Abstract
Spinal and bulbar muscular atrophy (SBMA) is an X-linked neuromuscular disease caused by a trinucleotide (CAG) repeat expansion in the androgen receptor gene. Patients with SBMA have weakness, atrophy, and fasciculations in the bulbar and extremity muscles. Individuals with CAG repeat lengths greater than 62 have not previously been reported. We evaluated a 29 year old SBMA patient with 68 CAGs who had unusually early onset and findings not seen in others with the disease. Analysis of the androgen receptor gene confirmed the repeat length of 68 CAGs in both peripheral blood and fibroblasts. Evaluation of muscle and sensory function showed deficits typical of SBMA, and in addition the patient had manifestations of autonomic dysfunction and abnormal sexual development. These findings extend the known phenotype associated with SBMA and shed new insight into the effects of the mutated androgen receptor.
Keywords: Motor neuron disease, Spinal bulbar muscular atrophy, Kennedy’s disease, Androgen receptor, Genetics
Introduction
Spinal and bulbar muscular atrophy (SBMA), also known as Kennedy’s disease [1], is an X-linked neuromuscular disease caused by a CAG repeat expansion in the androgen receptor gene. Affected males develop weakness, atrophy, and fasciculations in the limb and bulbar muscles during adulthood. In addition to the motor effects, degeneration of the dorsal root ganglia can result in a loss of sensory function in the distal extremities. Patients also often show evidence of androgen insensitivity, such as gynecomastia, oligospermia, and erectile dysfunction.
The disease manifestations likely result from a toxic gain of function of the androgen receptor that is dependent on ligand (testosterone and dihydrotestosterone) [2]. However, loss of normal receptor function also occurs, and contributes to the androgen insensitivity. The mutant protein has a tendency to aggregate, and it forms intracellular inclusions in susceptible cells [3, 4]. The principal cellular disturbance caused by the mutant AR is likely transcriptional dysregulation, with secondary changes occurring in cell signaling, mitochondrial activity, and axonal transport [5–8].
Neurological symptoms typically develop during the fourth or fifth decade of life, but the onset ranges from ages 18 to 64 [9]. The length of the CAG repeat in the AR gene correlates inversely with the age of disease onset, with longer repeats associated with earlier onset [10]. The disease has been reported with repeat lengths between 38 and 62 CAGs [8,10]. Here we report a 29 year old patient with SBMA who has a 68 CAG repeat expansion in the androgen receptor gene. This patient not only had early onset of disease, but also was found to have additional findings of autonomic dysfunction and abnormal sexual development.
Case Report
We report a 29-year-old male of Afro-Caribbean ancestry who has had muscle weakness in his thighs, fatigue after exercise, fasciculations, cramping, and tremor since age 18. He has also had burning neuropathic pain with dysesthesia in the distal lower extremities for the past two years. He occasionally takes ibuprofen to alleviate the pain in his feet. Over the past several months he has also experienced similar symptoms in his fingertips. His feet usually feel cool, and he reported decreased sweating. He has no difficulty with swallowing, however he does experience fatigue in the muscles of mastication after a meal. He occasionally experiences phlegm accumulating in his throat, although he does not report choking. In addition to the neurological features, he had a congenital abnormality of the penis (chordee), which was surgically corrected at age 7. He also has bilateral testicular atrophy and difficulties with ejaculation. By age 16 he developed gynecomastia, which became socially stigmatizing. He reported having decreased facial hair growth overall and only occasionally shaves two areas on his upper lip. He has a pertinent family history of a 19 year old maternal first cousin once-removed with exercise intolerance and no other symptoms who has not received genetic testing. He also had a maternal great grandfather without a diagnosis who had decreased mobility. His mother passed away at age 40, and did not have any neuromuscular symptoms.
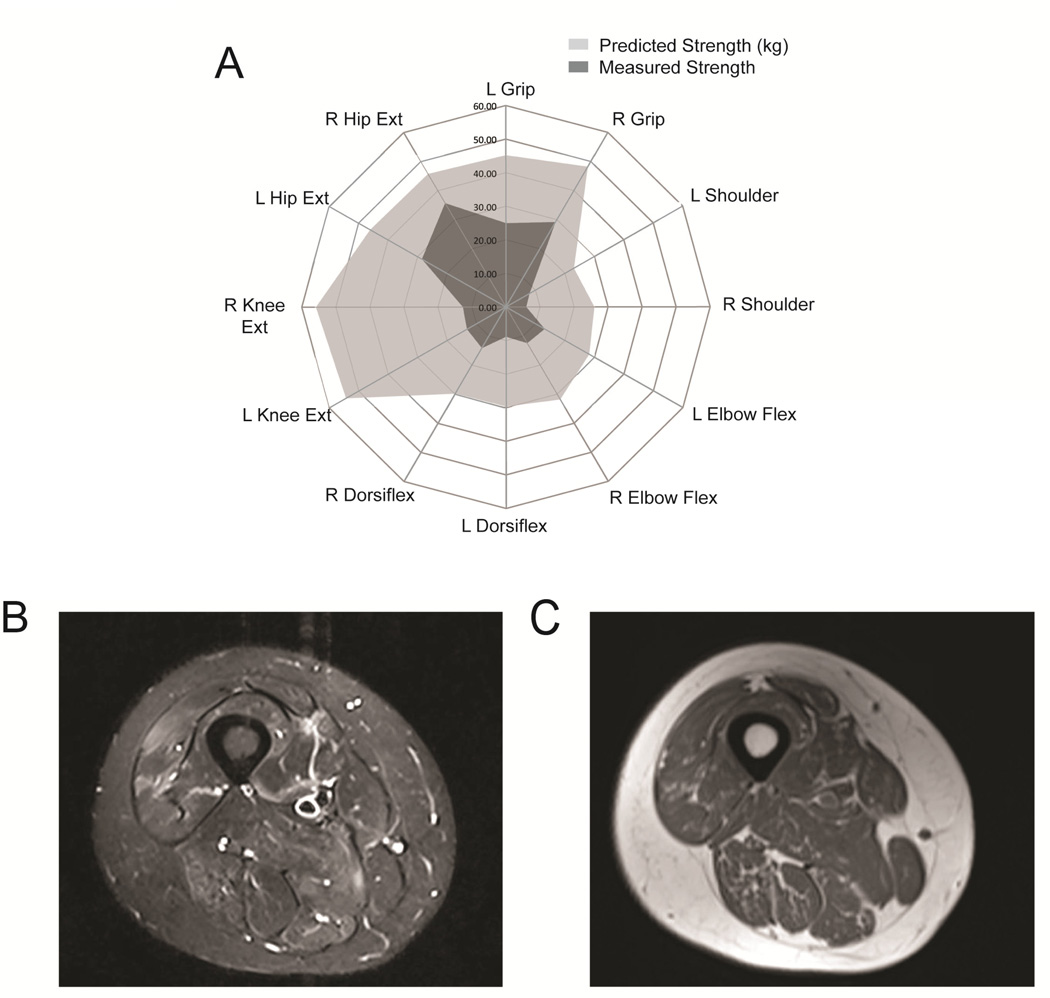
On examination the patient had pronounced gynecomastia bilaterally. Fibrillations and atrophy were noted in the tongue, and weakness was observed in the orbicularis oculi and orbicularis oris. He had prominent perioral fasciculations. Limb weakness was severe in the shoulders, thighs, and toes bilaterally. Sensory testing showed a loss of temperature and vibratory sensation in the fingers and toes with a length-dependent gradient. On gait testing, he had difficulty standing on his heels and ankles. Abnormalities were also found in quantitative muscle strength testing (figure 1A) [11] and muscle MRI (figure 1B and C), demonstrating the extent of disease involvement at a relatively early age.
Figure 1. Strength testing and muscle MRI findings.
Quantitative muscle strength testing in 12 different muscle groups shows the degree of muscle weakness (in kg) as compared to healthy controls matched for age, height, weight, and body side (A). STIR MRI image of the right thigh showing patchy areas of hyperintensity indicative of muscle injury and degeneration (B). T1 image of the same region shows muscle atrophy and evidence of increased fat signal within the muscle (C).
An evaluation of autonomic function showed evidence of sudomotor dysfunction, as negligible sweat reponses (less than 40 nL total each site) were detected at the forearm, calf, ankle, and foot. Tilt table testing showed evidence of orthostatic tachycardia, with the heart rate increasing by 35–40 beats per minute with prolonged upright position in the absence of orthostatic blood pressure changes or symptoms. No abnormalities were detected in valsava testing, heart rate during deep breathing and quantitative sensory testing of vibration and cool detection thresholds. Skin biopsies were performed in the distal leg (10 cm proximal to the lateral malleolus), distal thigh, and proximal thigh, and showed a normal epidermal nerve fiber (ENF) density compared to controls [12]. ENF density at the distal leg was 24.3 fibers/mm. Laboratory tests, including TSH, vitamin B12, anti-nuclear antibodies, fasting glucose, and hemaglobin A1c were normal. Electrocardiography was normal on two separate evaluations.
Detection of a 68 CAG repeat expansion in the androgen receptor
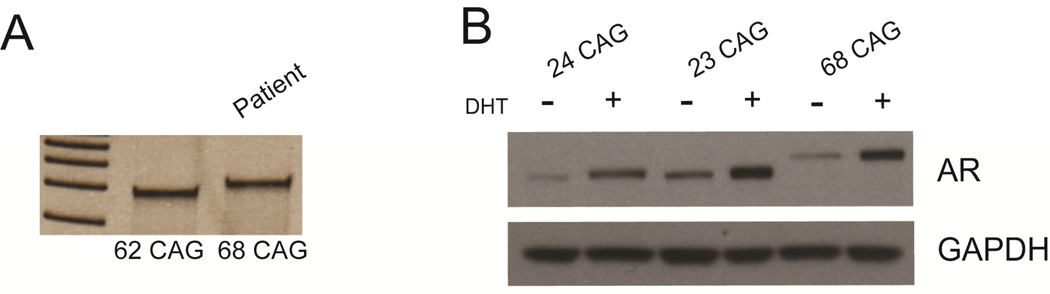
Sequencing and PCR analysis of genomic DNA isolated from the patient’s venous blood and forearm skin fibroblasts showed a repeat length of 68 CAGs in both samples. CAG repeat length was confirmed by clinical testing (Athena Diagnostics) of peripheral blood, and remained unchanged with repeated testing after one year. Figure 2A shows a DNA fragment obtained by PCR with primers flanking the CAG repeat run on a 20% acrylamide gel with a DNA fragment obtained from another patient with 62 CAGs. Sequencing of the remaining translated portion of the androgen receptor gene was done, and no other genetic variants were found. In particular, the glycine repeat length was in the normal range [13]. Androgen receptor protein levels in patient-derived fibroblasts were comparable to control samples with non-expanded androgen receptor (figure 2B).
Figure 2. Androgen receptor gene and protein.
PCR products from patient DNA were generated using primers that bracket the CAG expansion. Acrylamide gel electrophoresis shows the band shift relative to a 62 CAG sample from a different patient (A). Androgen receptor expression is equal in patient derived fibroblast cultures when compared to control samples on western blotting. Dihydrotestosterone (DHT) treatment results in similar upregulation of androgen receptor levels in all samples evaluated (B).
Discussion
Patients with SBMA usually present with the onset of weakness in their mid-forties and have an average androgen receptor repeat length of 47 CAGs [9]. Here we describe a patient with a 68 CAG repeat who had onset of weakness at age 18 and additional features of disease which have not previously been reported, including abnormalities in sexual development and sudomotor function. The identification of a patient with 68 CAGs expands the spectrum of disease features associated with SBMA, as the disease has previously been reported only in patients with 62 CAGs or less.
The association of a polyglutamine repeat expansion in the androgen receptor with a chordee deformity is novel but not unexpected, given the known expression of androgen receptor in sexually dimorphic tissues. Features of androgen insensitivity including infertility, gynecomastia, and reduced androgenic alopecia [14] have been described in SBMA. It is possible that the additional findings of chordee and autonomic involvement observed in this patient are due to the larger expansion of the polyglutamine repeat. Ogata et al. [15] reported the identification of a 44 CAG repeat in the androgen receptor of an 11 year old boy with under-masculinized genitalia and chordee without neuromuscular disease. The diagnosis of 5α-reductase deficiency has also been reported in a pediatric male patient with chordee and normal androgen receptor studies [16]. Treatment of this infant with dihydrotestosterone and surgical release of chordee resulted in improvement of symptoms.
Although findings of sensory neuropathy are typical in SBMA, the findings suggestive of small nerve fiber involvement seen in this subject have not been described in the disease. Previous studies have found axonal atrophy of large myelinated sensory fibers [17], and in a cohort of 54 SBMA subjects sensory nerve action potentials (SNAP) were reduced in at least 94% of subjects [9]. A study of small fiber involvement in two SBMA subjects found a reduction of epidermal nerve fiber density and evidence of autonomic skin denervation on skin biopsy [18]. These findings likely represent subclinical involvement of small diameter peripheral nerves. Subclinical involvement of the autonomic system has been demonstrated in four out of five subjects with SBMA previously [19]. Plasma levels of noradrenaline were found to be reduced in these patients, which also implies dysfunction of the sympathetic nervous system. Our patient has abnormal sudomotor function and complaints of anhidrosis, suggesting abnormal sympathetic post-ganglionic activity. In addition, the presence of orthostatic tachycardia suggests dysfunction of the small fiber sympathetic nervous system [20]. Altogether the presentation of pain in the distal extremities with abnormal post-ganglionic sympathetic activity suggests that small, unmyelinated nerve fibers are dysfunctional in this patient. The lack of a reduction in the epidermal nerve fiber density may be due to the relatively recent onset of his pain, or evidence of unmyelinated nerve dysfunction without degeneration. The normal ENF finding may also be related to the sampling location, as the biopsy site was proximal to the distal extremities where the pain was described. A normal ENF density has also been reported with chemotherapy-induced neuropathy in patients who were experiencing neuropathic pain [21].
Collectively, these findings emphasize that the CAG repeat expansion in the androgen receptor gene is capable of altering androgen-dependent signaling pathways. Our observations suggest that the CAG expansion mutation may result in dysfunction of unmyelinated nerve fibers and adversely affect sexual development.
Highlights.
A detailed clinical study was done on an SBMA patient with a large 68 CAG repeat.
He had burning neuropathic pain, anhidrosis, and abnormal sexual development.
Autonomic testing showed decreased sudomotor function and orthostatic tachycardia.
Acknowledgements
We thank Elizabeth Hartnett for her help with arranging the subject visits and evaluations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kennedy WR, Alter M, Sung JH. Progressive proximal spinal and bulbar muscular atrophy of late onset: a sex-linked recessive trait. Neurology. 1968;18:671–680. doi: 10.1212/wnl.18.7.671. [DOI] [PubMed] [Google Scholar]
- 2.Grunseich C, Rinaldi C, Fischbeck KH. Spinal and bulbar muscular atrophy: pathogenesis and clinical management. Oral Dis. 2014;20:6–9. doi: 10.1111/odi.12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walcott JL, Merry DE. Ligand promotes intranuclear inclusions in a novel cell model of spinal and bulbar muscular atrophy. J Biol Chem. 2002;277:50855–50859. doi: 10.1074/jbc.M209466200. [DOI] [PubMed] [Google Scholar]
- 4.Li M, Miwa S, Kobayashi Y, et al. Nuclear inclusions of the androgen receptor protein in spinal and bulbar muscular atrophy. Ann Neurol. 1998;44:249–254. doi: 10.1002/ana.410440216. [DOI] [PubMed] [Google Scholar]
- 5.McCampbell A, Taylor JP, Taye AA, et al. CREB-binding protein sequestration by expanded polyglutamine. Hum Molec Genet. 2000;9:2197–2202. doi: 10.1093/hmg/9.14.2197. [DOI] [PubMed] [Google Scholar]
- 6.Lieberman AP, Harmison G, Strand AD, Olson JM, Fischbeck KH. Altered transcriptional regulation in cells expressing the expanded polyglutamine androgen receptor. Hum Molec Genet. 2002;11:1967–1976. doi: 10.1093/hmg/11.17.1967. [DOI] [PubMed] [Google Scholar]
- 7.Ranganathan S, Harmison GG, Meyertholen K, Pennuto M, Burnett BG, Fischbeck KH. Mitochondrial abnormalities in spinal and bulbar muscular atrophy. Hum Molec Genet. 2009;18:27–42. doi: 10.1093/hmg/ddn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katsuno M, Adachi H, Minamiyama M, et al. Reversible disruption of dynactin 1-mediated retrograde axonal transport in polyglutamine-induced motor neuron degeneration. J Neurosci. 2006;26:12106–12117. doi: 10.1523/JNEUROSCI.3032-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rhodes LE, Freeman BK, Auh S, et al. Clinical features of spinal and bulbar muscular atrophy. Brain. 2009;132:3242–3251. doi: 10.1093/brain/awp258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atsuta N, Watanabe H, Ito M, et al. Natural history of spinal and bulbar muscular atrophy (SBMA): a study of 223 Japanese patients. Brain. 2006;129:1446–1455. doi: 10.1093/brain/awl096. [DOI] [PubMed] [Google Scholar]
- 11.The National Isometric Muscle Strength (NIMS) Database Consortium. Muscular Weakness Assessment: Use of Normal Isometric Strength Data. Arch Phys Med Rehabil. 1996;77:1251–1255. doi: 10.1016/s0003-9993(96)90188-4. [DOI] [PubMed] [Google Scholar]
- 12.McArthur JC, Stocks EA, Hauer P, Cornblath DR, Griffin JW. Epidermal Nerve Fiber Density. Arch Neurol. 1998;55:1513–1520. doi: 10.1001/archneur.55.12.1513. [DOI] [PubMed] [Google Scholar]
- 13.Ding D, Xu L, Menon M, et al. Effect of GGC (Glycine) repeat length polymorphism in the human androgen receptor on androgen action. Prostate. 2005;62:133–139. doi: 10.1002/pros.20128. [DOI] [PubMed] [Google Scholar]
- 14.Sinclair R, Greenland KJ, van Egmond S, et al. Men with Kennedy disease have a reduced risk of androgenetic alopecia. Br J Dermatol. 2007;157:290–294. doi: 10.1111/j.1365-2133.2007.08026.x. [DOI] [PubMed] [Google Scholar]
- 15.Ogata T, Muroya K, Ishii T, et al. Undermasculinized genitalia in a boy with an abnormally expanded CAG repeat length in the androgen receptor gene. Clin Endocrinol (Oxf) 2001;53:835–838. doi: 10.1046/j.1365-2265.2001.01205.x. [DOI] [PubMed] [Google Scholar]
- 16.Carpenter TO, Imperato-McGinley J, Boulware SD, et al. Variable expression of 5α-reductase deficiency: presentation with male phenotype in a child of greek origin. J Clin Endrocrinol Metab. 1990;71:318–322. doi: 10.1210/jcem-71-2-318. [DOI] [PubMed] [Google Scholar]
- 17.Li M, Sobue G, Doyu M, Mukai E, Hashizume Y, Mitsuma T. Primary sensory neurons in X-linked recessive bulbospinal neuronopathy: histopathology and androgen receptor gene expression. Muscle Nerve. 1995;18:301–308. doi: 10.1002/mus.880180306. [DOI] [PubMed] [Google Scholar]
- 18.Manganelli F, Iodice V, Provitera V, et al. Small-fiber involvement in spinobulbar muscular atrophy (Kennedy’s Disease) Muscle Nerve. 2007;36:816–820. doi: 10.1002/mus.20872. [DOI] [PubMed] [Google Scholar]
- 19.Rocchi C, Greco V, Urbani A, et al. Subclinical autonomic dysfunction in spinobulbar muscular atrophy (Kennedy Disease) Muscle Nerve. 2011;44:737–740. doi: 10.1002/mus.22159. [DOI] [PubMed] [Google Scholar]
- 20.Haensch CA, Tosch M, Katona I, Weis J, Isenmann S. Small-fiber neuropathy with cardiac denervation in postural tachycardia syndrome. Muscle Nerve. 2014 Mar 20; doi: 10.1002/mus.24245. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 21.Koskinen MJ, Kautio AL, Haanpaa ML, et al. Intraepidermal nerve fibre density in cancer patients receiving adjuvant chemotherapy. Anticancer Res. 2011;12:4413–4416. [PubMed] [Google Scholar]