Abstract
Over the past two decades, slow but deliberate progress has been made in understanding the genetics of CLL and how the surrounding microenvironment influences leukemia cell survival. The complexity of CLL with respect to different chromosomal aberrations, lack of a common aberrant signaling pathway activation, and associated immune suppression of the disease has been seen a major stumbling block for developing a single targeted therapy similar to imatinib used in chronic myeloid leukemia (CML). The upcoming therapeutic era we are entering with the B-cell receptor (BCR) tyrosine kinase inhibitors ibrutinib and idelalisib, appear to be overcoming this obstacle. Indeed, for the large majority of patients it appears that application of BCR kinase inhibitors can promote durable remissions without the need for chemotherapy. Where other very active targeted agents such as ABT-199, therapeutic antibodies, and chimeric antigen receptor-modified T-cells will be used in CLL also represents a major question that future clinical trials will answer.
Background
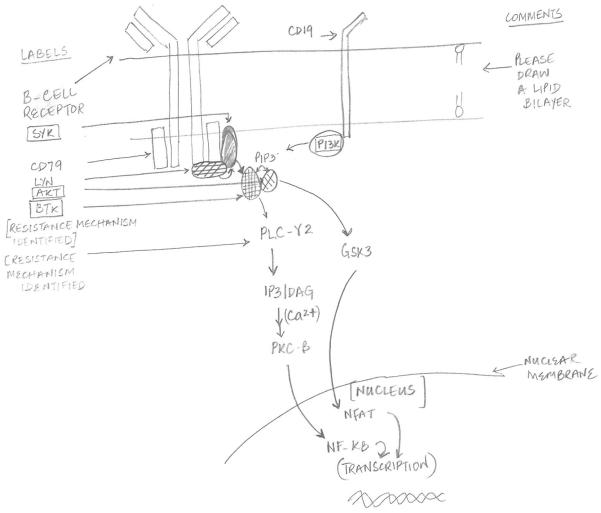
The advent of B-cell receptor kinase inhibitors signals a landmark event in the management of patients with chronic lymphocytic leukemia (CLL). Kinase inhibitors are not just another treatment option for these patients but have the potential to effectively transform the treatment paradigm for this disease. Early results with these agents including ibrutinib and idelalisib that target Bruton tyrosine kinase (BTK) and Phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K) respectively, have shown promising efficacy and excellent tolerability that allows for prolonged treatment. Moreover, discovery of novel prognostic markers and their significance with regards to disease progression and response to therapy has also improved personalized therapy for these patients (Fig. 1).
Figure 1.
Overview of Bruton's tyrosine kinase (BTK) and Phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K) signaling in CLL B-cell. Multiple kinases and second messengers are involved and regulate specific transcription factors as indicated. Inhibitors of targets indicated by boxes are currently in clinical trials for patients with CLL. PIP3, phosphatidylinositol-3,4,5,-trisphosphate; SYK, spleen tyrosine kinase; phospholipase Cγ2 (PLCγ2); DAG, diacylglycerol; PKC, protein kinase C; GSK, glycogen synthase kinase; nuclear factor-κB (NF-κB); nuclear receptor of activated T cells (NFAT).
BTK was first discovered as the defective protein kinase in the inherited disease X-linked agammaglobulinemia that is characterized by a severe immune deficient state (1). A deleterious mutation of the BTK results in severely impaired B-cell development and subsequent impaired B-cell immunity (2). Furthermore, stimulation of the B-cell receptor (BCR) results in induction of tyrosine phosphorylation and activation of BTK and subsequent triggering of multiple pathways involved in B-cell survival (2). CLL B-cells have been shown to have an increased level of BTK that can be activated by the autonomous BCR activation recognized in CLL B-cells (3). Ibrutinib is a first-in-class, irreversible inhibitor of BTK. It covalently binds to Cys-481 in the ATP binding domain of the BTK molecule and abrogates BCR mediated survival signals. Ibrutinib also has the ability to irreversibly target IL-2 inducible T-cell kinase (ITK) in T-cells potentiating Th-1 driven immune responses (4), thus potentially reversing tumor induced T-cell anergy and providing an alternative immune modulating role for this treatment.
Recent reports from early phase clinical trials of ibrutinib in patients with relapsed CLL showed on overall response (OR) rate of 71% with an additional 20% of patients experiencing partial response with lymphocytosis (PR+L). These responses were independent of conventional clinical and genetic factors and resulted in progression free survival of 75% at 26-months (5). Similar exciting results were reported in elderly, treatment naïve patients treated with ibrutinib with OR rate of 71% and 13% PR+L. These responses also appear to be sustained over time with progression free survival (PFS) of 96.1% at 2–years (6). Ibrutinib in general is well-tolerated with the most common side effects being mild diarrhea, nausea and fatigue. Interestingly PR+L does not appear to predict for inferior PFS (7).
PI3K are a family of enzymes involved in an extraordinarily diverse group of cellular functions, including cell growth, proliferation, differentiation, motility, survival and intracellular trafficking (8). Many of these functions relate to the ability of class I PI3Ks to activate the PI3K/AKT/mTOR pathway (9). The p110δ isoform regulates different aspects of cellular proliferation and survival and is constitutively over-expressed in CLL B-cells (10). Idelalisib is an orally bioavailable, first-in-class isoform selective PI3K-δ inhibitor that promoted apoptosis of CLL B-cells ex vivo. It was also shown to be successful in abrogating the survival signal provided by the micrenvironment (11).
In a recent report of a phase I trial (12), idelalisib was evaluated in patients with relapsed/refractory high-risk CLL patients and resulted in an OR rate of 72% (including PR + L). The median PFS was 15.8 months. Therapy was generally well-tolerated with the most commonly observed Grade ≥3 adverse events being pneumonia (20%), neutropenic fever (11%) and diarrhea (6%).
Based on these early results, multiple studies are currently underway to further improve the outcomes of CLL patients treated with kinase inhibitors. This review focuses on the ongoing development of these agents.
On the Horizon
Chemoimmunotherapy vs. kinase inhibitors
Despite the early excitement about the use of kinase inhibitors in the management of CLL, long-term data is lacking and multiple questions are still unanswered about the durability of their efficacy and long-term disease control. Early results however, indicate that in patients with treatment naïve CLL, PFS was 96.1% at 2-years despite the lower complete response (CR) and OR rates (6). This compares extremely favorably to a failure free survival of 51% at 6 years reported with the use of fludarabine, cyclophosphamide and rituximab (FCR) chemoimmunotherapy (13). However, long term data reported from the initial cohort of patients treated with FCR at M. D. Anderson cancer center reveals that patients with mutated IGHV and 13q deletion have extremely prolonged PFS with no relapses reported after 10-years (14). These issues are being addressed by two large intergroup trials currently underway to compare ibrutinib to chemoimmunotherapy as first line therapy.
A significant issue with the use of chemotherapy has been the incidence of infectious complications. Specifically, treatment with regimens like FCR results in prolonged significant cytopenias (grade 2–4), and resulting infectious complications ranging from 35% at 3 months to 12% at 9 months. Moreover, 38% of the patients develop infectious complications if they were cytopenic at 9 months (15). Fludarabine based regimens are also poorly tolerated in patients above 65–70 years of age, with multiple co-morbid conditions and patients with impaired renal function. This is in contrast to data reported from the ibrutinib trials that demonstrates a progressive decline in the incidence of infectious complications with ongoing therapy (5) with excellent tolerability in older patients and patients with co-morbidities. This may preclude the use of routine anti-microbial prophylaxis commonly utilized with chemoimmunotherapy. Moreover, improvements were also observed in stress, depressive symptoms, fatigue, and quality-of-life in patients treated with ibrutinib (16); factors that are generally adversely impacted while patients are undergoing chemotherapy. Likewise, a combination of idelalisib and rituximab was also found to be safe and effective in patients with compromised renal function and co-morbid conditions (17).
Chemotherapy use, especially when nucleoside analogues are combined with alkylating agents, is associated with a significant rate of therapy related secondary malignancies in up to 35% and more importantly, therapy related myeloid neoplasms in up to 10% of patients (13, 15, 18). Current follow-up of ongoing studies with kinase inhibitors is too short to make definitive conclusions about the risks of secondary malignancies in patients who will be exposed to these agents for an extended period. These effects would need to be evaluated in on going post-approval studies.
Early results from the use of combination therapy of kinase inhibitors with targeted therapies like rituximab have also resulted in encouraging early outcomes. The combination of ibrutinib and rituximab in patients with high-risk CLL was generally well-tolerated and resulted in an OR rate of 95% and a PFS of 78% at 18-months (19). Patients did experience a higher incidence of atrial fibrillation and bleeding diathesis possibly due to collagen-mediated platelets aggregation defect (20, 21). Similarly, the combination of idelalisib and rituximab resulted in an OR rate of 81% and PFS at 1 year in excess of 90%. Serious toxicities observed with idelalisib included transaminase elevations, diarrhea with colitis and pneumonitis that were primarily observed after continued drug exposure (12).
The question of whether kinase inhibitors will replace chemotherapy as first-line therapy for patients with CLL remains to be answered; but a vast majority of patients are likely to consider or be prescribed oral kinase inhibitors as these agents become more acceptable in the oncology community. They are more likely to be used in combination with other targeted therapies and patient and cost preference will probably dictate a limited treatment course.
Establishing endpoints for kinase inhibitor therapy
Another issue that might become more relevant in the future is the duration of therapy with kinase inhibitors. No consensus currently exists for the appropriate end-point of therapy with kinase inhibitors. There is also no validated clinical or laboratory endpoint that might be used as a surrogate for a stopping rule. Minimal residual disease (MRD) status has been established as a marker for predicting survival in patients treated with kinase inhibitors (17) and is likely to be utilized for making treatment discontinuation decisions. However, since monotherapy with kinase inhibitors have not been shown to result in MRD negative states, various combinations with different targeted therapies are being explored to achieve MRD negative status and potentially limit the duration of therapy and possibly the emergence of resistance to kinase inhibitors. Early results with ibrutinib in combination with rituximab show a small percentage of patients with MRD negative disease at 12 months of therapy (22) arguing for combining targeted therapies with kinase inhibitors to effect a deeper remission.
Kinase inhibitors in patients with high-risk CLL
Outcomes in patients with high-risk del(17p) CLL is an area of special interest, since these patients have rapidly progressive disease and limited effective therapeutic options. Recently reported FCR data reveals an OR rate of 33% with a median PFS of 14 months in this group of patients (23). Various other agents including alemtuzumab (24) and rituximab (25) in combination with high dose steroids and oxaliplatin, fludarabine, cytarabine and rituximab (OFAR) (26), flavopiridol (27) and lenalidomide (28) based combinations result in similar response rates and short PFS.
Early data from ibrutinib in patients with del(17p) reveals an OR rate of 55.9% with a median duration of response of 25 months. Ibrutinib was also well tolerated with gradual decline in the incidence of adverse events with progressive therapy (29). Our institutional historical comparison also revealed a significantly improved response rate and PFS when ibrutinib was compared to either cyclin-dependent kinase inhibitors or other conventional therapies (30). Similar exciting results have been preliminarily reported in patients treated with a combination of idelalisib and rituximab with OR rates around 80% and PFS at 1-year in excess of 75% (17). However, despite the fact that therapy with kinase inhibitors may result in improved response as compared to conventional therapies, the outcomes of patients with these high-risk mutations are still inferior to patients without these abnormalities. This suggests that kinase inhibitors are not able to completely overcome the adverse prognosis conferred by the presence of these factors (5). Efforts are currently underway to further improve outcomes in patients with this high-risk group by utilizing various combinations of kinase inhibitors with targeted therapies.
Kinase inhibitors in the initial treatment of high-risk patients not meeting criteria for therapy
Current International Workshop on Chronic Lymphocytic Leukemia (IWCLL) guidelines (31) recommend initiating therapy for CLL at the onset of symptoms or documentation of clinically significant progressive disease. This is based on historical data that failed to demonstrate a survival advantage with early treatment of patients with CLL (32). Recent attempts to treat patients early with high-risk disease on clinical trials were halted because of poor accrual. However, these treatments were primarily based on chemoimmunotherapeutic regimens with associated toxicities. Given the excellent tolerability and impressive responses observed in patients treated with kinase inhibitors, efforts are currently underway to evaluate their role when used in patients with high-risk disease at the time of diagnosis prior to them meeting the conventional criteria for initiating therapy. This approach has the potential to significantly prolong overall survival of patients with CLL and early disease control might limit the development of immune dysfunction and resulting infectious complications which is the leading cause of morbidity and mortality in patients with CLL (33).
Resistance to kinase inhibitors and strategies to overcome it
Chronic exposure to kinase inhibitors might result in clonal selection pressure resulting in the emergence of resistant malignant cell clones. Recent data derived from whole exome sequencing of paired samples at baseline and at the time of relapse while being treated with ibrutinib, identified a cysteine-to-serine mutation in BTK at the binding site of ibrutinib and three distinct mutations in PLCγ2 (34). Functional analysis revealed that the C481S mutation of BTK results in a protein that is only reversibly inhibited by ibrutinib. The R665W and L845F mutations in PLCγ2 are both potentially gain-of-function mutations that lead to autonomous B-cell-receptor activity. Trials are currently underway of innovative BTK inhibitors that bind to an alternative site in the BTK protein and may potentially be effective in patients who acquire resistance to ibrutinib through the binding site mutation. Interestingly, these mutations were not found in any of the patients with prolonged lymphocytosis who were taking ibrutinib, suggesting an alternative and as yet unidentified mechanism for the persistence of lymphocytosis in those patients. Efforts are currently under way to identify specific resistance mechanisms to other novel therapies and kinase inhibitors. This will enable us to develop specific agents that have the ability to overcome these resistance pathways and eventually develop therapeutic protocols that can be utilized in combination at the outset and limit the development of these resistance mechanisms.
New frontiers
Multiple therapies are currently at various stages of development for the treatment of patients with CLL. These include alternative kinase inhibitors including spleen tyrosine kinase (SYK) (35), cyclin dependent kinase (CDK) (36) and others; antibodies and antibody like molecules targeting various surface antigens on CLL B-cells including CD20 (37), CD19 (38, 39) and CD37 (40), etc. and various other molecules specifically designed either to directly target CLL B-cells or overcome the micro-environmental signals that provide the CLL B-cells a survival advantage, e.g. ABT-199 (41), lenalidomide (28).
One of the most significant advances in the treatment of patients with CLL has been the development of autologous chimeric antigen receptor-modified T-cells directed towards the CD19 antigen (42). Autologous lentiviral modified T-cells were able to persist in vivo for an extended period and were able to induce prolonged clinical responses in the majority of patients (42, 43). Therapy was however, associated with significant cytokine release syndrome necessitating intensive supportive care (44, 45). Moreover, treatment resulted in the elimination of normal B-cells and subsequent sustained hypogammaglobulinemia. Aggressive supportive care and infection prophylaxis can limit the incidence of infectious complications in these patients and result in prolonged disease control. Larger multi-institutional trials are currently underway to further develop and enhance this potentially curative therapy.
Therapy for CLL has undergone remarkable progress over the last few years with multiple new agents being approved by the FDA or in the process of obtaining approval. The majority of these agents are generally well-tolerated oral agents with remarkable efficacy. Together with antibodies and kinase inhibitors (46), various combinations of these therapies have the potential to transform care of patients with CLL and potentially affect the ever-elusive cure with limited adverse effects.
Acknowledgments
Grant Support F.T. Awan was supported by a Career Development Award from the Lymphoma Research Foundation. J.C. Byrd was supported by the NCI (P50-CA140158), the Specialized Center of Research from the Leukemia and Lymphoma Society, the D. Warren Brown Foundation, and the Four Winds Foundation.
Footnotes
Disclosure of Potential Conflicts of Interest No potential conflicts of interest were disclosed.
References
- 1.Buckley RH. Primary immunodeficiency diseases due to defects in lymphocytes. N Engl J Med. 2000;343:1313–24. doi: 10.1056/NEJM200011023431806. [DOI] [PubMed] [Google Scholar]
- 2.Herman SE, Gordon AL, Hertlein E, Ramanunni A, Zhang X, Jaglowski S, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood. 2011;117:6287–96. doi: 10.1182/blood-2011-01-328484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duhren-von Minden M, Ubelhart R, Schneider D, Wossning T, Bach MP, Buchner M, et al. Chronic lymphocytic leukaemia is driven by antigen-independent cell-autonomous signalling. Nature. 2012;489:309–12. doi: 10.1038/nature11309. [DOI] [PubMed] [Google Scholar]
- 4.Dubovsky JA, Beckwith KA, Natarajan G, Woyach JA, Jaglowski S, Zhong Y, et al. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood. 2013;122:2539–49. doi: 10.1182/blood-2013-06-507947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369:32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Brien S, Furman RR, Coutre SE, Sharman JP, Burger JA, Blum KA, et al. Ibrutinib as initial therapy for elderly patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma: an open-label, multicentre, phase 1b/2 trial. Lancet Oncol. 2014;15:48–58. doi: 10.1016/S1470-2045(13)70513-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woyach JA, Smucker K, Smith LL, Lozanski A, Zhong Y, Ruppert AS, et al. Prolonged lymphocytosis during ibrutinib therapy is associated with distinct molecular characteristics and does not indicate a suboptimal response to therapy. Blood. 2014;123:1810–7. doi: 10.1182/blood-2013-09-527853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.So L, Fruman DA. PI3K signalling in B- and T-lymphocytes: new developments and therapeutic advances. Biochem J. 2012;442:465–81. doi: 10.1042/BJ20112092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bunney TD, Katan M. Phosphoinositide signalling in cancer: beyond PI3K and PTEN. Nat Rev Cancer. 2010;10:342–52. doi: 10.1038/nrc2842. [DOI] [PubMed] [Google Scholar]
- 10.Chantry D, Vojtek A, Kashishian A, Holtzman DA, Wood C, Gray PW, et al. p110delta, a novel phosphatidylinositol 3-kinase catalytic subunit that associates with p85 and is expressed predominantly in leukocytes. J Biol Chem. 1997;272:19236–41. doi: 10.1074/jbc.272.31.19236. [DOI] [PubMed] [Google Scholar]
- 11.Herman SE, Gordon AL, Wagner AJ, Heerema NA, Zhao W, Flynn JM, et al. Phosphatidylinositol 3-kinase-delta inhibitor CAL-101 shows promising preclinical activity in chronic lymphocytic leukemia by antagonizing intrinsic and extrinsic cellular survival signals. Blood. 2010;116:2078–88. doi: 10.1182/blood-2010-02-271171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown JR, Byrd JC, Coutre SE, Benson DM, Flinn IW, Wagner-Johnston ND, et al. Idelalisib, an inhibitor of phosphatidylinositol 3-kinase p110delta, for relapsed/refractory chronic lymphocytic leukemia. Blood. 2014;123:3390–7. doi: 10.1182/blood-2013-11-535047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tam CS, O'Brien S, Wierda W, Kantarjian H, Wen S, Do KA, et al. Long-term results of the fludarabine, cyclophosphamide, and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood. 2008;112:975–80. doi: 10.1182/blood-2008-02-140582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strati P, Keating M, O'Brien S, Burger J, Ferrajoli A, Jain N, et al. Early bone marrow MRD negativity with first-line FCR in CLL may prompt consideration of stopping treatment. 15th International Workshop on Chronic Lymphocytic Leukaemia (iwCLL 2013); Cologne, Germany. 2013 Sep 9–11; p. 5. abstract. 2013. Abstract nr 21. [Google Scholar]
- 15.Strati P, Wierda W, Burger J, Ferrajoli A, Tam C, Lerner S, et al. Myelosuppression after frontline fludarabine, cyclophosphamide, and rituximab in patients with chronic lymphocytic leukemia: analysis of persistent and new-onset cytopenia. Cancer. 2013;119:3805–11. doi: 10.1002/cncr.28318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Godiwala N, Maddocks K, Westbrook TB, J, Andersen B, Johnson A. Covariation of psychological and inflammatory variables in patients with chronic lymphocytic leukemia receiving ibrutinib. J Clin Oncol. 2014;32(suppl):5s. abstr 7057. [Google Scholar]
- 17.Furman RR, Sharman JP, Coutre SE, Cheson BD, Pagel JM, Hillmen P, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370:997–1007. doi: 10.1056/NEJMoa1315226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou Y, Tang G, Medeiros LJ, McDonnell TJ, Keating MJ, Wierda WG, et al. Therapy-related myeloid neoplasms following fludarabine, cyclophosphamide, and rituximab (FCR) treatment in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma. Mod Pathol. 2012;25:237–45. doi: 10.1038/modpathol.2011.158. [DOI] [PubMed] [Google Scholar]
- 19.Burger JA, Keating MJ, Wierda WG, Hartmann E, Hoellenriegel J, Rosin NY, et al. Safety and activity of ibrutinib plus rituximab for patients with high-risk chronic lymphocytic leukaemia: a single-arm, phase 2 study. Lancet Oncol. 2014;15:1090–9. doi: 10.1016/S1470-2045(14)70335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamel S, Horton L, Ysebaert L, Levade M, Burbury K, Tan S, et al. Ibrutinib inhibits collagen-mediated but not ADP-mediated platelet aggregation. Leukemia. 2014 Aug 20; doi: 10.1038/leu.2014.247. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 21.Byrd JC, Brown JR, O'Brien S, Barrientos JC, Kay NE, Reddy NM, et al. Ibrutinib versus Ofatumumab in Previously Treated Chronic Lymphoid Leukemia. N Engl J Med. 2014;371:213–23. doi: 10.1056/NEJMoa1400376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burger JA, Keating MJ, Wierda WG, Hoellenriegel J, Ferrajoli A, Faderl S, et al. The Btk inhibitor ibrutinib (PCI-32765) in combination with rituximab is well tolerated and displays profound activity in high-risk chronic lymphocytic leukemia (CLL) patients. Blood (Ash Annual Meeting Abstracts) 2012;120:187. [Google Scholar]
- 23.Strati P, Keating MJ, O'Brien SM, Ferrajoli A, Burger J, Faderl S, et al. Outcomes of first-line treatment for chronic lymphocytic leukemia with 17p deletion. Haematologica. 2014;99:1350–5. doi: 10.3324/haematol.2014.104661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pettitt AR, Jackson R, Carruthers S, Dodd J, Dodd S, Oates M, et al. Alemtuzumab in combination with methylprednisolone is a highly effective induction regimen for patients with chronic lymphocytic leukemia and deletion of TP53: final results of the national cancer research institute CLL206 trial. J Clin Oncol. 2012;30:1647–55. doi: 10.1200/JCO.2011.35.9695. [DOI] [PubMed] [Google Scholar]
- 25.Castro JE, Sandoval-Sus JD, Bole J, Rassenti L, Kipps TJ. Rituximab in combination with high-dose methylprednisolone for the treatment of fludarabine refractory high-risk chronic lymphocytic leukemia. Leukemia. 2008;22:2048–53. doi: 10.1038/leu.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsimberidou AM, Wierda WG, Wen S, Plunkett W, O'Brien S, Kipps TJ, et al. Phase I–II clinical trial of oxaliplatin, fludarabine, cytarabine, and rituximab therapy in aggressive relapsed/refractory chronic lymphocytic leukemia or Richter syndrome. Clin Lymphoma Myeloma Leuk. 2013;13:568–74. doi: 10.1016/j.clml.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stephens DM, Ruppert AS, Maddocks K, Andritsos L, Baiocchi R, Jones J, et al. Cyclophosphamide, alvocidib (flavopiridol), and rituximab, a novel feasible chemoimmunotherapy regimen for patients with high-risk chronic lymphocytic leukemia. Leuk Res. 2013;37:1195–9. doi: 10.1016/j.leukres.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.James DF, Werner L, Brown JR, Wierda WG, Barrientos JC, Castro JE, et al. Lenalidomide and rituximab for the initial treatment of patients with chronic lymphocytic leukemia: a multicenter clinical-translational study from the chronic lymphocytic leukemia research consortium. J Clin Oncol. 2014;32:2067–73. doi: 10.1200/JCO.2013.51.5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Brien S, Furman R, Coutre S, Flinn I, Burger J, Blum K, et al. Independent evaluation of ibrutinib efficacy 3 years post-initiation of monotherapy in patients with chronic lymphocytic leukemia/small lymphocytic leukemia including deletion 17p disease. J Clin Oncol. 2014;32(suppl):5s. abstr 7014. [Google Scholar]
- 30.Stephens DM, Ruppert AS, Jones JA, Woyach J, Maddocks K, Jaglowski SM, et al. Impact of targeted therapy on outcome of chronic lymphocytic leukemia patients with relapsed del(17p13.1) karyotype at a single center. Leukemia. 2014;28:1365–8. doi: 10.1038/leu.2014.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Dohner H, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–56. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chemotherapeutic options in chronic lymphocytic leukemia: a meta-analysis of the randomized trials. CLL Trialists' Collaborative Group. J Natl Cancer Inst. 1999;91:861–8. doi: 10.1093/jnci/91.10.861. [DOI] [PubMed] [Google Scholar]
- 33.Morrison VA. Infectious complications in patients with chronic lymphocytic leukemia: pathogenesis, spectrum of infection, and approaches to prophylaxis. Clin Lymphoma Myeloma. 2009;9:365–70. doi: 10.3816/CLM.2009.n.071. [DOI] [PubMed] [Google Scholar]
- 34.Woyach JA, Furman RR, Liu TM, Ozer HG, Zapatka M, Ruppert AS, et al. Resistance mechanisms for the Bruton's tyrosine kinase inhibitor ibrutinib. N Engl J Med. 2014;370:2286–94. doi: 10.1056/NEJMoa1400029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klein L, Boxer M, Kolibaba KS, Hawkins MJ, Di Paolo JA, Hu J, et al. Phase 2 trial of GS-9973, a selective syk inhibitor, in chronic lymphocytic leukemia (CLL) and non-Hodgkin lymphoma (NHL) J Clin Oncol. 2014;32(suppl):5s. abstr 7059. [Google Scholar]
- 36.Blachly JS, Byrd JC. Emerging drug profile: cyclin-dependent kinase inhibitors. Leuk Lymphoma. 2013;54:2133–43. doi: 10.3109/10428194.2013.783911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goede V, Fischer K, Busch R, Engelke A, Eichhorst B, Wendtner CM, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014;370:1101–10. doi: 10.1056/NEJMoa1313984. [DOI] [PubMed] [Google Scholar]
- 38.Woyach JA, Awan F, Flinn IW, Enoch R, Foster PA, Byrd JC. Final results of a phase I study of the Fc engineered CD19 antibody XmAb(R)5574 (MOR00208) in patients with relapsed or refractory chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) Blood (ASH Annual Meeting Abstracts) 2012;120:2894. [Google Scholar]
- 39.Awan FT, Lapalombella R, Trotta R, Butchar JP, Yu B, Benson DM, Jr., et al. CD19 targeting of chronic lymphocytic leukemia with a novel Fc-domain-engineered monoclonal antibody. Blood. 2010;115:1204–13. doi: 10.1182/blood-2009-06-229039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Byrd JC, Pagel JM, Awan FT, Forero A, Flinn IW, Deauna-Limayo DP, et al. A phase 1 study evaluating the safety and tolerability of otlertuzumab, an anti-CD37 mono-specific ADAPTIR therapeutic protein in chronic lymphocytic leukemia. Blood. 2014;123:1302–8. doi: 10.1182/blood-2013-07-512137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19:202–8. doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- 42.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–33. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kalos M, Levine BL, Macatee TL, Kulikovskaya I, Suppa E, Jena B, et al. Sustained Functional T Cell Persistence and B Cell Aplasia Following CD19-Targeting Adoptive T Cell Immunotherapy for Relapsed, Refractory CD19+ Malignacy. ASH Annual Meeting Abstracts. 2012 Nov 16;120:756. 2012. [Google Scholar]
- 45.Grupp SA, Porter DL, Teachey DT, Barrett DM, Chew A, Suppa E, et al. CD19-redirected chimeric antigen receptor T (CART19) cells induce a cytokine release syndrome (CRS) and induction of treatable macrophage activation syndrome (MAS) that can be managed by the IL-6 antagonist tocilizumab (toc) Blood (ASH Annual Meeting Abstracts) 2012;120:2604. [Google Scholar]
- 46.Byrd JC, Brown JR, O'Brien S, Barrientos JC, Kay NE, Reddy NM, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371:213–23. doi: 10.1056/NEJMoa1400376. [DOI] [PMC free article] [PubMed] [Google Scholar]