Abstract
Background
Over the last decade, the incidence of ultraviolet B (UVB)-related skin problems has increased. Oxidative stress caused by UVB induces the secretion of melanocyte growth and activating factors from keratinocytes, which results in the formation of cutaneous hyperpigmentation. Therefore, increasing the antioxidant abilities of skin cells is thought to be a beneficial strategy for the development of sunscreen agents. Superoxide dismutase 1 (SOD1) is an antioxidant enzyme that is known to exhibit antioxidant properties.
Objective
The purpose of this study was to investigate the effect of SOD1 on alpha-melanocyte stimulating hormone (α-MSH) and UVB-induced melanogenesis in B16F10 melanoma cells and HRM-2 melanin-possessing hairless mice.
Methods
The inhibitory effect of SOD1 on tyrosinase activity was evaluated in a cell-free system. Additional experiments were performed using B16F10 melanoma cells to demonstrate the effects of SOD1 in vitro, and HRM-2 melanin-possessing hairless mice were used to evaluate the antimelanogenic effects of SOD1 in vivo.
Results
We found that SOD1 inhibited melanin production in a dose-dependent manner without causing cytotoxicity in B16F10 melanoma cells. SOD1 did not inhibit tyrosinase activity under cell-free conditions. The results indicate that SOD1 may reduce pigmentation by an indirect, nonenzymatic mechanism. We also found that SOD1 decreased UVB-induced melanogenesis in HRM-2 melanin-possessing hairless mice, as visualized through hematoxylin and eosin staining and Fontana-Masson staining.
Conclusion
Our results indicate that SOD1 has an inhibitory effect on α-MSH and UVB-induced melanogenesis, indicating that SOD1 may be a promising sunscreen agent.
Keywords: Alpha-MSH, HRM-2, Superoxide dismutase 1, Skin pigmentation, Ultraviolet radiation
INTRODUCTION
Ultraviolet (UV) radiation is divided into three categories: UVC (<290 nm), UVB (290~320 nm), and UVA (320~400 nm). Short-wavelength UVC and a portion of UVB are absorbed in the ozone layer of the atmosphere and do not reach the earth's surface1. Although the UVB that does reach the surface represents only 4% of the total environmental UV light2, it has a stronger phototoxic effect than does UVA because DNA shows an absorption maximum at about 260 nm with an absorption tail in the UVB region. As a result, UVB has many adverse effects on human skin3, including cancer4,5, immunosuppression6, erythema7, hyperpigmentation8, and photoaging9.
Skin susceptibility to UV depends on several factors, for example, melanin, which plays a key protective role. Two main types of melanin have been defined: eumelanin, a characteristic of darkly pigmented skin, and pheomelanin, which is mainly present in the epidermis of fair-skinned subjects. Eumelanin acts as a filter against UV irradiation and possesses scavenger properties toward UV-induced free radicals10,11. Pheomelanin is a less effective filter against UV irradiation and apparently acts as an endogenous photosensitizer12,13. Since at least some of the adverse effects of UV irradiation are related to the generation of free radicals14,15, antioxidant protection is clearly an important factor in protecting the skin16,17.
Melanogenesis has many important physiological functions, including photoprotection of human skin from UV irradiation18. Melanin synthesis19 is stimulated by various molecules such as alpha-melanocyte stimulating hormone (α-MSH)20 and cyclic adenosine monophosphate-elevating agents (including forskolin, glycyrrhizin, and isobutylmethylxanthine), UVB, and the placental total lipid fraction21. Melanin synthesis occurs in melanocytes and melanoma cells in an enzymatic process catalyzed by tyrosinase. Tyrosinase catalyzes the oxidation of L-tyrosine to 3,4-dihydroxyphenyl-L-alanine (L-DOPA), followed by the oxidation of L-DOPA to dopaquinone; oxidative polymerization of several dopaquinone derivatives then produces melanin. Thus, tyrosinase inhibitor is a candidate for reducing melanogenesis22. It has been reported that superoxide dismutase (SOD) is one of the key factors that reduces melanin production caused by UV irradiation23.
We studied the inhibitory effects of copper- and zinc-dependent SOD1 on α-MSH-induced melanogenesis in B16F10 melanoma cells and in a UVB-treated melanin-possessing hairless mouse model. We show that SOD1 leads to antimelanogenesis effects through α-MSH-induced melanogenesis and UVB-induced melanogenesis in HRM-2 melanin-possessing hairless mice.
MATERIALS AND METHODS
Compounds and reagents
SOD1 was provided by Nutrex Technology Corporation (Seoul, Korea). Mushroom tyrosinase, α-MSH, L-DOPA, and phenylthiourea were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Dulbecco's modified Eagle's medium (DMEM), trypsin-ethylenediaminetetraacetic acid, phosphate-buffered saline, and penicillin/streptomycin were purchased from WelGENE Biopharmaceuticals (Daegu, Korea). Fetal bovine serum (FBS) was purchased from Life Technologies (Gibco; Life Technologies, Grand Island, NY, USA).
Cell culture
B16F10 murine melanoma cells (CRL-6475) were purchased from the American Type Culture Collection (Rockville, MD, USA). Cells were maintained in DMEM supplemented with 10% FBS and 1% penicillin-streptomycin in 5% CO2 at 37℃. Cells were passaged every 3 days until a maximum passage number of 20 was achieved.
Animals and skin color determination
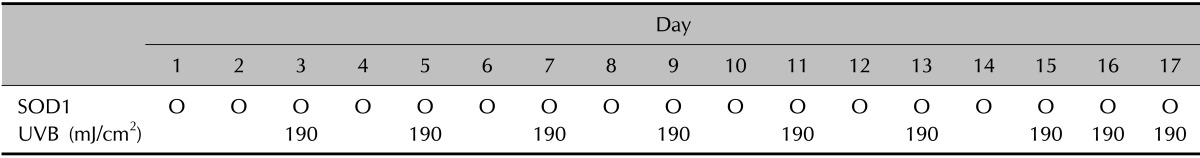
Male, 4-week-old, HRM-2 melanin-possessing hairless mice (total=9) were obtained from Hoshino Laboratory Animals (Saitama, Japan) and housed in an air-conditioned room maintained at 24℃±2℃ and 55%±15% humidity with a 12-hours light-dark cycle. The mice were placed individually in separate cages and fed ad libitum. All procedures involving animals were conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee of Chung-Ang University in Korea (IRB Number: 13-0013). After a 1-week stabilization period, mice were randomly divided into three groups of three animals each. The mice were anesthetized with Zoletil 50 (50 mg/kg) and xylazine (10 mg/kg). SOD1 was dissolved in a vehicle consisting of ethanol and propylene glycol at a ratio of 3:7, and then topically applied to a designated site on the dorsal skin of the back of the animal once a day. Mice were exposed to UVB using an NB-UVB lamp (Dermalight Psoracomb UV-B-311nm-narrowband; National Biological Corp., Twinsburg, OH, USA) and a UV-B Narrowband PL-L/PL-S lamp (Philips, Eindhoven, Netherlands) at 190 mJ/cm2. The experiment schedule is shown in Table 124,25. The color of the skin sites was measured using a spectrophotometer (CR-10; Konica Minolta Sensing Inc., Osaka, Japan), and the colors are described by L-values according to the Commission Internationale de l'Eclairage color system. The mice were killed and their skin was quickly removed and immediately frozen in liquid nitrogen and stored at -80℃.
Table 1.
The experimental schedule of UVB irradiation in HRM-2 melanin-possessing hairless mice
SOD1: superoxide dismutase 1, UVB: ultraviolet B.
Cell viability assay
B16F10 melanoma cells were seeded in 96-well plates (2×104 cells/well). After 24 hours incubation at 37℃, the medium was replaced with media containing SOD1 diluted to the appropriate concentration. Control cells were treated with distilled water (DW) at a final concentration of 0.1%. After 24 hours, the media containing the compounds or DW were replaced with media containing 10% EZ-CyTox (Daeil Lab Service, Seoul, Korea). The cells were then incubated at 37℃ for 30 minutes, and the absorbance was measured using an enzyme-linked immunosorbent assay (ELISA) reader (VERSAMax; Molecular Devices, Sunnyvale, CA, USA) at a wavelength of 450 nm. All assays were performed in triplicate. The cytotoxic effect of each treatment was expressed as a percentage of cell viability relative to the untreated control cells.
Cell-free enzymatic assay for tyrosinase activity
Tyrosinase activity was assayed as a function of DOPA oxidase activity. A total of 70 µl of 0.1 M phosphate buffer (pH 6.8) containing SOD1 was mixed with 20 µl of 10 µg/ml mushroom tyrosinase in a well of a 96-well plate, and 10 µl of 10 mM L-DOPA was added. Absorbance values were measured every 10 minutes for at least 1 hour at 475 nm using an ELISA reader at an incubation temperature of 37℃. The quantity of dopachrome formed in the reaction mixture was determined against a blank (solution without enzyme) at 475 nm in the ELISA reader. The percentage of tyrosinase activity was calculated as follows: tyrosinase activity (%)=[(A-B)/(C-D)]×100, where A is the absorbance of the reaction mixture containing the test sample and mushroom tyrosinase, B is the absorbance of the blank sample containing the test sample but no mushroom tyrosinase, C is the absorbance of the reaction mixture without the test sample and with mushroom tyrosinase, and D is the absorbance of the well lacking both the test sample and mushroom tyrosinase (L-DOPA alone).
Measurement of melanin content
B16F10 melanoma cells were incubated at a density of 1×105 cells in 6-well plates overnight. The α-MSH (1 µm) was then added, and the cells were treated with increasing concentrations of substance in phenol red-free DMEM for 3 days. Two hundred-microliter aliquots of medium were then placed in 96-well plates, and the optical density of each culture well was measured using an ELISA reader at 405 nm. The data were normalized to the protein content of the cell lysates. The cell lysates were subsequently processed for determining the protein concentration using a BCA Protein Assay Kit (Pierce Biotechnology, Rockford, IL, USA). Melanin production was expressed as the percentage of living cells compared to the controls.
Histological analysis
Skin samples were fixed in 4% paraformaldehyde overnight at room temperature and stained for melanin using a Fontana-Masson staining kit (American Mastertech Inc., Lodi, CA, USA) according to the manufacturer's instructions. Briefly, sliced skin samples were stained with ammoniacal silver solution for 60 minutes at 60℃, followed by incubation in 0.1% gold chloride and then in 5% sodium thiosulfate. The tissue was also stained with hematoxylin and eosin for morphological analyses. Staining was examined under a phase-contrast microscope (Eclipse TS100; Nikon Instruments Inc., Melville, NY, USA).
Statistical analysis
Data are presented as mean±standard deviation, and statistical comparisons between treated groups and the untreated group were performed using one-way ANOVA followed by post hoc Tukey tests for direct comparison between specific groups (PASW Statistics ver. 18.0; IBM Co., Armonk, NY, USA). p<0.05, p<0.01, and p<0.001 were considered statistically significant.
RESULTS
Effect of superoxide dismutase 1 on cell viability in B16F10 melanoma cells
To determine if SOD1 could inhibit melanogenesis, B16F10 melanoma cells were treated with the SOD1 before α-MSH stimulation. We first tested the cytotoxicity of SOD1 in B16F10 melanoma cells using the EZ-CyTox assay. B16F10 melanoma cells were treated with SOD1 at concentrations of 1 to 1,000 ng/ml for 24 hours. SOD1 was found to have no cytotoxic effect on B16F10 melanoma cells at the concentrations used (Fig. 1).
Fig. 1.
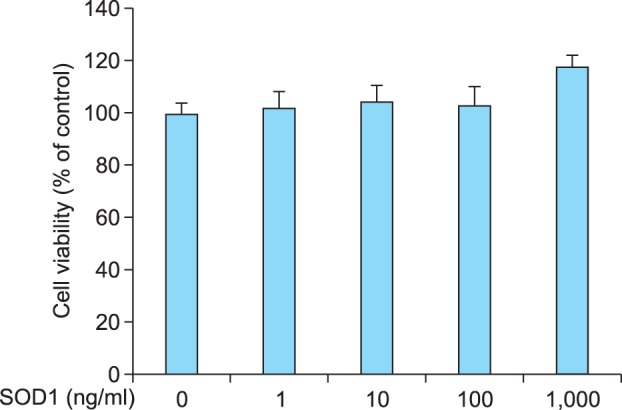
Cell viability of B16F10 melanoma cells treated with superoxide dismutase 1 (SOD1) at different concentrations. Cells were treated with SOD1 at 1 to 1,000 ng/ml for 24 hours. Cell viability was determined using EZ-CyTox assays. Results are expressed as the percent viability relative to controls. Most of the B16F10 melanoma cells were viable at concentrations in the range of 1 to 1,000 ng/ml. Each measurement was made in triplicate and data represent the mean±standard deviation.
Effects of superoxide dismutase 1 on tyrosinase activity in B16F10 melanoma cells
To investigate the direct effects of SOD1 on tyrosinase activity, we measured the tyrosinase activities of mushroom tyrosinase in a cell-free system, as described in the Materials and Methods. However, SOD1 was found to have no direct inhibitory effect on mushroom tyrosinase activity at concentrations ranging from 1 to 1,000 ng/ml (Fig. 2).
Fig. 2.
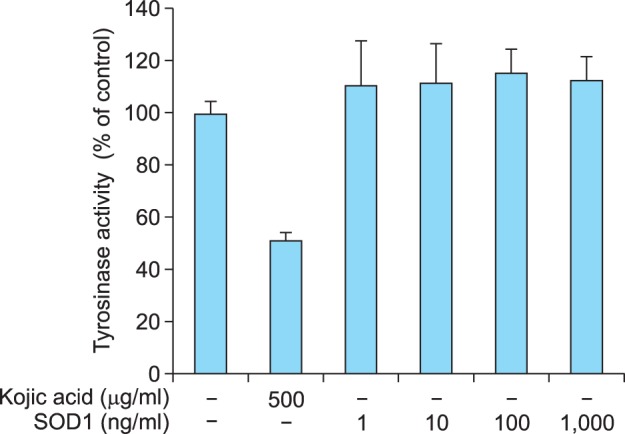
Inhibitory effects of superoxide dismutase 1 (SOD1) on tyrosinase activity in cell-free assays. Mushroom tyrosinase was treated with SOD1 at 1 to 1,000 ng/ml. SOD1 did not appear to inhibit mushroom tyrosinase activity. Each measurement was made in triplicate and data shown represent the mean±standard deviation.
Effect of superoxide dismutase 1 on melanogenesis in B16F10 melanoma cells
The effect of SOD1 on the melanogenesis of B16F10 melanoma cells was examined. To determine whether SOD1 inhibits α-MSH-induced melanin synthesis in B16F10 melanoma cells, we measured the α-MSH-induced melanin production of B16F10 melanoma cells after 3 days of SOD1 treatment at 10 to 1,000 ng/ml. SOD1 considerably inhibited melanin synthesis when used at concentrations greater than 10 ng/ml (Fig. 3). SOD1 treatment also reduced α-MSH-induced melanin release in a dose-dependent manner.
Fig. 3.
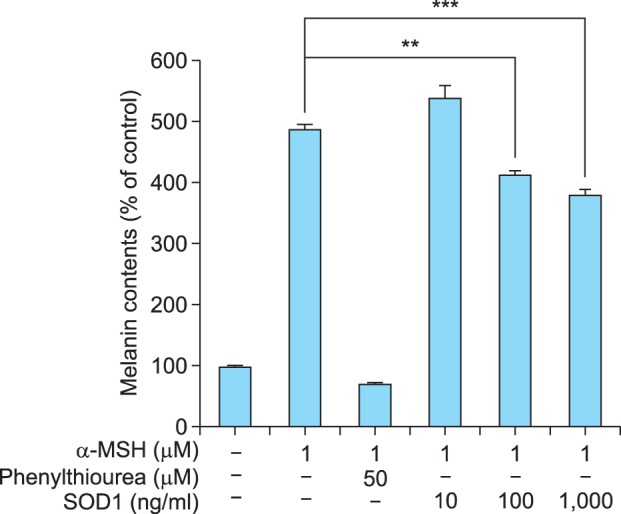
Effect of superoxide dismutase 1 (SOD1) on melanin content in B16F10 melanoma cells. SOD1 was tested at 10, 100, and 1,000 ng/ml in B16F10 melanoma cells. B16F10 melanoma cells were co-cultured with alpha-melanocyte stimulating hormone (α-MSH) (1 µm) and SOD1 at 10 to 1,000 ng/ml for 72 hours, and compared with untreated and α-MSH-stimulated control cells. Levels of inhibition of melanogenesis are expressed as percentages of the control. The results are averages of three independent experiments and the data are expressed as mean±standard deviation. Inhibition of melanin content was related to exposure to SOD1 in a dose-dependent manner. **p<0.05, ***p<0.001 as compared with α-MSH-treated controls.
Inhibitory effect of superoxide dismutase 1 on ultraviolet B-induced melanogenesis in HRM-2 melanin-possessing hairless mice
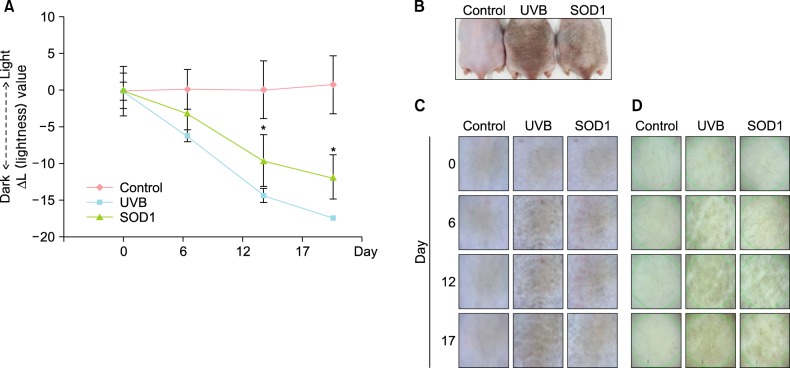
The effect of SOD1 on skin pigmentation was examined in vivo in HRM-2 melanin-possessing hairless mice treated according to the schedule shown in Table 1. Treatment of animals with SOD1 dissolved in vehicle did not cause any noticeable skin irritation. Exposure of mice to UVB led to decreased ΔL (lightness) values, which represented a pigmentation effect. The UVB-induced changes in ΔL (lightness) values were significantly reversed in SOD1-treated animals, and the color properties of the skin of SOD1-treated animals were comparable to those of the control groups (Fig. 4). The pigmentation pattern was photographed using a Dermoscope (DermLite Pro, California, USA) and a Folliscope (LeedM, Seoul, Korea) (Fig. 4). In addition, SOD1 was found to have a protective effect on UVB-induced epidermal and dermal changes (Fig. 5). To visualize the melanin, the skin samples were stained with Fontana-Masson stain and examined. As shown in Fig. 6, the amount of melanin granules was significantly increased by UVB irradiation (arrows in Fig. 6B). Topical application of SOD1 inhibited the UVB-induced increase in melanin generation (Fig. 6C).
Fig. 4.
Effect of superoxide dismutase 1 (SOD1) on the level of ultraviolet B (UVB)-induced melanogenesis in HRM-2 melanin-possessing hairless mice. HRM-2 melanin-possessing hairless mice were pretreated with vehicle or SOD1 on a designated site on the dorsal skin and then UVB-irradiated according to the indicated schedule. The picture represents the dorsal skin of the HRM-2 melanin-possessing hairless mice after treatment. For statistical analysis, ΔL (lightness) values of each animal were calculated as the average values after UVB exposure (day 17) minus the average baseline values before any treatment (day 0). Values represent the mean±standard deviation of five experiments. For statistical analysis, results of one-way ANOVA were used: *p<0.05, as compared with UVB-treated controls. (A) ΔL (lightness) values. (B) D3200. (C) Dermoscope. (D) Folliscope.
Fig. 5.
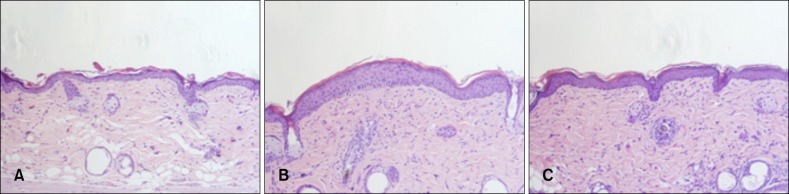
Effect of superoxide dismutase 1 (SOD1) on ultraviolet B (UVB) irradiation-induced histopathological changes in skin tissue. Change in histopathological features of the skin after repeated treatment with SOD1. Representative histopathological images of UVB-irradiated skin lesions stained by H&E (×200). (A) Control. (B) UVB 190 mJ/cm2. (C) 1,000 ng/ml SOD1+UVB 190 mJ/cm2.
Fig. 6.
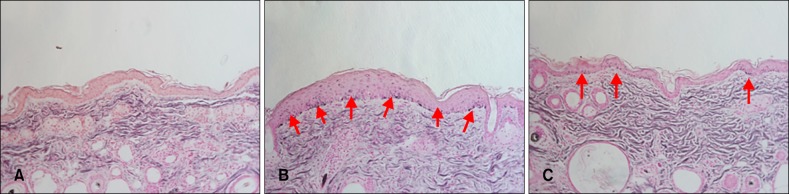
Effect of superoxide dismutase 1 (SOD1) on ultraviolet B (UVB) irradiation-induced hyperpigmentation changes in skin tissue. Change in histopathological features of the skin after repeated treatment with SOD1. Representative histopathological images of UVB-induced skin pigmentation stained by Fontana and Masson (×200). Melanin pigments (arrows) are stained black. (A) Control. (B) UVB 190 mJ/cm2. (C) 1,000 ng/ml SOD1+UVB 190 mJ/cm2.
DISCUSSION
Exposure of skin to UV irradiation generates reactive oxygen species that damage DNA, membranes, mitochondria, and proteins. To protect against such damage, skin cells have evolved antioxidant enzymes, including SOD1, glutathione peroxidase, mitochondrial manganese-dependent superoxide dismutase (SOD2), and catalase. It has been reported that SOD1 is significantly suppressed by a single dose of UVB irradiation26, whereas it is induced by repeated exposure to UVB in response to chronic photooxidative stress27. The UVB-mediated melanogenic response of melanocytes and melanoma cells is complex and remains unclear. While melanin is believed to exert photoprotective effects on human skin under physiological conditions, overstimulation of melanin production could be harmful and could possibly contribute to melanoma development; the biological implication of melanogenesis should thus be taken into account28. Since excess melanin under UVB-induced oxidative stress may become pro-oxidant and carcinogenic, blocking the aggravation of melanogenesis might therefore serve as a potential target for the prevention of hyperpigmentation and melanoma development induced by UVB29.
Hyperpigmentation is characterized by an abnormal increase in melanin in the skin30,31. Recently, much effort has been focused on understanding the mechanisms underlying melanogenesis in order to develop new therapeutic agents against skin pigmentation abnormalities. Tyrosinase is a key enzyme in melanogenesis and plays a regulatory role in the production of melanin32. In general, assays for mushroom tyrosinase have been applied to screening for putative depigmenting agents by assessing their abilities to inhibit tyrosinase-mediated L-DOPA oxidation33. SOD1 did not directly inhibit tyrosinase activity, indicating its involvement in alternative mechanisms (Fig. 2). These results suggest that reduced pigmentation by SOD1 might be attributed to the effect of SOD1 upon the signaling pathways regulating tyrosinase.
Melanin pigments produced in human melanocytes are classified into two categories: black-colored eumelanin and reddish-yellow pheomelanin. Stimulation of melanocytes with α-MSH, one of several melanogenic factors, has been reported to enhance eumelanogenesis to a greater degree than pheomelanogenesis, which contributes to hyperpigmentation. This study demonstrates the potent inhibitory effect of SOD1 on melanogenesis in α-MSH-induced B16F10 melanoma cells (Fig. 3). The inhibitory effects of SOD1 were dose-dependent and did not incur significant cytotoxicity (Fig. 1).
This study is valuable in its discovery that SOD1 exhibited consistent antimelanogenic activity in both in vitro and in vivo models. We used a murine skin model, since it is difficult to test the antimelanogenic effects of novel compounds in vivo in humans. The HRM-2 melanin-possessing hairless mouse, which was used in our study, was generated from a cross between HR-1 hairless mice, which have an albino phenotype, and the C3H/He strain produced by the Hoshino laboratory in 2007. The HRM-2 melanin-possessing hairless mouse is a widely used animal research model that can be used for the measurement of skin color, wrinkle formation, and damage, without requiring hair removal in long-term experiments.
In conclusion, our data showed that SOD1 suppressed α-MSH-induced melanogenesis. We propose that SOD1 prevents abnormal hyperpigmentation induced by UVB and may be a promising sunscreen agent for preventing esthetic problems related to hyperpigmentation.
ACKNOWLEDGMENT
This research was financially supported by the Ministry of Knowledge Economy (MKE), Korea Institute for Advancement of Technology (KIAT) through the Inter-ER Cooperation Projects.
References
- 1.Nole G, Johnson AW. An analysis of cumulative lifetime solar ultraviolet radiation exposure and the benefits of daily sun protection. Dermatol Ther. 2004;17(Suppl 1):57–62. doi: 10.1111/j.1396-0296.2004.04s1007.x. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez Maglio DH, Paz ML, Ferrari A, Weill FS, Czerniczyniec A, Leoni J, et al. Skin damage and mitochondrial dysfunction after acute ultraviolet B irradiation: relationship with nitric oxide production. Photodermatol Photoimmunol Photomed. 2005;21:311–317. doi: 10.1111/j.1600-0781.2005.00185.x. [DOI] [PubMed] [Google Scholar]
- 3.Matsumura Y, Ananthaswamy HN. Toxic effects of ultraviolet radiation on the skin. Toxicol Appl Pharmacol. 2004;195:298–308. doi: 10.1016/j.taap.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 4.Gloster HM, Jr, Brodland DG. The epidemiology of skin cancer. Dermatol Surg. 1996;22:217–226. doi: 10.1111/j.1524-4725.1996.tb00312.x. [DOI] [PubMed] [Google Scholar]
- 5.Fisher MS, Kripke ML. Suppressor T lymphocytes control the development of primary skin cancers in ultraviolet-irradiated mice. Science. 1982;216:1133–1134. doi: 10.1126/science.6210958. [DOI] [PubMed] [Google Scholar]
- 6.Ullrich SE. Mechanisms underlying UV-induced immune suppression. Mutat Res. 2005;571:185–205. doi: 10.1016/j.mrfmmm.2004.06.059. [DOI] [PubMed] [Google Scholar]
- 7.Mørch CD, Gazerani P, Nielsen TA, Arendt-Nielsen L. The UVB cutaneous inflammatory pain model: a reproducibility study in healthy volunteers. Int J Physiol Pathophysiol Pharmacol. 2013;5:203–215. [PMC free article] [PubMed] [Google Scholar]
- 8.Friedmann PS, Gilchrest BA. Ultraviolet radiation directly induces pigment production by cultured human melanocytes. J Cell Physiol. 1987;133:88–94. doi: 10.1002/jcp.1041330111. [DOI] [PubMed] [Google Scholar]
- 9.Gilchrest BA. A review of skin ageing and its medical therapy. Br J Dermatol. 1996;135:867–875. doi: 10.1046/j.1365-2133.1996.d01-1088.x. [DOI] [PubMed] [Google Scholar]
- 10.Kadekaro AL, Kavanagh RJ, Wakamatsu K, Ito S, Pipitone MA, Abdel-Malek ZA. Cutaneous photobiology. The melanocyte vs. the sun: who will win the final round? Pigment Cell Res. 2003;16:434–447. doi: 10.1034/j.1600-0749.2003.00088.x. [DOI] [PubMed] [Google Scholar]
- 11.Meredith P, Sarna T. The physical and chemical properties of eumelanin. Pigment Cell Res. 2006;19:572–594. doi: 10.1111/j.1600-0749.2006.00345.x. [DOI] [PubMed] [Google Scholar]
- 12.Hill HZ, Hill GJ. UVA, pheomelanin and the carcinogenesis of melanoma. Pigment Cell Res. 2000;13(Suppl 8):140–144. doi: 10.1034/j.1600-0749.13.s8.25.x. [DOI] [PubMed] [Google Scholar]
- 13.Rouzaud F, Kadekaro AL, Abdel-Malek ZA, Hearing VJ. MC1R and the response of melanocytes to ultraviolet radiation. Mutat Res. 2005;571:133–152. doi: 10.1016/j.mrfmmm.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 14.Yamaguchi Y, Takahashi K, Zmudzka BZ, Kornhauser A, Miller SA, Tadokoro T, et al. Human skin responses to UV radiation: pigment in the upper epidermis protects against DNA damage in the lower epidermis and facilitates apoptosis. FASEB J. 2006;20:1486–1488. doi: 10.1096/fj.06-5725fje. [DOI] [PubMed] [Google Scholar]
- 15.Miyamura Y, Coelho SG, Wolber R, Miller SA, Wakamatsu K, Zmudzka BZ, et al. Regulation of human skin pigmentation and responses to ultraviolet radiation. Pigment Cell Res. 2007;20:2–13. doi: 10.1111/j.1600-0749.2006.00358.x. [DOI] [PubMed] [Google Scholar]
- 16.Svobodová A, Zdarilová A, Malisková J, Mikulková H, Walterová D, Vostalová J. Attenuation of UVA-induced damage to human keratinocytes by silymarin. J Dermatol Sci. 2007;46:21–30. doi: 10.1016/j.jdermsci.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 17.Tobi SE, Gilbert M, Paul N, McMillan TJ. The green tea polyphenol, epigallocatechin-3-gallate, protects against the oxidative cellular and genotoxic damage of UVA radiation. Int J Cancer. 2002;102:439–444. doi: 10.1002/ijc.10730. [DOI] [PubMed] [Google Scholar]
- 18.Eller MS, Ostrom K, Gilchrest BA. DNA damage enhances melanogenesis. Proc Natl Acad Sci U S A. 1996;93:1087–1092. doi: 10.1073/pnas.93.3.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halaban R, Pomerantz SH, Marshall S, Lerner AB. Tyrosinase activity and abundance in Cloudman melanoma cells. Arch Biochem Biophys. 1984;230:383–387. doi: 10.1016/0003-9861(84)90121-8. [DOI] [PubMed] [Google Scholar]
- 20.Hunt G, Kyne S, Wakamatsu K, Ito S, Thody AJ. Nle4DPhe7 alpha-melanocyte-stimulating hormone increases the eumelanin: phaeomelanin ratio in cultured human melanocytes. J Invest Dermatol. 1995;104:83–85. doi: 10.1111/1523-1747.ep12613565. [DOI] [PubMed] [Google Scholar]
- 21.Wong G, Pawelek J. Melanocyte-stimulating hormone promotes activation of pre-existing tyrosinase molecules in Cloudman S91 melanoma cells. Nature. 1975;255:644–646. doi: 10.1038/255644a0. [DOI] [PubMed] [Google Scholar]
- 22.Matsuda H, Higashino M, Nakai Y, Iinuma M, Kubo M, Lang FA. Studies of cuticle drugs from natural sources. IV. Inhibitory effects of some Arctostaphylos plants on melanin biosynthesis. Biol Pharm Bull. 1996;19:153–156. doi: 10.1248/bpb.19.153. [DOI] [PubMed] [Google Scholar]
- 23.Tobin D, Thody AJ. The superoxide anion may mediate short- but not long-term effects of ultraviolet radiation on melanogenesis. Exp Dermatol. 1994;3:99–105. doi: 10.1111/j.1600-0625.1994.tb00266.x. [DOI] [PubMed] [Google Scholar]
- 24.Chung KW, Park YJ, Choi YJ, Park MH, Ha YM, Uehara Y, et al. Evaluation of in vitro and in vivo anti-melanogenic activity of a newly synthesized strong tyrosinase inhibitor (E)-3-(2,4 dihydroxybenzylidene)pyrrolidine-2,5-dione (3-DBP) Biochim Biophys Acta. 2012;1820:962–969. doi: 10.1016/j.bbagen.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 25.Song K, An SM, Kim M, Koh JS, Boo YC. Comparison of the antimelanogenic effects of p-coumaric acid and its methyl ester and their skin permeabilities. J Dermatol Sci. 2011;63:17–22. doi: 10.1016/j.jdermsci.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 26.Pence BC, Naylor MF. Effects of single-dose ultraviolet radiation on skin superoxide dismutase, catalase, and xanthine oxidase in hairless mice. J Invest Dermatol. 1990;95:213–216. doi: 10.1111/1523-1747.ep12478037. [DOI] [PubMed] [Google Scholar]
- 27.Okada K, Takahashi Y, Ohnishi K, Ishikawa O, Miyachi Y. Time-dependent effect of chronic UV irradiation on superoxide dismutase and catalase activity in hairless mice skin. J Dermatol Sci. 1994;8:183–186. doi: 10.1016/0923-1811(94)90052-3. [DOI] [PubMed] [Google Scholar]
- 28.Kvam E, Dahle J. Pigmented melanocytes are protected against ultraviolet-A-induced membrane damage. J Invest Dermatol. 2003;121:564–569. doi: 10.1046/j.1523-1747.2003.12418.x. [DOI] [PubMed] [Google Scholar]
- 29.Zbytek B, Carlson JA, Granese J, Ross J, Mihm MC, Jr, Slominski A. Current concepts of metastasis in melanoma. Expert Rev Dermatol. 2008;3:569–585. doi: 10.1586/17469872.3.5.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peng LH, Liu S, Xu SY, Chen L, Shan YH, Wei W, et al. Inhibitory effects of salidroside and paeonol on tyrosinase activity and melanin synthesis in mouse B16F10 melanoma cells and ultraviolet B-induced pigmentation in guinea pig skin. Phytomedicine. 2013;20:1082–1087. doi: 10.1016/j.phymed.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 31.Kumar KJ, Vani MG, Wang SY, Liao JW, Hsu LS, Yang HL, et al. in vitro and in vivo studies disclosed the depigmenting effects of gallic acid: a novel skin lightening agent for hyperpigmentary skin diseases. Biofactors. 2013;39:259–270. doi: 10.1002/biof.1064. [DOI] [PubMed] [Google Scholar]
- 32.Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 33.Lei TC, Virador VM, Vieira WD, Hearing VJ. A melanocytekeratinocyte coculture model to assess regulators of pigmentation in vitro. Anal Biochem. 2002;305:260–268. doi: 10.1006/abio.2002.5665. [DOI] [PubMed] [Google Scholar]