Abstract
Objective
Diabetes mellitus (DM) is a risk factor for endometrial cancer and is associated with poorer outcomes in breast and colon cancers. This association is less clear in epithelial ovarian cancer (EOC). We sought to examine the effect of DM on progression-free (PFS) and overall survival (OS) in women with EOC.
Methods
A retrospective cohort study of EOC patients diagnosed between 2004 and 2009 at a single institution was performed. Demographic, pathologic and DM diagnosis data were abstracted. Pearson chi-square test and t test were used to compare variables. The Kaplan-Meier method and the log rank test were used to compare PFS and OS between non-diabetic (ND) and DM patients.
Results
62 (17%) of 367 patients had a diagnosis of DM. No differences in age, histology, debulking status, or administration of intraperitoneal chemotherapy between ND and DM patients were present, although there were more stage I and IV patients in the ND group (p=0.04). BMI was significantly different between the two groups (ND vs. DM, 27.5 vs. 30.7 kg/m2, p < 0.001). While there were no differences in survival based on BMI, diabetic patients had a poorer PFS (10.3 vs. 16.3 months, p=0.024) and OS (26.1 vs. 42.2 months, p=0.005) compared to ND patients. Metformin use among diabetic patients did not appear to affect PFS or OS.
Conclusions
EOC patients with DM have poorer survival than patients without diabetes; this association is independent of obesity. Metformin use did not affect outcomes. The pathophysiology of this observation requires more inquiry.
Introduction
Greater than one-third of the adult population, in addition to almost one-fifth of youths, in the United States are obese based on estimates from the 2011-12 National Health and Nutrition Examination Survey (NHANES)1. Not surprisingly, secondary to this current obesity epidemic, there has been a consistent increase in cardiovascular disease, type II diabetes mellitus and cancer2. When specifically considering the impact of obesity on diabetes disease prevalence, currently almost 10% of the United States adult population is diabetic, and more than a quarter of individuals over the age of 65 have been diagnosed with diabetes3. DM is associated with many other diseases, most notably cardiovascular and renal disease, as well as upwards of 20% of cancers in the United States2.
The association between diabetes and cancer is complex. From a molecular standpoint, data suggests that elevated insulin-like growth factor I, increased cytokine and estrogen levels, adipokine imbalances and hyperinsulinemia likely contribute to both an increase risk of malignancy as well as leading to inferior cancer outcomes2. Data from multiple epidemiologic reports and meta-analyses support the postulation that diabetes increases the risk of colorectal, breast, and endometrial cancers, among others4, and may be associated with poorer survival in colon, pancreas, and breast cancers5. This effect seems to be independent of obesity5, which is a well-known risk factor for both the development of, and mortality from, cancer 6,7.
Obesity has been associated with ovarian cancer8,9 although results are conflicting10. Two recently published large meta-analyses came to differing conclusions regarding obesity and ovarian cancer risk. Olsen and colleagues examined studies from institutions participating in the Ovarian Cancer Association Consortium and found that elevated BMI was not associated with high- grade serous cancers10. Conversely, the Collaborative Group on Epidemiological Studies of Ovarian Cancer performed a meta-analysis of 47 studies (including 25,157 ovarian cancer patients and 81,311 patients without ovarian cancer) and found a 10% increase in risk per 5 kg/m2 8. A recent prospective study among 70,258 Chinese women found that women with a BMI ≥ 30 had over a two-fold increase in ovarian cancer development risk9. Moreover, there are data to suggest that obesity may also be associated with poorer overall survival in ovarian cancer patients11-13. Physiologically, obesity and diabetes share many of the same inflammatory mediators therefore biologic plausibility linking the two diseases to ovarian cancer exists; however, there is little information regarding the effect of diabetes on ovarian cancer survival. Therefore, the objective of our study was to evaluate the potential impact of diabetes mellitus on survival in patients with epithelial ovarian cancer.
Methods
Subjects
This retrospective cohort study was performed following approval and in accordance with the standards of the Institutional Human Subjects Protection Review Board at the University of Alabama at Birmingham (UAB). Eligible subjects were women diagnosed with epithelial ovarian cancer and treated between 2004-2009 at our institution with complete, evaluable records. The comprehensive cancer tumor registry, which captures all new cancer diagnoses within the UAB system, was used to identify patients.
Study Design
This was a retrospective cohort study designed to determine if there was a difference in progression-free survival (PFS) and overall survival (OS) between women with diabetes and those without. Records were reviewed for standard demographics, presence or absence of diabetes, use of metformin and other diabetic medications, pathologic and treatment data including chemotherapy administration approach (primary, intravenous vs. intravenous/intraperitoneal, and NACT), PFS and OS. PFS was calculated from the time of initiation of chemotherapy until disease recurrence or progression according to clinical assessment, rising CA-125, or radiographic evidence of recurrence. Overall survival was calculated from initiation of chemotherapy until last known follow-up or death from any cause.
Statistical Analysis
Chi-square (χ2) test or Fisher's exact test for categorical variables and the t-test or Wilcoxon rank sum test for continuous variables were used to assess the differences between patients with and without diabetes. Kaplan-Meier (KM) estimates were used to compute the survival functions for patients with and without diabetes and were compared using log-rank tests. Cox proportional hazards models were used to evaluate the association between progression-free and overall survival and diabetes, independent of potential confounders. A value of p<0.05 was considered statistically significant. Analyses were performed using either SAS version 9.2 (SAS Institute Inc., Cary, NC) or SPSS version 21 (IBM, Armonk, NY) with Kaplan-Meier survival curves constructed by SPSS.
Results
367 patients who were diagnosed with EOC between 2004 and 2009 met inclusion criteria. Of study patients, 62 women (17%) had a recorded diagnosis of diabetes. There was no difference in age, grade, histology, or debulking status between the two cohorts (Table 1). Administration of intraperitoneal (IP) chemotherapy was not different between the non-diabetic and diabetic patients (23 (7.5%) vs. 5 (8.1%); p=0.80). Moreover, a neoadjuvant treatment strategy was employed in a small but similar proportion of each group (non-diabetic, 13(4.3%) vs. diabetic, 4 (6.5%); p=0.50).
Table 1. Patient characteristics.
| Diabetic (N=62) | Non-diabetic (N=305) | p-value | |
|---|---|---|---|
| Age (years)* | 64.6 ± 10.4 | 63.2 ± 12.2 | 0.41 |
| Stage (number, %) | 0.04 | ||
| I | 1 (1.6) | 22 (7.2) | |
| II | 7 (11.3) | 57 (18.7) | |
| III | 51 (82.3) | 195 (63.9) | |
| IV | 3 (4.8) | 31 (10.2) | |
| Grade | 0.05 | ||
| 1 | 1 (1.6) | 9 (3.0) | |
| 2 | 8 (12.9) | 62 (20.3) | |
| 3 | 44 (71.0) | 217 (71.1) | |
| Unknown | 9 (14.5) | 17 (5.6) | |
| Histology | 0.75 | ||
| Papillary serous | 42 (67.7) | 199 (65.2) | |
| Endometrioid | 4 (6.5) | 39 (12.8) | |
| Other | 16 (25.8) | 67 (22.0) | |
| Debulking status | 0.69 | ||
| Optimal | 47 (75.8) | 219 (71.8) | |
| Suboptimal | 15 (24.2) | 84 (27.7) | |
| Unknown | 0 (0) | 2 (0.7) | |
| BMI (kg/m2)* | 30.7 ± 6.5 | 27.5 ± 6.2 | < 0.001 |
| NACT | 4 (6.5) | 13 (4.3) | 0.50 |
| IP chemotherapy | 5 (8.1) | 23 (7.5) | 0.80 |
Values presented are means ± standard deviation
BMI – body mass index
NACT – neoadjuvant chemotherapy
IP - intraperitoneal
Differences in stage distribution were present for the two groups. Diabetic patients were less likely to have either stage I or stage IV disease than the nondiabetic cohort (p=0.039). Not surprisingly, patients with DM had higher BMI, 30.7 vs. 27.5 kg/m2 (p < 0.001), than their non-diabetic cohorts (Table 1). Among the diabetic patients, 27 (43.5%) patients had recorded metformin use.
Patients with DM had a significantly shorter median progression-free survival compared to patients without diabetes (10.3 vs. 16.3 months, p=0.02, Figure 1a). Diabetic patients also had a significantly poorer median overall survival (26.1 vs. 42.2 months, p=0.005, Figure 1b). Interestingly, when the entire patient cohort was divided by BMI (< 30 kg/m2 vs. ≥ 30 mg/m2), although the point estimate was greater in patients whose BMI was < 30 kg/m2, there was no statistical difference in either PFS, 16.5 versus 14.0 months (p=0.082) nor OS, 41.0 versus 33.1 months (p=0.486) (Figure 2). Moreover, within the diabetic cohort, metformin use did not affect median PFS (use vs. non-use, 10.1 vs. 10.3 months, p=0.7) nor median OS (use vs. non-use, 23.9 vs. 26.1 months, p=0.6) (Figure 3). Further comparisons within this group were limited due to the small sample size.
Figure 1.
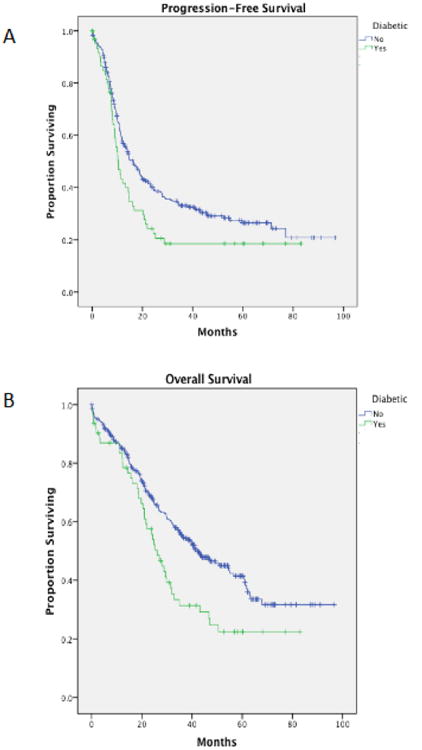
(A) Progression-free survival amongst ovarian cancer patients with versus without diabetes (p=0.024). (B) Overall survival amongst ovarian cancer patients with versus without diabetes (p=0.005).
Figure 2.
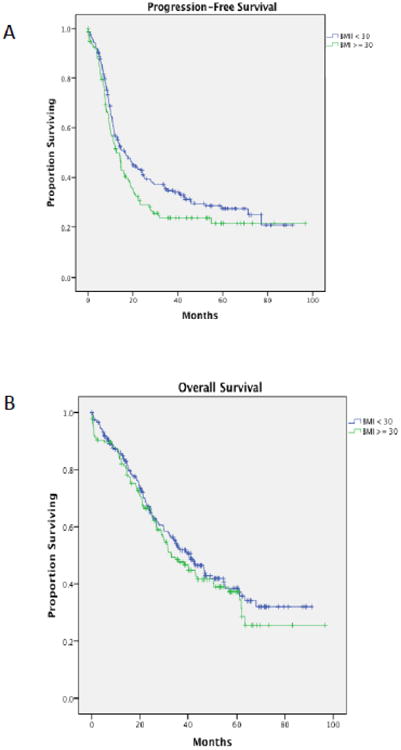
(A) Progression-free survival amongst ovarian cancer patients with a BMI < 30 versus a BMI ≥ 30 (p=0.07). (B) Overall survival amongst ovarian cancer patients with a BMI < 30 versus a BMI ≥ 30 (p=0.4).
Figure 3.
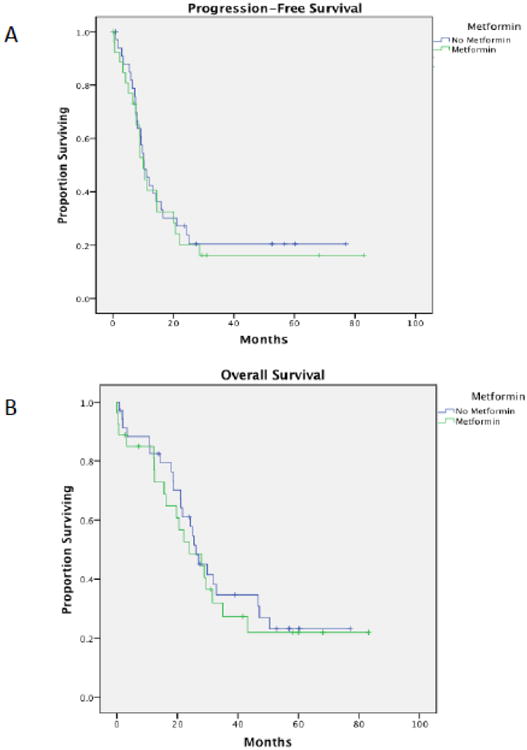
(A) Progression-free survival amongst diabetic ovarian cancer patients who used metformin versus those who did not (p=0.62). (B) Overall survival amongst diabetic ovarian cancer patients who used metformin versus those who did not (p=0.7).
The unadjusted hazard ratio (HR) for the association between PFS and diabetes was 1.44 (95% confidence interval [CI], 1.05-1.98). Following adjustment for age, stage, grade, histology, debulking status, BMI, neoadjuvant chemotherapy and IP chemotherapy the HR for progression in patients with diabetes was 1.29 (95% CI 0.91-1.84; p=0.15). This association was modified by stage, adjusted age, histology, debulking status, BMI, and IP chemotherapy. Among those with early-stage (1 and 2) disease, the PFS HR was 2.26 (p=0.17) whereas the PFS HR was 1.15 (p=0.44) in patients with advanced-stage (3 and 4) disease. The unadjusted and adjusted HRs for overall survival in patients with diabetes were similar (HR 1.62, 95% CI 1.16-2.28 and HR 1.64, 95% CI 0.12-2.40, respectively). As with PFS, the association for OS was modified by stage; the HRs for early and advanced-stage disease were 4.17 (95% CI 0.78-22.21; p=0.09) and 1.60 (95% CI 1.08-2.36; p=0.02), respectively.
Discussion
In our aging population, the incidence of both diabetes and cancer is increasing. The association between diabetes and cancer was noted as early as 191414, but the etiologies of both diseases are complex and heterogeneous. Both cancer and diabetes share common risk factors such as age, race and obesity, making simple conclusions very difficult.
Our retrospective cohort study suggests that there is an association between diabetes and survival. The univariate analysis demonstrated a significant hazard of progression and death in patients with diabetes; however, when adjusted for stage, grade, BMI and other known important clinical factors, the magnitude of effect was not significant. Interestingly, although not statistically significant there was a greater hazard for death in diabetic patients with early stage disease, HR 4.17 versus 1.60, potentially suggesting that diabetes had more of a negative influence in patients with early stage disease. In general, patients with advanced-stage disease have a more predictably aggressive and fatal course, potentially lessening the effect of any other given comorbidity. While our results are not statistically significant for early stage disease, this is likely due to the small sample size.
The etiology of the association between diabetes and ovarian cancer is not clear, but there is ample evidence for biologic plausibility. Several studies have evaluated the effect of increased insulin-like growth factor (IGF)15. IGF-I and -II are overexpressed in many cancers16, which may lead to increased proliferation as well as stimulation of pathways involved in invasion and metastasis17,18. Indeed, elevated IGF-I and –II levels have been associated with decreased survival in epithelial ovarian cancer19,20. Insulin resistance and diabetes are associated with decreased serum sex hormone binding globulin21,22, which can lead to elevated levels of free estrogen. While the evidence for elevated estrogen as a carcinogen is well-established in endometrial cancer, recent murine models suggest that it may also play a role in ovarian cancer23. Another compelling link between diabetes and cancer development and progression is through inflammatory pathways. Adipose metabolic dysregulation is a hallmark of diabetes24. This can lead to increased levels of inflammatory cytokines such as interleukin-6 and tumor necrosis factor-α24, in turn activating pathways involved in cell proliferation, invasion and evasion of antitumor immunity25.
Studies examining metformin use suggest an improvement in both cancer risk as well as survival26-29. The mechanism of action is unclear, although inhibition of the mTOR pathway may contribute to metformin's antiproliferative effects29,30. While there is observational and preclinical data suggesting a beneficial effect of metformin on ovarian cancer survival31,32, we could not confirm this with our data; however, our sample size was quite small which may have limited this analysis. Moreover, patients with diabetes in our cohort presented with more advanced stage disease than the nondiabetic group. This has been observed in urothelial33 and pancreatic34 cancers as well. While the etiology is not known, it may relate to the biologic mechanisms detailed above.
We acknowledge several important limitations of our study. Similar to studies examining diabetes and cancer, there are many common factors contributing to the development of both diseases that complicates the analysis. We do not routinely collect data regarding physical activity and diet, which certainly contributes to diabetes outcomes but also may contribute to cancer outcomes35-38. Patients were considered to have diabetes only if they had a recorded diagnosis or an antihyperglycemic medication was listed, as hemoglobin A1c levels and fasting glucose levels are not a standard part of our preoperative workup. Given that up to 27% of diabetes is undiagnosed3, this could have skewed our groups. Similarly, we had no information regarding degree of diabetic control, which also may impact outcomes39. Additionally, information regarding the length of diagnosis and burden of diabetes was not captured in our study. Finally, confounding and other sources of potential bias may be present. Nonetheless, the sample size from our study is fairly robust and clinical factors including staging at presentation, histology, and cytoreductive status are consistent with published norms40.
The relationship between ovarian cancer and diabetes is complex. While population data suggests that there may be an increased risk of ovarian cancer in women who are diabetic or hyperinsulinemic5, our data suggest that diabetes may in fact impact prognosis and survival. Given the increasing incidence of diabetes in our aging population, this is an important avenue of inquiry that requires more investigation.
Research highlights.
Diabetes mellitus (DM) is increasingly prevalent in our obese society.
DM confers poorer outcomes in some cancers, but there is little data regarding its relationship to ovarian cancer.
DM was associated with worse outcomes regardless of BMI in our population.
Acknowledgments
Funding support was provided in part by NIHT32-CA091078 to BKE and 5K12HD0012580-14 to CAL.
Footnotes
Disclosure: None of the authors have a conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311:806–14. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howe LR, Subbaramaiah K, Hudis CA, Dannenberg AJ. Molecular pathways: adipose inflammation as a mediator of obesity-associated cancer. Clin Cancer Res. 2013;19:6074–83. doi: 10.1158/1078-0432.CCR-12-2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. [Accessed April 5, 2014];Fast Facts: Data and Statistics about Diabetes. 2013 at http://professional.diabetes.org/admin/UserFiles/0%20-%20Sean/FastFacts%20March%202013.pdf.
- 4.Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R. Diabetes and cancer. Endocr Relat Cancer. 2009;16:1103–23. doi: 10.1677/ERC-09-0087. [DOI] [PubMed] [Google Scholar]
- 5.Coughlin SS, Calle EE, Teras LR, Petrelli J, Thun MJ. Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. Am J Epidemiol. 2004;159:1160–7. doi: 10.1093/aje/kwh161. [DOI] [PubMed] [Google Scholar]
- 6.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 7.Barone BB, Yeh HC, Snyder CF, et al. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta-analysis. JAMA. 2008;300:2754–64. doi: 10.1001/jama.2008.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ovarian cancer and body size: individual participant meta-analysis including 25,157 women with ovarian cancer from 47 epidemiological studies. PLoS Med. 2012;9:e1001200. doi: 10.1371/journal.pmed.1001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma X, Beeghly-Fadiel A, Shu XO, et al. Anthropometric measures and epithelial ovarian cancer risk among Chinese women: results from the Shanghai Women's Health Study. Br J Cancer. 2013;109:751–5. doi: 10.1038/bjc.2013.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olsen CM, Nagle CM, Whiteman DC, et al. Obesity and risk of ovarian cancer subtypes: evidence from the Ovarian Cancer Association Consortium. Endocr Relat Cancer. 2013;20:251–62. doi: 10.1530/ERC-12-0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diaz ES, Karlan BY, Li AJ. Obesity-associated adipokines correlate with survival in epithelial ovarian cancer. Gynecol Oncol. 2013;129:353–7. doi: 10.1016/j.ygyno.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Pavelka JC, Brown RS, Karlan BY, et al. Effect of obesity on survival in epithelial ovarian cancer. Cancer. 2006;107:1520–4. doi: 10.1002/cncr.22194. [DOI] [PubMed] [Google Scholar]
- 13.Protani MM, Nagle CM, Webb PM. Obesity and ovarian cancer survival: a systematic review and meta-analysis. Cancer Prev Res (Phila) 2012;5:901–10. doi: 10.1158/1940-6207.CAPR-12-0048. [DOI] [PubMed] [Google Scholar]
- 14.Greenwood M, Wood F. The Relation between the Cancer and Diabetes Death-rates. J Hyg (Lond) 1914;14:83–118. doi: 10.1017/s0022172400005702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. CA Cancer J Clin. 2010;60:207–21. doi: 10.3322/caac.20078. [DOI] [PubMed] [Google Scholar]
- 16.Malaguarnera R, Belfiore A. The Emerging Role of Insulin and Insulin-Like Growth Factor Signaling in Cancer Stem Cells. Front Endocrinol (Lausanne) 2014;5:10. doi: 10.3389/fendo.2014.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallagher EJ, LeRoith D. Minireview: IGF, Insulin, and Cancer. Endocrinology. 2011;152:2546–51. doi: 10.1210/en.2011-0231. [DOI] [PubMed] [Google Scholar]
- 18.Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev Cancer. 2012;12:159–69. doi: 10.1038/nrc3215. [DOI] [PubMed] [Google Scholar]
- 19.Sayer RA, Lancaster JM, Pittman J, et al. High insulin-like growth factor-2 (IGF-2) gene expression is an independent predictor of poor survival for patients with advanced stage serous epithelial ovarian cancer. Gynecol Oncol. 2005;96:355–61. doi: 10.1016/j.ygyno.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 20.Yu H, Rohan T. Role of the insulin-like growth factor family in cancer development and progression. J Natl Cancer Inst. 2000;92:1472–89. doi: 10.1093/jnci/92.18.1472. [DOI] [PubMed] [Google Scholar]
- 21.Ding EL, Song Y, Manson JE, et al. Sex hormone-binding globulin and risk of type 2 diabetes in women and men. N Engl J Med. 2009;361:1152–63. doi: 10.1056/NEJMoa0804381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wallace IR, McKinley MC, Bell PM, Hunter SJ. Sex hormone binding globulin and insulin resistance. Clin Endocrinol (Oxf) 2013;78:321–9. doi: 10.1111/cen.12086. [DOI] [PubMed] [Google Scholar]
- 23.Laws MJ, Kannan A, Pawar S, Haschek WM, Bagchi MK, Bagchi IC. Dysregulated estrogen receptor signaling in the hypothalamic-pituitary-ovarian axis leads to ovarian epithelial tumorigenesis in mice. PLoS Genet. 2014;10:e1004230. doi: 10.1371/journal.pgen.1004230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amato MC, Pizzolanti G, Torregrossa V, Misiano G, Milano S, Giordano C. Visceral Adiposity Index (VAI) Is Predictive of an Altered Adipokine Profile in Patients with Type 2 Diabetes. PLoS One. 2014;9:e91969. doi: 10.1371/journal.pone.0091969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Habib SL, Rojna M. Diabetes and risk of cancer. ISRN Oncol. 2013;2013:583786. doi: 10.1155/2013/583786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirsch HA, Iliopoulos D, Tsichlis PN, Struhl K. Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Res. 2009;69:7507–11. doi: 10.1158/0008-5472.CAN-09-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin M, Marais R. Metformin: a diabetes drug for cancer, or a cancer drug for diabetics? J Clin Oncol. 2012;30:2698–700. doi: 10.1200/JCO.2012.42.1677. [DOI] [PubMed] [Google Scholar]
- 29.Rattan R, Ali Fehmi R, Munkarah A. Metformin: an emerging new therapeutic option for targeting cancer stem cells and metastasis. J Oncol. 2012;2012:928127. doi: 10.1155/2012/928127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cantrell LA, Zhou C, Mendivil A, Malloy KM, Gehrig PA, Bae-Jump VL. Metformin is a potent inhibitor of endometrial cancer cell proliferation--implications for a novel treatment strategy. Gynecol Oncol. 2010;116:92–8. doi: 10.1016/j.ygyno.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dilokthornsakul P, Chaiyakunapruk N, Termrungruanglert W, Pratoomsoot C, Saokeaw S, Sruamsiri R. The effects of metformin on ovarian cancer: a systematic review. Int J Gynecol Cancer. 2013;23:1544–51. doi: 10.1097/IGC.0b013e3182a80a21. [DOI] [PubMed] [Google Scholar]
- 32.Gotlieb WH, Saumet J, Beauchamp MC, et al. In vitro metformin antineoplastic activity in epithelial ovarian cancer. Gynecol Oncol. 2008;110:246–50. doi: 10.1016/j.ygyno.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 33.Ozbek E, Otunctemur A, Dursun M, et al. Association between the Metabolic Syndrome and High Tumor Grade and Stage of Primary Urothelial Cell Carcinoma of the Bladder. Asian Pac J Cancer Prev. 2014;15:1447–51. doi: 10.7314/apjcp.2014.15.3.1447. [DOI] [PubMed] [Google Scholar]
- 34.Fan KY, Dholakia AS, Wild AT, et al. Baseline hemoglobin-A1c impacts clinical outcomes in patients with pancreatic cancer. J Natl Compr Canc Netw. 2014;12:50–7. doi: 10.6004/jnccn.2014.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Friedenreich CM, Neilson HK, Lynch BM. State of the epidemiological evidence on physical activity and cancer prevention. Eur J Cancer. 2010;46:2593–604. doi: 10.1016/j.ejca.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 36.Levine ME, Suarez JA, Brandhorst S, et al. Low Protein Intake Is Associated with a Major Reduction in IGF-1, Cancer, and Overall Mortality in the 65 and Younger but Not Older Population. Cell Metab. 2014;19:407–17. doi: 10.1016/j.cmet.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Makowski L, Zhou C, Zhong Y, et al. Obesity increases tumor aggressiveness in a genetically engineered mouse model of serous ovarian cancer. Gynecol Oncol. 2014;133:90–7. doi: 10.1016/j.ygyno.2013.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou Y, Chlebowski R, Lamonte MJ, et al. Body mass index, physical activity, and mortality in women diagnosed with ovarian cancer: Results from the Women's Health Initiative. Gynecol Oncol. 2014;133:4–10. doi: 10.1016/j.ygyno.2014.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Beer JC, Liebenberg L. Does cancer risk increase with HbA, independent of diabetes? Br J Cancer. 2014 doi: 10.1038/bjc.2014.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zivanovic O, Sima CS, Iasonos A, et al. The effect of primary cytoreduction on outcomes of patients with FIGO stage IIIC ovarian cancer stratified by the initial tumor burden in the upper abdomen cephalad to the greater omentum. Gynecol Oncol. 2010;116:351–7. doi: 10.1016/j.ygyno.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]


