Abstract
We report five cases of pattern alopecia in female patients who are undergoing hormonal anticancer therapy for the prevention of recurrence of breast cancer after surgery. Three patients demonstrated male pattern alopecia with receding frontal hairlines, and two patients demonstrated female pattern alopecia without receding hairlines. The detailed clinical history showed that the pattern alopecia of the patients developed after the full recovery of global hair loss of the entire scalp due to previous cytotoxic chemotherapy. All of the adjuvant hormonal anticancer drugs that were used in the patients are antiestrogenic agents, either aromatase inhibitors or selective estrogen receptor modulators. Considering androgen effect on the hair follicles of the fronto-parietal scalp, the androgen-estrogen imbalance caused by the drugs was thought to be the reason for the onset of pattern alopecia in the patients. In general, alopecia that develops during cytotoxic chemotherapy is well known to both physicians and patients; however, the diagnosis of pattern alopecia during hormonal anticancer therapy in breast cancer patients seems to be overlooked.
Keywords: Androgenetic alopecia, Aromatase inhibitors, Breast cancer, Estrogens, Selective estrogen receptor modulators
INTRODUCTION
Chemotherapy-induced alopecia involving the whole scalp is well documented in cancer patients in general, including those with breast cancer; however, pattern alopecia seen during the course of breast cancer treatment is rare in the medical literature. To date, only a few case reports of pattern alopecia associated with antiestrogen treatment have been published in the internal medicine and oncology literature1,2,3,4,5.
We report herein five cases of pattern alopecia that developed in breast cancer patients who had undergone surgical treatment followed by adjuvant hormonal anticancer therapy.
CASE REPORT
Case 1
A 51-year-old postmenopausal woman presented with frontal hair thinning occurring during 3 years. Four years before her presentation, she underwent modified radical mastectomy, chemotherapy (doxorubicin and cyclophosphamide), and radiotherapy (cumulative dose, 5,040 cGy) for her breast cancer. While receiving the chemotherapy, she showed total hair loss on the scalp, suggestive of anagen effluvium, from which she fully recovered after several months. To prevent the recurrence of the cancer, further hormonal anticancer therapy with selective estrogen receptor modulators (SERMs) (toremifene citrate, Fareston; Prostraka Inc., Somervillle, NJ, USA) was subsequently initiated. Two to three months later, she developed hair thinning limited to the frontal and parietal scalp. Dermatological examination showed typical male pattern alopecia with moderate fronto-parietal hair thinning and recession of the frontal hairline (Fig. 1). The hairs of the temporal and occipital scalp, as well as other body hairs, were normal. She had a family history of androgenetic alopecia on both paternal and maternal sides. She was treated with 3% minoxidil, 0.025% alfatradiol, and 0.025% tretinoin solution twice daily. Spironolactone (200 mg/d) and finasteride (1 mg/d) were added to the regimen at 1 month and 3 months after the initial treatment, respectively. After 4 months of follow-up, improvement in hair density and hair diameter in the fronto-parietal scalp was observed.
Fig. 1.
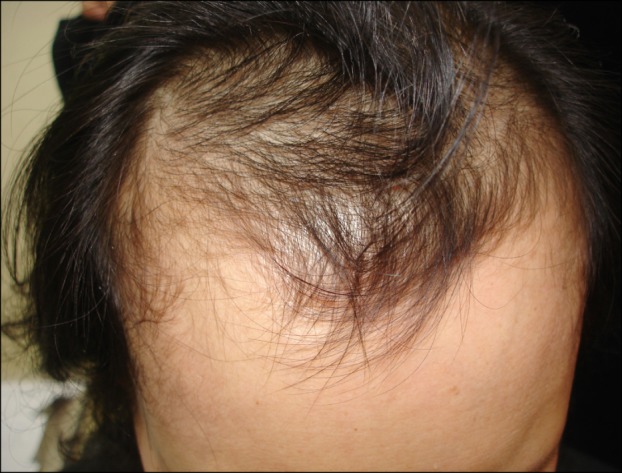
Clinical features of case 1 mimicking male pattern alopecia with recession of the anterior hairline.
Case 2
A 33-year-old premenopausal woman presented with a 1-year history of fronto-parietal hair thinning. Four years before presentation, she had undergone total mastectomy and chemotherapy with doxorubicin, cyclophosphamide, docetaxel, and tegafur-uracil. While receiving the chemotherapy, she showed total hair loss on the scalp and body, from which she fully recovered after several months. From 1 year before the presentation, she had undergone hormonal anticancer therapy with SERMs (tamoxifen citrate, Nolvadex; AstraZeneca Pharmaceuticals LP, Wilmington, DE, USA). One month later, she noted hair thinning on the top of her scalp. There was no family history of alopecia. Dermatological examination showed decreased hair density and hair diameter in the frontal and parietal scalp. She was treated with 3% minoxidil, 0.025% alfatradiol, and 0.025% tretinoin solution twice daily for the alopecia; however, she refused to continue the treatment.
Case 3
A 51-year-old postmenopausal woman presented with a 6-month history of fronto-parietal hair thinning. Three years before presentation, she had undergone wide local excision and axillary lymph node dissection, chemotherapy (doxorubicin, cyclophosphamide), and radiotherapy (cumulative dose, 6,000 cGy) for breast cancer. Additionally, after finishing all those treatments, she took aromatase inhibitors (AIs) (anastrozole, Arimidex; AstraZeneca Pharmaceuticals LP) for 9 months before her visit to our clinic. The hair loss that developed initially was recovered but the fronto-parietal hair thinning persisted. She had a family history of androgenetic alopecia on the paternal side. On dermatological examination, she had frontal recession with decreased hair density and hair diameter limited to the frontal and parietal scalp. She was subsequently treated with 3% minoxidil and 0.025% alfatradiol solution twice daily. After 6 months, a slight improvement in hair density and hair diameter in the frontal and parietal scalp was observed.
Case 4
A 50-year-old postmenopausal woman visited our dermatology clinic complaining of hair thinning on the fronto-parietal scalp occurring during the 10 months before her visit. Two years before presentation, she had undergone modified radical mastectomy, chemotherapy (doxorubicin, cyclophosphamide, and docetaxel), and radiotherapy (cumulative dose, 5,040 cGy) for breast cancer. Then, AIs (anastrozole, Arimidex) were subsequently administered to prevent the recurrence of the cancer. She had a family history of androgenetic alopecia on the paternal side. Dermatological examination revealed female pattern alopecia showing diffuse hair thinning limited to the fronto-parietal scalp with a preserved frontal hairline (Fig. 2). She was prescribed with 3% minoxidil and 0.025% alfatradiol solution twice daily. However, she wanted treatment only for her primary concern (i.e., breast cancer), and discontinued the dermatological care.
Fig. 2.
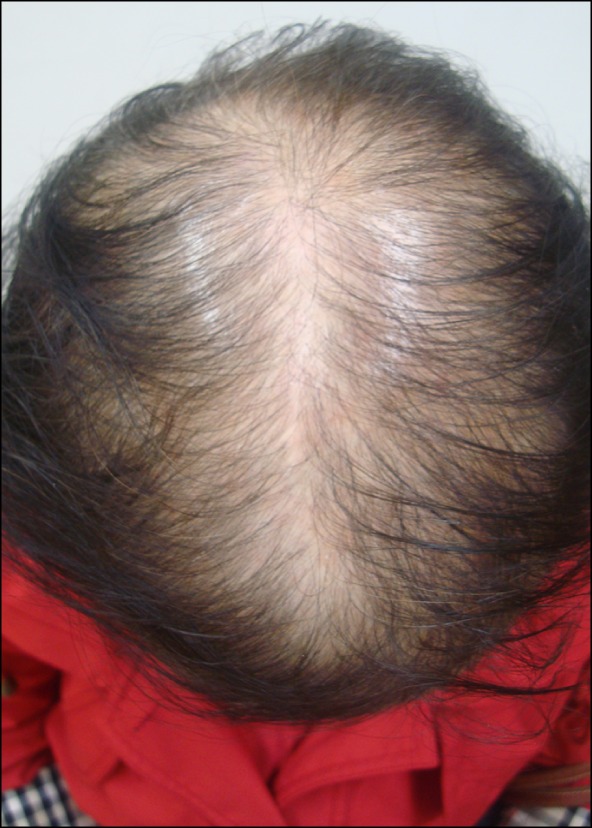
Clinical features of case 4 showing typical advanced female pattern hair loss with fronto-parietal thinning and preservation of the frontal hairline.
Case 5
A 50-year-old postmenopausal woman presented with a 1-year history of scalp hair loss, particularly of the frontal and parietal scalp. Six years before presentation, she had undergone breast cancer treatment that was similar to that of case 4. To prevent recurrence, SERMs (toremifene citrate, Fareston; tamoxifen citrate, Nolvadex) were administered for up to 1 year before her visit to our clinic. Switching from SERMs, she was subsequently given AIs (letrozole, Femara; Novartis Pharmaceuticals Corp., East Hanover, NJ, USA). Two months after those medications, she developed hair thinning limited to the parietal scalp without recession of the frontal hairline. She reported no family history of hair disorders. She was treated with 5% minoxidil and 0.025% alfatradiol solution twice daily, and slight improvement in hair thickness was observed after 3 months of follow-up.
DISCUSSION
To prevent the recurrence of breast cancer, adjuvant hormonal (antiestrogen) therapy is widely used after the initial surgery and primary cytotoxic chemotherapy. Antiestrogen therapy was introduced because of the role of estrogen in breast cancer (i.e., its carcinogenesis effect)6. Antiestrogen therapy reduces the risk of recurrence of breast cancer by lowering the estrogen level6. The antiestrogen drugs are largely divided into three groups; SERMs, AIs, and estrogen receptor downregulators. At present, SERMs and AIs are commonly used. Tosti and Pazzaglia7 reported that SERMs and AIs are related to hair thinning, discoloration, and androgenetic alopecia. In the study by Simpson et al.8, 3% and 5% of breast cancer patients who took SERMs (tamoxifen) and AIs (letrozole), respectively, exhibited hair loss.
In this report, we presented five cases of pattern alopecia in women with breast cancer who were treated with SERMs or nonsteroidal AIs (Table 1). The alopecia developed after the recovery of hair loss of the entire scalp from the previous cytotoxic chemotherapy in three patients. In other words, the scalp hairs were normal just before the treatment with antiestrogenic drugs. Two other patients also showed nonrecovery of alopecia limited on the fronto-parietal area after the previous global hair loss. Although three of the five patients had a family history of pattern alopecia, it would be difficult to ascribe their pattern alopecia to family predisposition. It was apparent that, chronologically, the pattern alopecia developed because of the treatment with antiestrogens.
Table 1.
Summary of five cases of pattern alopecia following anti-estrogen therapy in patients with breast cancer
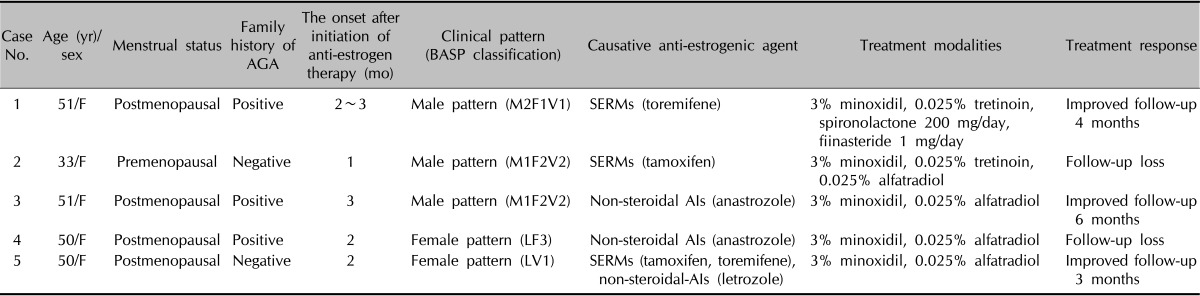
AGA: androgenetic alopecia, BASP classification: basic and specific classification, F: female, SERMs: selective estrogen receptor modulators, AIs: aromatase inhibitors.
The plausible mechanism of pattern alopecia in those cases is thought to be estrogen-androgen imbalance. It has been reported that estrogen-androgen imbalance, relative and absolute hyperandrogenism, is associated with female pattern hair loss and hirsutism9. Also, the androgenic effect on hair follicular cells is the key factor in the pathogenesis of male and female pattern alopecia, and even in women, hairline recession can occur when the androgen level is high. In the present five cases, considering the three cases that showed a receding hairline, which is rare in female pattern alopecia, the estrogen-androgen imbalance or relative hyperandrogenism caused by the drugs seem to be considerably high. However, there were no biochemical signs of androgen excess in any of the five patients.
There is no specific therapy for drug-associated pattern alopecia other than the discontinuation of the causal agent. If discontinuation of the offending agent is impossible, topical minoxidil5 and alfatradiol can be used, as in the treatment of general pattern hair loss. Alfatradiol (synthetic 17α-estradiol), which lowers dihydrotestosterone levels and stimulates aromatase10, is theoretically more effective than minoxidil for pattern alopecia caused by antiestrogenic drugs. Oral antiandrogens (spironolactone) and 5α-reductase inhibitors are not generally recommended for breast cancer patients because breast lumps, tenderness, and, rarely, breast cancer have been reported in patients taking these drugs, although there is no evidence suggesting a definite association between antiandrogens and breast cancer11,12,13. Wearing a wig to disguise hair loss could also be a cosmetic option during long periods of antiestrogen therapy1,2. Our patients were treated with topical 3% minoxidil and 0.025% alfatradiol, and three of the five patients showed clinical improvement.
The incidence of the pattern alopecia in breast cancer patients is not known. Considering the prevalence of breast cancer and the common use of antiestrogen therapy in these patients, the incidence of pattern alopecia during antiestrogen therapy is likely to be higher than that reported in the literature. The most important reason for the rarity of this case is that patients are not seeing dermatologists and their attending physicians are not interested in the diagnosis of pattern alopecia. The attending physicians may be not be concerned about hair loss and do not make referrals for a dermatological consultation, which could then lead the cancer patients under treatment to take hair loss for granted. However, we found that the pattern hair loss of those patients is a chronic, long-standing condition because antiestrogen drugs are prescribed usually longer than several years, and the patients themselves are unhappy about not having any information about disease course and solution of the hair loss.
ACKNOWLEDGMENT
This paper was supported by the Fund of Biomedical Research Institute, Chonbuk National University Hospital.
References
- 1.Ayoub JP, Valero V, Hortobagyi GN. Tamoxifen-induced female androgenetic alopecia in a patient with breast cancer. Ann Intern Med. 1997;126:745–746. doi: 10.7326/0003-4819-126-9-199705010-00033. [DOI] [PubMed] [Google Scholar]
- 2.Gateley CA, Bundred NJ. Alopecia and breast disease. BMJ. 1997;314:481. doi: 10.1136/bmj.314.7079.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Puglisi F, Aprile G, Sobrero A. Tamoxifen-induced total alopecia. Ann Intern Med. 2001;134:1154–1155. doi: 10.7326/0003-4819-134-12-200106190-00029. [DOI] [PubMed] [Google Scholar]
- 4.Evrenkaya TR, Atasoyu EM, Unver S, Tulbek MY. Tamoxifen-induced androgenetic alopecia in a dialysis patient with sclerosing encapsulating peritonitis (SEP) Indian J Nephrol. 2004;14:28–29. [Google Scholar]
- 5.Carlini P, Di Cosimo S, Ferretti G, Papaldo P, Fabi A, Ruggeri EM, et al. Alopecia in a premenopausal breast cancer woman treated with letrozole and triptorelin. Ann Oncol. 2003;14:1689–1690. doi: 10.1093/annonc/mdg444. [DOI] [PubMed] [Google Scholar]
- 6.Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354:270–282. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- 7.Tosti A, Pazzaglia M. Drug reactions affecting hair: diagnosis. Dermatol Clin. 2007;25:223–231. doi: 10.1016/j.det.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Simpson D, Curran MP, Perry CM. Letrozole: a review of its use in postmenopausal women with breast cancer. Drugs. 2004;64:1213–1230. doi: 10.2165/00003495-200464110-00006. [DOI] [PubMed] [Google Scholar]
- 9.Tulchinsky D, Chopra IJ. Estrogen-androgen imbalance in patients with hirsutism and amenorrhea. J Clin Endocrinol Metab. 1974;39:164–169. doi: 10.1210/jcem-39-1-164. [DOI] [PubMed] [Google Scholar]
- 10.Hoffmann R, Niiyama S, Huth A, Kissling S, Happle R. 17alpha-estradiol induces aromatase activity in intact human anagen hair follicles ex vivo. Exp Dermatol. 2002;11:376–380. doi: 10.1034/j.1600-0625.2002.110413.x. [DOI] [PubMed] [Google Scholar]
- 11.Shenoy NK, Prabhakar SM. Finasteride and male breast cancer: does the MHRA report show a link? J Cutan Aesthet Surg. 2010;3:102–105. doi: 10.4103/0974-2077.69022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loube SD, Quirk RA. Breast cancer associated with administration of spironolactone. Lancet. 1975;1:1428–1429. doi: 10.1016/s0140-6736(75)92645-8. [DOI] [PubMed] [Google Scholar]
- 13.Danielson DA, Jick H, Hunter JR, Stergachis A, Madsen S. Nonestrogenic drugs and breast cancer. Am J Epidemiol. 1982;116:329–332. doi: 10.1093/oxfordjournals.aje.a113416. [DOI] [PubMed] [Google Scholar]


