Abstract
Objectives
Men treated with androgen deprivation therapy (ADT) or radiation therapy (RT) for prostate cancer have an increased risk for fractures. Given uncertainty as to whether specific clinical factors can identify men at increased risk, we sought to develop a prognostic index for risk of fracture in this population.
Materials and methods
We used the Surveillance, Epidemiology, and End Results-Medicare database to identify men who received ADT or RT after being diagnosed with localized prostate cancer in 2007-2009. Cox proportional hazards models tested the association of potential risk factors with fracture. In a derivation group, hazard ratios were used to assign points for factors independently related to fracture. The prognostic index was then applied to a validation group.
Results
The sample of 5,824 men had a median age of 73.0 years; 82.9% were white and 8.6% had a fracture within 2 years of treatment for prostate cancer. The Cox model identified 8 variables (age, race, hormone treatment, Elixhauser score, anxiety, Parkinson's, fall-inducing medications and disability status) independently associated with fracture. In the derivation cohort, 4.3% of the sample experienced a fracture in the low-risk group, 8.9% in the intermediate group, and 19.2% in the high-risk group (C statistic, 0.749). The index was applied to the validation cohort (C statistic, 0.782).
Conclusion
The prognostic index can help to identify patients at increased risk for fracture. This underscores the importance of identifying risk factors for fracture, given the substantial variation in fracture risk in men treated with ADT or RT.
Keywords: Prostate cancer, Androgen deprivation therapy, Radiation therapy, Risk score, Fractures
INTRODUCTION
Prostate cancer is primarily a disease of aging.(1) The mean age of patients with prostate cancer is 73 years, and about 85% of patients are diagnosed after age 65 years.(2) Aging is associated with a progressive decrease of physiologic reserves that affects older patients’ tolerance for cancer therapy, and, in some cases, can restrict the options for cancer treatment.(3)
Fractures are a relatively common and clinically significant adverse outcome among older men with prostate cancer.(4-6) Additionally, fractures are associated with severe bone pain, limited mobility, and hospitalization for treatment, as well as negatively associated with overall survival independent of pathological stage.(4, 7, 8) In addition to (lower) bone density, factors associated with an increased risk of fracture include: older age, multiple coexisting conditions, history of falls and previous fractures, lower body mass index, poor functional status, and lifestyle factors (such as physical inactivity, smoking and alcohol use).(9-15) Hence, a better understanding of which patients are at increased risk for fracture should be considered when selecting cancer treatments.
Androgen deprivation therapy (ADT) and radiation therapy (RT) are two forms of cancer treatment that are implicated in promoting fractures,(5, 15-17) and are widely used therapeutic treatment modalities for prostate cancer.(18, 19) ADT, often given concomitantly with RT for men with non-metastatic disease or rising prostate-specific antigen, has been shown to reduce morbidity and possibly increase survival in men with locally advanced disease.(20-22) Although these forms of therapy exhibit cancer control benefits they can also produce negative side effects, such as weakening of the skeleton.(5, 9, 15, 18) For example, a rapid loss of bone-mineral density due to hypogonadism occurs within the first 6 to 12 months of ADT.(16, 17, 23) Prior studies have demonstrated that RT also increases the risk of fractures by damaging the bone matrix.(15, 24) Fracture risk-stratification for men treated with ADT or RT therefore has particular importance.(5, 15, 16, 25)
In addition to cancer treatments, older men with prostate cancer take, on average, five different medications. Polypharmacy has been linked to increased risk of falls and fractures, as well as decline in cognitive and physical functioning.(3, 26) Patients taking multiple medications have an especially high risk of fractures, through several potential mechanisms. For instance, some medications have been demonstrated to decrease bone density and subsequently increase fracture risk.(27-31) Some antihypertensive medications are frequently associated with falls due to postural hypotension.(32, 33) Benzodiazepines have negative effects on cognition, gait, and balance, and are also associated with a high risk of falling.(28, 29)
Although studies have identified risk factors for fractures in men with prostate cancer, the combined effect of these factors on fracture risk has not been adequately addressed.(5, 16-19, 24, 25) These factors alone may not be sufficient for identifying patients at increased risk, because they fail to take into account additional significant predictors of fracture, such as poor functional status. Valid and effective prognostic indices are greatly needed—specifically, risk stratification systems for fracture designed or tested exclusively in the older population. Moreover, the recent availability of Medicare Part D data allows us to incorporate medications in addition to cancer treatment in the prognostic index to investigate specific coexisting conditions in greater detail.
To address these knowledge gaps, we performed a claims-based observational study to identify factors associated with fracture among Medicare beneficiaries receiving ADT and/or RT for prostate cancer, as this is a group of patients who face an increased risk of fracture as a result of their prostate cancer treatment. We then developed a prognostic index to assess fracture risk in these patients. Stratifying patients treated with ADT or RT for prostate cancer—based on clinical characteristics, coexisting conditions, and medication use—has the potential to identify men with increased fracture risk, thus allowing for targeted treatment interventions of the high-risk populations.
MATERIALS AND METHODS
Study Overview and Data Source
We conducted a retrospective cohort study of men with prostate cancer who received ADT and/or RT, using the Surveillance, Epidemiology, and End Results (SEER)-Medicare database. SEER-Medicare is a database consisting of patient demographics and cancer characteristics from 17 tumor registries linked to Medicare claims that include date of service, diagnoses, and procedures from care billed by hospitals, outpatient facilities, and physicians.(34) We also used First Databank's MedKnowledge database, which contains drug information including National Drug Codes (NDC). The Yale Human Investigation Committee approved the protocol, determining that this study did not involve human subjects.
Study Sample
We identified patients, 67 years or older, who started ADT and/or RT from April 2007 through June 2009. We restricted the study to patients with clinical tumor stage I or II who lived at least 6 months after starting ADT and/or RT. Patients who received at least one dose of medical ADT after prostate cancer diagnosis either in the form of gonadotropin-releasing hormone (GnRH) agonist, steroidal or non-steroidal anti-androgens, or who underwent orchiectomy within 9 months of prostate cancer diagnosis, were classified as ADT users. Patients who received ADT, RT or orchiectomy in the two years prior to prostate cancer diagnosis were excluded. We also excluded patients who had a fracture claim in the year prior to receipt of ADT or RT to ensure that the fracture captured in the claims data is a new or incident fracture.
Construction of Variables
We assessed the primary outcome of interest (fracture) using International Classification of Diseases, 9th revision (ICD-9) and Healthcare Common Procedure Coding System (HCPCS) codes (Appendix 1). We identified diagnosis and procedure codes indicative of fracture from a Mr OS study assessing osteoporosis in men and from studies investigating fractures from Medicare claims and consolidated the codes into a master list by fracture site.(35-42) Patients were followed up for outcomes from the start of treatment until end of follow-up (December 31, 2010).
We grouped the risk factors that we hypothesized to be associated with fracture into four categories: demographic variables, clinical risk factors (tumor grade, tumor stage, comorbid conditions, disability), cancer treatment, and medication use. The demographic characteristics in our analysis included age, race, and median household income at the census tract or zip code level. Comorbidity status was calculated according to conditions used by Elixhauser, et al., that we previously found were associated with survival in a sample of patients without cancer.(43) Three additional specific coexisting conditions (anxiety, dementia and Parkinson's disease) that are not a part of the Elixhauser index were selected a priori because of their association with falls.(32) All comorbid conditions were identified by searching the inpatient, outpatient, and physician claims in the interval from 24 through 3 months prior to diagnosis, for specific ICD-9 diagnosis codes. Codes were only included if they were associated with a hospital claim or appeared on at least two outpatient/physician claims that were billed at least 30 days apart. Osteoporosis was identified by a combination of diagnosis code 733.0× or the receipt of medication used to treat osteoporosis. For diagnosis code 733.0×, we applied the same requirements used for the Elixhauser conditions, such that a patient had to have this diagnosis code recorded on at least one inpatient claim or >2 outpatient/physician claims billed >30 days apart. We also utilized the Medicare part D database to identify patients receiving medicationsused to treat osteoporosis (bisphosphonates and selective estrogen receptor modulators) for a minimum of 60 days in the four months prior to starting ADT and/or RT.
We also included disability status as a measure of functional status. The original disability status prediction model was created using data from a representative sample of the Medicare beneficiary population age 66 and over to generate a weighted prediction of the probability that a beneficiary has poor functional status.(44) The disability status measure is a marker of poor functional status linking self-reported measures of functional status, strength, stamina, and exercise to various functional dimensions and degrees of limitations. We categorized the disability status into quartiles and created a dichotomous variable based on the highest quartile (i.e. most disabled) vs. the remaining three quartiles.
We ascertained receipt of radiation by searching claims for HCPCS codes indicating the delivery of standard external beam radiation therapy (EBRT), intensity-modulated radiation therapy (IMRT), stereotactic radiosurgery, or proton beam therapy. Patients who received EBRT or IMRT must have received at least four treatments to be considered treated. Patients were categorized based on the dose frequency of ADT [1-3 doses, 4-8 doses or ≥ 9 doses] taken during the time period. The study included osteoporosis-promoting medications (calcineurin inhibitors and steroids) as well as medications that increase fall risk (antihypertensive medications and central nervous system (CNS)-active medications; Appendix 2). To be considered a medication user the patient must have received a minimum of 60 days of medication in the four months prior to starting treatment.
Statistical Analysis
We used Cox proportional-hazards regression to determine which covariates were independently associated with the occurrence of fracture, adjusting for sociodemographic and clinical characteristics, cancer treatment received, and medication use.
To create the risk score, we randomly divided the sample into two cohorts: derivation (n=2,912) and validation samples (n=2,912). We used unadjusted Cox proportional hazards models to determine which covariates were significantly associated with the outcome of any fracture in the derivation cohort. Covariates had to be significant at the level of p<.20 to be included in the multivariable model and at the level of p<.10 to be retained in the final set of risk factors. We then constructed a risk score using a method similar to the Framingham Risk Score.(45) We divided the regression coefficients for the various risk factors by the lowest coefficient, and rounded the resulting coefficients to the nearest integer; the overall risk score was calculated by adding up the points for each of the final set of risk factors present. A risk score was calculated for each patient by adding the points of each risk factor that was present. For example, a white male (2 points), 75 years old (2 point), treated with 6 months of ADT (1 point), greater than 3 Elixhauser conditions (4 points) and taking CNS active medications (2 points) would have a risk score of 11 points. After Winsorizing to the 5th and 95th percentiles, derivation cohort risk scores ranged from 2 to 12 points. We divided the risk score into 3 groups representing low, intermediate, and high risk of any fracture. Model performance in both cohorts was measured with the C statistic. All analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary NC).
RESULTS
We identified 5,824 men who fulfilled our inclusion criteria. The median age was 73.0 years, the majority of the cohort was white (82.9%), and approximately 15% of the patient population had ≥3 coexisting conditions (Table 1). Twenty percent of men received ADT only, 43% of men received RT only, whereas approximately 36% of men received both forms of therapy.
Table 1.
Patient Characteristics
| Characteristic | N | % |
|---|---|---|
| Total | 5824 | |
| Age (in years) | ||
| 67-69 | 1155 | 19.8 |
| 70-74 | 2193 | 37.7 |
| 75-79 | 1521 | 26.1 |
| 80-84 | 738 | 12.7 |
| 85-94 | 217 | 3.7 |
| Race | ||
| White | 4830 | 82.9 |
| Black | 533 | 9.2 |
| Other | 461 | 7.9 |
| Income | ||
| <$33K | 1441 | 24.7 |
| $33K - <$40K | 942 | 16.2 |
| $40K - <$50K | 1107 | 19.0 |
| $50K - <$63K | 958 | 16.5 |
| ≥$63K | 1376 | 23.6 |
| Year of Diagnosis | ||
| 2007 | 2635 | 45.2 |
| 2008 | 2512 | 43.1 |
| 2009 | 677 | 11.6 |
| Radiation therapy | ||
| No | 1200 | 20.6 |
| Yes | 4624 | 79.4 |
| ADT | ||
| None | 2534 | 43.5 |
| Hormone (1-3 units) | 599 | 10.3 |
| Hormone (4-8 units) | 1881 | 32.3 |
| Hormone (≥9 units) | 810 | 13.9 |
| Tumor Grade | ||
| Well-differentiated | 29 | 0.5 |
| Moderately-differentiated | 2256 | 38.7 |
| Poorly-differentiated | 3517 | 60.4 |
| Undifferentiated | 22 | 0.4 |
| Clinical T Stage | ||
| I | 3457 | 59.4 |
| II | 2367 | 40.6 |
| Elixhauser Score | ||
| 0 | 2532 | 43.5 |
| 1-2 | 2376 | 40.8 |
| ≥3 | 916 | 15.7 |
| Anxiety | ||
| No | 5712 | 98.1 |
| Yes | 112 | 1.9 |
| Dementia | ||
| No | 5764 | 99.0 |
| Yes | 60 | 1.0 |
| Parkinson's | ||
| No | 5749 | 98.7 |
| Yes | 75 | 1.3 |
| Osteoporosis | ||
| No | 5690 | 97.7 |
| Yes | 134 | 2.3 |
| Medications that cause osteoporosis* | ||
| No | 5725 | 98.3 |
| Yes | 99 | 1.7 |
| Medications that cause falls (CNS)† | ||
| No | 5448 | 93.5 |
| Yes | 376 | 6.5 |
| Medications that cause falls (Antihypertensive)‡ | ||
| No | 3855 | 66.2 |
| Yes | 1969 | 33.8 |
| Disability score | ||
| Q1 | 1456 | 25.0 |
| Q2-Q4 | 4368 | 75.0 |
Medications that cause osteoporosis include: calcineurin inhibitors and glucocorticoids.
Central nervous system-active medications include: tricyclic agents, antipsychotics, and atypical antipsychotics).
Antihypertensive medications include: peripheral alpha-1 receptor blockers, central alpha-2 receptor agonists, cardiac and non-cardiac selective medications and alpha-beta blockers.
At 2-years post-treatment, 8.6% of the sample experienced a fracture (3.1% with fracture of the hip, humerus, or elbow; Table 2). Advancing age was associated with risk of fracture; men over the age of 85 had almost twice the risk of fracture compared to men under 69 years (HR 2.23; 95% CI, 1.55-3.22). Men with greater than three coexisting conditions had a significant increase in fracture risk (HR 2.56; 95% CI, 2.07-3.15). Specifically, we found that men with anxiety, Parkinson's or osteoporosis had a significantly increased risk for fracture. Increased doses of ADT were associated with fracture risk compared to men who did not receive this form of therapy. Likewise, men treated with CNS active medications or antihypertensive medications had an increased risk of fracture compared to men not taking these medications (HR 1.74; 95% CI, 1.35-2.26, HR 1.19; 95% CI, 1.07-1.33).
Table 2.
Patient Characteristics and Fracture Risk
| % experiencing fracture within 2 years* |
Unadjusted |
Adjusted |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Overall | % 8.6 | p-value† | Hazard Ratio | 95% CI | p-value | Hazard Ratio | 95% CI | p-value | ||
| Characteristic | ||||||||||
| Age (in years) | <.001 | <.001 | <.001 | |||||||
| 67-69 | 6.5 | 1.00 | -- | -- | 1.00 | -- | -- | |||
| 70-74 | 6.9 | 1.04 | 0.79 | 1.36 | 1.02 | 0.79 | 1.33 | |||
| 75-79 | 10.6 | 1.39 | 1.02 | 1.89 | 1.30 | 0.97 | 1.74 | |||
| 80-84 | 12.4 | 1.85 | 1.33 | 2.57 | 1.57 | 1.13 | 2.17 | |||
| 85-94 | 10.9 | 2.23 | 1.55 | 3.22 | 1.82 | 1.30 | 2.56 | |||
| Race | .04 | <.001 | <.001 | |||||||
| White | 9.1 | 1.00 | -- | -- | 1.00 | -- | -- | |||
| Black | 6.2 | 0.57 | 0.42 | 0.79 | 0.57 | 0.43 | 0.75 | |||
| Other | 6.3 | 0.66 | 0.45 | 0.95 | 0.60 | 0.41 | 0.87 | |||
| Income | .34 | <.001 | <.001 | |||||||
| <$33K | 8.6 | 1.00 | -- | -- | 1.00 | -- | -- | |||
| $33K - <$40K | 7.2 | 0.80 | 0.71 | 0.90 | 0.80 | 0.69 | 0.94 | |||
| $40K - <$50K | 7.8 | 0.97 | 0.77 | 1.23 | 0.96 | 0.74 | 1.24 | |||
| $50K - <$63K | 9.8 | 1.04 | 0.81 | 1.34 | 1.08 | 0.82 | 1.42 | |||
| ≥$63K | 9.5 | 0.99 | 0.80 | 1.23 | 1.02 | 0.80 | 1.29 | |||
| Radiation therapy | .03 | .002 | .43 | |||||||
| No | 10.5 | 1.00 | -- | -- | 1.00 | -- | -- | |||
| Yes | 8.2 | 0.74 | 0.62 | 0.90 | 1.08 | 0.89 | 1.31 | |||
| ADT | .002 | <.001 | <.001 | |||||||
| None | 6.9 | 1.00 | -- | -- | 1.00 | -- | -- | |||
| Hormone (1-3 doses) | 8.7 | 1.21 | 0.94 | 1.56 | 1.23 | 0.98 | 1.55 | |||
| Hormone (4-8 doses) | 9.8 | 1.55 | 1.32 | 1.82 | 1.44 | 1.21 | 1.71 | |||
| Hormone (≥9 doses) | 11.1 | 1.75 | 1.39 | 2.20 | 1.46 | 1.17 | 1.83 | |||
| Elixhauser Score | <.001 | <.001 | <.001 | |||||||
| 0 | 6.5 | 1.00 | -- | -- | 1.00 | -- | -- | |||
| 1-2 | 8.1 | 1.46 | 1.26 | 1.68 | 1.33 | 1.16 | 1.53 | |||
| ≥3 | 16.3 | 2.56 | 2.07 | 3.15 | 2.18 | 1.76 | 2.70 | |||
| Anxiety | <.001 | <.001 | <.001 | |||||||
| No | 8.4 | 1.00 | -- | -- | 1.00 | -- | -- | |||
| Yes | 22.8 | 2.22 | 1.70 | 2.89 | 1.70 | 1.36 | 2.12 | |||
| Dementia | .68 | .42 | ||||||||
| No | 8.6 | 1.00 | -- | -- | ||||||
| Yes | 10.5 | 1.28 | 0.70 | 2.33 | ||||||
| Parkinson's | <.001 | <.001 | <.001 | |||||||
| No | 8.4 | 1.00 | -- | -- | 1.00 | -- | -- | |||
| Yes | 27.3 | 3.54 | 2.58 | 4.86 | 2.13 | 1.44 | 3.14 | |||
| Osteoporosis | .04 | .20 | .46 | |||||||
| No | 8.5 | 1.00 | -- | -- | 1.00 | -- | -- | |||
| Yes | 14.2 | 1.65 | 0.77 | 3.56 | 1.34 | 0.62 | 2.88 | |||
| Medications that cause osteoporosis‡ | <.001 | .003 | .06 | |||||||
| No | 8.5 | 1.00 | -- | -- | 1.00 | -- | -- | |||
| Yes | 19.7 | 1.78 | 1.20 | 2.64 | 1.37 | 0.99 | 1.90 | |||
| Medications that cause falls (CNS)§ | .02 | <.001 | .009 | |||||||
| No | 8.4 | 1.00 | -- | -- | 1.00 | -- | -- | |||
| Yes | 12.6 | 1.74 | 1.35 | 2.26 | 1.41 | 1.09 | 1.82 | |||
| Medications that cause falls (Antihypertensive)¶ | .03 | .002 | .69 | |||||||
| No | 8.0 | 1.00 | -- | -- | 1.00 | -- | -- | |||
| Yes | 9.9 | 1.19 | 1.07 | 1.33 | 1.02 | 0.91 | 1.15 | |||
| Disability score | .79 | .009 | .02 | |||||||
| Q1 | 8.5 | 1.00 | -- | -- | 1.00 | -- | -- | |||
| Q2-Q4 | 8.7 | 1.21 | 1.05 | 1.39 | 1.18 | 1.03 | 1.36 | |||
Among patients with at least 2 years of follow-up
p-value is for chi-square test of the association between each covariate and 2-year fracture
Medications that cause osteoporosis include: calcineurin inhibitors and glucocorticoids.
Central nervous system-active medications include: tricyclic agents, antipsychotics, and atypical antipsychotics.
Antihypertensive medications include: peripheral alpha-1 receptor blockers, central alpha-2 receptor agonists, cardiac and non-cardiac selective medications and alpha-beta blockers.
Multivariable Analysis
In multivariable analyses, advanced age, race, and number of GnRH doses were independently associated with greater risk for fracture. Men aged 80-84 and 85-94 had a higher risk for fracture compared to men aged 67-69 (HR 1.57; 95% CI, 1.13-2.17 and HR 1.82; 95% CI, 1.30-2.56), respectively. Black men had significantly lower risk for fracture than white men (HR 0.57; 95% CI, 0.43-0.75). A dose-dependent relationship between ADT and risk for fracture was also found, increasing steadily with the increasing number of doses of GnRH agonist received (HR for 1-3 doses: 1.23; 95% CI, 0.98-1.55; 4-8 doses: 1.44; 95% CI, 1.21-1.71; ≥9 doses: 1.46; 95% CI, 1.17-1.83). Patients with anxiety, Parkinson's and poorer functional status, based on the disability status measure also had an increased risk for fracture (HR 1.70; 95% CI, 1.36-2.12, HR 2.13; 95% CI, 1.44-3.14 and HR 1.18; 95% CI, 1.03-1.36), respectively. After controlling for other covariates, men treated with CNS active medications had an increased risk for fracture (HR 1.41; 95% CI, 1.09-1.82). The use of antihypertensive medications or osteoporosis-promoting medications was not associated with risk of fracture in our multivariable analysis.
Risk stratification system
Our risk score was based on 8 significant risk factors identified in the multivariable analysis, which include: age, race, number of ADT units, Elixhauser score, whether the patient had anxiety, Parkinson's disease, use of CNS active medications, and disability status (Table 3). Patients were divided by risk scores into 3 risk groups, with 1,323 patients in the lowest risk group (risk score 2-5), 1,234 patients in the intermediate group (risk score 6-9) and 355 patients in the highest risk group (risk score ≥10).
Table 3.
Prognostic index for risk of fracture
| Characteristic | Points |
|---|---|
| Age (in years) | |
| 67-74 | 0 |
| 75-79 | 2 |
| 80-84 | 3 |
| 85-94 | 5 |
| Race | |
| White | 2 |
| Black/other | 0 |
| ADT | |
| None | 0 |
| Hormone (1-3 doses) | 1 |
| Hormone (4-8 doses) | 1 |
| Hormone (≥9 doses) | 2 |
| Elixhauser Score | |
| 0 | 0 |
| 1-2 | 1 |
| ≥3 | 4 |
| Anxiety | |
| No | 0 |
| Yes | 5 |
| Parkinson's | |
| No | 0 |
| Yes | 4 |
| Medications that cause falls (CNS) | |
| No | 0 |
| Yes | 2 |
| Disability status | |
| Q1 | 0 |
| Q2-Q4 | 2 |
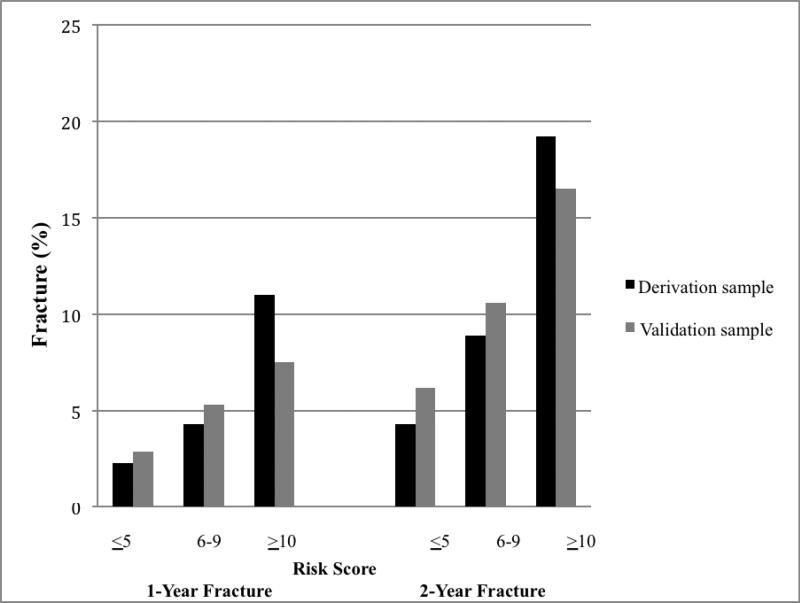
In the derivation cohort, the 2-year fracture rate for the low-risk group was 4.3% (95% CI, 3.1-5.7; Figure 1) while patients in the high-risk group had a fracture rate of 19.2% (95% CI, 14.5-24.7), representing a 4.5-fold increase in fracture between the low-risk and high-risk groups. Applying the risk stratification system to the validation cohort, the 2-year fracture rates for the low-, intermediate-, and high-risk groups were 6.2% (95% CI, 4.7-7.9), 10.6% (95% CI, 8.7-12.8), and 16.5% (95% CI 12.3-21.5), respectively, representing a 2.6-fold increase in fracture between the low-risk and high-risk groups. The model predicted well in both the derivation (C statistic, 0.749) and validation (C statistic, 0.782) cohorts.
Figure 1.
1- and 2-year fracture in derivation and validation cohorts by risk strata
DISCUSSION
In this study, we developed and validated a prognostic index to identify older men with localized prostate cancer at high risk for fracture based on demographic characteristics, comorbidities, and medication use in addition to cancer treatment. The proposed index could help to stratify patients into fracture risk groups.
We identified a group of patients receiving treatment for prostate cancer at highest risk of fracture. ADT and RT are a routine part of the management for many men with nonmetastatic prostate cancer.(21, 46) These forms of treatment result in significant bone loss, thus increasing the risk of fractures. Fractures are associated with increased morbidity, hospitalizations and reduced survival in men with prostate cancer independent of metastasis.(4, 47) Men with prostate cancer treated with ADT and/or RT are particularly susceptible to fractures because these patients are most likely elderly and may have a multitude of risk factors that contribute to the increased risk of fracture. Prior studies have reported that measures to prevent osteoporosis are not routinely utilized in this population.(48-51) Based on clinic audits and surveys, few urologists and radiation oncologists would order bone mineral density tests or start bisphosphonates or vitamin D to prevent fractures.(48, 50)
Our study builds upon prior reports by identifying modifiable risk factors independently associated with fractures. We found that treatment with CNS-active medications increases fracture risk in this patient population. Of the 8 risk factors included in our prognostic index, medications can be one of the simplest risk factors to modify. Consistent with our results, a case-control study among elderly Medicaid enrollees reported an increased risk of hip fracture associated with use of hypnotics-anxiolytics, tricyclic antidepressants and antipsychotics.(52) A previous retrospective study in patients without cancer reported a moderate association between CNS-active medications and fracture,(53) whereas we found a slightly stronger association between CNS-active medications and fracture risk among men treated with ADT or RT. This finding could be attributed to the fact that we grouped antipsychotics in addition to antidepressants and benzodiazepines in the CNS-active medication category. The results could also reflect the inherent increased fracture risk among patients treated with ADT or RT.
We found a relationship between cancer treatment and fracture risk. Studies of prescription claims databases have suggested that GnRH-agonist treatment is associated with a 1.5-fold greater risk of fracture.(5) This observation is consistent with prior work that found pelvic three-dimensional EBRT was associated with a 76% increased risk of hip fracture.(15) Furthermore, they report that the risk was increased further by the addition of short-course ADT to EBRT.(15) Our findings also support the dose-response relationship between dosage of ADT administered and the risk of fracture seen in a prior study.(5) In this study, however, investigators did not explore whether common risk factors for fractures, such as coexisting conditions specific to the geriatric population or medication use, potentiate this risk. In addition, previous studies have found that patients who received orchiectomy are at an increased risk of fracture.(25, 54) We were unable to evaluate this association reliably due to the low number of patients who received orchiectomy in our sample.
Our study has limitations, including the inability to assess all variables that may contribute to fracture risk as the SEER-Medicare database does not include potentially important fracture risk factors such as BMI, bone mineral density, balance, gait, alcohol consumption, nicotine usage, use of vitamin D supplements or over-the-counter sedative/hypnotic drugs. We excluded men with prior fracture, in order to capture new fractures associated with cancer treatment. Because prior fracture is an important risk factor for subsequent fracture, it is possible that the results of our prognostic index could be different in this population. In addition, although we excluded men with metastatic disease, some of the fracture risk observed in men treated with ADT or RT may have been related to the progression of the underlying malignancy. Accurate assessment of dementia diagnosis remains challenging using administrative data. The severity of dementia is unknown from Medicare claims and early dementia was found to be under-reported in claims data. (55, 56) Our study is also limited in that we only included data from 2007-2010, so we were unable to assess long-term outcomes.
In summary, our data suggest that the assessment of fracture risk for patients with localized prostate cancer cannot be based on cancer treatment alone, given that a combination of factors can substantially elevate a man's risk of fracture. Fractures are an important cause of morbidity and mortality in these patients, and may be preventable with effective prophylactic strategies. Current National Comprehensive Cancer Network clinical guidelines recommend dual-energy X-ray absorptiometry scan for risk assessment for men with prostate cancer treated with ADT alone or in combination with radiation therapy; however, a recent study reports that few men receiving therapy for prostate cancer undergo bone density screening.(57, 58) Based on our findings, clinicians prescribing ADT or initiating radiation should use risk factors such as concurrent use of CNS-active medications, increasing age, and history of anxiety or Parkinson's to guide decision making for fracture prevention strategies. Clinicians can use this index as a basis to counsel patients on their increased risk for fracture and also as a means to decide who should be screened for bone mineral density. Acknowledging the limitations of administrative data, our prognostic index performed well for 1-and 2-year fracture prediction in two independent cohorts of men receiving treatment for prostate cancer. Future work is needed to validate this prediction system using clinical data abstracted from electronic medical records or physical examination. This future work will be vital to assure the validity and generalizability of the proposed index. The model provides a practical system that we hope will ultimately prove to be a useful tool for risk stratification in older patients with prostate cancer.
Supplementary Material
Acknowledgements
The collection of the California cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute's Surveillance, Epidemiology and End Results Program under contract N01-PC-35136 awarded to the Northern California Cancer Center, contract N01-PC-35139 awarded to the University of Southern California, and contract N02-PC-15105 awarded to the Public Health Institute; and the Centers for Disease Control and Prevention's National Program of Cancer Registries, under agreement #U55/CCR921930-02 awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California, Department of Public Health the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database. The interpretation and reporting of the SEER-Medicare data are the sole responsibility of the authors.
Funding: This study was supported by the National Cancer Institute (R01CA149045) (C.P.G). T.G-S was supported by a T32 fellowship training grant (T32AG1934) from the National Institute on Aging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
Study Concept and Design: TR Graham-Steed, PR Soulos, N Dearing, J Concato, ME Tinetti, CP Gross
Data Acquisition: PR Soulos, CP Gross
Quality Control of Data and Algorithms: PR Soulos, CP Gross
Data Analysis and Interpretation: TR Graham-Steed, PR Soulos, N Dearing, J Concato, ME Tinetti, CP Gross
Statistical Analysis: PR Soulos
Manuscript Preparation: TR Graham-Steed, PR Soulos
Manuscript Editing and Review: TR Graham-Steed, PR Soulos, N Dearing, J Concato, ME Tinetti, CP Gross
Disclosures and Conflict of Interest Statements
The authors have no conflicts of interest to disclose.
Disclaimer
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number R01CA149045. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Brawley O. Prostate cancer epidemiology in the United States. World J Urol. 2012;30(2):195–200. doi: 10.1007/s00345-012-0824-2. [DOI] [PubMed] [Google Scholar]
- 2.Grönberg H. Prostate cancer epidemiology. Lancet. 2003;361(9360):859–64. doi: 10.1016/S0140-6736(03)12713-4. [DOI] [PubMed] [Google Scholar]
- 3.Terret C, Zulian GB, Naiem A, Albrand G. Multidisciplinary Approach to the Geriatric Oncology Patient. J Clin Oncol. 2007;25(14):1876–81. doi: 10.1200/JCO.2006.10.3291. [DOI] [PubMed] [Google Scholar]
- 4.Oefelein MG, Ricchiuti V, Conrad W, Resnick MI. Skeletal Fractures Negatively Correlate With Overall Survival in Men With Prostate Cancer. J Urol. 2002;168(3):1005–7. doi: 10.1016/S0022-5347(05)64561-2. [DOI] [PubMed] [Google Scholar]
- 5.Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352(2):154–64. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- 6.Onukwugha E, Yong C, Mullins CD, Seal B, McNally D, Hussain A. Skeletal-related events and mortality among older men with advanced prostate cancer. J Geriatr Oncol. 2014 doi: 10.1016/j.jgo.2014.03.002. Epub 2014/04/15. [DOI] [PubMed] [Google Scholar]
- 7.Saad F, Olsson C, Schulman CC. Skeletal Morbidity in Men with Prostate Cancer: Quality-of-Life Considerations throughout the Continuum of Care. Eur Urol. 2004;46(6):731–40. doi: 10.1016/j.eururo.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 8.Tinetti ME, Baker DI, King M, Gottschalk M, Murphy TE, Acampora D, et al. Effect of Dissemination of Evidence in Reducing Injuries from Falls. N Engl J Med. 2008;359(3):252–61. doi: 10.1056/NEJMoa0801748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alibhai SMH, Duong-Hua M, Cheung AM, Sutradhar R, Warde P, Fleshner NE, et al. Fracture Types and Risk Factors in Men With Prostate Cancer on Androgen Deprivation Therapy: A Matched Cohort Study of 19,079 Men. The Journal of Urology. 2010;184(3):918–24. doi: 10.1016/j.juro.2010.04.068. [DOI] [PubMed] [Google Scholar]
- 10.Alibhai SMH, Duong-Hua M, Sutradhar R, Fleshner NE, Warde P, Cheung AM, et al. Impact of Androgen Deprivation Therapy on Cardiovascular Disease and Diabetes. Journal of Clinical Oncology. 2009;27(21):3452–8. doi: 10.1200/JCO.2008.20.0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith MR. Osteoporosis during androgen deprivation therapy for prostate cancer. Urology. 2002;60(3, Supplement 1):79–85. [PubMed] [Google Scholar]
- 12.Smith MR. Osteoporosis and Other Adverse Body Composition Changes during Androgen Deprivation Therapy for Prostate Cancer. Cancer and Metastasis Reviews. 2002;21(2):159–66. doi: 10.1023/a:1020840311573. [DOI] [PubMed] [Google Scholar]
- 13.Conde FA, Sarna L, Oka RK, Vredevoe DL, Rettig MB, Aronson WJ. Age, body mass index, and serum prostate-specific antigen correlate with bone loss in men with prostate cancer not receiving androgen deprivation therapy. Urology. 2004;64(2):335–40. doi: 10.1016/j.urology.2004.03.036. [DOI] [PubMed] [Google Scholar]
- 14.Housman DM, Savage CJ, Zelefsky MJ, Elkin EB. Pelvic Fracture after Radiation Therapy for Localized Prostate Cancer: A Population Based Study. International journal of radiation oncology, biology, physics. 2010;78(3):S64. [Google Scholar]
- 15.Elliott SP, Jarosek SL, Alanee SR, Konety BR, Dusenbery KE, Virnig BA. Three-dimensional external beam radiotherapy for prostate cancer increases the risk of hip fracture. Cancer. 2011;117(19):4557–65. doi: 10.1002/cncr.25994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mittan D, Lee S, Miller E, Perez RC, Basler JW, Bruder JM. Bone Loss following Hypogonadism in Men with Prostate Cancer Treated with GnRH Analogs. J Clin Endocrinol Metab. 2002;87(8):3656–61. doi: 10.1210/jcem.87.8.8782. [DOI] [PubMed] [Google Scholar]
- 17.Daniell HW, Dunn SR, Ferguson DW, Lomas G, Niazi Z, Stratte PT. Progressive osteoporosis during androgen deprivation therapy for prostate cancer. J Urol. 2000;163(1):181–6. [PubMed] [Google Scholar]
- 18.Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA. 2005;294(2):238–44. doi: 10.1001/jama.294.2.238. Epub 2005/07/15. [DOI] [PubMed] [Google Scholar]
- 19.Fowler FJ, Barry MJ, Lu-Yao G, Wasson JH, Bin L. Outcomes of external-beam radiation therapy for prostate cancer: a study of Medicare beneficiaries in three surveillance, epidemiology, and end results areas. J Clin Oncol. 1996;14(8):2258–65. doi: 10.1200/JCO.1996.14.8.2258. [DOI] [PubMed] [Google Scholar]
- 20.Bolla M, Collette L, Blank L, Warde P, Dubois JB, Mirimanoff R-O, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet. 2002;360(9327):103–8. doi: 10.1016/s0140-6736(02)09408-4. [DOI] [PubMed] [Google Scholar]
- 21.Bolla M, Gonzalez D, Warde P, Dubois JB, Mirimanoff R-O, Storme G, et al. Improved Survival in Patients with Locally Advanced Prostate Cancer Treated with Radiotherapy and Goserelin. N Engl J Med. 1997;337(5):295–300. doi: 10.1056/NEJM199707313370502. [DOI] [PubMed] [Google Scholar]
- 22.Higano C. Androgen deprivation therapy: monitoring and managing the complications. Hematol Oncol Clin North Am. 2006;20(4):909–23. doi: 10.1016/j.hoc.2006.03.013. Epub 2006/07/25. [DOI] [PubMed] [Google Scholar]
- 23.Wei JT, Gross M, Jaffe CA, Gravlin K, Lahaie M, Faerber GJ, et al. Androgen deprivation therapy for prostate cancer results in significant loss of bone density. Urology. 1999;54(4):607–11. doi: 10.1016/s0090-4295(99)00301-5. [DOI] [PubMed] [Google Scholar]
- 24.Hopewell JW. Radiation-therapy effects on bone density. Med Pediatr Oncol. 2003;41(3):208–11. doi: 10.1002/mpo.10338. [DOI] [PubMed] [Google Scholar]
- 25.Melton LJ, Alothman KI, Khosla S, Achenbach SJ, Oberg AL, Zincke H. Fracture Risk Following Bilateral Orchiectomy. J Urol. 2003;169(5):1747–50. doi: 10.1097/01.ju.0000059281.67667.97. [DOI] [PubMed] [Google Scholar]
- 26.Maggiore RJ, Gross CP, Hurria A. Polypharmacy in Older Adults with Cancer. Oncologist. 2010;15(5):507–22. doi: 10.1634/theoncologist.2009-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gosch M, Jeske M, Kammerlander C, Roth T. Osteoporosis and polypharmacy. Z Gerontol Geriatr. 2012;45(6):450–4. doi: 10.1007/s00391-012-0374-7. [DOI] [PubMed] [Google Scholar]
- 28.Cumming RG. Epidemiology of medication-related falls and fractures in the elderly. Drugs Aging. 1998;12(1):43–53. doi: 10.2165/00002512-199812010-00005. Epub 1998/02/19. [DOI] [PubMed] [Google Scholar]
- 29.Ensrud KE, Blackwell T, Mangione CM, Bowman PJ, Bauer DC, Schwartz A, et al. Central nervous system active medications and risk for fractures in older women. Arch Intern Med. 2003;163(8):949–57. doi: 10.1001/archinte.163.8.949. Epub 2003/04/30. [DOI] [PubMed] [Google Scholar]
- 30.Steinbuch M, Youket T, Cohen S. Oral glucocorticoid use is associated with an increased risk of fracture. Osteoporos Int. 2004;15(4):323–8. doi: 10.1007/s00198-003-1548-3. [DOI] [PubMed] [Google Scholar]
- 31.Mazziotti G, Canalis E, Giustina A. Drug-induced Osteoporosis: Mechanisms and Clinical Implications. Amer J Med. 2010;123(10):877–84. doi: 10.1016/j.amjmed.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 32.Moylan KC, Binder EF. Falls in Older Adults: Risk Assessment, Management and Prevention. Amer J Med. 2007;120(6):493, e1–e6. doi: 10.1016/j.amjmed.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 33.Woolcott JC, Richardson KJ, Wiens MO, et al. Meta-analysis of the impact of 9 medication classes on falls in elderly persons. Arch Intern Med. 2009;169(21):1952–60. doi: 10.1001/archinternmed.2009.357. [DOI] [PubMed] [Google Scholar]
- 34.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(8 Suppl):IV–3-18. doi: 10.1097/01.MLR.0000020942.47004.03. Epub 2002/08/21. [DOI] [PubMed] [Google Scholar]
- 35.Jutberger H, Lorentzon M, Barrett-Connor E, Johansson H, Kanis JA, Ljunggren Ö , et al. Smoking predicts incident fractures in elderly men: Mr OS Sweden. J Bone Miner Res. 2010;25(5):1010–6. doi: 10.1359/jbmr.091112. [DOI] [PubMed] [Google Scholar]
- 36.Tseng VL, Yu F, Lum F, Coleman AL. Risk of fractures following cataract surgery in medicare beneficiaries. JAMA. 2012;308(5):493–501. doi: 10.1001/jama.2012.9014. [DOI] [PubMed] [Google Scholar]
- 37.McAdam-Marx C, Unni S, Ye X, Nelson S, Nickman NA. Effect of Medicare reimbursement reduction for imaging services on osteoporosis screening rates. J Am Geriatr Soc. 2012;60(3):511–6. doi: 10.1111/j.1532-5415.2011.03837.x. Epub 2012/02/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blume SW, Curtis JR. Medical costs of osteoporosis in the elderly Medicare population. Osteoporos Int. 2011;22(6):1835–44. doi: 10.1007/s00198-010-1419-7. Epub 2010/12/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roudsari BS, Ebel BE, Corso PS, Molinari NA, Koepsell TD. The acute medical care costs of fall-related injuries among the U.S. older adults. Injury. 2005;36(11):1316–22. doi: 10.1016/j.injury.2005.05.024. Epub 2005/10/11. [DOI] [PubMed] [Google Scholar]
- 40.Baron JA, Barrett J, Malenka D, Fisher E, Kniffin W, Bubolz T, et al. Racial Differences in Fracture Risk. Epidemiology. 1994;5(1):42–7. doi: 10.1097/00001648-199401000-00008. [DOI] [PubMed] [Google Scholar]
- 41.Sporer SM, Weinstein JN, Koval KJ. The Geographic Incidence and Treatment Variation of Common Fractures of Elderly Patients. J Am Acad Orthop Surg. 2006;14(4):246–55. doi: 10.5435/00124635-200604000-00006. [DOI] [PubMed] [Google Scholar]
- 42.Miller M, Stürmer T, Azrael D, Levin R, Solomon DH. Opioid Analgesics and the Risk of Fractures in Older Adults with Arthritis. J Am Geriatr Soc. 2011;59(3):430–8. doi: 10.1111/j.1532-5415.2011.03318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity Measures for Use with Administrative Data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 44.Davidoff AJ, Zuckerman IH, Pandya N, Hendrick F, Ke X, Hurria A, et al. A novel approach to improve health status measurement in observational claims-based studies of cancer treatment and outcomes. J Geriatr Oncol. 2013;4(2):157–65. doi: 10.1016/j.jgo.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sullivan LM, Massaro JM, D'Agostino RB. Presentation of multivariate data for clinical use: The Framingham Study risk score functions. Stat Med. 2004;23(10):1631–60. doi: 10.1002/sim.1742. [DOI] [PubMed] [Google Scholar]
- 46.Smith MR, Eastham J, Gleason DM, Shasha D, Tchekmedyian S, Zinner N. Randomized Controlled Trial of Zoledronic Acid to Prevent Bone Loss in Men Receiving Androgen Deprivation Therapy for Nonmetastatic Prostate Cancer. J Urol. 2003;169(6):2008–12. doi: 10.1097/01.ju.0000063820.94994.95. [DOI] [PubMed] [Google Scholar]
- 47.Center JR, Nguyen TV, Schneider D, Sambrook PN, Eisman JA. Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet. 1999;353(9156):878–82. doi: 10.1016/S0140-6736(98)09075-8. [DOI] [PubMed] [Google Scholar]
- 48.Alibhai SMH, Rahman S, Warde PR, Jewett MAS, Jaffer T, Cheung AM. Prevention and management of osteoporosis in men receiving androgen deprivation therapy: A survey of urologists and radiation oncologists. Urology. 2006;68(1):126–31. doi: 10.1016/j.urology.2006.01.054. [DOI] [PubMed] [Google Scholar]
- 49.Panju AH, Breunis H, Cheung AM, Leach M, Fleshner N, Warde P, et al. Management of decreased bone mineral density in men starting androgen-deprivation therapy for prostate cancer. BJU International. 2009;103(6):753–7. doi: 10.1111/j.1464-410X.2008.08156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tanvetyanon T. Physician practices of bone density testing and drug prescribing to prevent or treat osteoporosis during androgen deprivation therapy. Cancer. 2005;103(2):237–41. doi: 10.1002/cncr.20766. [An erratum to this Article has been published in Cancer 2006;106(11):2530].
- 51.Yee ET, White R, Murata G, Handanos C, Hoffman R. Osteoporosis Management in Prostate Cancer Patients Treated with Androgen Deprivation Therapy. Journal of General Internal Medicine. 2007;22(9):1305–10. doi: 10.1007/s11606-007-0291-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ray WA, Griffin MR, Schaffner W, Baugh DK, Melton LJ. Psychotropic Drug Use and the Risk of Hip Fracture. N Engl J Med. 1987;316(7):363–9. doi: 10.1056/NEJM198702123160702. [DOI] [PubMed] [Google Scholar]
- 53.Bolton JM, Metge C, Lix L, Prior H, Sareen J, Leslie WD. Fracture Risk From Psychotropic Medications: A Population-Based Analysis. J Clin Psychopharmacol. 2008;28(4):384–91. doi: 10.1097/JCP.0b013e31817d5943. [DOI] [PubMed] [Google Scholar]
- 54.Daniell HW. Osteoporosis After Orchiectomy for Prostate Cancer. The Journal of Urology. 157(2):439–44. [PubMed] [Google Scholar]
- 55.Taylor DH, Jr, Fillenbaum GG, Ezell ME. The accuracy of medicare claims data in identifying Alzheimer's disease. J Clin Epidemiol. 2002;55(9):929–37. doi: 10.1016/s0895-4356(02)00452-3. [DOI] [PubMed] [Google Scholar]
- 56.Bynum JPW, Rabins PV, Weller W, Niefeld M, Anderson GF, Wu AW. The Relationship Between a Dementia Diagnosis, Chronic Illness, Medicare Expenditures, and Hospital Use. J Amer Geriatr Soc. 2004;52(2):187–94. doi: 10.1111/j.1532-5415.2004.52054.x. [DOI] [PubMed] [Google Scholar]
- 57.Guise TA. Bone Loss and Fracture Risk Associated with Cancer Therapy. The Oncologist. 2006;11(10):1121–31. doi: 10.1634/theoncologist.11-10-1121. [DOI] [PubMed] [Google Scholar]
- 58.Morgans AK, Smith MR, O'Malley AJ, Keating NL. Bone density testing among prostate cancer survivors treated with androgen-deprivation therapy. Cancer. 2013;119(4):863–70. doi: 10.1002/cncr.27830. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.