Abstract
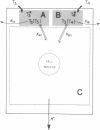
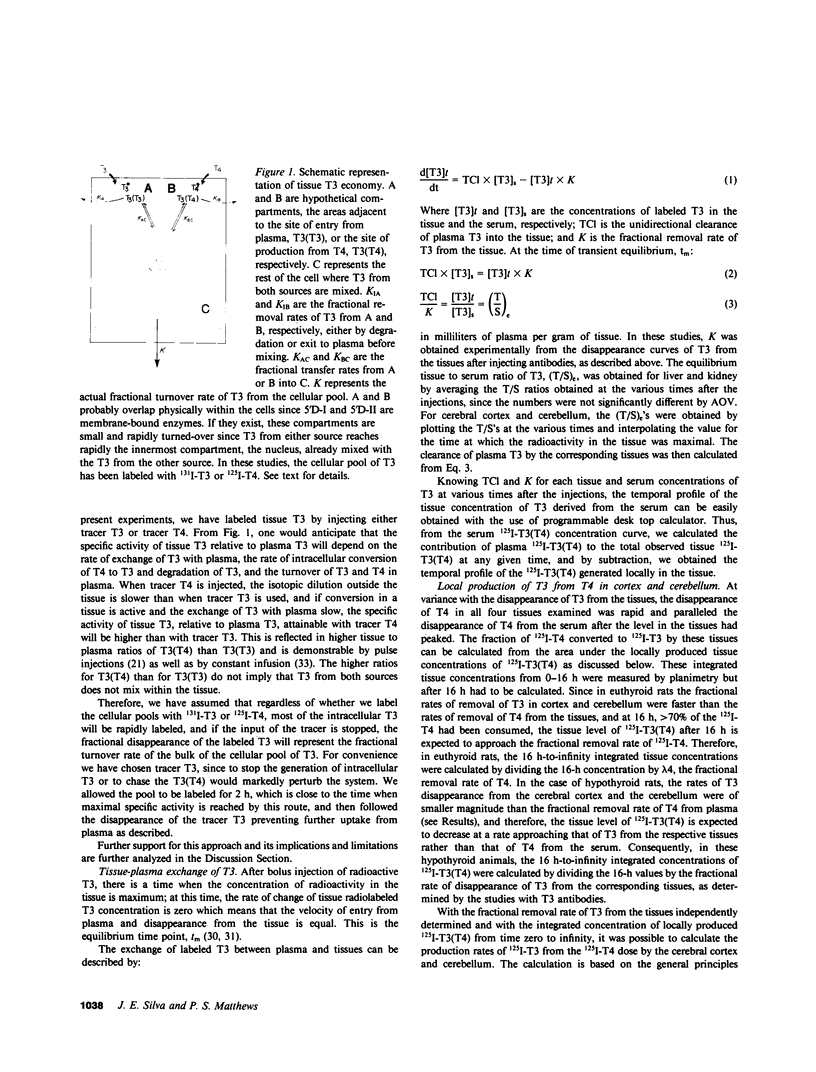
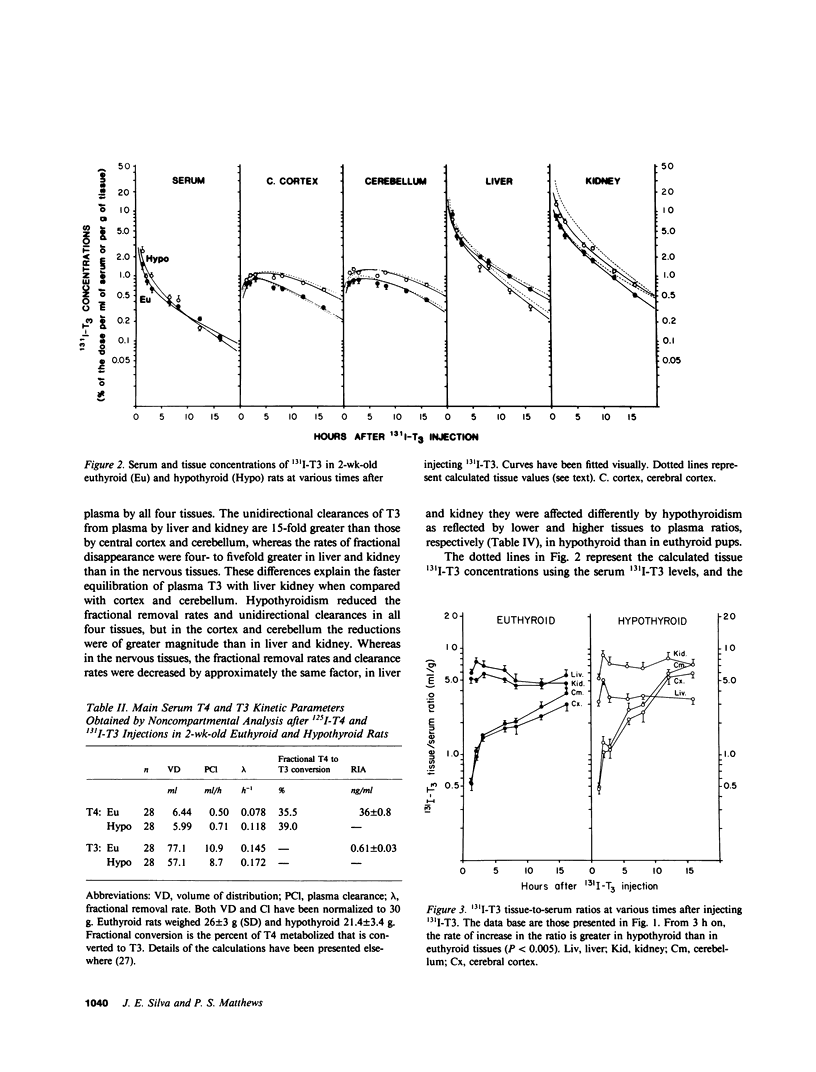
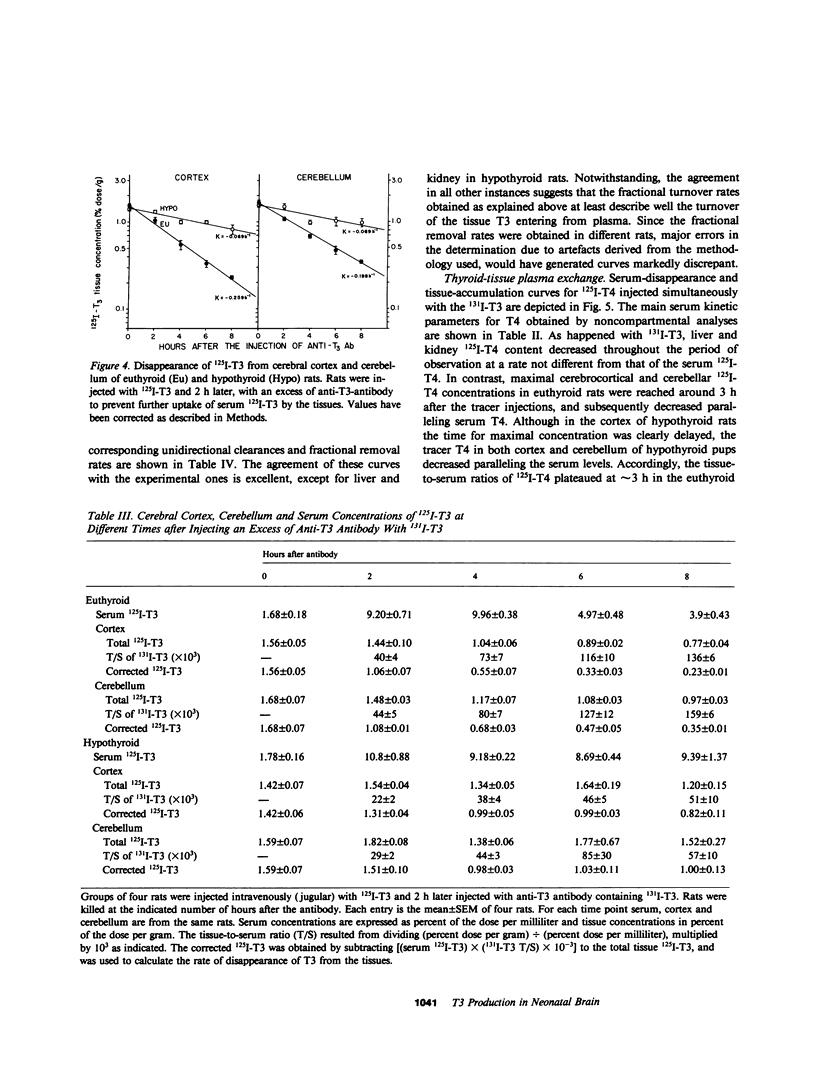
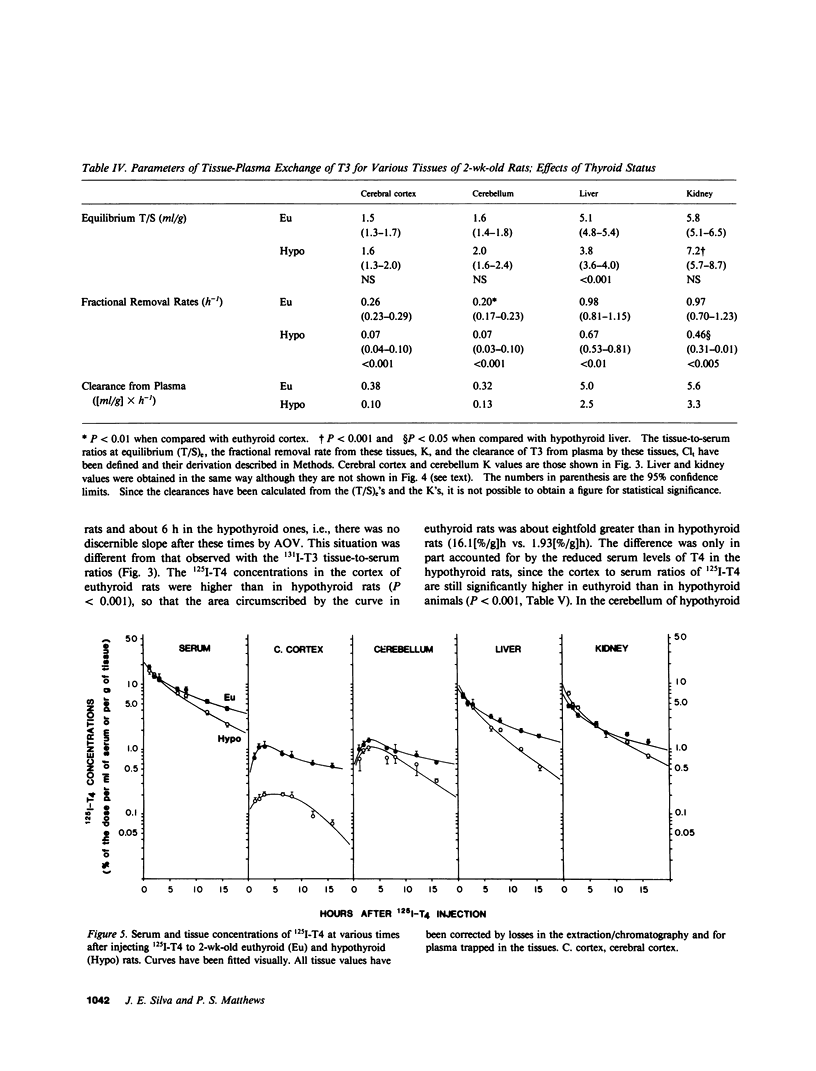
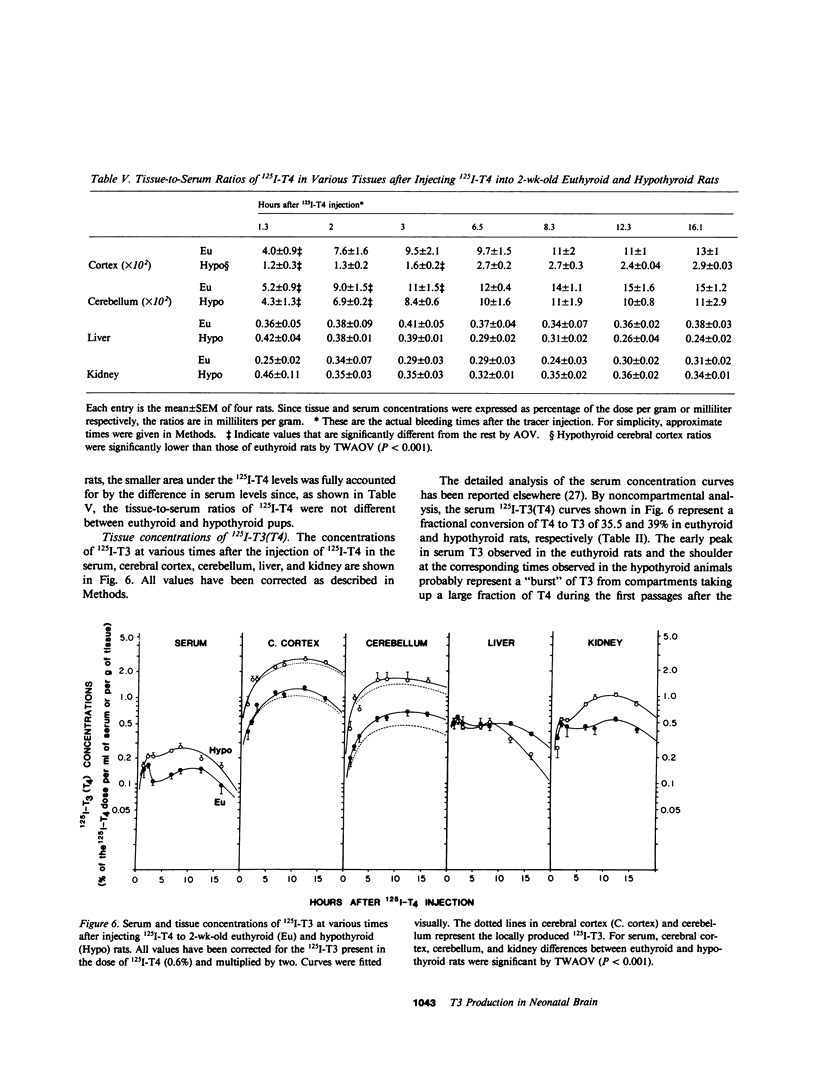
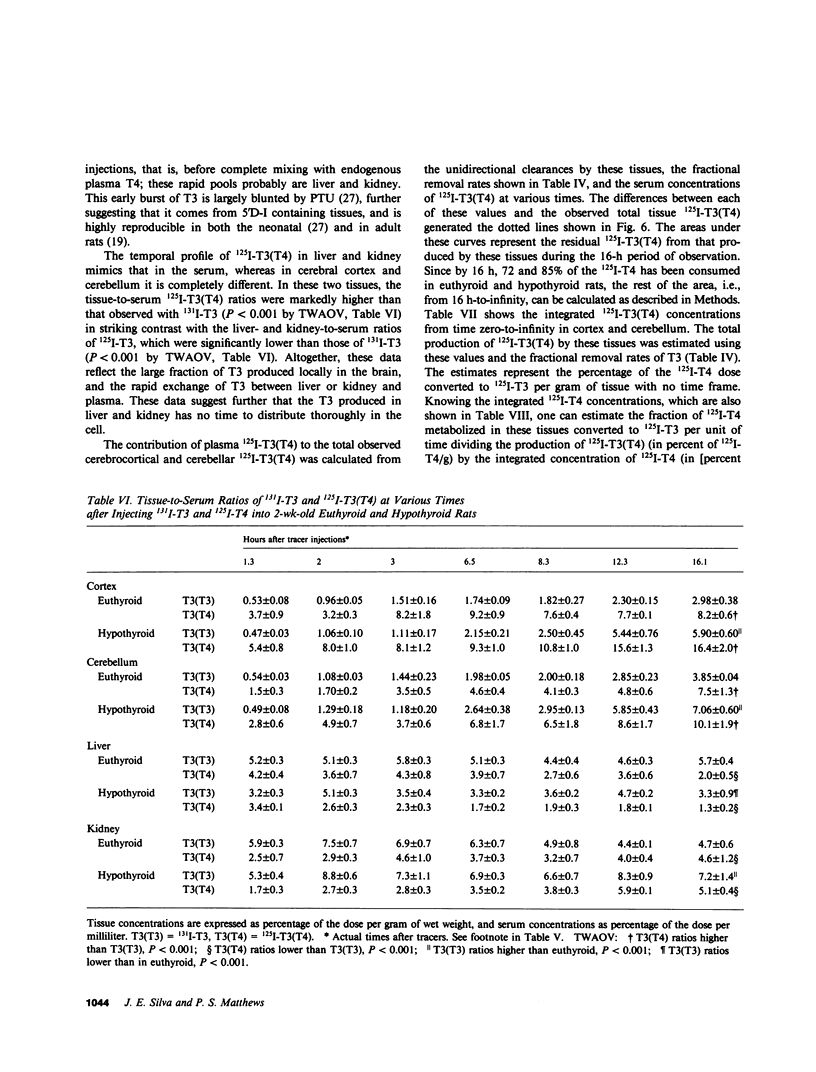
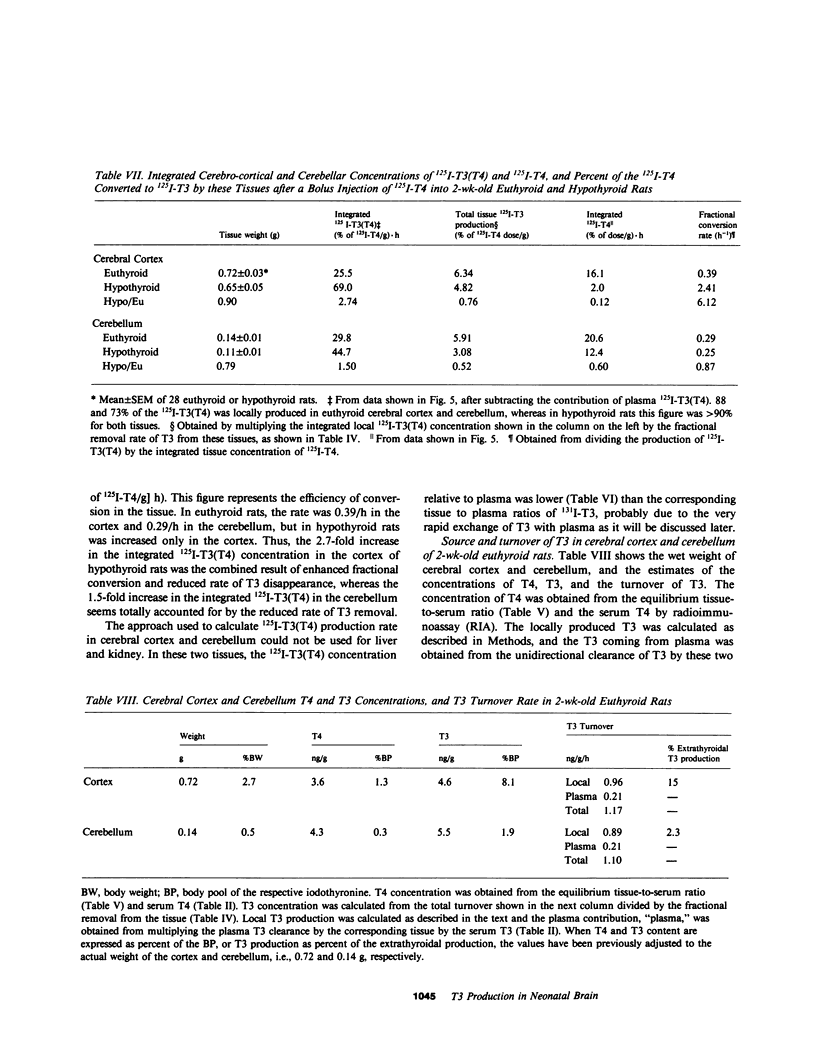
Local 5'-deiodination of serum thyroxine (T4) is the main source of triiodothyronine (T3) for the brain. Since we noted in previous studies that the cerebral cortex of neonatal rats tolerated marked reductions in serum T4 without biochemical hypothyroidism, we examined the in vivo T4 and T3 metabolism in that tissue and in the cerebellum of euthyroid and hypothyroid 2-wk-old rats. We also assessed the contribution of enhanced tissue T4 to T3 conversion and decreased T3 removal from the tissues to the T3 homeostasis in hypothyroid brain. Congenital and neonatal hypothyroidism was induced by adding methimazole to the drinking water. Serum, cerebral cortex (Cx), cerebellum (Cm), liver (L) and kidney (R) concentrations of 125I-T4, 125I-T3(T4), and 131I-T3 were measured at various times after injecting 125I-T4 and 131I-T3. The rate of T3 removal from the tissues was measured after injecting an excess of anti-T3-antibody to rats previously injected with tracer T3. In euthyroid rats, fractional turnover rates of T3 per hour were: Cx, 0.26 +/- 0.02 (SE); Cm, 0.20 +/- 0.02; L, 0.98 +/- 0.07; R, 0.97 +/- 0.12; and the calculated unidirectional plasma T3 clearance by these tissues were, in milliliters per gram per hour: Cx = 0.38, Cm = 0.32, L = 5.0, and R = 5.6. In hypothyroidism, the fractional removal rates and clearances were reduced in all tissues, in cortex and cerebellum by 70%, and in liver and kidney ranging from 30 to 50%. While greater than 80% of the 125I-T3(T4) in the brain tissues of euthyroid rats was locally produced, in hypothyroid cerebral cortex and cerebellum the integrated concentrations of 125I-T3(T4) were 2.7- and 1.5-fold greater than in euthyroid rats. In the Cx, this response resulted from an approximately sixfold increase in fractional conversion and an approximately fourfold decrease in T3 removal rate hampered by a decreased uptake of T4 from plasma, whereas in Cm the response resulted only from the reduced T3 removal rate. In euthyroid rats, the calculated production rate of T3 in nanograms per gram per hour by the Cx was 0.96 and 0.89 by the Cm, which on a per organ basis equals 15 and 2%, respectively, of the extrathyroidal production rate as assessed in the body pool exchanging with plasma. Several conclusions can be drawn: Production of T3 by developing brain is a very active process in agreement with the need of thyroid hormones during this period. (b) The brain-plasma exchange of T3 is slow compared with that of L or R. (c) This, along with the active local production, explains the predominant role of the latter as a source of T3 for the brain. (d) In hypothyroidism, the Cx is protected by an increase in the efficiency of T4 to T3 conversion and a prolong residence time of T3 in the tissue, whereas the Cm is protected only by the latter. Because of the large fraction of the T3 produced locally and the active turnover rate of T3 in the brain, reductions in T3 removal rate are of utmost importance for T3 homeostasis in these tissues.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balsam A., Sexton F., Ingbar S. H. The effect of thyroidectomy, hypophysectomy, and hormone replacement on the formation of triiodothyronine from thyroxine in rat liver and kidney. Endocrinology. 1978 Nov;103(5):1759–1767. doi: 10.1210/endo-103-5-1759. [DOI] [PubMed] [Google Scholar]
- Bellabarba D., Peterson R. E., Sterling K. An improved method for chromatography of iodothyronines. J Clin Endocrinol Metab. 1968 Feb;28(2):305–307. doi: 10.1210/jcem-28-2-305. [DOI] [PubMed] [Google Scholar]
- Chopra I. J. A study of extrathyroidal conversion of thyroxine (T4) to 3,3',5-triiodothyronine (T3) in vitro. Endocrinology. 1977 Aug;101(2):453–463. doi: 10.1210/endo-101-2-453. [DOI] [PubMed] [Google Scholar]
- Crantz F. R., Larsen P. R. Rapid thyroxine to 3,5,3'-triiodothyronine conversion and nuclear 3,5,3'-triiodothyronine binding in rat cerebral cortex and cerebellum. J Clin Invest. 1980 Apr;65(4):935–938. doi: 10.1172/JCI109749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crantz F. R., Silva J. E., Larsen P. R. An analysis of the sources and quantity of 3,5,3'-triiodothyronine specifically bound to nuclear receptors in rat cerebral cortex and cerebellum. Endocrinology. 1982 Feb;110(2):367–375. doi: 10.1210/endo-110-2-367. [DOI] [PubMed] [Google Scholar]
- Harris A. R., Fang S. L., Vagenakis A. G., Braverman L. E. Effect of starvation, nutriment replacement, and hypothyroidism on in vitro hepatic T4 to T3 conversion in the rat. Metabolism. 1978 Nov;27(11):1680–1690. doi: 10.1016/0026-0495(78)90290-1. [DOI] [PubMed] [Google Scholar]
- Kaplan M. M. Thyroxine 5'-monodeiodination in rat anterior pituitary homogenates. Endocrinology. 1980 Feb;106(2):567–576. doi: 10.1210/endo-106-2-567. [DOI] [PubMed] [Google Scholar]
- Kaplan M. M., Utiger R. D. Iodothyronine metabolism in rat liver homogenates. J Clin Invest. 1978 Feb;61(2):459–471. doi: 10.1172/JCI108957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan M. M., Yaskoski K. A. Effects of congenital hypothyroidism and partial and complete food deprivation on phenolic and tyrosyl ring iodothyronine deiodination in rat brain. Endocrinology. 1982 Mar;110(3):761–767. doi: 10.1210/endo-110-3-761. [DOI] [PubMed] [Google Scholar]
- Kaplan M. M., Yaskoski K. A. Maturational patterns of iodothyronine phenolic and tyrosyl ring deiodinase activities in rat cerebrum, cerebellum, and hypothalamus. J Clin Invest. 1981 Apr;67(4):1208–1214. doi: 10.1172/JCI110136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan M. M., Yaskoski K. A. Phenolic and tyrosyl ring deiodination of iodothyronines in rat brain homogenates. J Clin Invest. 1980 Sep;66(3):551–562. doi: 10.1172/JCI109887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochupillai N., Yalow R. S. Preparation, purification, and stability of high specific activity 125I-labeled thyronines. Endocrinology. 1978 Jan;102(1):128–135. doi: 10.1210/endo-102-1-128. [DOI] [PubMed] [Google Scholar]
- Larsen P. R. Direct immunoassay of triiodothyronine in human serum. J Clin Invest. 1972 Aug;51(8):1939–1949. doi: 10.1172/JCI107000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen P. R., Dockalova J., Sipula D., Wu F. M. Immunoassay of thyroxine in unextracted human serum. J Clin Endocrinol Metab. 1973 Aug;37(2):177–182. doi: 10.1210/jcem-37-2-177. [DOI] [PubMed] [Google Scholar]
- Larsen P. R., Silva J. E., Kaplan M. M. Relationships between circulating and intracellular thyroid hormones: physiological and clinical implications. Endocr Rev. 1981 Winter;2(1):87–102. doi: 10.1210/edrv-2-1-87. [DOI] [PubMed] [Google Scholar]
- Leonard J. L., Mellen S. A., Larsen P. R. Thyroxine 5'-deiodinase activity in brown adipose tissue. Endocrinology. 1983 Mar;112(3):1153–1155. doi: 10.1210/endo-112-3-1153. [DOI] [PubMed] [Google Scholar]
- Leonard J. L., Rennke H., Kaplan M. M., Larsen P. R. Subcellular distribution of iodothyronine 5'-deiodinase in cerebral cortex from hypothyroid rats. Biochim Biophys Acta. 1982 Sep 17;718(1):109–119. doi: 10.1016/0304-4165(82)90015-0. [DOI] [PubMed] [Google Scholar]
- Leonard J. L., Rosenberg I. N. Subcellular distribution of thyroxine 5'-deiodinase in the rat kidney: a plasma membrane location. Endocrinology. 1978 Jul;103(1):274–280. doi: 10.1210/endo-103-1-274. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Koerner D., Surks M. I. Limited binding capacity sites for L-triiodothyronine in rat liver nuclei. Nuclear-cytoplasmic interrelation, binding constants, and cross-reactivity with L-thyroxine. J Clin Invest. 1974 Mar;53(3):768–777. doi: 10.1172/JCI107615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Surks M. I. Determination of common parameters fo iodothyronine metabolism and distribution in man by noncompartmental analysis. J Clin Endocrinol Metab. 1975 Aug;41(2):319–324. doi: 10.1210/jcem-41-2-319. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Surks M. I. Letter: (to the editor). Erratum: revised calculations of common parameters of iodothyronine metabolism and distribution by noncompartmental analysis. J Clin Endocrinol Metab. 1975 Dec;41(06):1172–1173. doi: 10.1210/jcem-41-6-1172. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Surks M. I. Tissue differences in the concentration of triiodothyronine nuclear binding sites in the rat: liver, kidney, pituitary, heart, brain, spleen, and testis. Endocrinology. 1974 Sep;95(3):897–903. doi: 10.1210/endo-95-3-897. [DOI] [PubMed] [Google Scholar]
- Pardridge W. M. Transport of protein-bound hormones into tissues in vivo. Endocr Rev. 1981 Winter;2(1):103–123. doi: 10.1210/edrv-2-1-103. [DOI] [PubMed] [Google Scholar]
- Silva J. E., Gordon M. B., Crantz F. R., Leonard J. L., Larsen P. R. Qualitative and quantitative differences in the pathways of extrathyroidal triiodothyronine generation between euthyroid and hypothyroid rats. J Clin Invest. 1984 Apr;73(4):898–907. doi: 10.1172/JCI111313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J. E., Larsen P. R. Comparison of iodothyronine 5'-deiodinase and other thyroid-hormone-dependent enzyme activities in the cerebral cortex of hypothyroid neonatal rat. Evidence for adaptation to hypothyroidism. J Clin Invest. 1982 Nov;70(5):1110–1123. doi: 10.1172/JCI110699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J. E., Leonard J. L., Crantz F. R., Larsen P. R. Evidence for two tissue-specific pathways for in vivo thyroxine 5'-deiodination in the rat. J Clin Invest. 1982 May;69(5):1176–1184. doi: 10.1172/JCI110554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J. E., Matthews P. Thyroid hormone metabolism and the source of plasma triiodothyronine in 2-week-old rats: effects of thyroid status. Endocrinology. 1984 Jun;114(6):2394–2405. doi: 10.1210/endo-114-6-2394. [DOI] [PubMed] [Google Scholar]
- Silva J. E., Silva S. Interrelationships among serum thyroxine, triiodothyronine, reverse triiodothyronine, and thyroid-stimulating hormone in iodine-deficient pregnant women and their offspring: effects of iodine supplementation. J Clin Endocrinol Metab. 1981 Apr;52(4):671–677. doi: 10.1210/jcem-52-4-671. [DOI] [PubMed] [Google Scholar]
- Surks M. I., Schadlow A. R., Stock J. M., Oppenheimer J. H. Determination of iodothyronine absorption and conversion of L-thyroxine (T 4 ) to L-triiodothyronine (T 3 ) using turnover rate techniques. J Clin Invest. 1973 Apr;52(4):805–811. doi: 10.1172/JCI107244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser T. J., Kaplan M. M., Leonard J. L., Larsen P. R. Evidence for two pathways of iodothyronine 5'-deiodination in rat pituitary that differ in kinetics, propylthiouracil sensitivity, and response to hypothyroidism. J Clin Invest. 1983 Apr;71(4):992–1002. doi: 10.1172/JCI110854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser T. J., Leonard J. L., Kaplan M. M., Larsen P. R. Kinetic evidence suggesting two mechanisms for iodothyronine 5'-deiodination in rat cerebral cortex. Proc Natl Acad Sci U S A. 1982 Aug;79(16):5080–5084. doi: 10.1073/pnas.79.16.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeke J., Orskov H. Synthesis of 125I monolabelled 3, 5, 3'-triiodothyronine and thyroxine of maximum specific activity for radioimmunoassay. Scand J Clin Lab Invest. 1973 Dec;32(4):357–360. doi: 10.3109/00365517309084359. [DOI] [PubMed] [Google Scholar]
- Zimmerman C. J., Izumi M., Larsen P. R. Isolation of labeled triiodothyronine from serum using affinity chromatography: application to the extimation of the peripheral T4 to T3 conversion in rats. Metabolism. 1978 Mar;27(3):303–313. doi: 10.1016/0026-0495(78)90110-5. [DOI] [PubMed] [Google Scholar]
- van Doorn J., van der Heide D., Roelfsema F. Sources and quantity of 3,5,3'-triiodothyronine in several tissues of the rat. J Clin Invest. 1983 Nov;72(5):1778–1792. doi: 10.1172/JCI111138. [DOI] [PMC free article] [PubMed] [Google Scholar]