Abstract
Background
Krüppel-like factor 4 (KLF4) is a transcription factor that regulates a diverse array of cellular processes, including development, differentiation, proliferation, and apoptosis. Although its function in keratinocytes has been widely studied, its exact role in psoriasis has not been elucidated.
Objective
We designed this study to investigate epidermal expression levels of KLF4 and the change in KLF4 expression after treatment in patients with psoriasis.
Methods
We compared the expression levels of KLF4 in the basal, suprabasal, and superficial epidermal layers, in psoriatic lesional, non-lesional, and normal skin, using an immunoreactivity intensity distribution index (IRIDI). In addition, we measured the change in KLF4 expression on the basis of the IRIDI and by reverse transcription polymerase chain reaction (RT-PCR) analysis after treatment.
Results
The combined IRIDI scores in psoriatic lesional skin were significantly higher than the scores in both non-lesional and normal skin. The psoriatic epidermis, particularly the suprabasal layer, showed a significantly increased IRIDI score compared to that of non-lesional and normal skin, which was significantly decreased after treatment. RT-PCR analysis exhibited a slight increase in KLF4 mRNA expression level after treatment; however, this increase was not significant.
Conclusion
These data indicate that KLF4 could regulate epidermal proliferation and differentiation. Moreover, we believe that KLF4 may play an important role in the physiological reaction to counteract abnormal differentiation and proliferation of keratinocytes.
Keywords: Krüppel-like factor 4, Psoriasis
INTRODUCTION
Krüppel-like factors (KLFs) are named based on their sequence homology with the Krüppel protein of the Drosophila embryonic pattern regulator1. KLFs are zinc finger-containing transcription factors that regulate a diverse array of cellular processes, including development, differentiation, proliferation, and apoptosis2. For the KLF family, 17 members have been identified in mammalian cells, and they are referred to as KLF1-171,3. KLF4 is highly expressed in epithelial tissues, including the skin and gastrointestinal tract4,5,6, and plays an important role in differentiation and specification of the skin epithelium7. Recent studies8,9 revealed a correlation between KLF4 and psoriasis. However, currently, no data are available on the differences in KLF4 expression between lesional and non-lesional skin of patients with psoriasis or on the change in KLF4 expression after treatment. In the present study, we aim to elucidate evidence supporting the in vivo association between KLF4 and psoriasis.
MATERIALS AND METHODS
Subjects and skin samples
We studied patients with psoriasis (aged >18 years), who visited the Department of Dermatology at the Hallym University Sacred Heart Hospital from July 2010 to December 2012. In total, 31 patients with moderate- to severe psoriasis (defined by a psoriasis area and severity index [PASI] score ≥12 or a total body surface area involvement ≥10%) were included. None of the patients had received any psoriasis treatment except for emollient for at least 3 weeks prior to the study. Two punch biopsies (diameter: 3 mm) were obtained under local anesthesia from each patient before initiating treatment: one from lesional skin (n=31) and the other from adjacent non-lesional skin (n=9). After 2 months of treatment, skin biopsies were again obtained from a site adjacent to the previous biopsy sites in 15 of the 31 patients, who showed a reduction in the baseline PASI score of ≥50%. The specimens were subjected to immunohistochemical (n=15) and reverse transcription polymerase chain reaction (RT-PCR) (n=11) analyses. Additionally, skin samples were taken from healthy volunteers with no personal or family history of psoriasis (n=4, control group). The study was approved by the institutional review board of Hallym University Sacred Heart Hospital (IRB No. 2010-I029).
Immunohistochemistry
Sections were cut from paraffinized specimens, deparaffinized in xylene for 5 min and sequentially rehydrated in 100%, 95%, and 75% alcohol solutions for 3 min each, washed with water, and boiled twice for 10 min each in 10 mM citrate buffer by using an ultrashort wave. To inhibit intrinsic enzymatic activation, samples were treated with 0.3% hydrogen peroxide for 10 min and washed with phosphate-buffered saline (PBS). For immunohistochemistry, samples were incubated with approximately 0.7 mg/ml of anti-KLF4 antibody (Millipore, Billerica, MA, USA) for 1 hour at 37℃, washed with PBS, and treated with a secondary, biotinylated antibody for 20 min. After washing in PBS, the sections were incubated with streptavidin peroxidase for 20 min. Diaminobenzidine was used as a chromogenic substrate, and the sections were lightly counterstained with 10% hematoxylin.
Immunoscoring
The semiquantitative evaluation method by Chaiyarit et al.10, which involves scoring the percentage of positive cells and the staining intensity, was used to assess KLF4 expression. On microscopic examination, the epidermis was divided into three layers: basal, suprabasal, and superficial. Each layer was evaluated for the proportion of positive cells and the intensity of overall staining. The percentage of positive cells was graded as follows (percentage scores): 0=no stained cells; 1=<25% stained cells; 2=25%~50% stained cells; and 3=>50% stained cells. The intensity of the immunostaining was graded as follows: 0=non-reactive; 1=weakly stained; 2=intermediately stained; and 3=intensely stained. The immunoreactivity intensity distribution index (IRIDI) was then computed as follows: the percentage score for each layer was multiplied by the intensity score of that layer to provide the IRIDI of the layer; the resulting scores for the three layers were then added to provide a combined IRIDI for each specimen.
Reverse transcription polymerase chain reaction
Total RNA was isolated from whole tissue specimens (before and after treatment) by using TRIzol reagent (Invitrogen, Carlsbad, CA, USA), following the manufacturer's protocol. Two micrograms of extracted RNA were reverse-transcribed using reverse transcriptase (Invitrogen). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a control to correct for variation in mRNA extraction and reverse transcription. KLF4 and GAPDH transcripts were amplified using an RT-PCR PreMix Kit (Intron Biotechnology, Seongnam, Korea). The primer sequences were as follows: KLF4 forward: 5'-CCTACCTCGGAGAGAGACCG-3', KLF4 reverse: 5'-GGACTCCCTGCCATAGAGGA-3' and GAPDH forward: 5'-CGTCTTCACCACCATGGAGA-3', GAPDH reverse: 5'-CGGCCATCACGCCACAGTTT-3'. PCR products were electrophoresed on an agarose gel, stained with ethidium bromide, and visualized under ultraviolet light. The results are reported as the ratio of relative intensity of KLF4/GAPDH. All experiments were performed in triplicate.
Knockdown of KLF4 expression
For this procedure, we used KLF4 Trilencer-27 Human siRNA (Cat. No. SR306162; Origene Technologies, Rockville, MD, USA) with Lipofectamine (Invitrogen), according to the manufacturer's protocol. Trilencer-27 Universal Scrambled Negative Control siRNA Duplex (Cat. No. SR30004; Origene Technologies) was used as a negative control. HaCaT cells were seeded at a density of 5,000 cells/well in 96-well plates and transfected with siRNA. Methyl-thiazolyl- tetrazolium (MTT; Amresco, Solon, OH, USA) was added to each well 24 hours after the transfection. After additional incubation for 2 hours, the medium was removed, dimethyl sulfoxide (DMSO; Amresco) was added to each well, the plate was mixed thoroughly for 10 min, and optical density (OD) was measured on a microplate reader (Multiskan GO; Thermo Scientific, Waltham, MA, USA) at 540 nm. Proliferation index (PI) was calculated from the formula PI=OD of the KLF4 knockdown group/OD of the control.
Statistical analysis
Lesional and non-lesional skin samples from patients with psoriasis and normal skin samples from healthy donors were compared to identify differences in KLF4 staining. IRIDI scores for each layer were compared using the Kruskal-Wallis test. If the difference was significant, as determined by the Kruskal-Wallis test, the Bonferroni post hoc test was used to identify the difference between each group. Statistical analysis of the RT-PCR results and the MTT assay was performed using Student's t-test for paired samples. IBM SPSS Statistics 20.0 (IBM Co., Armonk, NY, USA) was used for all statistical analysis, and a p-value <0.05 was considered statistically significant.
RESULTS
KLF4 expression in the epidermis
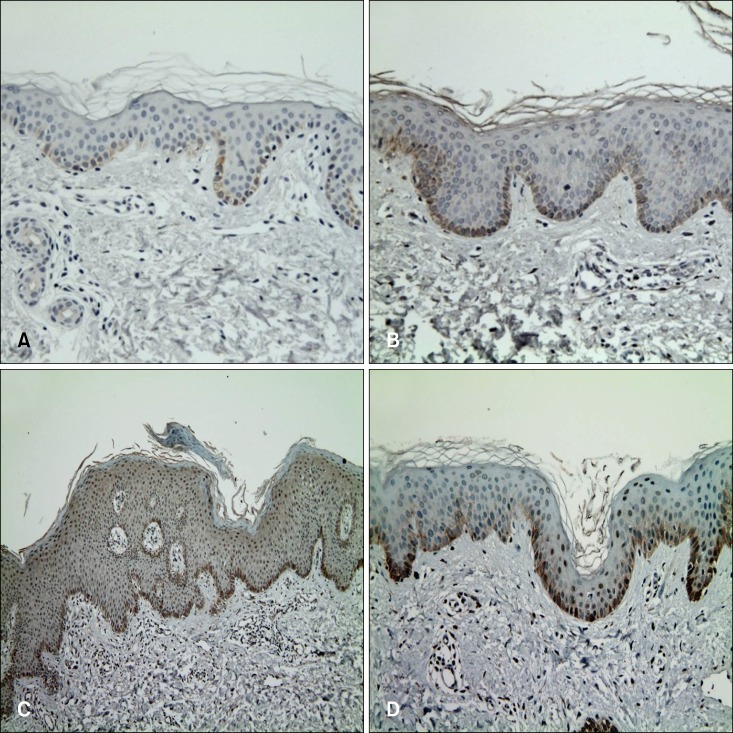
In all groups, immunoreactivity for KLF4 was noted in the basal and suprabasal layers, and no stained cells were observed in the superficial layer (Fig. 1A~C, Table 1). Moreover, KLF4 positive cells were predominantly located in the basal layer; however, no significant difference was observed among the groups. For the suprabasal layer, the mean score in psoriatic lesional skin was significantly higher than that in both non-lesional and normal skin samples. The combined IRIDI scores in psoriatic lesional skin were significantly higher than the scores of both non-lesional and normal skin (Table 2). While normal skin showed a lower level than did non-lesional skin, both IRIDI score of each layer and combined IRIDI score were not significantly different.
Fig. 1.
Immunohistochemical staining for KLF4. (A) Normal skin specimen showing KLF4 expression in the basal layer. (B) Non-lesional skin of a patient with psoriasis, showing an increased number of KLF4-positive cells compared to that in a normal skin specimen. (C) Psoriasis plaque showing increased KLF4 expression. KLF4 expression in psoriatic lesional skin was significantly higher compared to that in both non-lesional and normal skin. (D) After treatment, KLF4 expression decreased in suprabasal layer (A, B, D: ×200; C: ×100).
Table 1.
The KLF4 expression in each layer of epidermis
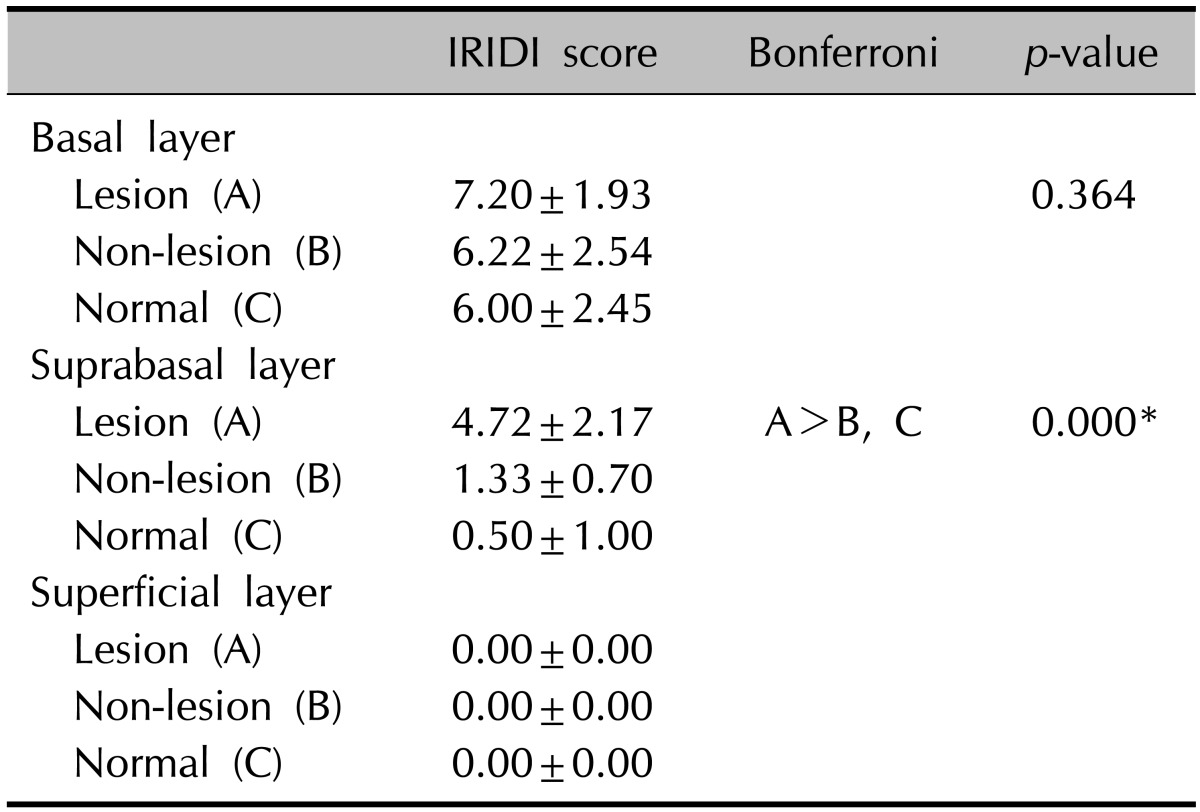
Values are presented as mean±standard deviation.
KLF4: Krüppel-like factor 4, IRIDI: immunoreactivity intensity distribution index.
*p<0.001.
Table 2.
The KLF4 expression in epidermis
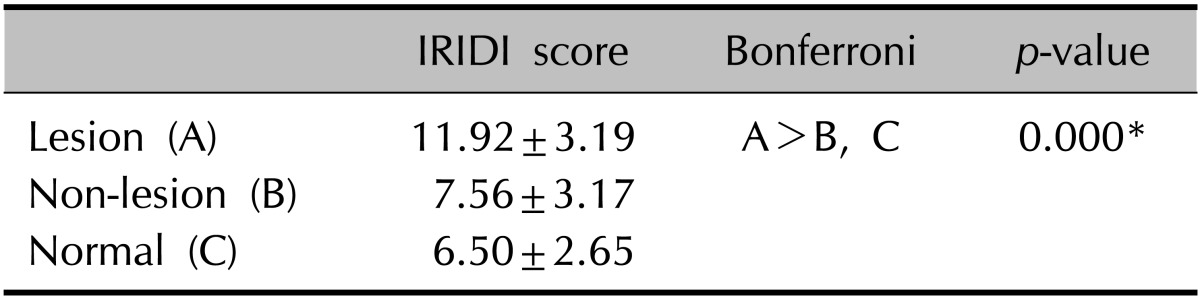
Values are presented as mean±standard deviation.
KLF4: Krüppel-like factor 4, IRIDI: immunoreactivity intensity distribution index.
*p<0.001.
Effect of treatment on KLF4 expression
The combined IRIDI score before and after treatment was 11.92±3.45 and 11.52±3.85, respectively, which are not significantly different (Table 3). For the basal layer, the difference was not significant, although it showed a tendency to increase slightly after treatment. However, a significant decrease in the IRIDI score was noted in the suprabasal layer (Fig. 1C, D). In addition, mRNA expression of KLF4 before and after treatment was evaluated by RT-PCR. The relative intensity of KLF4 mRNA expression compared to that of GAPDH (control) was slightly, but not significantly, increased after treatment (Fig. 2).
Table 3.
The change in KLF4 expression after treatment
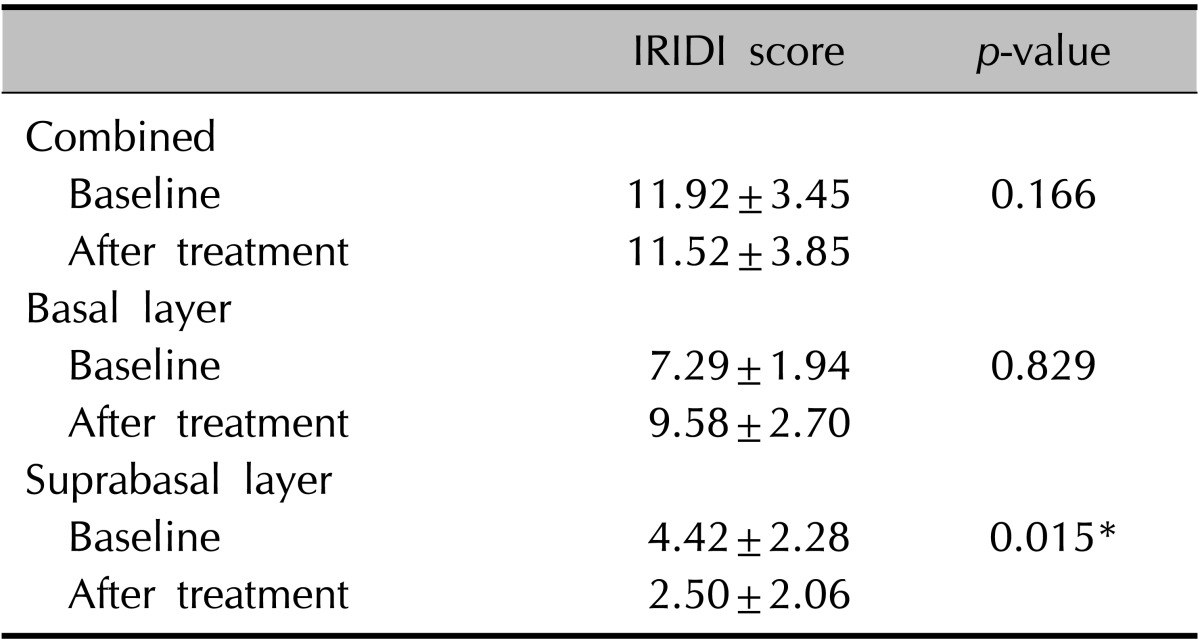
Values are presented as mean±standard deviation.
KLF4: Krüppel-like factor 4, IRIDI: immunoreactivity intensity distribution index.
*p<0.05.
Fig. 2.
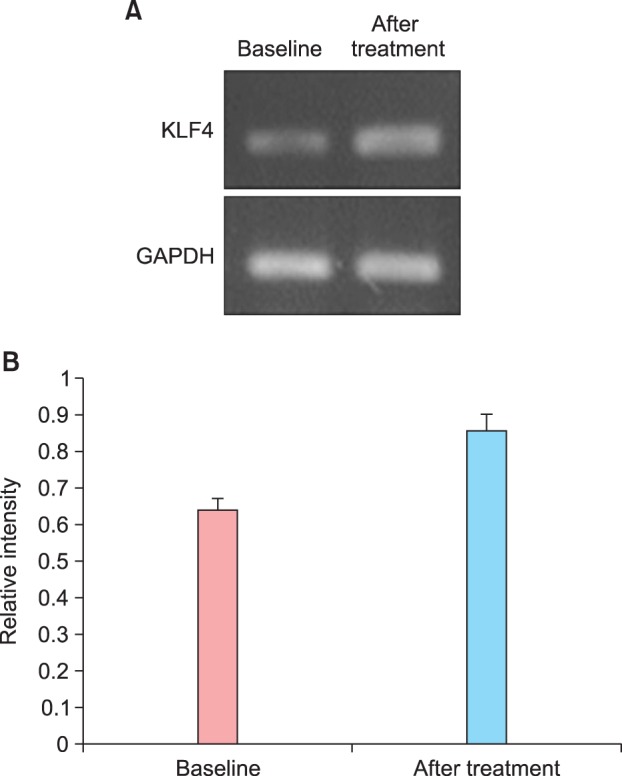
(A) Representative RT-PCR analysis of KLF4 and GAPDH (control) before and after treatment. After treatment, the intensity of KLF4 mRNA expression was increased compared to that in the control. (B) The relative intensity of KLF4 mRNA expression was slightly, but not significantly, increased.
The effect of KLF4 expression on the proliferation of HaCaT cells
The siRNA probes did not lead to a significant change in PI, although a decreasing tendency in PI was noted (p=0.37). Three siRNA probes (A, B, and C) were evaluated; however, they did not result in significantly different PIs. We achieved the highest level of inhibition by using siRNA probe B, which reduced the proliferation of HaCaT cells from 100% to 73.3% (Fig. 3).
Fig. 3.
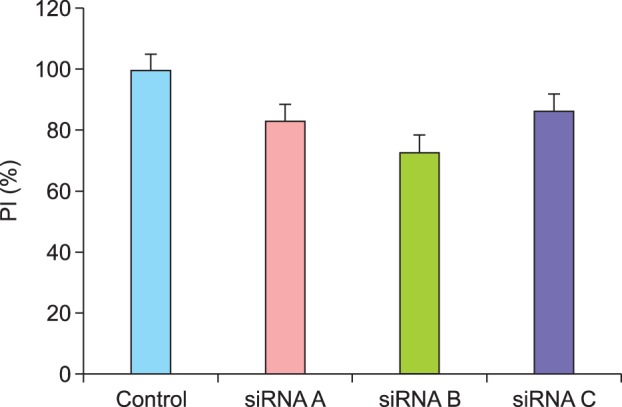
Reduction in the proliferation of HaCaT cells after KLF4 knockdown. Three siRNA probes (A, B, and C) were evaluated, all of which showed a decreasing tendency in proliferation index (PI); however, this decrease and the margin of decrease between the groups were not statistically significant.
DISCUSSION
Evidence for the role of KLF4 in regulating epithelial differentiation has been demonstrated by gene targeting experiments that confirmed the effect of KLF4 on the differentiation and development of epidermal keratinocytes. KLF4-/- knockout mice died from the rapid loss of body fluids as a result of defects in the barrier function of the skin, suggesting that KLF4 plays an important role in the differentiation and specification of the skin epithelium7. Conversely, in transgenic mice that are induced to ectopically express KLF4 in their epidermis, KLF4 has been shown to accelerate terminal differentiation11. In addition to regulating differentiation, KLF4 may have an important function in the regulation of cell proliferation12. Induction of KLF4 results in G1/S cell-cycle arrest; this arrest is accompanied by the activation of p21WAF1/Cip1, a potent suppressor of cell proliferation13. Although psoriasis is a common skin disease, featuring abnormal differentiation and proliferation of keratinocytes, few studies8,9 have reported an association between KLF4 and psoriasis. Tsoi et al.8 identified KLF4 as a new psoriasis susceptibility locus, and Madonna et al.9 demonstrated the expression of KLF4 in psoriatic keratinocytes.
We found that the KLF4 expression level was significantly higher in psoriatic lesional skin than in non-lesional and normal skin. The increased expression of KLF4 in psoriatic skin is likely a physiological reaction to maintain homeostasis. KLF4 is markedly induced in response to IFN-γ and TNF-α, which have been associated with the pathogenesis of psoriasis14. Furthermore, the ETS-related transcription factor ELF4 directly activates KLF4 downstream of T-cell receptor signaling to induce cell-cycle arrest in naïve CD8+ T cells, and eventually regulates the proliferation of naïve CD8+ T cells15. Because psoriasis is characterized by T cell-mediated hyperproliferation of keratinocytes and CD8+ T cells are predominantly located in the psoriatic epidermis, this inhibitory effect on CD8+ T cell proliferation may be a physiological reaction to counteract the T-cell effect.
The epidermis is composed of a basal layer of proliferating cells and a suprabasal layer of terminally differentiating cells. We found that the psoriatic epidermis, particularly the suprabasal layer, showed significantly increased expression of KLF4 compared to that in normal and non-lesional skin, which changed significantly after treatment. This finding suggests that the enhanced expression of KLF4 in the suprabasal layer is a physiological reaction to counteract the abnormal differentiation rather than the proliferation of keratinocytes.
Through RT-PCR analysis, we found that KLF4 mRNA expression was slightly, but not significantly, increased after treatment, suggesting that the total amount of expression increased as the psoriatic plaque normalized. Similarly, Madonna et al.9 reported that KLF4 levels, as determined by RT-PCR, were significantly reduced in psoriatic keratinocytes compared with healthy keratinocytes. They suggested that reduction of KLF4 might be responsible for the enhanced expression of the suppressor of cytokine signaling 1 (SOCS1) in psoriatic cells, which plays an important anti-inflammatory and self- protective role. The increased KLF4 expression levels detected by RT-PCR after treatment correspond with the findings of previous reports9. In contrast, IRIDI analysis indicates a decrease in KLF4 expression after treatment. While the present results appear to contradict formerly published data, previous reports had not assessed psoriatic keratinocytes after treatment; therefore, those results cannot be directly compared to the present findings. Moreover, it was difficult to compare the expression levels assessed with the IRIDI method, which involved only the epidermis, and RT-PCR analysis, which involved the dermis as well as the epidermis. Regarding the reduction of proliferation after KLF4 knockdown, our results seem to be contradictory to those of a previous report, which showed that KLF4 inhibits cell proliferation by blocking G1/S progression of the cell cycle13. However, the decreased proliferation after KLF4 knockdown can be explained by the fact that KLF4 depletion caused p53-dependent apoptosis, as reported in breast cancer cells by Rowland et al.16.
Our study had several limitations such as the small number of tissue specimens evaluated and the determination of KLF4 expression levels by using immunohistochemical staining and RT-PCR only; thus, we could not find a definitive explanation for the role of KLF4 in the development or maintenance of psoriasis. However, unlike previous studies, we were able to show that KLF4 expression is significantly increased in the suprabasal layer of psoriatic epidermis compared to non-lesional and normal skin. A few data on KLF4 are available, except cancer. In particular, little is known regarding the association between KLF4 and psoriasis; therefore, further studies are needed.
In conclusion, we found that KLF4 expression levels in psoriatic lesional skin were significantly higher than those in non-lesional and normal skin. KLF4-positive cells were predominantly located in the basal layer; however, no significant differences were observed among the three groups. For the suprabasal layer, the KLF4 expression level in the psoriatic lesional skin was significantly higher than that in the other skin samples, and the scores were significantly reduced after treatment. RT-PCR analysis showed slightly increased KLF4 mRNA expression after treatment; however, this increase was not statistically significant. We believe that KLF4 may play an important role in the physiological reaction to counteract abnormal differentiation and proliferation of keratinocytes.
References
- 1.Kaczynski J, Cook T, Urrutia R. Sp1- and Krüppel-like transcription factors. Genome Biol. 2003;4:206. doi: 10.1186/gb-2003-4-2-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McConnell BB, Ghaleb AM, Nandan MO, Yang VW. The diverse functions of Krüppel-like factors 4 and 5 in epithelial biology and pathobiology. Bioessays. 2007;29:549–557. doi: 10.1002/bies.20581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pearson R, Fleetwood J, Eaton S, Crossley M, Bao S. Krüppel-like transcription factors: a functional family. Int J Biochem Cell Biol. 2008;40:1996–2001. doi: 10.1016/j.biocel.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 4.Garrett-Sinha LA, Eberspaecher H, Seldin MF, de Crombrugghe B. A gene for a novel zinc-finger protein expressed in differentiated epithelial cells and transiently in certain mesenchymal cells. J Biol Chem. 1996;271:31384–31390. doi: 10.1074/jbc.271.49.31384. [DOI] [PubMed] [Google Scholar]
- 5.Shields JM, Christy RJ, Yang VW. Identification and characterization of a gene encoding a gut-enriched Krüppellike factor expressed during growth arrest. J Biol Chem. 1996;271:20009–20017. doi: 10.1074/jbc.271.33.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conkright MD, Wani MA, Anderson KP, Lingrel JB. A gene encoding an intestinal-enriched member of the Krüppel-like factor family expressed in intestinal epithelial cells. Nucleic Acids Res. 1999;27:1263–1270. doi: 10.1093/nar/27.5.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Segre JA, Bauer C, Fuchs E. Klf4 is a transcription factor required for establishing the barrier function of the skin. Nat Genet. 1999;22:356–360. doi: 10.1038/11926. [DOI] [PubMed] [Google Scholar]
- 8.Tsoi LC, Spain SL, Knight J, Ellinghaus E, Stuart PE, Capon F, et al. Identification of 15 new psoriasis susceptibility loci highlights the role of innate immunity. Nat Genet. 2012;44:1341–1348. doi: 10.1038/ng.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madonna S, Scarponi C, Sestito R, Pallotta S, Cavani A, Albanesi C. The IFN-gamma-dependent suppressor of cytokine signaling 1 promoter activity is positively regulated by IFN regulatory factor-1 and Sp1 but repressed by growth factor independence-1b and Krüppel-like factor-4, and it is dysregulated in psoriatic keratinocytes. J Immunol. 2010;185:2467–2481. doi: 10.4049/jimmunol.1001426. [DOI] [PubMed] [Google Scholar]
- 10.Chaiyarit P, Kafrawy AH, Miles DA, Zunt SL, Van Dis ML, Gregory RL. Oral lichen planus: an immunohistochemical study of heat shock proteins (HSPs) and cytokeratins (CKs) and a unifying hypothesis of pathogenesis. J Oral Pathol Med. 1999;28:210–215. doi: 10.1111/j.1600-0714.1999.tb02026.x. [DOI] [PubMed] [Google Scholar]
- 11.Jaubert J, Cheng J, Segre JA. Ectopic expression of kruppel like factor 4 (Klf4) accelerates formation of the epidermal permeability barrier. Development. 2003;130:2767–2777. doi: 10.1242/dev.00477. [DOI] [PubMed] [Google Scholar]
- 12.Chen X, Whitney EM, Gao SY, Yang VW. Transcriptional profiling of Krüppel-like factor 4 reveals a function in cell cycle regulation and epithelial differentiation. J Mol Biol. 2003;326:665–677. doi: 10.1016/S0022-2836(02)01449-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen X, Johns DC, Geiman DE, Marban E, Dang DT, Hamlin G, et al. Krüppel-like factor 4 (gut-enriched Krüppel-like factor) inhibits cell proliferation by blocking G1/S progression of the cell cycle. J Biol Chem. 2001;276:30423–30428. doi: 10.1074/jbc.M101194200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feinberg MW, Cao Z, Wara AK, Lebedeva MA, Senbanerjee S, Jain MK. Kruppel-like factor 4 is a mediator of proinflammatory signaling in macrophages. J Biol Chem. 2005;280:38247–38258. doi: 10.1074/jbc.M509378200. [DOI] [PubMed] [Google Scholar]
- 15.Yamada T, Park CS, Mamonkin M, Lacorazza HD. Transcription factor ELF4 controls the proliferation and homing of CD8+ T cells via the Krüppel-like factors KLF4 and KLF2. Nat Immunol. 2009;10:618–626. doi: 10.1038/ni.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rowland BD, Bernards R, Peeper DS. The KLF4 tumour suppressor is a transcriptional repressor of p53 that acts as a context-dependent oncogene. Nat Cell Biol. 2005;7:1074–1082. doi: 10.1038/ncb1314. [DOI] [PubMed] [Google Scholar]