Abstract
Background
Alopecia areata (AA) is a common dermatologic condition with a broad spectrum of clinical features and age of onset, classically characterized by nonscarring patches of hair loss. In the past, early-onset (before adolescence) AA has been associated with various autoimmune diseases, especially atopic diseases and lupus erythematosus and demonstrates a worse prognosis compared with late onset AA.
Objective
To evaluate the differences in the comorbidity profile of AA with regard to age at onset.
Methods
We completed a retrospective study of 871 Korean AA patients seen at our department within the last 10 years. After these patients were subdivided according to onset before or after age 13 years, the two groups were compared on the basis of their comorbid disorders, family history of AA, and hematologic test results.
Results
Our results demonstrate that significantly more patients in the early-onset group had a personal history of atopic dermatitis or family history of AA. These findings are consistent with previous reports associating early-onset AA with autoimmune diseases and a family history of AA in different ethnic populations. Most of the serologic test values showed no significant differences between the groups and the results were considerably affected by age.
Conclusion
This study is significant because it is a large group study in Korean AA patients, and Korean AA patients with an onset age before adolescence show similar clinical manifestations to other ethnic populations.
Keywords: Alopecia areata, Autoimmune diseases, Comorbidity, Onset age
INTRODUCTION
Alopecia areata (AA) is a common inflammatory disease characterized by a sudden-onset, nonscarring hair loss that has a significant cosmetic impact on patients1. The prevalence of AA has been reported as approximately between 0.1% and 0.2%, and may occur at any age2. As AA has a widely variable clinical course, it is difficult for clinicians to predict the prognosis and clinical outcome of any individual AA patient. Previously, some reports have suggested that early-onset (before adolescence) AA has a poorer prognosis than middle age onset AA, which has been associated with a more favorable outcome3,4,5. Although variations in the onset age of AA lead to distinct clinical consequences, the underlying mechanisms remain unknown. To date, many epidemiologic studies have found an association between AA and multiple atopic diseases-specifically asthma, atopic dermatitis (AD), and allergic rhinitis-as well as various autoimmune diseases, particularly thyroid disease and vitiligo 6,7,8,9,10. Other case reports also suggest an association between AA and psoriasis and lupus erythematosus (LE)6,11. Notably, one prior epidemiologic study demonstrated that AA cases with an age of onset before 10 years are more frequently associated with atopic diseases and LE, indicating a need for screening for these diseases in this age group12. According to this study, the different onset age of AA reflects the specific atopic or autoimmune diseases, and clinicians should consider these comorbid disorders and treat these diseases in a timely manner. While its underlying pathogenesis has not been fully characterized, AA may represent an organ-specific autoimmune disease resulting from a genetic predisposition and certain environmental triggers2,12. However, the relation between the age of onset of AA and the resulting clinical presentation remains unclear. Therefore, the purpose of this study is to characterize the differences in clinical manifestations of early-onset and late-onset AA patients by means of a chart review.
MATERIALS AND METHODS
Patients
In total, 871 patients with AA were enrolled in this study between 2003 and 2012 from the Department of Dermatology at Yonsei University Wonju Severance Christian Hospital. In all cases, a diagnosis of AA was reached through clinical examination by a group of experienced dermatologists. All medical records were reviewed retrospectively. The subjects were subdivided into two groups specifically, an early-onset group and a late-onset groupon the basis of whether the age of AA onset was before or after the subject's 13th birthday. The age of the patients at the time of visit to our department was not considered in the grouping of patients. The patients presented variable subtypes and severities of AA, and the subtypes were not considered in subdividing them into groups.
Clinical profiles
The following clinical characteristics were recorded for all patients: age at AA onset, family history of AA (including parents, grandparents, and siblings); history of hypertension, diabetes, hypercholesterolemia, thyroid disease, and AD. All serologies (performed on the initial clinic visit) were also recorded, including all hematologic tests, thyroid function tests (TFTs), routine chemistry panel, lipid profile, and antinuclear antibody (ANA) test.
Statistical methods
For all data analysis, a statistics analysis package (SPSS for Windows, version 18.0; SPSS Inc., Chicago, IL, USA) was used. For continuous data (i.e., serologies), the format mean±standard deviation was used. For all comparisons between groups, Student's t-test was used. For all comparisons of non-continuous data (i.e., medical history data and serologic abnormalities), the Pearson χ2 test was used. To estimate the magnitude of atopic disease and the probability of abnormal laboratory findings, we calculated the odds ratio (OR) by using logistic regression. In all cases, p-values <0.05 were considered statistically significant.
RESULTS
Demographics
Of the 871 AA patients, 98 patients (11.3%) were in the early-onset group, whereas 773 patients (88.7%) were in the late-onset group. No significant difference in sex was observed between the early-onset and late-onset groups (Table 1).
Table 1.
Patient demographics and family history of AA
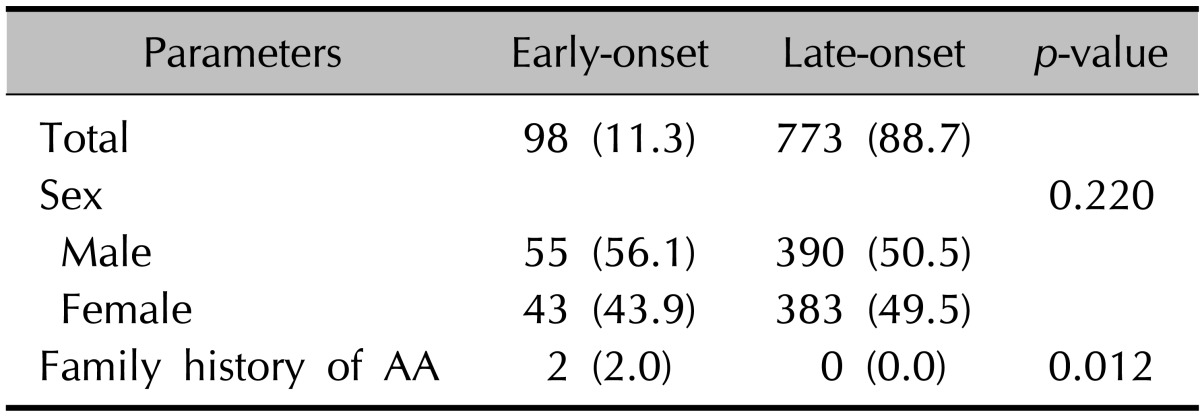
Values are presented as number (%). AA: alopecia areata.
Family history of alopecia areata
When compared with the late-onset group (0%, p=0.00), significantly more patients (2.0%) in the early-onset group had a positive family history of AA (Table 1).
Comorbid disorders
We assessed the patients' medical histories and compared the comorbid disorders of the early-onset and late-onset groups. AD was observed to be the most common comorbid disorder in the early-onset group, whereas thyroid disease including hyperthyroidism, hypothyroidism, goiter, and thyroiditis, was the most common in the late-onset group. Significantly more patients in the late-onset group had a medical history of hypertension, diabetes and thyroid disease (p=0.014, 0.047, and 0.000, respectively). Conversely, AD was significantly more common in the early-onset group (p=0.008) (Table 2). By calculating the OR of AD, the early-onset group tended to have a significantly higher chance of associated AD (OR, 2.795; 95% confidence interval [CI], 1.27~6.13).
Table 2.
Comorbid disorders
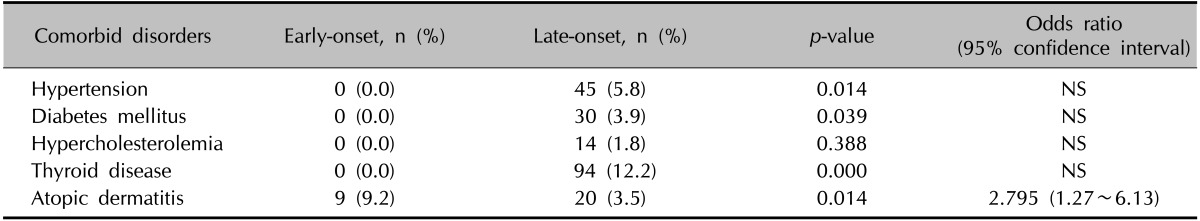
NS: non-statistically significant odds ratio.
Serum test results
To compare the other clinical manifestations of early-onset and late-onset AA patients, we compared the average levels of serum parameters that were measured on the first day of the patients' visit to the clinic. Most of the values showed no significant differences. The average hemoglobin, hematocrit, creatinine (Cr), aspartate aminotransferase, triglyceride (TG), and cholesterol were all significantly greater in the late-onset group than in the early-onset group (Table 3); however, notably, the mean levels from all the above tests fell into the reference ranges for both groups. No significant differences were observed in TFTs. The probability of abnormal serum test results was then compared between groups. Specifically, an abnormal test result was defined as low hematologic test results, abnormal TFT results, elevated blood urea nitrogen, elevated Cr, elevated glucose, elevated liver enzymes, elevated lipid panel, and/or positive ANA with s titer >1:80, as per the laboratory reference values. Both age groups showed no significant differences of probabilities of having abnormal laboratory results except for lipid profiles. The late-onset group showed a significantly greater number of patients with high TG or cholesterol according to reference values. By calculating the OR, the late-onset group showed a higher chance of having abnormally high lipid profile levels (Table 4). However, the OR calculated after adjusting for age showed no significance, which implies that these results are considerably influenced by age. Although there was no significance, the early-onset group had a higher probability of having a positive ANA test than the late-onset group.
Table 3.
Serologic test results
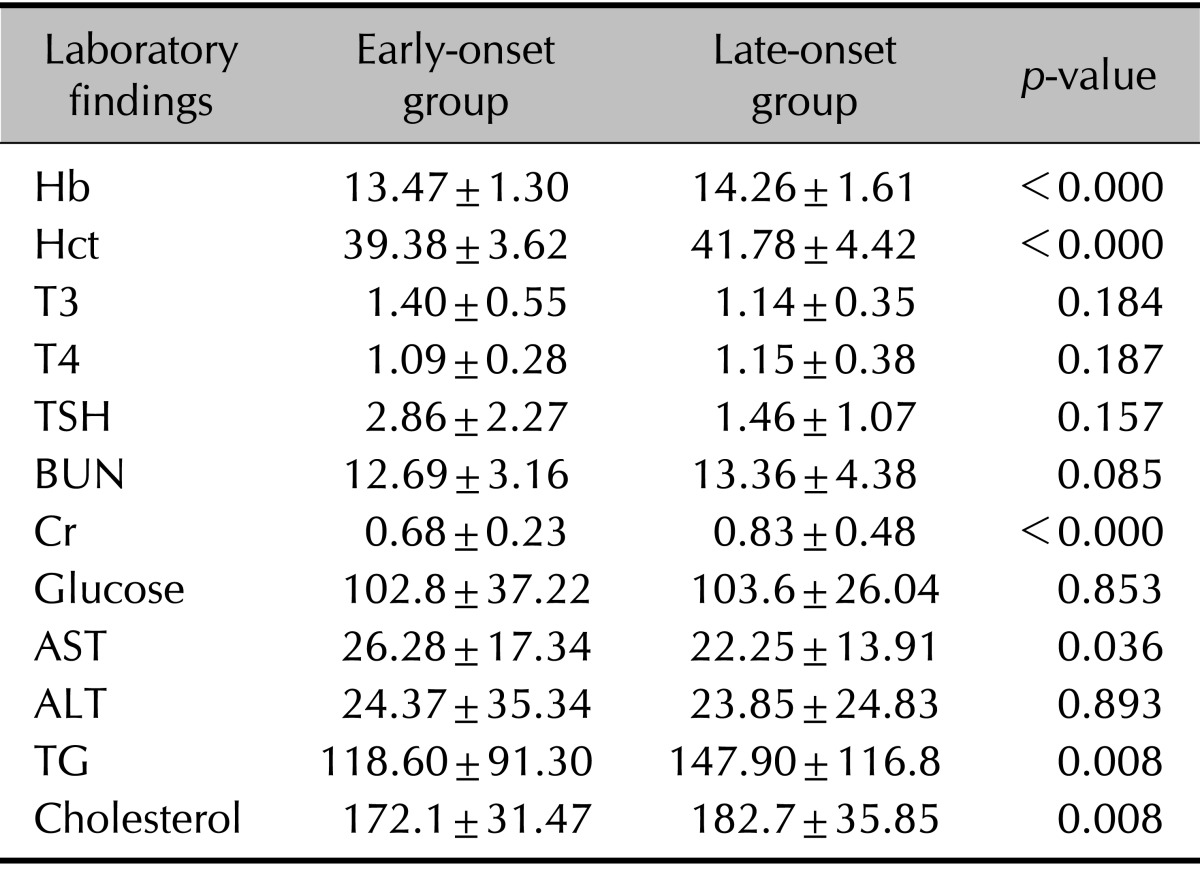
Values are presented as mean±standard deviation. Hb: hemoglobin, Hct: hematocrit, T3: triiodothyronine, T4: thyroxine, TSH: thyroid-stimulating hormone, BUN: blood urea nitrogen, Cr: creatinine, AST: aspartate aminotransferase, ALT: alanine aminotransferase, TG: triglyceride.
Table 4.
Probability of abnormal laboratory findings
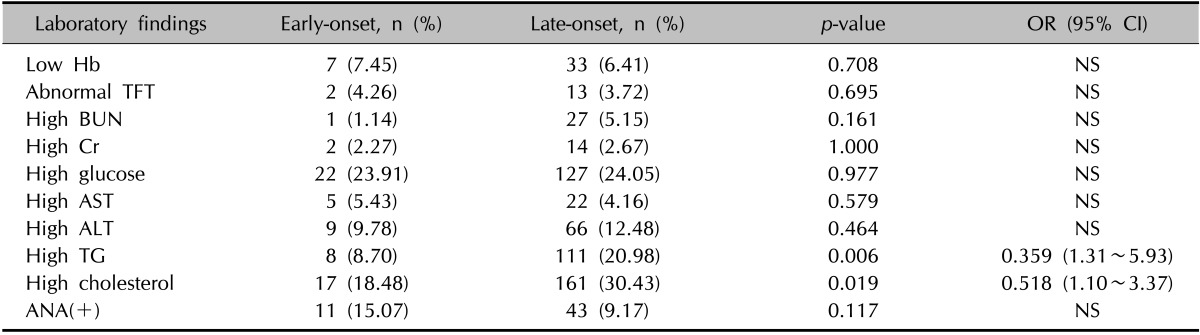
OR: odds ratio, CI: confidence interval, Hb: hemoglobin, TFT: thyroid function test, BUN: blood urea nitrogen, Cr: creatinine, AST: aspartate aminotransferase, ALT: alanine aminotransferase, TG: triglyceride, ANA: antinuclear antibody, NS: non-statistically significant odds ratio.
DISCUSSION
AA is a common disease with a broad spectrum of clinical features and a variable age of onset. The lifetime risk of AA has been estimated to be about 1.7%, with childhood onset occurring in 20% to 50% of AA patients13,14,15. Previously, AA has been linked to other autoimmune diseases, primarily atopic disease, thyroid disease, and vitiligo8,13,16,17,18,19,20. Moreover, some previous reports suggest that early-onset AA has a stronger association with a positive family history, a worse prognosis, and an increased risk of other concomitant medical disorders, particularly atopic diseases and LE4,10,12. A recent epidemiologic study about the difference between adult and pediatric AA21, claimed that there was no statistically significant difference in the frequencies of autoimmune and atopic disease. Therefore, this issue remains controversial. Little is known about the relation between the age of onset and clinical presentation of AA, particularly in Asian populations. To characterize whether any clinical differences exist between early- and late-onset AA, we defined early-onset as occurring before age 13 years, as this age roughly represents the beginning of puberty4,22.
As evident in our results, the late-onset group had a significantly higher prevalence of comorbid disorders, including hypertension, diabetes, and thyroid disease, although these results may be skewed by the higher average age of the late-onset group, and OR was not significant. On the other hand, the early-onset group had a significantly higher prevalence of AD (OR, 2.795; 95% CI 1.27~6.13) and positive family history of AA, results that are consistent with previous reports18,23 in other ethnic populations. We sub-divided the patients into the early-onset and late-onset groups according to the onset age obtained from medical history taking in the patients. However, most of the patients recalled that their condition appeared not long before the visit to the clinic, and therefore, in most cases, the onset age was similar to the age at the time of clinic visit.
Serologic testing was also used as an endpoint in this study. However, although the mean levels of certain serologic tests varied significantly between groups, these data could not be interpreted as all average levels were within the laboratory ranges. The probability of having an abnormal laboratory finding varied significantly between the two groups in lipid profiles. However, these results were proved to be influenced by the older age of the late-onset group. Although no significance was found, more patients in the early-onset group showed positive ANA test, with titer >1 : 80 compared with late-onset group. This implies that in further studies, history of autoimmune diseases and laboratory autoimmune tests must be carefully checked in early-onset AA patients to search for a higher prevalence of autoimmune diseases.
Although the pathogenesis of AA is not yet fully understood, the prevalence of comorbid atopic and autoimmune diseases suggests that an immune mechanism is somehow essential to the etiology24,25. Previous reports indicate that both Th1 and Th2 cytokines participate in AA pathogenesis7. Furthermore, although Th2 cytokines usually outweigh Th1 in relevance in atopic diseases, Th1 also plays an important role in the later stages of AD26. Accordingly, a similar biphasic Th pattern may explain the increased prevalence of AD in AA patients7. The higher frequency of AD in the early-onset AA group in our study may lend weight to the idea that AA and atopy share a Th2 cytokine pattern7. On the other hand, the difference in the extent of AA between the early-onset and late-onset groups may have contributed to this result. As a history of AD and hypothyroidism is significantly associated with alopecia totalis or alopecia universalis7,16, and early-onset AA is known to have a tendency to be more severe and less favourable prognosis27, the early-onset AA patients in our study may have had more severe subtypes of AA, including alopecia totalis and universalis.
This study has several notable limitations. First, because of both the retrospective nature of the study and the fact that all family history data were obtained from patients' statements, the prevalence of comorbid disorders and family history may have been underestimated. Second, as all serologic tests were performed at the time of the initial visit, the results may be biased by the patients' age. As mentioned above, the onset age did not differ considerably from the age at the time of visit; thus, the age of the patients may have had a significant influence on the comparison of comorbidity profiles between the two groups. Finally, owing to the retrospective design of the study, we could not determine if further evaluations for abnormal serum test results, for example, evaluation for thyroid function abnormality, anemia, or autoimmune disease, were done subsequently. If appropriate follow-up was done, it would have been much more helpful in clarifying the clinical manifestations of the two groups. For a further, more comprehensive evaluation of clinical manifestations among early-onset AA patients, a prospective study comparing a large group of AA patients with healthy controls is clearly needed.
In summary, we have done a large group study on Korean AA patients, and compared the comorbidity profiles of the early-onset and late-onset groups. The early-onset group (before age 13 years) had significantly more patients with AD as a comorbid disorder and with a family history of AA, which is consistent with the previous reports on different ethnic populations. Although this study presents limited knowledge that has been previously reported, it is significant in that it is an epidemiologic study done on a large group of Korean AA patients, comparing two groups differentiated by onset age. Moreover, early-onset AA patients show similar clinical characteristics to other ethnic populations.
References
- 1.Safavi K. Prevalence of alopecia areata in the First National Health and Nutrition Examination Survey. Arch Dermatol. 1992;128:702. doi: 10.1001/archderm.1992.01680150136027. [DOI] [PubMed] [Google Scholar]
- 2.Xiao FL, Yang S, Yan KL, Cui Y, Liang YH, Zhou FS, et al. Association of HLA class I alleles with aloplecia areata in Chinese Hans. J Dermatol Sci. 2006;41:109–119. doi: 10.1016/j.jdermsci.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 3.Rebora A. Acute diffuse and total alopecia of the female scalp: a new subtype of diffuse alopecia areata that has a favorable prognosis--a reply. Dermatology. 2003;207:339. doi: 10.1159/000073108. author reply 340. [DOI] [PubMed] [Google Scholar]
- 4.Cho HH, Jo SJ, Paik SH, Jeon HC, Kim KH, Eun HC, et al. Clinical characteristics and prognostic factors in early-onset alopecia totalis and alopecia universalis. J Korean Med Sci. 2012;27:799–802. doi: 10.3346/jkms.2012.27.7.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiao FL, Yang S, Liu JB, He PP, Yang J, Cui Y, et al. The epidemiology of childhood alopecia areata in China: a study of 226 patients. Pediatr Dermatol. 2006;23:13–18. doi: 10.1111/j.1525-1470.2006.00161.x. [DOI] [PubMed] [Google Scholar]
- 6.Hordinsky M, Ericson M. Autoimmunity: alopecia areata. J Investig Dermatol Symp Proc. 2004;9:73–78. doi: 10.1111/j.1087-0024.2004.00835.x. [DOI] [PubMed] [Google Scholar]
- 7.Barahmani N, Schabath MB, Duvic M National Alopecia Areata Registry. History of atopy or autoimmunity increases risk of alopecia areata. J Am Acad Dermatol. 2009;61:581–591. doi: 10.1016/j.jaad.2009.04.031. [DOI] [PubMed] [Google Scholar]
- 8.Tan E, Tay YK, Goh CL, Chin Giam Y. The pattern and profile of alopecia areata in Singapore--a study of 219 Asians. Int J Dermatol. 2002;41:748–753. doi: 10.1046/j.1365-4362.2002.01357.x. [DOI] [PubMed] [Google Scholar]
- 9.Kakourou T, Karachristou K, Chrousos G. A case series of alopecia areata in children: impact of personal and family history of stress and autoimmunity. J Eur Acad Dermatol Venereol. 2007;21:356–359. doi: 10.1111/j.1468-3083.2006.01931.x. [DOI] [PubMed] [Google Scholar]
- 10.Chu SY, Chen YJ, Tseng WC, Lin MW, Chen TJ, Hwang CY, et al. Comorbidity profiles among patients with alopecia areata: the importance of onset age, a nationwide population-based study. J Am Acad Dermatol. 2011;65:949–956. doi: 10.1016/j.jaad.2010.08.032. [DOI] [PubMed] [Google Scholar]
- 11.Barber HW. Case of vitiligo, with (formerly) alopecia areata and lupus erythematosus. Proc R Soc Med. 1925;18:51. [PMC free article] [PubMed] [Google Scholar]
- 12.Alkhalifah A, Alsantali A, Wang E, McElwee KJ, Shapiro J. Alopecia areata update: part I. Clinical picture, histopathology, and pathogenesis. J Am Acad Dermatol. 2010;62:177–188. doi: 10.1016/j.jaad.2009.10.032. quiz 189-190. [DOI] [PubMed] [Google Scholar]
- 13.Sharma VK, Kumar B, Dawn G. A clinical study of childhood alopecia areata in Chandigarh, India. Pediatr Dermatol. 1996;13:372–377. doi: 10.1111/j.1525-1470.1996.tb00703.x. [DOI] [PubMed] [Google Scholar]
- 14.Sharma VK, Dawn G, Kumar B. Profile of alopecia areata in Northern India. Int J Dermatol. 1996;35:22–27. doi: 10.1111/j.1365-4362.1996.tb01610.x. [DOI] [PubMed] [Google Scholar]
- 15.Muller SA, Winkelmann RK. Alopecia areata. An evaluation of 736 patients. Arch Dermatol. 1963;88:290–297. doi: 10.1001/archderm.1963.01590210048007. [DOI] [PubMed] [Google Scholar]
- 16.Goh C, Finkel M, Christos PJ, Sinha AA. Profile of 513 patients with alopecia areata: associations of disease subtypes with atopy, autoimmune disease and positive family history. J Eur Acad Dermatol Venereol. 2006;20:1055–1060. doi: 10.1111/j.1468-3083.2006.01676.x. [DOI] [PubMed] [Google Scholar]
- 17.Shellow WV, Edwards JE, Koo JY. Profile of alopecia areata: a questionnaire analysis of patient and family. Int J Dermatol. 1992;31:186–189. doi: 10.1111/j.1365-4362.1992.tb03932.x. [DOI] [PubMed] [Google Scholar]
- 18.Tan E, Tay YK, Giam YC. A clinical study of childhood alopecia areata in Singapore. Pediatr Dermatol. 2002;19:298–301. doi: 10.1046/j.1525-1470.2002.00088.x. [DOI] [PubMed] [Google Scholar]
- 19.Cunliffe WJ, Hall R, Stevenson CJ, Weightman D. Alopecia areata, thyroid disease and autoimmunity. Br J Dermatol. 1969;81:877–881. doi: 10.1111/j.1365-2133.1969.tb15967.x. [DOI] [PubMed] [Google Scholar]
- 20.Ahmed I, Nasreen S, Bhatti R. Alopecia areata in children. J Coll Physicians Surg Pak. 2007;17:587–590. doi: 10.2007/JCPSP.587590. [DOI] [PubMed] [Google Scholar]
- 21.Serarslan G, Savaş N, Yenin JZ. Is atopy and autoimmunity more prevalent in patients with alopecia areata? A comparative study. J Eur Acad Dermatol Venereol. 2012;26:720–723. doi: 10.1111/j.1468-3083.2011.04152.x. [DOI] [PubMed] [Google Scholar]
- 22.Al Shobaili HA. The impact of childhood atopic dermatitis on the patients' family. Pediatr Dermatol. 2010;27:618–623. doi: 10.1111/j.1525-1470.2010.01215.x. [DOI] [PubMed] [Google Scholar]
- 23.Tosti A, Bellavista S, Iorizzo M. Alopecia areata: a long term follow-up study of 191 patients. J Am Acad Dermatol. 2006;55:438–441. doi: 10.1016/j.jaad.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 24.Bertolini M, Gilhar A, Paus R. Alopecia areata as a model for T cell-dependent autoimmune diseases. Exp Dermatol. 2012;21:477–479. doi: 10.1111/j.1600-0625.2011.01427.x. [DOI] [PubMed] [Google Scholar]
- 25.Alexis AF, Dudda-Subramanya R, Sinha AA. Alopecia areata: autoimmune basis of hair loss. Eur J Dermatol. 2004;14:364–370. [PubMed] [Google Scholar]
- 26.Ker J, Hartert TV. The atopic march: what's the evidence? Ann Allergy Asthma Immunol. 2009;103:282–289. doi: 10.1016/S1081-1206(10)60526-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Waard-van der Spek FB, Oranje AP, De Raeymaecker DM, Peereboom-Wynia JD. Juvenile versus maturity-onset alopecia areata--a comparative retrospective clinical study. Clin Exp Dermatol. 1989;14:429–433. doi: 10.1111/j.1365-2230.1989.tb02604.x. [DOI] [PubMed] [Google Scholar]


