Abstract
Sex steroid hormones secreted by gonads influence development and expression of many behaviors including parental behaviors. The capacity to display many behaviors develops under the influence of sex steroid hormones; it begins with gonadal differentiation and lasts through puberty. The timing of gonadectomy may have important and long lasting effects on the organization and activation of neural circuits regulating the expression of different behaviors. The present study investigated the importance of exposure to endogenous gonadal steroid hormones during pubertal period/adolescence on parental behavior in adult mice. Male and female WT mice were gonadectomized either before puberty (25 days of age) or after puberty (60 days of age) and tested for parental behavior with and without estradiol benzoate (EB) replacement in adulthood. Additional groups of mice were gonadectomized at P25 and supplemented with estradiol (females) or testosterone (males) during puberty. Female mice gonadectomized after puberty or gonadectomized before puberty and supplemented with estradiol during puberty, displayed better pup directed parental behaviors in comparison to mice gonadectomized at 25 days of age regardless treatment with estradiol in adulthood. However, mice treated with EB in adulthood displayed better non-pup directed nest building behavior than when they were tested without EB treatment regardless of sex and time of gonadectomy. To examine whether the sensitivity to sex steroid hormones was altered due to differences in time without gonads prior to the testing, mice were also tested for female sex behavior and there were no differences between mice gonadectomized at P25 or P60, although this could not completely rule out the possibility that parental behavior is more sensitive to prolonged absence of steroid hormones than female sex behavior. These results suggest that the absence of gonads and thereby the absence of appropriate gonadal steroid hormones during puberty/adolescence may have a profound effect on pup directed parental behaviors in adult mice.
Keywords: mice, parental behavior, gonadal hormones, puberty/adolescence
Introduction
Gonadal steroid hormones influence the development of brain and consequently behavior. Steroid hormones affect brain development and activity through two relatively separable processes characterized as organizational versus activational (Arnold and Breedlove, 1985; Schulz et al., 2009; Sisk and Foster, 2004). Usually, more permanent organizational effects occur perinatally, while activational effects occur later in life when gonadal hormones act on specific neural circuits to trigger specific aspects of physiology or the expression of various adult behaviors (reviewed in (Arnold, 2009; Majdic and Tobet, 2011; McCarthy and Arnold, 2011). A growing number of studies also show that gonadal hormones can have “organizational” effects on the brain later in life, during puberty and possibly in adult life (Ahmed et al., 2008; De Lorme et al., 2012; Romeo, 2003; Romeo et al., 2000; Schulz and Sisk, 2006; Schulz et al., 2009). Puberty is a period during which the hypothalamic-pituitary-gonadal axis reactivates with the elevated secretion of gonadal steroid hormones (Sisk and Zehr, 2005). Many brain regions are sensitive to the action of gonadal steroid hormones, including parts of the brain that are thought to be involved in the regulation of parental behavior (Kalinichev et al., 2000).
Parental behavior is broadly defined as any behavior performed in the relation to one’s offspring, or as any behavior that contributes directly to the survival of fertilized eggs or newborns. In mammals, maternal care is more common than paternal, although paternal behavior is also present in several mammalian species including mice and humans (Nelson, 2005). In many species, maternal behavior is triggered by the exposure to steroid hormones during pregnancy. However, nulliparous (virgin) female rats and mice exhibit parental behavior when presented with foster pups even without circulating gonadal steroids (Numan and Insel, 2003). By contrast, male rats and mice are commonly infanticidal (Lonstein and De Vries, 2000), although a recent study by Tachikawa et al (Tachikawa et al., 2013) demonstrated that male mice are not aggressive toward pups if cohabited with female mice for two weeks after mating. In this study, it was suggested that pheromonal cues are responsible for aggressive behavior of males toward pups and this response is diminished if fathers are cohabited with pregnant females or the vomeronasal organ in sexually naïve males has been surgically removed. Many studies of parental behavior in mice and rats, and influences of gonadal steroid hormones on parental behavior, have been done in parturient rodents (e.g. (Lonstein et al., 1999; Stolzenberg and Rissman, 2011) or in virgin, gonadally intact or gonadectomized rodents in adulthood (e.g. (Gatewood et al., 2006; Koch and Ehret, 1989; Lonstein et al., 1999; Ogawa et al., 1998a; Okabe et al., 2013; Okabe et al., 2010; Stolzenberg and Rissman, 2011). Gonadectomy in adult life seems to have little effect on parental behavior in adult virgin female mice (Ogawa et al., 1998a) and rats (Lonstein et al., 1999) while in male mice, published results differ with one study reporting improvement of paternal behavior after gonadectomy (Okabe et al., 2013) and another finding no influence of adult gonadectomy on paternal behavior (Gatewood et al., 2006). Although pup retrieval behavior can improve with gonadectomy (or social experience) in adulthood in mice of both sexes (Okabe et al., 2010), we were not aware of reports concerning the possible consequences of juvenile gonadectomy on the development of parental behavior in mice. Therefore, the present study examined parental behavior in mice of both sexes that were gonadectomized at 25 days of age (before puberty) or at 60 days of age (after puberty) to explore whether the prolonged absence of gonads during pubertal/adolescent period can impact parental behaviors directed towards foster pups in adult mice. To check whether exposure to sex steroid hormones during puberty is necessary for the development of parental behavior directed towards foster pups in adult mice, some mice were also gonadectomized before puberty and supplemented with sex steroid hormones during puberty.
Materials and Methods
Animals
C57BL/6J male and female mice were originally obtained from Harlan Italy and bred at the University of Ljubljana, Veterinary Faculty, in standard conditions with 12–12 LD cycle (lights on at 3 am and off at 3 pm) and food (phytoestrogen free diet; Harlan Teklad Diet 2016, Harlan, Milan, Italy) and water ad libitum. Mice were weaned at 21 days of age and mice from the same litters were group-housed (3 mice of same sex per cage). Mice were housed in 15 cm high cages with floor area of 37.5 × 22 cm. First groups of male (n = 11) and female mice (n = 10) were gonadectomized (GDX) at 25 days of age (before puberty), the second groups of male (n = 5) and female (n = 8) mice were gonadectomized at 60 days of age (after puberty) and additional groups of male (n = 4) and female mice (n = 4) were gonadectomized at 25 days of age (before puberty) and had inserted testosterone (T) or estradiol benzoate (EB) silastic implants, respectively, from 25 to 60 days of age (during puberty).
Foster pups for assessing parental behavior were obtained from the mice of the same strain, bred in the same conditions.
Sexually experienced stimulus males (C57BL/6J), previously used for mating (at least 3 successful mating with weaned litters), were used for assessing sexual behavior in female mice. Stimulus males were housed individually in 13 cm high cages with 28.5 × 10.5 cm floor area and bred also in the same conditions.
All animal experiments were approved by Veterinary Administration of Slovenia (licence no. 34401-32/2012/8) and were done according to ethical principles, EU Directive 2010/63/EU and NIH guidelines.
Surgery and hormone replacement
Male and female mice were gonadectomized bilaterally at 25 or 60 days of age to remove endogenous gonadal steroids. Mice were anesthetized with the mixture of ketamine (Vetoquinol Biowet, Gorzowie, Poland; 100 µg/g BW), acepromazine (Fort Dodge Animal Health, Fort Dodge, IA, USA; 2 µg/g BW) and xylazine (Chanelle Pharmaceuticals Ltd., Loughrea, Ireland; 10 µg/g BW) and gonads were excised through small incisions. Incisions were stitched and mice received two injections of butorfanol (Turbogesic, Fort Dodge Animal Health, Fort Dodge, IA, USA; 2 µg/g BW) after surgery to ease any potential pain. The additional group of males and females, gonadectomized at 25 days of age, received during gonadectomy subcutaneous silastic implants (1.02 mm inner diameter, 2.16 mm outer diameter) filled with sex steroid hormones according to sex. Males received implants filled 10 mm in length with crystalline testosterone (T; Sigma, Taufkirchen, Germany; (Gatewood et al., 2006) and females received implants filled 5 mm in length with crystalline 17β-estradiol 3-benzoate (EB; Sigma, Taufkirchen, Germany) diluted 1:1 with cholesterol (Wersinger et al., 1999) and closed on both ends by medical silastic adhesive (Dow Corning, Lakeside, AZ, USA). Implants were inserted subcutaneously in the midscapular region. Mice from this group had silastic implants removed in general anesthesia at 60 days of age, after 35 days of hormone exposure. Mice were left to recover for at least 10 days (Seitz et al., 2010) before behavior assessments were performed. Mice were tested as hormonally naïve first and then later after priming with estradiol benzoate (EB; only mice without gonadal hormones priming during puberty). All mice were initially tested twice on two consequent days for parental behavior between 70 and 75 days of age. Two to 3 days after the second test, mice without gonadal hormone priming during puberty received subcutaneous implants of EB and were tested again 10 days after implantation. Silastic implants (1.02 mm inner diameter, 2.16 mm outer diameter) were again filled 5 mm in length with crystalline 17β-estradiol 3-benzoate (EB; Sigma, Taufkirchen, Germany) (diluted 1:1 with cholesterol) (Wersinger et al., 1999) and closed on both ends by medical silastic adhesive (Dow Corning, Lakeside, AZ, USA). Implants were inserted subcutaneously in the midscapular region under anesthesia. Similar implants filled with 17β-estradiol have yielded plasma estradiol levels within the physiological range in female mice (Bakker et al., 2002) and rats (Seale et al., 2004). For female sex behavior tests, implants inserted in adulthood were left in situ and mice were injected subcutaneously with 0.8 mg of progesterone (P; Sigma) approximately 4 to 8 hours before each test. All mice were initially tested for female sexual behavior after conclusion of parental behavior tests between 90 and 100 days of age.
Parental behavior test
Each mouse was tested in a standard parental behavior test 4 times, twice without hormone supplement and twice primed with EB (only mice without gonadal hormones priming during puberty) to examine potential effect of estradiol on parental behavior directed towards foster pups, although this is not mimicking physiological situation during pregnancy where mice are exposed first to high levels of progesterone followed by high estradiol exposure. First two (without hormones) and last two trials (with hormones) were performed on two consecutive days; on the first day the first trial lasted 20 min and the next day the second trial lasted for 15 min as mice were experienced and had shorter latencies to initiate parental behavior. Mice were transferred to smaller test-cages (14 cm high with 35 × 15 cm floor area) with at least three day-old bedding from their home cage 24 h prior to behavioral testing. After the first and last two trials, mice were returned to their larger home cages in their respective social groups.
Parental behavior tests were performed during the light cycle 1 to 2 h before the start of the dark cycle as described by Gatewood et al. (Gatewood et al., 2006). This is supported by previously published study reporting that parental behavior in mice has been reported to be more frequent during the light phase (Shoji and Kato, 2006). Three male foster pups 1 to 3 days old and torn tissue paper for nest building were placed in separate corners of the test-cage on the sides furthest from the nest of the test mouse. Male foster pups were chosen due to their potentially greater attraction to mothers in comparison to neonatal female rats (Moore, 1981, 1985), although in mice differences in maternal behavior, depending on the sex of pups have not been observed (Keller et al., 2010). If pups were attacked, all pups were immediately removed and infanticidal behavior was recorded. During testing, the following parental activities were recorded: latency to visit and lift pups, latency to retrieve the first, second and third pup and the number of pups retrieved into the nest, latency and total time licking and sniffing pups, latency and total time spend crouching over pups, aggression toward pups, latency, number and total time spend building the nest and the quality (zero to three points) of the nest.
All tests were video recorded and an observer (J.K.), blinded to the group assignment at the time of testing, scored all behaviors directly using ‘stopwatch’ software (Center for Behavioral Neurosciences, Atlanta, GA).
Female sexual behavior test
Female sexual behavior was tested to check the hormone sensitivity of mice with extended absence of endogenous steroids. Behavior tests were performed in a clear glass aquarium (17 cm high with 41.5 × 26 cm floor area) with a mirror positioned under the testing arena to obtain views of all facets of sexual behaviors (Wersinger et al., 1997). Females were tested during the first 2 to 4 hours of the beginning of the dark period of the circadian cycle, under dim red light illumination, and the test sessions were videotaped for subsequent scoring. The timing of testing was chosen to mimic the timing of the normal occurrence of mating in mice. As ovulation in intact female mice tend to occur during the late hours of the dark or early hours of the light period (Bingel and Schwartz, 1969) and the female is sexually receptive in the late proestrus/early estrus portion of the cycle (e.g. (Zinck and Lima, 2013), mating in mice normally occurs during the first half of the dark period. Each female was tested 6 times; every 4 to 5 days to mimic the timing of the normal physiological estrous cycle. The first trial served to provide the animals with sexual experience prior to testing the next 5 trials were scored. The stimulus males were placed into the aquarium at least 4 hours prior to behavior testing with at least 3 day old bedding and food and water ad libitum to acclimate to the novel environment. Food and water were removed during the behavior testing.
Hormonally-primed females (EB implants & subcutaneous injection of P 4 to 8 hours prior to behavioral assessment) were placed in the middle of aquaria with stimulus males for 20 minutes (Park, 2011), or until the females received an ejaculation and the following behaviors were recorded: lordosis posture, total number and latency of attempted mounts, successful mounts, pelvic thrusts and intromissions, and the latency of ejaculation. Behaviors were recorded by 'stopwatch' software (Center for Behavioral Neuroscience, Atlanta, GA) and were performed always by the same investigator (J.K.) who was blinded to the group assignment at the time of testing.
If the stimulus male did not try to mount the female after 5 min of testing, the female was transferred to a different cage with new stimulus male. Ejaculating males were not used again for the remainder of the trial day. Mounts were counted when the female had all four limbs on the floor, exhibited lordosis posture and the male successfully clasped its hindquarters with forepaws. Attempted mounts were counted when the female resisted and failed to display lordosis postures when males were clearly trying to mount. Female lordosis posture as an index of sexual receptivity was scored from 0 (no receptive behavior with no lordosis reflex) to 5 (completely receptiveness with strongest lordosis reflex; modification of the 4 scale protocol in (Bakker et al., 2002). Lordosis was defined using the following stipulations: all four paws are grounded, hind region is elevated off the floor of the test chamber, and the back is slightly arched (Kudwa et al., 2007; Takasugi et al., 1983). A lordosis quotient (LQ) was calculated by the following formula: number of mounts during which the female stood still (lordosis 4 and 5)/ total number of attempted and successful mounts × 100.
Blood collection and hormone assay
Four days after the last sexual behavior test, around 125 days of age or approximately 45 days after receiving the EB implants, females were anesthetized with the mixture of ketamine (Vetoquinol Biowet, Gorzowie, Poland; 100 µg/g BW), acepromazine (Fort Dodge Animal Health, Fort Dodge, IA, USA; 2 µg/g BW) and xylazine (Chanelle Pharmaceuticals Ltd., Loughrea, Ireland; 10 µg/g BW) and a blood sample was collected by cardiac puncture. Plasma estradiol concentration was determined by commercial IBL (Hamburg, Germany) ELISA kit following the instructions for users and performed at the Institute of Physiology, Pharmacology and Toxicology, Veterinary faculty, University of Ljubljana. Intra and inter assay coefficients of variation (CVs) were 7.91% and 10.12% for low (62 ± 0.34 pg/mL) and 4.65% and 8.57 % for high (430 ± 0.81 pg/mL) estradiol values, respectively.
Statistical analyses
All statistical analyses were done using NCSS software (NCSS statistical software, Kaysville, UT). To test differences between groups in parental behavior, repeated measures ANOVA was performed with sex and gonadectomy as independent variables, and tests as repeated measures, followed by post hoc Fisher LSD test. To test differences between groups in female sexual behavior, repeated measures ANOVA was performed with gonadectomy/ovariectomy as an independent variable, and tests as a repeated measure, followed by post hoc Fisher LSD test. At least four mice (4 to 9) of each sex were tested in both parental and female sexual behavior tests. Size effect was estimated by calculating eta squared (η2) using formula η2 = SSbetween/SStotal. Differences were considered statistically significant with p < 0.05.
Results
Parental behavior
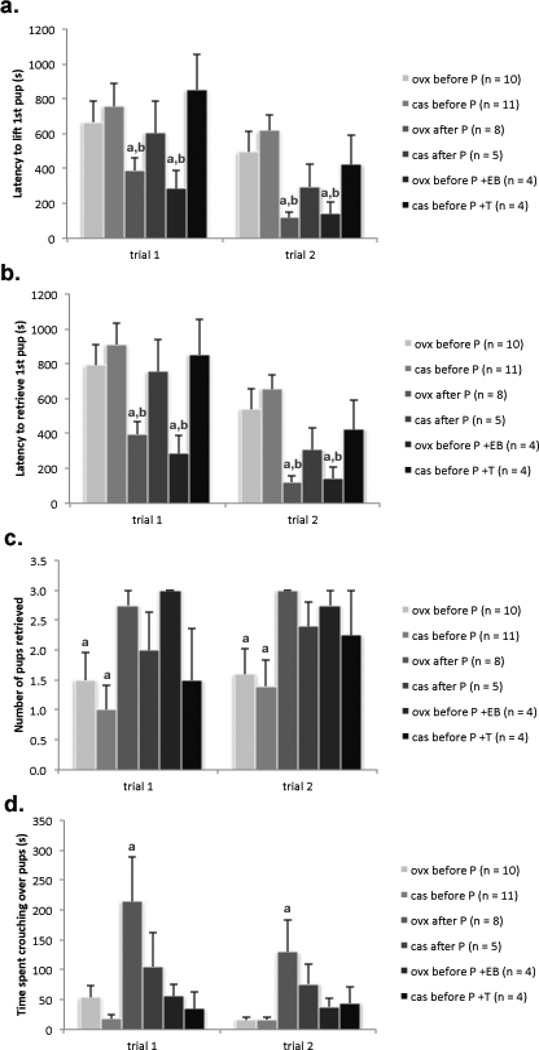
Most of mice tested showed parental behavior directed towards foster pups at some point. The analyses indicated that earlier gonadectomies led to less parental behavior in females and this could be rescued in females by pubertal treatment with estradiol, but not in males treated pubertally with testosterone. Components of a 3-way ANOVA (time of gonadectomy × sex and test as a repeated measure) revealed a significant effect of time of gonadectomy/pubertal EB treatment [F(8, 83) = 5.39, p < 0.01; η2 = 0.13] on latency to lift the first pup (Figure 1a) and a significant effect of time of gonadectomy/pubertal EB treatment [F(8, 83) = 7.06, p < 0.01; η2 = 0.16] on the latency to retrieve the first pup into the nest (Figure 1b). Statistical analysis also revealed a significant effect of sex [F(8, 83) = 6.35, p < 0.05; η2 = 0.08] on the latency to lift the first pup (Figure 1a) and on the latency to retrieve the first pup into the nest [F(8, 83) = 8.89, p < 0.01; η2 = 0.10] (Figure 1b). The post hoc test revealed that male and female mice gonadectomized at P25 had significantly longer latencies to lift and to retrieve the first pup into the nest in comparison to females gonadectomized at P60 and females gonadectomized at P25 and supplemented with EB, suggesting that treatment during puberty could restore pup lifting and retrieving latency to the levels observed in P60 gonadectomized female mice. Males gonadectomized at P60 or gonadectomized at/treated with T during puberty needed almost twice as long to lift and to retrieve the first pup into the nest in comparison to females from the same group (Figure 1b).
Figure 1.
Mice gonadectomized at P25 displayed reduced parental behavior in comparison to female mice gonadectomized at P60 and female mice gonadectomized at P25 and treated with EB during puberty in most parental behavior activities directed toward pups: a. latency to lift the first pup (ap < 0.01; bp < 0.05), b. latency to the retrieval of the first pup into the nest (ap < 0.01; bp < 0.05), c. number of retrieved pups into the nest (ap < 0.01) and d. total time spent crouching over the pups (ap < 0.01). Data are reported as mean + SEM; aSignificant difference between mice gonadectomized before puberty, after puberty, or mice gonadectomized before puberty and treated with EB during puberty. bSignificantly different from all three groups of males.
Statistical analyses also revealed a significant effect of time of gonadectomy [F(8, 83) = 5.44, p < 0.01; η2 = 0.18] on the number of retrieved pups with female mice gonadectomized at P60/gonadectomized at P25 and treated with EB during puberty retrieving almost twice as many pups into the nest in comparison to male and female mice gonadectomized at P25 (Fig. 1c). There was a trend toward statistical significant effect of sex [F(8, 83) = 3.52, p = 0.07; η2 = 0.06] in the number of retrieved pups into the nest (Figure 1c) with females retrieving more pups than males.
The post hoc analysis revealed that mice of both sexes gonadectomized at P25 retrieved significantly fewer pups into the nest than female mice gonadectomized at P60 and gonadectomized at P25 and supplemented with EB during puberty (Figure 1c). However, there was no difference between males and females gonadectomized at P25 and males gonadectomized at P60 and males gonadectomized at P25 and treated with testosterone through puberty.
A significant effect of time of gonadectomy [F(8, 83) = 8.00, p < 0.01; η2 = 0.19] was found also in the time spent crouching over the pups (Figure 1d) with mice gonadectomized after puberty spending almost four times as much time crouching over pups than mice gonadectomized before puberty. Post hoc analyses revealed that mice gonadectomized at P25 regardless of pubertal sex steroid treatment spent significantly less time crouching over the pups than female mice gonadectomized at P60 (Figure 1d).
EB treatment in adulthood did not affect a majority of pup-directed parental behaviors in mice of both sexes with the exception of time spend crouching over pups which was reduced in mice treated with EB in both sexes (34.5± 10.8 s in P25 females without adult EB treatment, 24.7 ± 10.3 s in P25 GDX females with adult EB treatment, 171.7 ±45.9 s in P60 GDX females without EB treatment, 49.3 ± 24.0 s in P60 females with adult EB treatment, 17.0 ± 3.9 s in P25 GDX males without adult EB treatment, 9.9 ±4.8 s in P25 GDX males with EB treatment, 90.6 ± 31.6 s in P60 GDX males without EB treatment and 8.9 ± 3.7 s in P60 GDX males with adult EB treatment; [F(8, 108) = 7.23, p < 0.05; η2 = 0.07].
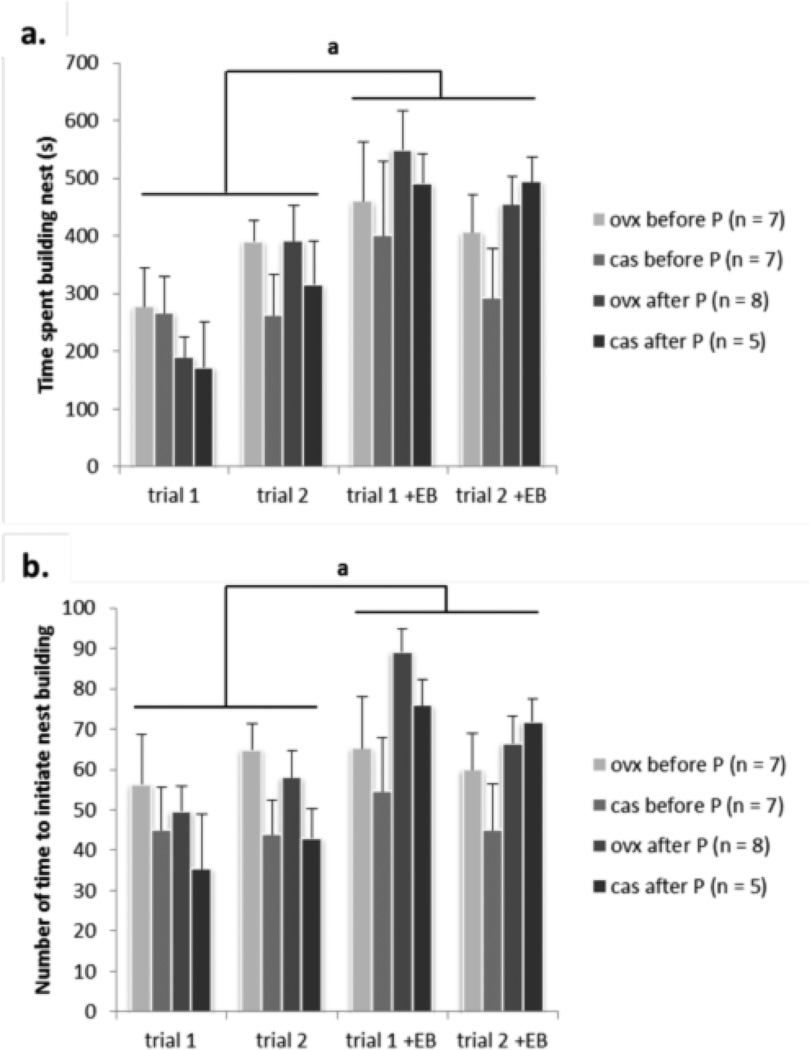
There was an effect of EB treatment on non-pup oriented behaviors. Statistical analyses revealed a significant effect of EB treatment on the duration of nest building (non-pup-directed behavior) in males and females [F(8, 108) = 12.40, p < 0.01; η2 = 0.15] with mice treated with EB spending almost twice as much time building nests as mice without EB supplementation (Figure 2a). Similarly, there was a significant difference in the number of times to initiate building the nest (non-pup-directed behavior) [F(8, 108) = 7.06, p < 0.05; η2 = 0.09] regardless of sex (Figure 2b) with mice supplemented with EB initiating nest building up to twice as often as mice without EB treatment. Post hoc test revealed that mice of both sexes without EB treatment spent less time to build nest and less often initiated this non-pup-directed behavior than mice of both sexes treated with EB.
Figure 2.
Mice without adult EB treatment displayed less nest building behavior than mice supplemented with EB in adulthood (during testing)regardless of sex or time of gonadectomy: a. total time spent building the nest (ap < 0.01), b. the number of the time to initiate nest building (ap < 0.05). Data are reported as mean + SEM. aSignificant difference between mice without and with EB treatment.
Seven of total 49 mice tested displayed aggressive behaviors and there were no significant differences in the number of aggressive mice between groups (data not shown).
Female sexual behavior
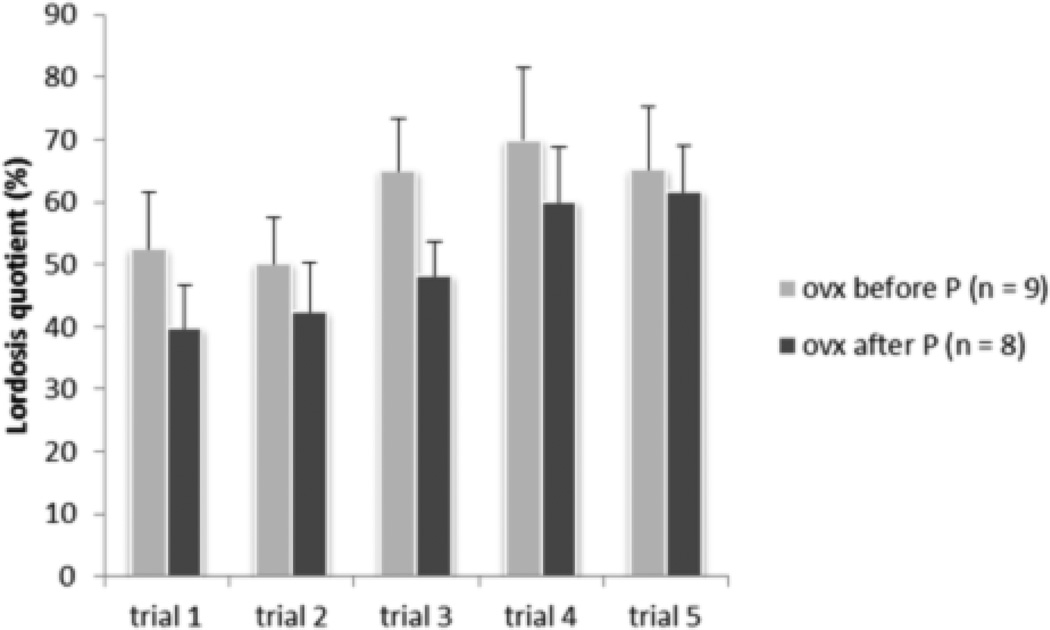
When female mice from the parental behavior tests were tested for female sexual behavior, there were no differences between female mice ovariectomized at P25 versus those ovariectomized at P60 (Figure 3). Lordosis quotients (LQs) were similar for both groups suggesting that long absence of exposure to sex steroid hormones did not affect the sensitivity to EB and progesterone for the induction of female sexual behavior.
Figure 3.
Female mice gonadectomized at P25 displayed similar lordosis quotient as females, gonadectomized at P60 after EB and progesterone treatment in adulthood. Data are reported as mean ± SEM.
Plasma estradiol levels
Plasma estradiol level in ovariectomized (before & after puberty) females (n = 14) after behavior assessments (~ 125 days of age) and after approximately 45 days of 17β-estradiol 3-benzoate supplementation in the form of subcutaneous implants was 224 ± 19 pg/mL. This was higher than previously reported levels for implants with crystalline 17β-estradiol without benzoate esterification: from ~ 80 pg/mL (Wersinger et al., 1999) to ~130 pg/mL (Bakker et al., 2002) and also from levels obtained using EB implants in rats that were approximately 140 pg/mL (Febo et al., 2002). However, the levels were within the range reported in a previous study measuring levels of estradiol during pregnancy, which were around 200 pg/ml on day 6 of pregnancy (with individual levels up to 250 pg/ml; Jacquet et al., 1977).
Discussion
Parental behavior is expressed in most mammalian species from marsupials (Russell, 1982), monotremes (Nicol, 2013) to eutherians. The widest variety of parental care patterns has evolved among the eutherians with significant differences in the amount and quality of parental behavior, and which sex engages in care of the offspring (e.g. (Dwyer, 2008; Lonstein and De Vries, 2000; Root Kustritz, 2005; Saltzman and Maestripieri, 2011). Although it is well established that parental behavior is provoked by exposure to hormones during pregnancy in rats (reviewed in (Rosenblatt et al., 1988) and sheep (reviewed in (Dwyer, 2008), the development of capacity to express parental behavior in mice is not yet fully understood (Kuroda et al., 2011). The current study examined whether the timing of gonadectomy during the adolescent period affects parental behavior in adult mice. In general, several measures of parental behavior were more prominent in mice that were gonadectomized after puberty and tested shortly thereafter in comparison to mice gonadectomized before puberty and tested after 7 weeks, mostly regardless of sex. Interestingly, only estradiol but not testosterone treatment during puberty could restore several measures of pup-directed parental behavior to the levels observed in P60 gdx mice. The results in the current study affirm the importance of gonads and thereby the continuous presence of appropriate gonadal steroid hormones in young mice for normal parental behaviors. This is consistent with the suggestion that gonadal steroids have organizational effects on the brain during puberty in addition to their activational roles, as has been described for other behaviors like sexual, agonistic (aggressive behavior and flank marking, submissive behavior) and anxiety- related (social interaction in a novel environment, open-field ambulation) behaviors (reviewed in (Romeo, 2003; Sisk and Zehr, 2005).
Most studies of parental behavior in mice were conducted with mice that were gonadectomized postpubertally (e.g. (Gatewood et al., 2006; Koch and Ehret, 1989; Ogawa et al., 1998a; Ogawa et al., 1998b; Okabe et al., 2013; Okabe et al., 2010). In virgin mice, gonadectomy after puberty seems to have little or no effect on parental behavior in adult females (Ogawa et al., 1998a) and males (Gatewood et al., 2006; Ogawa et al., 1998b), although gonadectomy in male mice may moderately improve parental behavior in adulthood (Okabe et al., 2013). To examine the importance of continuous exposure to gonadal sex steroids during the pubertal period on development of parental behavior in adult virgin mice of both sexes, mice in the present study were gonadectomized at 25 days of age (before puberty) or at 60 days of age, after the normal pubertal period. An additional group of male and female mice was gonadectomized at 25 days of age and treated either with testosterone (males) or EB (females) in the form of subcutaneous implants only during puberty. Results revealed that mice of both sexes gonadectomized before puberty had significantly longer latencies to lift a pup, retrieve pups to the nest, retrieved less pups into the nest and spent less time crouching over pups in comparison to mice gonadectomized after puberty. In most of these pup-directed parental behaviors, with the exception of the time crouching over pups, estradiol given to prepubertal ovariectomized females rescued the behavior, but testosterone given to prepubertally castrated males had no discernible effect. These results suggest that the continuous presence of gonadal hormones (especially estradiol) during puberty is likely to be important for the development of the capacity to display these behaviors in adulthood either by causing permanent alterations in brain circuitry responsible for parental behavior (Kalinichev et al., 2000) or perhaps maintaining the sensitivity to sex steroid hormones by continuous exposure to such hormones. Although estradiol levels measured in mice in the current study were higher than previously reported preovulatory levels, these levels were within the physiological range of late pregnancy stages (Jacquet et al., 1977) when capacity to display maternal behavior is thought to develop at least partially under the influence of high levels of estradiol.
Sex differences were detected in the latency to lift and to retrieve the first pup into the nest, similar to a previous report (Gatewood et al., 2006) where it was also reported that females were faster to retrieve the first pup into the nest in comparison to males. However, there was no statistically significant effect of sex in some other behaviors. In particular, there was very little infanticidal behavior in males, suggesting that in mice, unlike rats, parental behavior is not strongly sex-dependent and is present in both sexes, although a recent study by Tachikawa shows that pup priming is necessary to display paternal behavior (Tachikawa et al., 2013). However, in the study by Tachikawa, gonadally intact males were used while in the present study, gonadectomized males were tested.
In comparison to the time of gonadectomy, EB treatment in adulthood did not have a major effect on parental behavior in mice of both sexes, although previous studies have suggested that EB stimulates maternal behavior in adult female mice (Koch and Ehret, 1989; McCarthy, 1995) and rats (Sheehan and Numan, 2002; Siegel and Rosenblatt, 1975). In contrast, a recent study by Okabe and coworkers (Okabe et al., 2013) reported no significant effect of estrogen implants (presumably estradiol, but not stated) after gonadectomy in adult female mice on retrieving behavior or pup sensitization, which is in agreement with the results in the current study. The only significant effect of EB treatment in the current study was found in the duration of nest building, in the number of times tested mice initiated nest building and in the time spent crouching over pups, suggesting that EB treatment in adulthood mainly affects non-pup oriented behavior, while EB is not necessary to provoke the onset of parental behavior directed directly towards pups even in gonadectomized mice. Interestingly, estrogens have been implicated in the thermoregulation and perhaps nest building behavior reflects some effects of estrogens on this mechanism, especially as two previous studies have suggested that in mice and rats, estrogens lower tail skin temperature (Opas et al., 2004, Opas et al., 2006). It has to be noted that mice in the present study were treated only with EB, while during normal pregnancy mice are exposed to both progesterone and estradiol. Therefore, the hormone “replacement” conditions in the present study were not identical to the hormonal status during pregnancy.
The timing of gonadectomy relative to testing is an important question. The time after gonadectomy and before behavioral testing can be a significant factor as sex steroid hormones can have long lasting effects (Becker et al., 2008). Although circulating plasma levels of testosterone or estradiol rapidly decline within 1 to 3 days after gonadectomy, testosterone or estradiol are cleared from the bloodstream by about 14 days after gonadectomy in male (Wichmann et al., 1996) and female (Ingberg et al., 2012) adult mice. Therefore, in hormonally naïve mice, the time between gonadectomy and testing (at least 10 to 15 days), especially in mice gonadectomized after puberty, could influence the results of such studies. Therefore, it is possible that in the current study, the observed alterations in the parental behavior resulted through 2 mechanisms. First, they could have arisen due to the alteration of neural circuits that develop during puberty. Alternatively, they could result as a consequence of the longer-term absence of exposure to sex steroid hormones (~50 days versus ~15 days). Post-castration deficiency in sex steroid sensitivity has previously been reported in rats (Clark et al., 1995). However, it seems less likely that sensitivity to the steroid hormones in a context of parental behavior was different between the groups in the present study. Treatment with EB did not result in any significant differences in parental behavior between mice gonadectomized before or after pubertal period. Furthermore, testing females for sexual behavior did not reveal any differences between mice ovariectomized before or after puberty. As female sex behavior is dependent on estrogen exposure, this could suggest that the female mice in the current study did not differ in their sensitivity to sex steroid hormones. Although of course, female sexual behavior and parental behavior are two different behaviors, regulated differently and therefore, similar sensitivity to estradiol in female sex behavior does not conclusively suggest that effects would be the same in parental behavior.
In conclusion, the current study suggests that the absence of gonadal steroid hormones during puberty/adolescence can have a significant effect on several aspects of pup directed parental behaviors in adult mice of both sexes, that can be reversed by estradiol supplementation of GDX mice during puberty. Steroid hormone supplementation in adulthood could not reverse the effects of a hormonal deficit during puberty on parental behavior. Adult hormone supplementation was equally effective in parental and sexual behaviors between groups, gonadectomized before or after puberty, suggesting that sensitivity to estradiol was not altered due to a longer absence of sex steroid hormones. These results therefore highlight a requirement of a pubertal period with appropriate gonadal hormonal stimulation for adequate development of parental behavior in mice.
Highlights.
Spontaneous parental behavior is present in both male and female adult mice, but stronger in female mice.
Absence of sex hormones during puberty impairs pup directed behaviors in adulthood.
Estradiol treatment in adulthood does not improve parental behavior, but estradiol treatment of females during puberty restore parental behavior.
Non-pup directed nest building behavior is improved by estradiol exposure in adulthood.
Sensitivity to sex hormones is not altered by prolonged absence of gonads.
Acknowledgement
This study was supported by ARRS grant P4-0053 (G.M.) and NIH grant MH61376 (S.A.T. and G.M.). We are thankful to Nina Sterman for animal husbandry and technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed EI, Zehr JL, Schulz KM, Lorenz BH, DonCarlos LL, Sisk CL. Pubertal hormones modulate the addition of new cells to sexually dimorphic brain regions. Nat Neurosci. 2008;11:995–997. doi: 10.1038/nn.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP. The organizational-activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Horm Behav. 2009;55:570–578. doi: 10.1016/j.yhbeh.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP, Breedlove SM. Organizational and activational effects of sex steroids on brain and behavior: a reanalysis. Horm Behav. 1985;19:469–498. doi: 10.1016/0018-506x(85)90042-x. [DOI] [PubMed] [Google Scholar]
- Bakker J, Honda S, Harada N, Balthazart J. The aromatase knock-out mouse provides new evidence that estradiol is required during development in the female for the expression of sociosexual behaviors in adulthood. J Neurosci. 2002;22:9104–9112. doi: 10.1523/JNEUROSCI.22-20-09104.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Berkley KJ, Geary N, Hampson E, Herman JP, Young EA. Sex differences in the brain: from genes to behavior. New York: Oxford University Press; 2008. [Google Scholar]
- Bingel AS, Schwartz NB. Timing of LH release and ovulation in the cyclic mouse. J Reprod Fertil. 1969;19:223–229. doi: 10.1530/jrf.0.0190223. [DOI] [PubMed] [Google Scholar]
- Clark JT, Micevych PE, Panossian V, Keaton AK. Testosterone-induced copulatory behavior is affected by the postcastration interval. Neurosci Biobehav Rev. 1995;19:369–376. doi: 10.1016/0149-7634(94)00049-7. [DOI] [PubMed] [Google Scholar]
- De Lorme KC, Schulz KM, Salas-Ramirez KY, Sisk CL. Pubertal testosterone organizes regional volume and neuronal number within the medial amygdala of adult male Syrian hamsters. Brain Res. 2012;1460:33–40. doi: 10.1016/j.brainres.2012.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer CM. Individual variation in the expression of maternal behaviour: a review of the neuroendocrine mechanisms in the sheep. J Neuroendocrinol. 2008;20:526–534. doi: 10.1111/j.1365-2826.2008.01657.x. [DOI] [PubMed] [Google Scholar]
- Febo M, Jimenez-Rivera CA, Segarra AC. Estrogen and opioids interact to modulate the locomotor response to cocaine in the female rat. Brain Res. 2002;943:151–161. doi: 10.1016/s0006-8993(02)02748-8. [DOI] [PubMed] [Google Scholar]
- Gatewood JD, Wills A, Shetty S, Xu J, Arnold AP, Burgoyne PS, Rissman EF. Sex chromosome complement and gonadal sex influence aggressive and parental behaviors in mice. J Neurosci. 2006;26:2335–2342. doi: 10.1523/JNEUROSCI.3743-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingberg E, Theodorsson A, Theodorsson E, Strom JO. Methods for long-term 17beta-estradiol administration to mice. Gen Comp Endocrinol. 2012;175:188–193. doi: 10.1016/j.ygcen.2011.11.014. [DOI] [PubMed] [Google Scholar]
- Jacquet P, Gerber GB, Leonard A, Maes J. Plasma hormone levels in normal and lead-treated pregnant mice. Experientia. 1977;33:1375–1377. doi: 10.1007/BF01920190. [DOI] [PubMed] [Google Scholar]
- Kalinichev M, Rosenblatt JS, Nakabeppu Y, Morrell JI. Induction of c-fos-like and fosB-like immunoreactivity reveals forebrain neuronal populations involved differentially in pup-mediated maternal behavior in juvenile and adult rats. J Comp Neurol. 2000;416:45–78. doi: 10.1002/(sici)1096-9861(20000103)416:1<45::aid-cne5>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Keller M, Pawluski JL, Brock O, Douhard Q, Bakker J. The alpha-fetoprotein knock-out mouse model suggests that parental behavior is sexually differentiated under the influence of prenatal estradiol. Horm Behav. 2010;57:434–440. doi: 10.1016/j.yhbeh.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M, Ehret G. Estradiol and parental experience, but not prolactin are necessary for ultrasound recognition and pup-retrieving in the mouse. Physiol Behav. 1989;45:771–776. doi: 10.1016/0031-9384(89)90293-x. [DOI] [PubMed] [Google Scholar]
- Kudwa AE, Boon WC, Simpson ER, Handa RJ, Rissman EF. Dietary phytoestrogens dampen female sexual behavior in mice with a disrupted aromatase enzyme gene. Behav Neurosci. 2007;121:356–361. doi: 10.1037/0735-7044.121.2.356. [DOI] [PubMed] [Google Scholar]
- Kuroda KO, Tachikawa K, Yoshida S, Tsuneoka Y, Numan M. Neuromolecular basis of parental behavior in laboratory mice and rats: with special emphasis on technical issues of using mouse genetics. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1205–1231. doi: 10.1016/j.pnpbp.2011.02.008. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, De Vries GJ. Sex differences in the parental behavior of rodents. Neurosci Biobehav Rev. 2000;24:669–686. doi: 10.1016/s0149-7634(00)00036-1. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Wagner CK, De Vries GJ. Comparison of the "nursing" and other parental behaviors of nulliparous and lactating female rats. Horm Behav. 1999;36:242–251. doi: 10.1006/hbeh.1999.1544. [DOI] [PubMed] [Google Scholar]
- Majdic G, Tobet S. Cooperation of sex chromosomal genes and endocrine influences for hypothalamic sexual differentiation. Front Neuroendocrinol. 2011;32:137–145. doi: 10.1016/j.yfrne.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM. Estrogen modulation of oxytocin and its relation to behavior. Adv Exp Med Biol. 1995;395:235–245. [PubMed] [Google Scholar]
- McCarthy MM, Arnold AP. Reframing sexual differentiation of the brain. Nat Neurosci. 2011;14:677–683. doi: 10.1038/nn.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CL. An olfactory basis for maternal discrimination of sex of offspring in rats (Rattus norvegicus) Anim Behav. 1981;29:383–386. [Google Scholar]
- Moore CL. Sex differences in urinary odors produced by young laboratory rats (Rattus norvegicus) J Comp Psychol. 1985;99:336–341. [PubMed] [Google Scholar]
- Nelson RJ. An introduction to behavioral endocrinology. third ed. Sunderland: Sinauer Associates; 2005. [Google Scholar]
- Nicol SC. Behaviour and ecology of monotremes. In: Ashwell KWS, editor. Neurobiology of monotremes. Brain evolution in our distant mammalian cousins. Collingwood: CSIRO Publishing; 2013. pp. 17–30. [Google Scholar]
- Numan M, Insel TR. The neurobiology of parental behavior. New York: Springer-Verlag; 2003. [Google Scholar]
- Ogawa S, Eng V, Taylor J, Lubahn DB, Korach KS, Pfaff DW. Roles of estrogen receptor-alpha gene expression in reproduction-related behaviors in female mice. Endocrinology. 1998a;139:5070–5081. doi: 10.1210/endo.139.12.6357. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Washburn TF, Taylor J, Lubahn DB, Korach KS, Pfaff DW. Modifications of testosterone-dependent behaviors by estrogen receptor-alpha gene disruption in male mice. Endocrinology. 1998b;139:5058–5069. doi: 10.1210/endo.139.12.6358. [DOI] [PubMed] [Google Scholar]
- Okabe S, Kitano K, Nagasawa M, Mogi K, Kikusui T. Testosterone inhibits facilitating effects of parenting experience on parental behavior and the oxytocin neural system in mice. Physiol Behav. 2013;118:159–164. doi: 10.1016/j.physbeh.2013.05.017. [DOI] [PubMed] [Google Scholar]
- Okabe S, Nagasawa M, Kihara T, Kato M, Harada T, Koshida N, Mogi K, Kikusui T. The effects of social experience and gonadal hormones on retrieving behavior of mice and their responses to pup ultrasonic vocalizations. Zoolog Sci. 2010;27:790–795. doi: 10.2108/zsj.27.790. [DOI] [PubMed] [Google Scholar]
- Park JH. Assessment of male sexual behavior in mice. In: Gould TD, editor. Mood and anxiety related phenotypes in mice: Characterization using behavioral tests. New York: Humana Press; 2011. pp. 357–373. [Google Scholar]
- Romeo RD. Puberty: a period of both organizational and activational effects of steroid hormones on neurobehavioural development. J Neuroendocrinol. 2003;15:1185–1192. doi: 10.1111/j.1365-2826.2003.01106.x. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Diedrich SL, Sisk CL. Effects of gonadal steroids during pubertal development on androgen and estrogen receptor-alpha immunoreactivity in the hypothalamus and amygdala. J Neurobiol. 2000;44:361–368. [PubMed] [Google Scholar]
- Root Kustritz MV. Reproductive behavior of small animals. Theriogenology. 2005;64:734–746. doi: 10.1016/j.theriogenology.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Rosenblatt JS, Mayer AD, Giordano AL. Hormonal basis during pregnancy for the onset of maternal behavior in the rat. Psychoneuroendocrinology. 1988;13:29–46. doi: 10.1016/0306-4530(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Russell EM. Patterns of parental care and parental investment in marsupials. Biol Rev Camb Philos Soc. 1982;57:423–486. doi: 10.1111/j.1469-185x.1982.tb00704.x. [DOI] [PubMed] [Google Scholar]
- Saltzman W, Maestripieri D. The neuroendocrinology of primate maternal behavior. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1192–1204. doi: 10.1016/j.pnpbp.2010.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KM, Sisk CL. Pubertal hormones, the adolescent brain, and the maturation of social behaviors: Lessons from the Syrian hamster. Mol Cell Endocrinol. 2006:254–255. 120–126. doi: 10.1016/j.mce.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Zehr JL, Salas-Ramirez KY, Sisk CL. Testosterone programs adult social behavior before and during, but not after, adolescence. Endocrinology. 2009;150:3690–3698. doi: 10.1210/en.2008-1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale JV, Wood SA, Atkinson HC, Harbuz MS, Lightman SL. Gonadal steroid replacement reverses gonadectomy-induced changes in the corticosterone pulse profile and stress-induced hypothalamic-pituitary-adrenal axis activity of male and female rats. J Neuroendocrinol. 2004;16:989–998. doi: 10.1111/j.1365-2826.2004.01258.x. [DOI] [PubMed] [Google Scholar]
- Seitz O, Schurmann C, Hermes N, Muller E, Pfeilschifter J, Frank S, Goren I. Wound healing in mice with high-fat diet- or ob gene-induced diabetes-obesity syndromes: a comparative study. Exp Diabetes Res. 2010;2010:476969. doi: 10.1155/2010/476969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan T, Numan M. Estrogen, progesterone, and pregnancy termination alter neural activity in brain regions that control maternal behavior in rats. Neuroendocrinology. 2002;75:12–23. doi: 10.1159/000048217. [DOI] [PubMed] [Google Scholar]
- Shoji H, Kato K. Maternal behavior of primiparous females in inbred strains of mice: a detailed descriptive analysis. Physiol Behav. 2006;89:320–328. doi: 10.1016/j.physbeh.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Siegel HI, Rosenblatt JS. Estrogen-induced maternal behavior in hysterectomized-overiectomized virgin rats. Physiol Behav. 1975;14:465–471. doi: 10.1016/0031-9384(75)90012-8. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nat Neurosci. 2004;7:1040–1047. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinol. 2005;26:163–174. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Stolzenberg DS, Rissman EF. Oestrogen-independent, experience-induced maternal behaviour in female mice. J Neuroendocrinol. 2011;23:345–354. doi: 10.1111/j.1365-2826.2011.02112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachikawa KS, Yoshihara Y, Kuroda KO. Behavioral transition from attack to parenting in male mice: a crucial role of the vomeronasal system. J Neurosci. 2013;33:5120–5126. doi: 10.1523/JNEUROSCI.2364-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasugi N, Tanaka M, Kato H. Occurrence of lordosis-like posture in male and female mice given manual stimulation during the early period of anesthesia. Endocrinol Jpn. 1983;30:29–33. doi: 10.1507/endocrj1954.30.29. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Haisenleder DJ, Lubahn DB, Rissman EF. Steroid feedback on gonadotropin release and pituitary gonadotropin subunit mRNA in mice lacking a functional estrogen receptor alpha. Endocrine. 1999;11:137–143. doi: 10.1385/ENDO:11:2:137. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Sannen K, Villalba C, Lubahn DB, Rissman EF, De Vries GJ. Masculine sexual behavior is disrupted in male and female mice lacking a functional estrogen receptor alpha gene. Horm Behav. 1997;32:176–183. doi: 10.1006/hbeh.1997.1419. [DOI] [PubMed] [Google Scholar]
- Wichmann MW, Zellweger R, DeMaso CM, Ayala A, Chaudry IH. Mechanism of immunosuppression in males following trauma-hemorrhage. Critical role of testosterone. Arch Surg. 1996;131:1186–1191. doi: 10.1001/archsurg.1996.01430230068012. discussion 1191–1182. [DOI] [PubMed] [Google Scholar]
- Zinck L, Lima SQ. Mate choice in Mus musculus is relative and dependent on the estrous state. PLoS One. 2013;8:e66064. doi: 10.1371/journal.pone.0066064. [DOI] [PMC free article] [PubMed] [Google Scholar]