Abstract
Background
Whole lung scaffolds can be created by perfusion decellularization of cadaveric donor lungs. The resulting matrices can then be recellularized to regenerate functional organs. This study evaluates the capacity of acellular lung scaffolds to support recellularization with human induced pluripotent stem cell (iPSC)-derived lung progenitors.
Methods
Whole rat and human lungs were decellularized by constant-pressure perfusion with 0.1% SDS solution. Resulting lung scaffolds were either cryosectioned into slices or left intact. Human iPSCs were differentiated to definitive endoderm, anteriorized to a foregut fate, and then ventralized to an Nkx2.1-expressing population. Cells were seeded onto slices and whole lungs, which were maintained under constant-perfusion biomimetic culture. Lineage specification was assessed by quantitative PCR and immunofluorescent staining. Regenerated left lungs were transplanted in orthotopic position.
Results
Activin-A treatment followed by TGF-β inhibition induced differentiation of human iPSCs to anterior foregut endoderm as confirmed by FOXA2, SOX17, and SOX2 expression. Cells cultured on decellularized lung slices demonstrated proliferation and lineage commitment after 5 days. Nkx2.1-expressing cells were identified at 40–60% efficiency. Within whole lung scaffolds and under perfusion culture, cells further up-regulated Nkx2.1 expression. After orthotopic transplantation, grafts were perfused and ventilated via host vasculature and airways.
Conclusions
Decellularized lung matrix supports the culture and lineage commitment of human iPSC-derived lung progenitor cells. Whole organ scaffolds and biomimetic culture enable co-seeding of iPSC-derived endothelial and epithelial progenitors and enhance early lung fate. Orthotopic transplantation may enable further in vivo graft maturation.
Keywords: Stem cells, Cell biology, Bioengineering, Transplantation
Introduction
At present, transplantation is the only therapeutic option for many patients with end-stage lung disease; yet, limitations in organ availability and complications of immunosuppression and chronic graft dysfunction remain (1). The successful translation of organ engineering strategies to regenerate lungs for transplantation would provide a novel organ source and increase the number of lungs available for patients. The ability to create patient-specific organs would be of further benefit, reducing the need for immunosuppression and potentially improving graft survival.
Acellular lung scaffolds can be created from cadaveric organs by multiple approaches, all aiming to remove cellular material while retaining the extracellular matrix scaffold upon which cells can later be re-introduced (2). Our decellularization methodology utilizes constant-pressure detergent perfusion through the native lung vasculature, and results in a biocompatible whole-organ scaffold (3). This scaffold can support primary epithelial and endothelial recellularization to regenerate transplantable constructs that could be perfused and ventilated in vivo for up to one week (4, 5).
There is great interest in the use of induced pluripotent stem cell (iPSC)-derived populations as tools for patient-specific lung regeneration (6). Derivation of these cells requires recapitulation of the sequential steps in early lung specification. During development, germ layer restriction first occurs during gastrulation, with cells exposed to strong nodal signalling becoming specified to definitive endoderm (DE) (7). In culture, nodal signalling is mimicked with high dose Activin-A to generate an enriched DE population marked by the transcription factors Sox17 and Foxa2 (8). The endoderm layer then forms the primitive gut tube from which the respiratory and digestive systems develop through an anterior-posterior division. Both lungs and trachea arise from the anterior foregut endoderm, marked by the transcription factor Sox2 and preservation of Foxa2 (9, 10). Timed inhibition of the TGF-β signalling pathway is sufficient to anteriorize iPSC-derived endoderm in vitro (11).
The transcription factor Nkx2.1 is the earliest marker of lung-specified endoderm (12), as well as early thyroid and brain. Mouse embryos carrying a homozygous disruption in the Nkx2.1 locus form a primitive lung structure, but fail to undergo branching morphogenesis or epithelial development, and cannot support ventilation (13). Specific growth factors, including WNT, BMP, and FGF, are critical to ventralization and the generation of Nkx2.1-expressing cells (9). From this ventral wall, the primary lung buds emerge and separate from the dorsal esophagus, driven by signalling from the surrounding mesenchyme (14).
Directed, step-wise in vitro differentiation protocols have successfully generated airway epithelium and lung pneumocytes (11, 15–17) from human iPSCs by recapitulation of these developmental stages.
The role of matrix-cell interactions in mediating cellular fate decisions is an important, but incompletely understood element of lung development. Basement membrane attachment can direct type II pneumocytes to adopt a differentiated type I phenotype (18), and matrix interactions can enhance surfactant protein-C expression by differentiating embryonic stem cells (ESCs) (19). The three-dimensional structure of lung matrix (20), along with mechanical stimuli, such as stretch, also provide developmental cues (21, 22). Moreover, the cross-talk between endogenous cells and the extracellular matrix during tissue repair, involving both growth factor production and matrix modification, further highlights this complex relationship (23).
By combining principles of lung development and bioengineering, the aim of patient-specific therapies for end-stage lung disease may be realised through the regeneration of whole-organ scaffolds (24). The use of patient-derived stem cells and the elucidation of the key developmental stages for recellularization of native scaffolds are important milestones toward functional organ regeneration and transplantation.
Material and Methods
Human iPSC Culture
All experiments utilized iPSCs generated by mRNA transfection (Klf4, c-Myc, Oct4, Sox2, LIN28) of post-natal fibroblasts (BJ-RiPSCs) (25). Undifferentiated cells were maintained in feeder-free culture in mTeSR media (Stemcell Technologies) on Geltrex-coated plates (Life Technologies), and passaged with Accutase (Stemcell Technologies) at 70% confluencey. Differentiation was initiated at 80% confluencey.
Human iPSC-derived endothelium
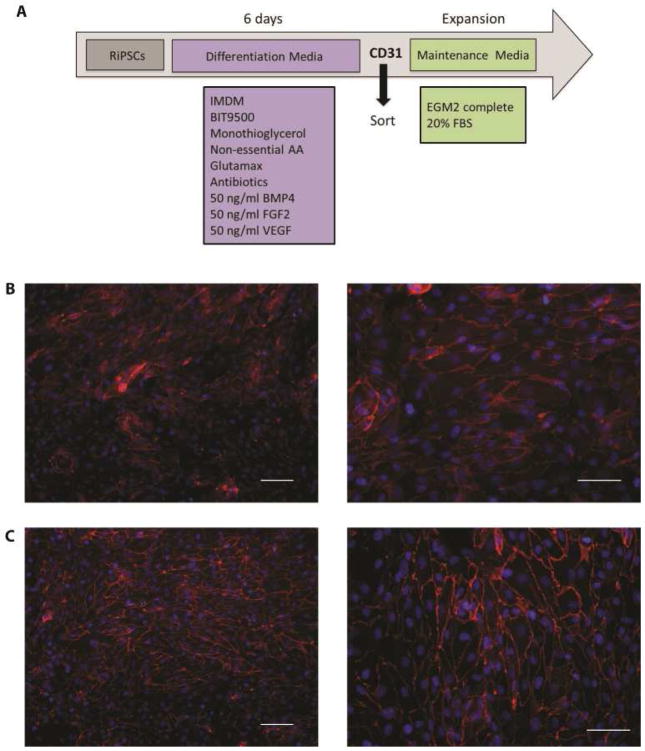
Endothelial differentiation utilized a basal media of Iscove’s modified Dulbecco’s medium, BIT 9500 Serum Substitute (Stemcell Technologies) Non-Essential Amino Acids (Gibco), L-Glutamine (Gibco), Monothioglycerol (10%, Sigma), and Penicillin/Streptomycin. Complete differentiation media was supplemented with BMP-4 (50ng/ml, PeproTech), FGF-basic (50ng/ml, PeproTech) and VEGF165 (50ng/ml, PeproTech) for 6 days. Differentiated endothelial cells were expanded in EGM2 (Lonza) supplemented with 20% fetal bovine serum (HyClone) on 0.1% Gelatin-coated plates (Appendix Figure 1).
Human iPSC-derived epithelial progenitors
A basal media of RPMI 1640 with Glutamax (Life Technologies) plus 2% B27 serum supplement minus insulin (Invitrogen) was supplemented to mimic three developmental stages: (1) induction of definitive endoderm with 50ng/ml Activin-A (Peprotech) for 4 days, (2) anteriorization of endoderm with 1μM A8301 (Tocris) for 4 days, and (3) ventralization with 10ng/ml BMP4 (Peprotech), 100ng/ml FGF2 (Peprotech), and 100nM CHIR 99021 (Stemgent) for 4–7 days.
Histology and Immunofluorescence
Paraformaldehyde-fixed, paraffin-embedded tissue sections were stained by haematoxylin-eosin or underwent antigen retrieval and permeabilization with 0.1% Triton-X100. Cultured cells were fixed with 100% methanol.
Immunofluorescent staining included (1) blocking by 5% donkey serum, (2) primary antibodies (1:200) overnight at 4°C, (3) washing with 0.1% Tween-20, (4) secondary antibodies (1:400; AlexFluor 488 or 594, Life Technologies) for 1 hour at room temperature, and (5) counterstaining with DAPI for 5 minutes. Images were captured with a Nikon Ti-PFS inverted microscope.
Quantitative Real-Time PCR
Samples were stored in RNALater (Qiagen) until RNA isolation by RNeasy-Plus Mini Kit (Qiagen). RNA was transcribed to cDNA by SuperScript® III Reverse Transcriptase (Life Technologies). Gene expression was quantified by Taqman® assays using the OneStepPlus system (Applied Biosystems). Gene expression was analysed by ΔΔCt method with normalization to 18S gene expression.
Lung Tissue Slice Experiments
Decellularized human lung tissue was fixed in 30% sucrose, embedded in optimum cutting temperature compound, cut at 100μm, and immobilized on tissue culture plates. Slices were washed with 100% and 95% ethanol and then air-dried for 30 minutes. Sections were incubated overnight in PBS to remove residual detergents, then pre-conditioned with media for 3 hours before seeding.
On day 2 of ventralization, cells were re-seeded directly to lung slices and cultured for 5 days. For cell localization, 10x images were captured on 6 separate cell-matrix cultures. ImageJ was used to quantify DAPI+ cells localized on or off the matrix. Counts were normalized to total matrix area.
Decellularization, Recellularization, and Whole-Organ Culture
Animal experiments were approved by the Massachusetts General Hospital Institutional Animal Care and Use Committee and performed in compliance with the Animal Welfare Act. Cadaveric rat lungs were perfusion decellularized as previously described (4). In brief, cadaveric lungs were explanted from Sprague-Dawley rats (260–280g, Charles River Labs). The pulmonary artery (PA) was cannulated with an 18G dispensing needle (McMaster-Carr), and perfused (constant pressure, 50 cmH2O) sequentially with heparinized (10 units/ml) saline, 0.1% sodium dodecyl sulfate (2 hours), deionized water (15 minutes), and 1% Triton-X-100 (10 minutes). Scaffolds were washed with PBS containing antibiotics for 72 hours (Sigma Aldrich) to eliminate residual detergent. Removal of cells and residual DNA was routinely confirmed by hematoxylin-eosin staining and by quantitative assay (Quant-iT™ kit, Invitrogen) (3).
Decellularized lungs were intubated with 16G intra-tracheal cannulae and placed in a custom bioreactor facilitating continuous perfusion through the PA (2ml/min). Human iPSC-derived endothelial cells were delivered to the PA (15–20×106) by syringe in a 10ml volume at 3–5ml/min. At day 10, a confluent 10cm plate of ventralized iPSC-derived epithelial progenitor cells was collected and delivered to the airways by gravity in a 30ml volume.
Orthotopic Lung Transplantation
Recipient Sprague-Dawley rats (260–300g, Charles River) were anesthetized with 5% isoflurane (Abbott), intubated with a 16G endotracheal tube (Becton-Dickinson), and ventilated with a rodent ventilator (Harvard Apparatus) supplying 100% supplemental oxygen. Regenerated lungs (n = 5) were removed from the bioreactor and the left lung was prepared for transplantation using a modified non-suture external cuff technique (26). In brief, the donor hilar vessels were isolated and transected to mount cuffs prepared from Venisystems Abbocaths (Hospira). Arterial and venous vessels were secured with 8-0 silk to the 18G cuff, while the bronchus was secured to a 16G cuff. A left anterior thoracotomy was performed on the non-heparinized recipient rats. The hilar vessels were dissected circumferentially and individually clamped with micro-Serrefines clamps. Donor vessels and cuffs were inserted into the recipient vessels and secured with 6-0 silk.
Blood gases were analyzed at 20 and 60 minutes after left lung reperfusion. Samples were drawn from the left pulmonary vein at the anastomosis and analyzed using an iStat Portable Analyzer with CG4+ Cartridges (Abbott). Lungs were then explanted and fixed in 10% formalin or stored in RNAlater.
Results
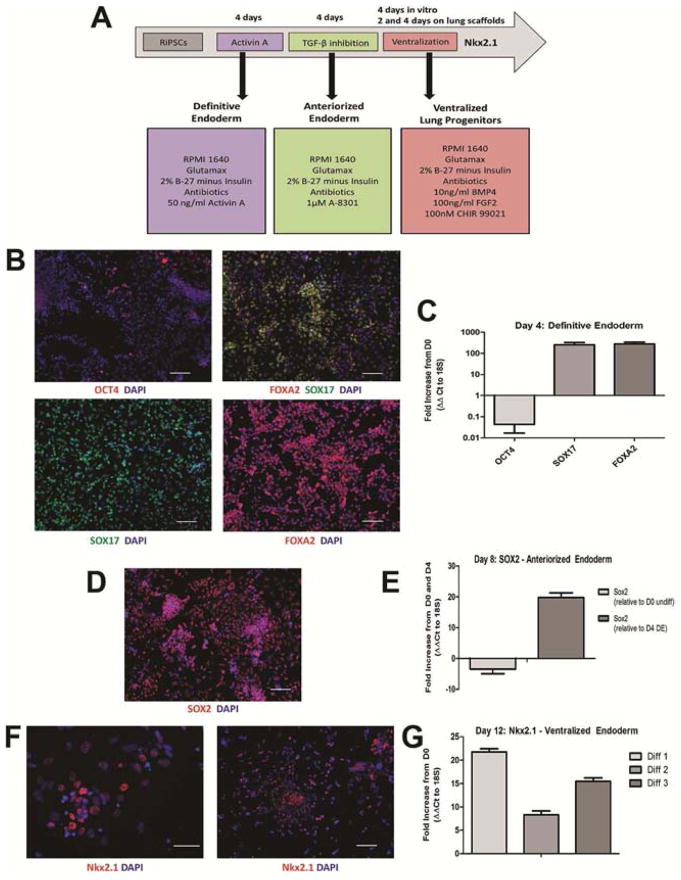
Human fibroblast-derived iPSCs were differentiated in vitro toward a lung epithelial progenitor phenotype through defined developmental stages (Figure 1A). First, Activin A treatment generated definitive endoderm at day 4, as confirmed by loss of OCT4 and induction SOX17 and FOXA2 (Figure 1B–C). Next, inhibition of TGF-β signalling for four days facilitated anteriorization to a foregut fate, indicated by SOX2 up-regulation (Figure 1D–E). Stimulation with FGF2 and BMP4 in combination with GSK3 pathway inhibition successfully ventralized the anteriorized population toward a lung epithelial progenitor phenotype. Approximately 40–60% of cells expressed Nkx2.1, the marker of lung specification, after twelve days of differentiation (Figure 1F); corresponding to an 8 to 21-fold increase in mRNA expression over that of undifferentiated iPSCs (Figure 1G).
Figure 1. Differentiation of human iPSCs to lung epithelial progenitors.
(A) In vitro differentiation protocol. (B) Generation of definitive endoderm indicated by loss of OCT4 (upper right), SOX 17 (lower right) and FOXA2 (upper left), and dual-immunofluorescence indicating single-cell coexpression (yellow, upper left). (C) qPCR analysis on day 4 of differentiation relative to undifferentiated day 0 cells. (D) Anteriorized endoderm population indicated by SOX2 expression on day 8 of differentiation by immunofluorescent staining (red) and (E) qPCR analysis relative to day 0 and day 4. (F) Differentiation to a lung epithelial progenitor population on day 12 indicated by nuclear Nkx2.1 expression (red), and (G) qPCR (n=3 separate experiments), expressed as fold increase from day 0 undifferentiated population. Gene expression all normalized to 18S expression (ΔΔCt), n=3 samples/differentiation in duplicate. Error bars represent standard deviation. Scale bars 100μm.
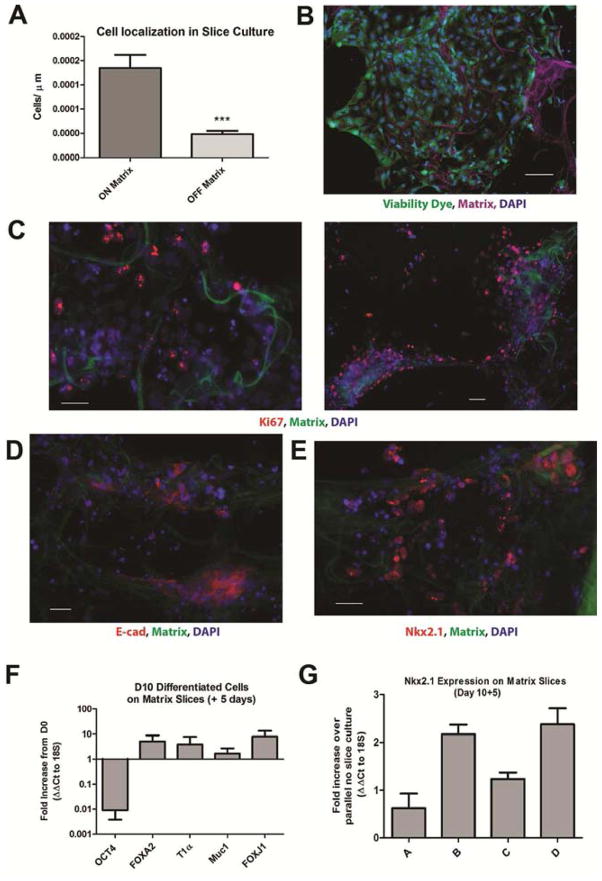
Next, ventralized iPSCs (day 10) were seeded onto human decellularized lung slices and maintained in ventralizing media for 5 days. A greater number of seeded cells was found localized to the matrix slice than on tissue culture plastic (Figure 2A). Cell viability (>90%) was observed (Figure 2B) and maintenance of a proliferative capacity confirmed by Ki67 expression (Figure 2C). Expression of E-cadherin demonstrated cell-cell interaction and predominance of an epithelial phenotype (Figure 2D). Lung progenitor phenotype was confirmed by Nkx2.1 expression in a subset of cells (Figure 2E). Loss of OCT4 and maintenance of FOXA2 expression was confirmed for the ventralized cell population on day 5 of cell-matrix culture. Additionally, expression of mature alveolar epithelial cell markers T1α and Mucin-1 was detected along with the ciliated cell marker FOXJ1 (Figure 2F). Compared to controls, an increase in Nkx2.1 expression was measured in cell-matrix co-cultures (Figure 2G).
Figure 2. In vitro differentiation of iPSC-derived lung progenitor cells on human decellularized lung slices.
(A) Cell localization relative to matrix slice, normalized to total matrix area per image, n=6. p=0.0006, Student’s T-test. Error bars represent standard deviation. (B) Viability of day 10 differentiated iPSCs seeded onto lung slices and cultured for 5 days. CalceinAM dye (green), matrix autofluorescence (magenta), DAPI (blue). 100μm scale bar. (C) Proliferation of Day 10+5 cell-matrix cultures by Ki67 (red). (D) Lung epithelial marker E-cadherin (red). (E) Lung progenitor marker Nkx2.1 (red). Matrix slice identified by autofluorescence (green), nucleus by DAPI (blue). 50μm scale bar. (F–G) Gene expression of Day 10+5 cells on matrix slices. (F) Fold increase normalized to 18S expression and relative to undifferentiated cells (ΔΔCt). (G) Fold increase in Nkx2.1 normalized to 18S expression and relative to parallel no-slice cultures (ΔΔCt). Four independent cultures, analyzed in duplicate. Error bars represent standard deviation.
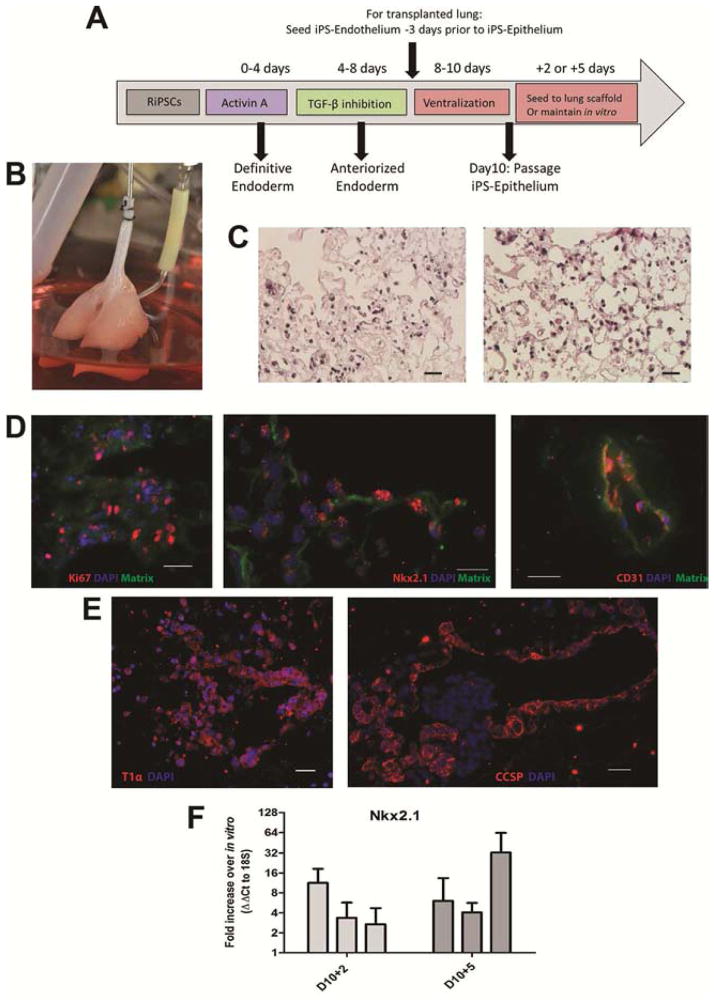
Whole lung constructs were prepared for transplantation by first recellularizing the vasculature with human iPSC-derived endothelium (Appendix Figure 1). Cells were delivered via the pulmonary artery and cultured for three days under constant perfusion with endothelial media. Next, ventralized (day 10) iPSC were delivered to the airways. Biomimetic culture with ventralizing media containing VEGF was maintained for two or five days (Figure 3A–B). Following culture, heterogeneous cell distribution was observed within the scaffold, with some areas remaining relatively acellular and others displaying notable cell attachment and retention (Figure 3C). Seeding efficiency was approximately 40–50% scaffold coverage, based on histological assessment. Cell proliferation within whole lung cultures was confirmed. Conservation of the epithelial progenitor phenotype was demonstrated by nuclear Nkx2.1 expression and the endothelial phenotype confirmed by CD31 expression (Figure 3D). Further specification toward mature lung phenotypes was noted by expression of the alveolar type I pneumocyte marker T1α/podoplanin and Clara cell secretory protein/CC10 thereby indicating an upper airway phenotype (Figure 3E). Quantification of Nkx2.1 revealed increased expression in cells cultured on whole lung scaffolds (Figure 3F). For recellularized lungs cultured for 2 days in the bioreactor, an average increase in Nkx2.1 expression of 5.8-fold ± 2.8 was measured when compared to cells maintained in vitro. For lungs cultured for five days, the average expression increased 14.2-fold ± 9.2 (n=3 lungs/time point).
Figure 3. Whole Lung Scaffold Recellularization and Biomimetic culture.
(A) Experimental design. (B) Re-seeded rat lung scaffold in the bioreactor. (C) Cell proliferation by Ki67 staining (red, left panel, 48μm scale bar), Nkx2.1 expression (red, middle panel, 24μm scale bar) and endothelial phenotype by CD31 (red, right panel, 24μm scale bar) within cultured lung constructs. Matrix outlined by autofluorescence (green) and nucleus by DAPI (blue). (D) Expression of T1α (red, left panel) and Clara Cell Secretory Protein (red, right panel) in lung constructs on day 10+5. 32.5μm scale bar (E) PCR quantification of Nkx2.1 expression in lung constructs normalized to 18S and relative to parallel in vitro cultured cells (ΔΔCt). 4 tissue pieces/lung, analyzed in duplicate. n=3 regenerated lungs. Error bars are standard error.
After bioreactor culture, single orthotopic left lung transplant was performed (n=5, Figure 4A, B). Following reperfusion, blood gases were sampled from the left pulmonary vein just proximal to the anastomosis; representing a mixed venous sample. Samples demonstrated adequate ventilation in the presence of the transplanted graft as indicated by a PCO2 maintained within normal limits over the study period; observed PO2 values were compatible with animal survival (Figure 4B). Histologic analysis of the explanted lungs showed red blood cell perfusion throughout the alveolar capillary network with occasional alveolar haemorrhage (Figure 4C).
Figure 4. Orthotopic Left Lung Transplantation of Recellularized Constructs.
(A) Experimental flow from cadaveric lung explant to transplantation of recellularized construct. (B) Implantation and reperfusion of recellularized lung construct. Right panel identifies the custom cuffs and sites of anastomosis. (B) Blood gases of recipient animal at 20 and 60 minutes post-reperfusion with positive pressure ventilation with 100% oxygen. Results from two separate transplants are presented. (C) Histology of the transplanted, recellularized constructs demonstrating blood perfusion of the alveolar capillaries. 25μm scale bars.
Comment
There is continued need for alternate and novel therapies for patients with end-stage lung disease. While advances in organ preservation, recipient management, and immunosuppression represent significant therapeutic advancements, lung transplantation remains the only effective treatment option (1, 27). Progress in both donor management and organ reconditioning have increased the usable fraction of the donor pool to nearly 50% (28), yet half of all donor lungs are not transplanted, often for logistical reasons. Decellularization and regeneration would impart new value to these organs from otherwise healthy donors with structurally-undamaged matrix.
Stem cell-based therapies for lung repair is an area of much research (29), involving both endogenous and exogenous mechanisms of cellular action (30). However, effective engraftment or direct enhancement of stem cell populations or their derivatives has not yet been successful in patients. As directed stem cell differentiation toward mature lung phenotype advances (31), the ability to therapeutically engraft stem cell-derived epithelial populations into diseased lungs remains a challenge. We propose that acellular scaffolds can optimally accommodate delivered cells and support the requisition of function. This goal presents many challenges, including the generation of sufficient cell numbers for regenerating clinical-scale organs and spatially delivering them to the regionally-distinct proximal and distal lung. For these reasons, we hypothesize that recellularization with multi-potent progenitor cells at the stage of lung specification would facilitate ex vivo expansion and localized differentiation following scaffold seeding. The Nkx2.1-expressing population can then be directed toward mature epithelial cell phenotypes by both exogenous growth factors and intrinsic matrix-derived cues. We demonstrate that induction of the Nkx2.1-expressing progenitor population is enhanced in cell-matrix culture when compared to differentiation in traditional culture.
Advancement toward a transplantable, fully-regenerated organ would likely require seeding of purified progenitor or improving in-situ control of cell fate to exclude off-target lineages. Gene modification of the source iPSCs may be required for lineage selection, which has been reported for ESC lines (32). Yet, these modifications also present the risk of introducing additional genetic mutations, and alternate techniques may be required.
In the advancing field of organ engineering a requirement for small-scale models remains. The high-throughput matrix slice model and rodent-scale whole-organ scaffolds allows for testing of specific cell populations, variable seeding techniques, and biomimetic culture conditions in a reproducible fashion. Matrix reseeding efficacy at various stages of differentiation can also be tested to assess expansion requirements. An additional benefit of whole-organ seeding is the recapitulation of endothelial and epithelial cross-talk across their respective physiological niches – a critical phenomenon in lung development (33, 34) and repair (35). The efficient differentiation of human iPSCs toward lung cell populations is also beneficial for patient-specific disease modelling (36), facilitating the study of cell biology and the testing of therapeutics in many diseases that currently lack strong animal models.
Successful transplantation and reperfusion of the dual epithelial and endothelial recellularized construct is an important proof-of-principle. While these experimental results do not directly reflect normal lung graft physiology, they provide foundational evidence to support the aim of clinical application. Following ex vivo cell delivery and culture, an “in vivo culture” period may be required for further regeneration. Complete recapitulation of the cellular components of the airways and alveoli will likely be aided by the recipient’s endogenous environment and repair program (37). At minimum, the implanted construct must permit adequate perfusion, with or without ventilation, but could otherwise be ‘cultured’ in vivo for an extended period of time prior to the reacquisition of full function. These concepts outline the critical next steps in the development of a regenerated, transplantable lung for therapeutic use.
Supplementary Material
Acknowledgments
This study was supported by the United Therapeutics Corporation, the National Institutes of Health Director’s New Innovator Award (DP2-OD008749-01), and the National Institutes of Health Research Project Grant (5R01-HL108678-03).
Footnotes
This paper was presented at the Society of Thoracic Surgeons 50th Annual Meeting. Orlando, Florida. January 25–29, 2014.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yeung JC, Keshavjee S. Overview of clinical lung transplantation. Cold Spring Harb Perspect Med. 2014;4(1) doi: 10.1101/cshperspect.a015628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32(12):3233–3243. doi: 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilpin SE, Guyette JP, Gonzalez G, Ren X, Asara JM, Mathisen DJ, et al. Perfusion decellularization of human and porcine lungs: bringing the matrix to clinical scale. J Heart Lung Transplant. 2014;33(3):298–308. doi: 10.1016/j.healun.2013.10.030. [DOI] [PubMed] [Google Scholar]
- 4.Ott HC, Clippinger B, Conrad C, Schuetz C, Pomerantseva I, Ikonomou L, et al. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med. 2010;16(8):927–933. doi: 10.1038/nm.2193. [DOI] [PubMed] [Google Scholar]
- 5.Song JJ, Kim SS, Liu Z, Madsen JC, Mathisen DJ, Vacanti JP, et al. Enhanced in vivo function of bioartificial lungs in rats. Ann Thorac Surg. 2011;92(3):998–1005. doi: 10.1016/j.athoracsur.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 6.Wagner DE, Bonvillain RW, Jensen T, Girard ED, Bunnell BA, Finck CM, et al. Can stem cells be used to generate new lungs? Ex vivo lung bioengineering with decellularized whole lung scaffolds. Respirology. 2013;18(6):895–911. doi: 10.1111/resp.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zorn AM, Wells JM. Vertebrate endoderm development and organ formation. Annu Rev Cell Dev Biol. 2009;25:221–251. doi: 10.1146/annurev.cellbio.042308.113344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nostro MC, Keller G. Generation of beta cells from human pluripotent stem cells: Potential for regenerative medicine. Semin Cell Dev Biol. 2012;23(6):701–710. doi: 10.1016/j.semcdb.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green MD, Chen A, Nostro MC, d’Souza SL, Schaniel C, Lemischka IR, et al. Generation of anterior foregut endoderm from human embryonic and induced pluripotent stem cells. Nat Biotechnol. 2011;29(3):267–272. doi: 10.1038/nbt.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Que J, Okubo T, Goldenring JR, Nam KT, Kurotani R, Morrisey EE, et al. Multiple dose-dependent roles for Sox2 in the patterning and differentiation of anterior foregut endoderm. Development. 2007;134(13):2521–2531. doi: 10.1242/dev.003855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mou H, Zhao R, Sherwood R, Ahfeldt T, Lapey A, Wain J, et al. Generation of multipotent lung and airway progenitors from mouse ESCs and patient-specific cystic fibrosis iPSCs. Cell Stem Cell. 2012;10(4):385–397. doi: 10.1016/j.stem.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lazzaro D, Price M, de Felice M, Di Lauro R. The transcription factor TTF-1 is expressed at the onset of thyroid and lung morphogenesis and in restricted regions of the foetal brain. Development. 1991;113:1093–1104. doi: 10.1242/dev.113.4.1093. [DOI] [PubMed] [Google Scholar]
- 13.Minoo P, Su G, Drum H, Bringas P, Kimura S. Defects in tracheoesophageal and lung morphogenesis in Nkx2. 1(−/−) mouse embryos. Dev Biol. 1999;209(1):60–71. doi: 10.1006/dbio.1999.9234. [DOI] [PubMed] [Google Scholar]
- 14.Morrisey EE, Hogan BL. Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev Cell. 2010;18(1):8–23. doi: 10.1016/j.devcel.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Longmire TA, Ikonomou L, Hawkins F, Christodoulou C, Cao Y, Jean JC, et al. Efficient derivation of purified lung and thyroid progenitors from embryonic stem cells. Cell Stem Cell. 2012;10(4):398–411. doi: 10.1016/j.stem.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong AP, Bear CE, Chin S, Pasceri P, Thompson TO, Huan LJ, et al. Directed differentiation of human pluripotent stem cells into mature airway epithelia expressing functional CFTR protein. Nat Biotechnol. 2012;30(9):876–882. doi: 10.1038/nbt.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghaedi M, Calle EA, Mendez JJ, Gard AL, Balestrini J, Booth A, et al. Human iPS cell-derived alveolar epithelium repopulates lung extracellular matrix. J Clin Invest. 2013;123(11):4950–4962. doi: 10.1172/JCI68793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lwebuga-Mukasa JS, Ingbar DH, Madri JA. Repopulation of a human alveolar matrix by adult rat type II pneumocytes in vitro. A novel system for type II pneumocyte culture. Exp Cell Res. 1986;162(2):423–435. doi: 10.1016/0014-4827(86)90347-2. [DOI] [PubMed] [Google Scholar]
- 19.Lin YM, Zhang A, Rippon HJ, Bismarck A, Bishop AE. Tissue engineering of lung: the effect of extracellular matrix on the differentiation of embryonic stem cells to pneumocytes. Tissue Eng Part A. 2010;16(5):1515–1526. doi: 10.1089/ten.TEA.2009.0232. [DOI] [PubMed] [Google Scholar]
- 20.Faulk DM, Johnson SA, Zhang L, Badylak SF. Role of the Extracellular Matrix in Whole Organ Engineering. J Cell Physiol. 2013 doi: 10.1002/jcp.24532. [DOI] [PubMed] [Google Scholar]
- 21.Joe P, Wallen LD, Chapin CJ, Lee CH, Allen L, Han VK, et al. Effects of mechanical factors on growth and maturation of the lung in fetal sheep. Am J Physiol. 1997;272:L95–105. doi: 10.1152/ajplung.1997.272.1.L95. [DOI] [PubMed] [Google Scholar]
- 22.Sanchez-Esteban J, Cicchiello LA, Wang Y, Tsai SW, Williams LK, Torday JS, et al. Mechanical stretch promotes alveolar epithelial type II cell differentiation. J Appl Physiol (1985) 2001;91(2):589–595. doi: 10.1152/jappl.2001.91.2.589. [DOI] [PubMed] [Google Scholar]
- 23.Sacco O, Silvestri M, Sabatini F, Sale R, Defilippi AC, Rossi GA. Epithelial cells and fibroblasts: structural repair and remodelling in the airways. Paediatr Respir Rev. 2004;5:S35–40. doi: 10.1016/s1526-0542(04)90008-5. [DOI] [PubMed] [Google Scholar]
- 24.Badylak SF, Taylor D, Uygun K. Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds. Annu Rev Biomed Eng. 2011;13:27–53. doi: 10.1146/annurev-bioeng-071910-124743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Warren L, Manos PD, Ahfeldt T, Loh YH, Li H, Lau F, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7(5):618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mizobuchi T, Sekine Y, Yasufuku K, Fujisawa T, Wilkes DS. Comparison of surgical procedures for vascular and airway anastomoses that utilize a modified non-suture external cuff technique for experimental lung transplantation in rats. J Heart Lung Transplant. 2004;23(7):889–893. doi: 10.1016/j.healun.2003.06.009. [DOI] [PubMed] [Google Scholar]
- 27.Christie JD, Edwards LB, Kucheryavaya AY, Benden C, Dipchand AI, Dobbels F, et al. The Registry of the International Society for Heart and Lung Transplantation: 29th adult lung and heart-lung transplant report-2012. J Heart Lung Transplant. 2012;31(10):1073–1086. doi: 10.1016/j.healun.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 28.Snell GI, Paraskeva M, Westall GP. Donor selection and management. Semin Respir Crit Care Med. 2013;34(3):361–370. doi: 10.1055/s-0033-1348464. [DOI] [PubMed] [Google Scholar]
- 29.Stripp BR, Shapiro SD. Stem cells in lung disease, repair, and the potential for therapeutic interventions: State-of-the-art and future challenges. Am J Respir Cell Mol Biol. 2006;34(5):517–518. doi: 10.1165/rcmb.F315. [DOI] [PubMed] [Google Scholar]
- 30.Rock J, Konigshoff M. Endogenous lung regeneration: potential and limitations. Am J Respir Crit Care Med. 2012;186(12):1213–1219. doi: 10.1164/rccm.201207-1151PP. [DOI] [PubMed] [Google Scholar]
- 31.Moodley Y, Thompson P, Warburton D. Stem cells: a recapitulation of development. Respirology. 2013;18(8):1167–1176. doi: 10.1111/resp.12186. [DOI] [PubMed] [Google Scholar]
- 32.Goulburn AL, Alden D, Davis RP, Micallef SJ, Ng ES, Yu QC, et al. A targeted NKX2.1 human embryonic stem cell reporter line enables identification of human basal forebrain derivatives. Stem Cells. 2011;29(3):462–473. doi: 10.1002/stem.587. [DOI] [PubMed] [Google Scholar]
- 33.Zhao L, Wang K, Ferrara N, Vu TH. Vascular endothelial growth factor co-ordinates proper development of lung epithelium and vasculature. Mech Dev. 2005;122:877–886. doi: 10.1016/j.mod.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 34.Yamamoto H, Yun EJ, Gerber HP, Ferrara N, Whitsett JA, Vu TH. Epithelial-vascular cross talk mediated by VEGF-A and HGF signaling directs primary septae formation during distal lung morphogenesis. Dev Biol. 2007;308(1):44–53. doi: 10.1016/j.ydbio.2007.04.042. [DOI] [PubMed] [Google Scholar]
- 35.Lahm T, Crisostomo PR, Markel TA, Wang M, Lillemoe KD, Meldrum DR. The critical role of vascular endothelial growth factor in pulmonary vascular remodeling after lung injury. Shock. 2007;28(1):4–14. doi: 10.1097/shk.0b013e31804d1998. [DOI] [PubMed] [Google Scholar]
- 36.Somers A, Jean JC, Sommer CA, Omari A, Ford CC, Mills JA, et al. Generation of transgene-free lung disease-specific human induced pluripotent stem cells using a single excisable lentiviral stem cell cassette. Stem Cells. 2010;28(10):1728–1740. doi: 10.1002/stem.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiao H, Li DX, Liu M. Knowledge translation: airway epithelial cell migration and respiratory diseases. Cell Mol Life Sci. 2012;69(24):4149–4162. doi: 10.1007/s00018-012-1044-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.