi: 10.1207/S15402010BSM0102_1. [DOI] [PubMed] [Google Scholar]
- 19.Erlik Y, Tataryn I, Meldrum D, Lomax P, Bajorek J, Judd H. Association of waking episodes with menopausal hot flushes. JAMA. 1981;245:1741–1744. [PubMed] [Google Scholar]
- 20.Gonen R, Sharf M, Lavie P. The association between mid-sleep waking episodes and hot flushes in post-menopausal women. Journal of Psychosomatic Obstetrics & Gynecology. 1986;5:113–117. [Google Scholar]
- 21.Woodward S, Freedman R. The thermoregulatory effects of menopausal hot flashes on sleep. Sleep. 1994;17:497–501. doi: 10.1093/sleep/17.6.497. [DOI] [PubMed] [Google Scholar]
- 22.Savard J, Davidson J, Ivers H, Quesnel C, Rioux D, Dupéré V, et al. The association between nocturnal hot flashes and sleep in breast cancer survivors. J Pain Symptom Manage. 2004;27:513–522. doi: 10.1016/j.jpainsymman.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 23.Savard M, Savard J, Caplette-Gingras A, Ivers H, Bastien C. Relationship between objectively recorded hot flashes and sleep disturbances among breast cancer patients: investigating hot flash characteristics other than frequency. Menopause. 2013;20:997–1005. doi: 10.1097/GME.0b013e3182885e31. [DOI] [PubMed] [Google Scholar]
- 24.Freedman R, Roehrs T. Lack of sleep disturbance from menopausal hot flashes. Fertil Steril. 2004;82:138–144. doi: 10.1016/j.fertnstert.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 25.Freedman R, Roehrs T. Effects of REM sleep and ambient temperature on hot flash16 induced sleep disturbance. Menopause. 2006;13:576–583. doi: 10.1097/01.gme.0000227398.53192.bc. [DOI] [PubMed] [Google Scholar]
- 26.Regestein Q. Do hot flashes disturb sleep? Menopause. 2012;19:715–718. doi: 10.1097/gme.0b013e318258dd40. [DOI] [PubMed] [Google Scholar]
- 27.Carpenter J, Yu M. Hot flashes and sleep: curious or spurious link? Menopause. 2013;20:991–992. doi: 10.1097/GME.0b013e3182a29bf2. [DOI] [PubMed] [Google Scholar]
- 28.Shaver J. Hot flashes and sleep: pieces of the puzzle. Menopause. 2013;20:877–880. doi: 10.1097/GME.0b013e3182a02bb5. [DOI] [PubMed] [Google Scholar]
- 29.Joffe H, White D, Crawford S, McCurnin K, Economou N, Connors S, et al. Adverse effects of induced hot flashes on objectively recorded and subjectively reported sleep: results of a gonadotropin-releasing hormone agonist experimental protocol. Menopause. 2013;20:905–914. doi: 10.1097/GME.0b013e31828292d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soules M, Sherman S, Parrott E, Rebar R, Santoro N, Utian W, et al. Executive summary: Stages of Reproductive Aging Workshop (STRAW) Climacteric. 2001;4:267–272. [PubMed] [Google Scholar]
- 31.Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM manual for the scoring of sleep and associated events: rules, terminology, and technical specification. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 32.Dormire S, Carpenter J. An alternative to Unibase/glycol as an effective nonhydrating electrolyte medium for the measurement of electrodermal activity. Psychophysiology. 2002;39:423–426. doi: 10.1017.S0048577201393149. [DOI] [PubMed] [Google Scholar]
- 33.Lykken DT, Venables PH. Direct measurement of skin conductance: A proposal for standardization. Psychophysiology. 1971;8:656–672. doi: 10.1111/j.1469-8986.1971.tb00501.x. [DOI] [PubMed] [Google Scholar]
- 34.Freedman R. Laboratory and ambulatory monitoring of menopausal hot flashes. Psychophysiology. 1989;26:573–579. doi: 10.1111/j.1469-8986.1989.tb00712.x. [DOI] [PubMed] [Google Scholar]
- 35.Carpenter J, Andrykowski M, Freedman R, Munn R. Feasibility and psychometrics of an ambulatory hot flash monitoring device. Menopause. 1999;6:209–215. doi: 10.1097/00042192-199906030-00006. [DOI] [PubMed] [Google Scholar]
- 36.Baker F, Sassoon S, Kahan T, Palaniappan L, Nicholas C, Trinder J, et al. Perceived poor sleep quality in the absence of polysomnographic sleep disturbance in women with severe premenstrual syndrome. J Sleep Res. 2012;21:535–545. doi: 10.1111/j.1365-2869.2012.01007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carpenter J. State of the science: hot flashes and cancer. Part 1: definition, scope, impact, physiology, and measurement. Oncol Nurs Forum. 2005;32:959–968. doi: 10.1188/05.ONF.959-968. [DOI] [PubMed] [Google Scholar]
- 38.Joffe H, Massler A, Sharkey K. Evaluation and management of sleep disturbance during the menopause transition. Semin Reprod Med. 2010;28:404–421. doi: 10.1055/s-0030-1262900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Freedman R, Roehrs T. Sleep disturbance in menopause. Menopause. 2007;14:826–829. doi: 10.1097/GME.0b013e3180321a22. [DOI] [PubMed] [Google Scholar]
- 40.de Zambotti M, Colrain I, Sassoon S, Nicholas C, Trinder J, Baker F. Vagal withdrawal during hot flashes occurring in undisturbed sleep. Menopause. 2013;20:1147–1153. doi: 10.1097/GME.0b013e31828aa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thurston R, Christie I, Matthews K. Hot flashes and cardiac vagal control: a link to cardiovascular risk? Menopause. 2010;17:456–461. doi: 10.1097/gme.0b013e3181c7dea7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Freedman R, Norton D, Woodward S, Cornélissen G. Core body temperature and circadian rhythm of hot flashes in menopausal women. J Clin Endocrinol Metab. 1995;80:2354–2358. doi: 10.1210/jcem.80.8.7629229. [DOI] [PubMed] [Google Scholar]
- 43.Thurston RC, Sutton-Tyrrell K, Everson-Rose SA, Hess R, Matthews KA. Hot Flashes and Subclinical Cardiovascular Disease Findings From the Study of Women’s Health Across the Nation Heart Study. Circulation. 2008;118:1234–1240. doi: 10.1161/CIRCULATIONAHA.108.776823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roth T. Insomnia: definition, prevalence, etiology, and consequences. J Clin Sleep Med. 2007;3:S7–S10. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.